Abstract
Differences in the way human and mouse fibroblasts experience senescence in culture had long puzzled researchers. While senescence of human cells is mediated by telomere shortening, Parrinello et al. demonstrated that senescence of mouse cells is caused by extreme oxygen sensitivity. It was hypothesized that the striking difference in oxygen sensitivity between mouse and human cells explains their different rates of aging. To test if this hypothesis is broadly applicable, we cultured cells from 16 rodent species with diverse lifespans in 3% and 21% oxygen and compared their growth rates. Unexpectedly, fibroblasts derived from laboratory mouse strains were the only cells demonstrating extreme sensitivity to oxygen. Cells from hamster, muskrat, woodchuck, capybara, blind mole rat, paca, squirrel, beaver, naked mole rat and wild-caught mice were mildly sensitive to oxygen, while cells from rat, gerbil, deer mouse, chipmunk, guinea pig and chinchilla showed no difference in the growth rate between 3% and 21% oxygen. We conclude that, although the growth of primary fibroblasts is generally improved by maintaining cells in 3% oxygen, the extreme oxygen sensitivity is a peculiarity of laboratory mouse strains, possibly related to their very long telomeres, and fibroblast oxygen sensitivity does not directly correlate with species' lifespan.
Keywords: fibroblasts, oxygen, senescence, human, rodents
INTRODUCTION
Maximum lifespan varies greatly between mammalian species. Humans can live over 100 years, while mice, the most widely used research models, rarely live longer than 3 years, with most laboratory mice becoming ill by the age of two years. There are also multiple species of mammals with lifespans ranging from as short as 2 years in a shrew to 211 years in a bowhead whale [1]. Identifying physiological parameters that correlate with these drastic differences in lifespan can lead to insight into the molecular mechanisms of longevity.
Human and mouse primary fibroblasts are commonly used by biogerontologists to study cellular senescence. However, human and mouse cells behave very differently during routine culture [2]. Human fibroblasts undergo 50-70 population doublings and then enter an irreversible state of replicative senescence. Replicative senescence of human fibroblasts is triggered by telomere shortening, and these cells never spontaneously bypass senescence arrest. Mouse fibroblasts, in contrast, undergo only 10-15 population doublings and then enter a phase of slow growth, also referred to as senescence or crisis. However, after a few days, fast growing clones of mouse cells emerge and continue to proliferate. Mouse fibroblasts express telomerase and senescence in such cells is not related to telomere shortening. This difference between human and mouse cell behavior had caused a great deal of controversy in the field until Parrinello et al. [3] demonstrated that the slow proliferation of mouse fibroblast cultures results from a high sensitivity to atmospheric oxygen. Mouse cells grown in 3% oxygen proliferated faster and did not display the characteristic slow growth or senescence state as the same cells cultured in 21% oxygen. In addition, mouse cells in 21% oxygen accumulated more oxidative damage than human cells suggesting that the shorter-lived mice cannot efficiently counteract chronic oxidative stress.
Oxygen can be damaging to cells and tissues through the production of reactive oxygen species (ROS) during oxidative phosphorylation in the mitochondria. ROS react with and damage macromolecules such as enzymes, membrane lipids, and nucleic acids, thereby compromising the integrity of the cell. Early studies with cultured cells already noted that high oxygen levels slow down cell proliferation [4-6]. It was suggested that gradual accumulation of macromolecular damage due to ROS is the causal factor in aging [7, 8]. Studies of long-lived mutants with dampened insulin/IGF-1 signaling, such as C. elegans [9-12] and mice [13, 14], suggest that there is a correlation between resistance to oxidative stress and longevity. The finding that mouse cells are strikingly more sensitive to oxidative stress than human cells led to the hypothesis that the ability to prevent and/or repair oxidative damage contributes to the dramatic difference in the rate of aging between humans and mice [3].
To further investigate the possible correlation between oxygen sensitivity and aging, we compared the growth rate of primary fibroblasts under atmospheric (21%) and low oxygen (3%), conditions for 16 different rodent species whose maximum lifespans ranged from 4 to 32 years. In agreement with previous reports, fibroblasts derived from laboratory mice were highly sensitive to oxygen. However, fibroblasts from 9 other species of rodents and fibroblasts from wild-caught mice proliferated only slightly slower in atmospheric oxygen, while fibroblasts from 6 other species proliferated with the same rate in 3% and 21% oxygen. Hence, there was no correlation between species lifespan and oxygen sensitivity. These surprising results suggest that high oxygen sensitivity is a peculiarity of cells from laboratory mice, which possibly arose when wild mice were selectively bred to produce the common inbred laboratory strains.
RESULTS AND DISCUSSION
To test whether sensitivity of fibroblasts to atmospheric oxygen correlates with lifespan, we cultured fibroblasts from 16 rodent species with lifespans ranging from 4 to 32 years in 3% and 21% oxygen. The list of species and their maximum lifespans are shown in Table 1. Primary fibroblasts were isolated from either skin or lung at 3% oxygen and frozen at PD<5. Only cells at low PD were used for the experiments to avoid selection of mutated clones. For each species, at least three cell lines, including both lung and skin fibroblasts, from three individual animals were examined. Lung and skin fibroblasts from the same species responded similarly to oxygen, therefore we pooled the results for lung and skin cells. Each cell line was taken out of liquid nitrogen storage, passaged once at 3% oxygen and then split into 3% and 21% oxygen conditions. Next, we compared the rate of growth under these conditions. We chose to compare the rate of growth rather than appearance of “senescence”, because the transient slowing of proliferation observed in mouse cells in 21% oxygen is highly variable among different cell lines.
Table 1. Maximum lifespan and body mass of the rodent species used in the study.
| Common name | Latin name | Maximum lifespan1, yr | Body mass1, g | Oxygen sensitivity1(3%O2 / 21%O2) |
|---|---|---|---|---|
| American beaver | Castor canadensis | 24 | 20,250 | 1.30 ± 0.4 |
| Blind mole rat | Nannospalax ehrenbergi | 21 | 160 | 1.42 ± 0.05 |
| Capybara | Hydrochaeris hydrochaeris | 15 | 55,000 | 2.23 ± 0.46 |
| Chinchilla | Chinchilla lanigera | 17 | 640 | 0.81 ± 0.18 |
| Deer mouse | Peromyscus maniculatus | 8 | 20 | 0.98 ± 0.13 |
| E. chipmunk | Tamias striatus | 10 | 100 | 1.0 ± 0.09 |
| E. grey squirrel | Sciurus carolinensis | 24 | 530 | 2.23 ± 0.32 |
| Golden hamster | Mesocricetus auratus | 4 | 100 | 2.11 ± 0.31 |
| Guinea pig | Cavia porcellus | 12 | 730 | 1.15 ± 0.14 |
| House mouse (laboratory) | Mus musculus | 4 | 30 | 4.45 ± 0.52 |
| House mouse (wild) | Mus musculus | 4 | 30 | 1.63 ± 0.11 |
| Mongolian gerbil | Meriones unguiculatus | 6 | 50 | 1.05 ± 0.06 |
| Muskrat | Ondatra zibethicus | 10 | 1,360 | 1.42 ± 0.27 |
| Naked mole rat | Heterocephalus glaber | 32 | 35 | 2.02 ± 0.4 |
| Norway rat (laboratory) | Rattus norvegicus | 4 | 400 | 1.0 ± 0.08 |
| Norway rat (wild) | Rattus norvegicus | 4 | 400 | 1.02 ± 0.03 |
| Paca | Cuniculus paca | 16 | 9,000 | 1.52 ± 0.42 |
| Woodchuck | Marmota monax | 14 | 4,000 | 2.21 ± 0.32 |
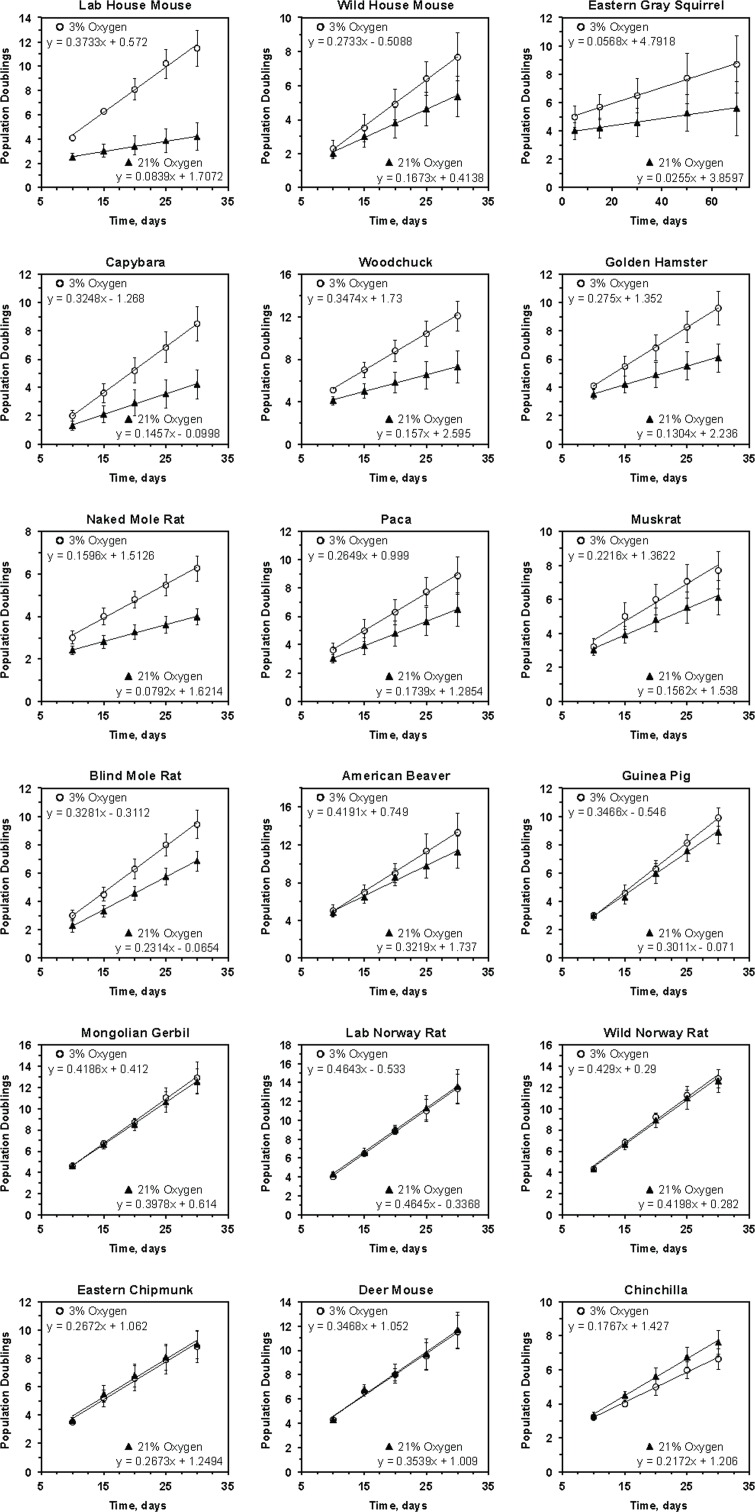
In agreement with previous reports [3], we observed that cells derived from laboratory mice proliferated much slower (4.5-fold) in 21% oxygen than in 3% oxygen. Interestingly, cells isolated from wild-caught mice were markedly less sensitive to culture in atmospheric oxygen (Figure 1). Although the wild mouse cells proliferated slower in atmospheric oxygen the difference in growth rate was only 1.6-fold.
Figure 1. Growth rate of primary rodent fibroblasts in 3% and 21% oxygen.
The cells were cultured in either 3% or 21% oxygen atmosphere. The regression lines were fitted for the initial phase of linear growth, before the cultures became clonal. For each species the data is an average of at least 3 cultures, including both lung and skin fibroblasts, isolated from separate individuals, and error bars show s.d.
Cells from 9 species, including squirrel, capybara, woodchuck, hamster, naked mole rat, paca, muskrat, blind mole rat, and beaver, showed mild sensitivity to atmospheric oxygen, proliferating between 1.3- and 2-fold faster in 3% oxygen than in 21% oxygen. Cells from 6 species, including guinea pig, gerbil, rat, chipmunk, deer mouse, and chinchilla, showed no difference in proliferation rates between high and low oxygen conditions.
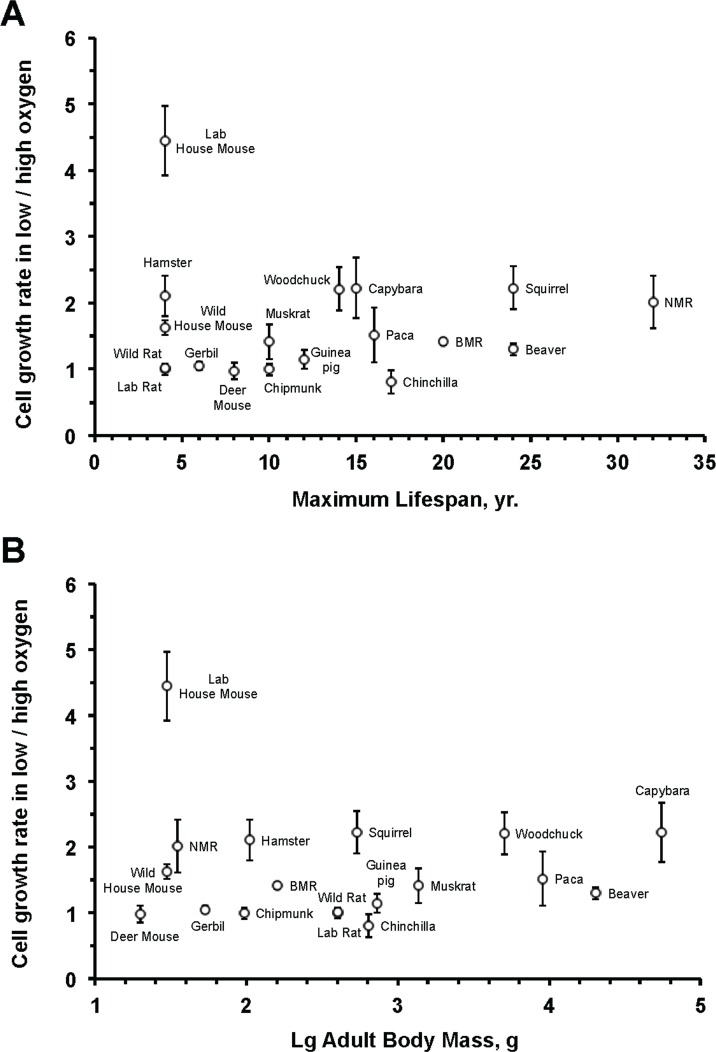
To test whether oxygen sensitivity correlates with species lifespan, we calculated the slope of the initial logarithmic segment of the cell growth curve for each species under high and low oxygen conditions. The ratio between cell proliferation rates in low versus high oxygen showed no significant correlation to species maximum lifespan (r2 = 0.0001; P = 0.97) (Figure 2A) or body mass (r2 = 0.006; P = 0.76) (Figure 2B). The correlation remained non-significant after an outlier (laboratory mouse) was excluded: oxygen sensitivity and maximum lifespan (r2 = 0.135; P = 0.15); oxygen sensitivity and body mass (r2 = 0.075; P = 0.29) (Figure 2B).
Figure 2. Fibroblast sensitivity to oxygen does not correlate with maximum lifespan (A) or body mass (B).
The sensitivity to oxygen environment is reflected by the ratio between the growth rate at 3% and 21% oxygen. The rate of cell proliferation in 3% or 21% oxygen is the slope of the regression line shown in Figure 1. No significant correlation was observed between oxygen sensitivity and maximum lifespan (r2 = 0.0001; P = 0.97) or oxygen sensitivity and body mass (r2 = 0.006; P = 0.76). The correlation remained non‐significant after an outlier (laboratory mouse) was excluded: oxygen sensitivity and maximum lifespan (r2 = 0.135; P = 0.15); oxygen sensitivity and body mass (r2 = 0.075; P = 0.29).
With the exception of the house mouse, species in the oxygen-sensitive group tend to be long-lived. Thus, the slower growth in atmospheric oxygen for these species is unlikely to result from deficiency in their ability to repair oxidative damage, and may reflect more stringent cell cycle checkpoints. Human fibroblasts, similarly, show mild sensitivity to oxygen and tend to grow faster in 3% oxygen and can reach higher PDs prior to entering telomere-mediated senescence. These results suggest that resistance to oxidative stress is not necessarily associated with longevity, rather the cells from long-lived species may be more sensitive leading to more efficient elimination of damaged cells. Indeed higher resistance of human lung fibroblasts to oxygen has been associated with a disease condition [15].
Three of the species in the oxygen-sensitive group, capybara, paca and beaver, have a body mass greater than 8,000 g, their fibroblasts do not express telomerase activity and eventually enter telomere-mediated senescence [16, 17]. Two other species sensitive to oxygen, the naked mole rat and the blind mole rat, are very long-lived [18, 19], cancer-resistant [20-23], subterranean rodents. Considering their cancer resistance, these species are likely to have stringent cell cycle checkpoints. Furthermore, due to their sub-terranean lifestyle, these species are exposed to ambient oxygen concentrations lower than 21%, which may explain some of the sensitivity.
The species in the oxygen non-sensitive group, such as rat, gerbil, and deer mouse, tend to be shorter-lived. These animals may be better equipped than house mouse for counteracting oxidative damage and lack the stringent cell cycle checkpoints, resulting in identical growth rates in high and low oxygen.
Cells from wild-caught house mice were more resistant to 21% oxygen than cells from two laboratory mouse strains, C57BL/6 and 129/SvJ. This peculiar finding may be explained by the loss or silencing of certain antioxidant defense mechanisms in laboratory mice as a result of artificial selection and accumulation of deleterious alleles. An alternative explanation may be related to the telomere biology unique to laboratory mice. It has been shown [24] that laboratory mouse strains evolve much longer telomeres (∼100 kb), while the wild mice have more modest telomeres ranging between 10-30 kb in length. It is tempting to speculate that the very long telomeres of laboratory mice serve as a sink for oxidative damage during in vitro culture and result in the senescent phenotype displayed by mouse fibroblasts. Indeed, telomeric sequences are sensitive to oxidative stress [25] and damage to DNA in these sequences is repaired more slowly than the rest of the genome [26]. Another argument in support of this hypothesis is that laboratory rats do not exhibit longer telomeres than those of wild rats, and laboratory and wild rats do not differ in their sensitivity to oxygen (Figure 1). In conclusion, based on the analysis of multiple rodent species, the sensitivity of primary fibroblasts to culture in atmospheric oxygen does not significantly correlate with species lifespan.
METHODS
Animal samples
Capybaras were obtained from Bio Fau Assesoria e Comercio (São Paulo, Brazil). Pacas were from the animal facility at São Paulo State University. Outbred multicolored guinea pigs were purchased from Elmhill Labs. Chinchillas were purchased from Molton Chinchilla Ranch. Naked mole rats and blind mole rats were from the University of Rochester colonies. Two laboratory mice were C57BL/6, and one mouse was 129SvJ strain. Three mice (Mus musculus) were caught in a dormitory and cafeteria in New York state. Two rats were BN, two rats were F344, and three rats were wild caught in New York state. Outbred Mongolian gerbils Crl:Mon(Tum) and outbred hamsters Crl:LVG(Syr) were purchased from Charles River Laboratories. Beavers, deer mice, muskrats, chipmunks, woodchucks and squirrels were wild-trapped in New York state. All animals used in this study were young adults. Exact age was known for laboratory animals and was estimated for wild-caught animals from body measurements and fur color. Live animals were euthanized according to the University of Rochester Animal Care and Use Committee guidelines. Care was taken to minimize pain and discomfort to the animals.
Tissue culture
Primary fibroblasts were isolated from lungs and under-arm skin as described [27]. The aliquots of cells were frozen in liquid nitrogen at PD<5. For the experiments, rodent fibroblasts were grown at 37°C and 3% CO2 in both high oxygen (21%) and low oxygen (3%) incubators on treated polystyrene culture dishes (Corning) using Eagle's Minimum Essential Medium (EMEM) supplemented with 15% FBS (Gibco) and 1% Penicillin-Streptomycin solution (Gibco). Cells were passaged regularly to avoid reaching confluency. During each passage, cells were counted using an automated cell counter and replated at 5×105 cells/plate for all rodent cells, except naked mole rat which were plated at 2×105 cells/plate.
PD calculations
Using the cell count recorded at the time of passage, the number of times the plated cell population doubled (population doublings or PD) before being passaged was determined. This was calculated each time the cells were split using the following equation:
In order to determine the number of times the original cell population (at the start of the experiment) had doubled at the time of each splitting, this PD was then added to the previous PD (PDinitial) using the following equation:
The final PD (PDfinal) was then graphed against the number of days the cells had been growing in order to quantify cell growth rate.
Footnotes
Funding
This work was supported by grants from US National Institutes of Health grant AG047200 and Life Extension Foundation to V. G. and A.S.
Conflict of interest statement
The authors have no conflict of interests to declare.
REFERENCES
- 1.de Magalhaes JP, Budovsky A, Lehmann G, Costa J, Li Y, Fraifeld V, Church GM. The Human Ageing Genomic Resources: online databases and tools for biogerontologists. Aging Cell. 2009;8:65–72. doi: 10.1111/j.1474-9726.2008.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright W, Shay J. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nature Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- 3.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper PD, Wilson JN, Burt AM. The bulk growth of animal cells in continuous suspension culture. Journal of general microbiology. 1959;21:702–720. doi: 10.1099/00221287-21-3-702. [DOI] [PubMed] [Google Scholar]
- 5.Cooper PD, Burt AM, Wilson JN. Critical effect of oxygen tension on rate of growth of animal cells in continuous suspended culture. Nature. 1958;182:1508–1509. doi: 10.1038/1821508b0. [DOI] [PubMed] [Google Scholar]
- 6.Balin AK, Goodman BP, Rasmussen H, Cristofalo VJ. The effect of oxygen tension on the growth and metabolism of WI-38 cells. J Cell Physiol. 1976;89:235–249. doi: 10.1002/jcp.1040890207. [DOI] [PubMed] [Google Scholar]
- 7.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 8.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 11.Ishii N, Goto S, Hartman PS. Protein oxidation during aging of the nematode Caenorhabditis elegans. Free radical biology & medicine. 2002;33:1021–1025. doi: 10.1016/s0891-5849(02)00857-2. [DOI] [PubMed] [Google Scholar]
- 12.Vanfleteren JR. Oxidative stress and ageing in Caenorhabditis elegans. Biochem J. 1993;292:605–608. doi: 10.1042/bj2920605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- 14.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 15.Yanai H, Shteinberg A, Porat Z, Budovsky A, Braiman A, Ziesche R, Fraifeld VE. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY) 2015;7:664–672. doi: 10.18632/aging.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seluanov A, Hine C, Bozzella M, Hall A, Sasahara TH, Ribeiro AA, Catania KC, Presgraves DC, Gorbunova V. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell. 2008;7:813–823. doi: 10.1111/j.1474-9726.2008.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seluanov A, Chen Z, Hine C, Sasahara TH, Ribeiro AA, Catania KC, Presgraves DC, Gorbunova V. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6:45–52. doi: 10.1111/j.1474-9726.2006.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 19.Edrey YH, Casper D, Huchon D, Mele J, Gelfond JA, Kristan DM, Nevo E, Buffenstein R. Sustained high levels of neuregulin-1 in the longest-lived rodents; a key determinant of rodent longevity. Aging Cell. 2012;11:213–222. doi: 10.1111/j.1474-9726.2011.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 22.Delaney MA, Nagy L, Kinsel MJ, Treuting PM. Spontaneous Histologic Lesions of the Adult Naked Mole Rat (Heterocephalus glaber): A Retrospective Survey of Lesions in a Zoo Population. Vet Pathol. 2013;50:607–621. doi: 10.1177/0300985812471543. [DOI] [PubMed] [Google Scholar]
- 23.Gorbunova V, Hine C, Tian X, Ablaeva J, Gudkov AV, Nevo E, Seluanov A. Cancer resistance in the blind mole rat is mediated by concerted necrotic cell death mechanism. Proc Natl Acad Sci U S A. 2012;109:19392–19396. doi: 10.1073/pnas.1217211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000;28:4474–4478. doi: 10.1093/nar/28.22.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 26.Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc Natl Acad Sci U S A. 1995;92:258–262. doi: 10.1073/pnas.92.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seluanov A, Vaidya A, Gorbunova V. Establishing primary adult fibroblast cultures from rodents. Journal of visualized experiments. 2010;44 doi: 10.3791/2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacutu R, Craig T, Budovsky A, Wuttke D, Lehmann G, Taranukha D, Costa J, Fraifeld VE, de Magalhaes JP. Human Ageing Genomic Resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 2013;41:D1027–1033. doi: 10.1093/nar/gks1155. [DOI] [PMC free article] [PubMed] [Google Scholar]