Abstract
Prolonged fetal exposure to opioids results in neonatal abstinence syndrome (NAS), a major medical problem requiring intensive care and increased hospitalization times for newborns with NAS. Multiple strategies are currently available to alleviate withdrawal in infants with NAS. To prevent NAS caused by opioid maintenance programs in pregnant women, blocking fetal dependence without compromising the mother’s opiate therapy is desirable. Here we tested in pregnant mice whether a peripherally selective opioid antagonist can preferentially enter the fetal brain and, thereby, in principle, selectively protect the fetus. We show using mass spectrometry that 6β-naltrexol, a neutral opioid antagonist with very limited ability to cross the blood-brain barrier (BBB), readily crosses the placental barrier and enters the fetal brain at high levels, although it is relatively excluded from the maternal brain. Furthermore, owing to the late development of the BBB in postnatal mice, we show that 6β-naltrexol can readily enter the juvenile mouse brain until at least postnatal day 14. Taking advantage of this observation, we show that long-term exposure to morphine starting in the second postnatal week causes robust and quantifiable dependence behaviors that are suppressed by concomitant administration of 6β-naltrexol with much greater potency (ID50 0.022–0.044 mg/kg, or 1/500 the applied dose of morphine) than previously demonstrated for either the suppression of central nervous system opioid effects or the induction of withdrawal in adults. These results indicate that peripherally selective opioid antagonists capable of penetrating the placenta may be beneficial for preventing or reducing neonatal dependence and NAS in a dose range that should not interfere with maternal opioid maintenance.
Introduction
Illicit drug use by pregnant women is a growing national concern, with an estimated prevalence of ∼16% in pregnant teens and ∼7% in women 18–25 years old (Patrick et al., 2012). The frequency of infants with opiate dependence at birth, evidenced as neonatal abstinence syndrome (NAS), has been estimated at 3.39 per 1000 births as of 2009 (Patrick et al., 2012). A large proportion of such births are from mothers engaged in managed methadone or buprenorphine treatment under the care of a physician (Jones et al., 2010; Patrick et al., 2012), providing a target group for the introduction of a prenatal therapy, could one be devised. Infants with NAS are often born prematurely and display a range of symptoms: underweight, breathing and feeding difficulties, irritability, feeding intolerance, emesis, and seizures (Dryden et al., 2009; Patrick et al., 2012). NAS presents a huge financial burden for society because of long intensive care retention times, and the likely effects on long-term cognitive, emotional, and social development of affected children. The consensus strategy for the treatment of newborns with NAS is oral methadone. Although stringent protocol-based treatment is a key factor in reducing length of hospital stay (Hall et al., 2014), national standards governing the therapeutic weaning strategy are lacking. A common goal of current strategies is to alleviate withdrawal only after the newborn has already become dependent, whereas antepartum strategies preventing the development of neonatal dependence in the first place are lacking. We test here the concept that NAS can be prevented with the use of opioid antagonists that are relatively excluded from the adult brain, but that pass through the placenta and readily enter the fetal brain because of an immature blood-brain barrier (BBB), thereby protecting the fetus from opioid agonist exposure, while, in principle, allowing uninterrupted opioid maintenance therapy of the mother.
Much effort has led to the development of effective opioid compounds that are peripherally selective [i.e., preferentially act on the peripheral nervous system compared with the central nervous system (CNS) in adults due to the BBB]. Peripheral agonists, such as loperamide and asimadoline, for example, have been developed for the treatment of diarrhea and irritable bowel syndrome, respectively (asimadoline: Mangel et al., 2008; Loperamide: Mainguet and Fiasse, 1977; Shannon and Lutz, 2002). Peripherally acting antagonists, on the other hand, such as methylnaltrexone (Yuan and Foss, 2000) and naloxegol, a pegylated form of naloxol (Chey et al., 2014), can block some of the peripheral side effects of centrally acting agonists. We have described a neutral antagonist of the μ-opioid receptor 6β-naltrexol, which can alleviate opiate-induced constipation in rodents and humans (Yancey-Wrona et al., 2009, 2011). This drug is orally available but has poor access to the CNS, presumably due to extrusion by the multidrug resistance protein MDR1 (also called P-glycoprotein transporter; ABCB1 gene; Xie et al., 1999). Furthermore, as a neutral opioid antagonist, 6β-naltrexol causes less severe withdrawal compared with traditional antagonists such as naloxone or naltrexone, which act as inverse agonists in the dependent state (Raehal et al., 2005).
We previously described the relative exclusion of 6β-naltrexol from the brain compared with naltrexone in adult mice (Wang et al., 2004), and the peripheral selectivity of 6b-naltrexol in humans (Yancey-Wrona et al., 2011). We note that 6β-naltrexol is a main metabolite of naltrexone in humans, whereas metabolic conversion is much reduced in mice. The intermediate polarity of 6β-naltrexol, enabling oral bioavailability, suggested that this compound could penetrate the placenta, which is less well protected by extrusion pumps compared with the BBB. Such compounds might be used to selectively target the fetal CNS compared with the maternal CNS because the fetal BBB is relatively undeveloped.
This concept of selective targeting of the fetal brain offers an opportunity for preventive pharmacotherapy of neonatal opioid dependence as part of a maternal opioid addiction management plan. Lack of interference with ongoing maternal addiction management is a critical aspect of any preventive approach with opioid antagonists, achieved by the use of peripherally selective antagonists with sufficient ability to penetrate cellular membranes. An additional therapeutic advantage may be gained with use of a neutral antagonist with reduced withdrawal effects (Wang et al., 2004; Raehal et al., 2005). Meeting all preferred characteristics, 6β-naltrexol, as a neutral antagonist and main metabolite of the Food and Drug Administration (FDA)-approved drug naltrexone, which has low withdrawal-inducing activity, represents a strong drug candidate for the treatment and prevention of NAS during pregnancy. Here, we tested whether 6β-naltrexol can cross the mouse placenta and compared drug levels in fetal versus adult brains after 6β-naltrexol administration to the pregnant mouse. We also used behavioral tests to measure the ability of 6β-naltrexol to prevent the development of morphine dependence in juvenile mice before the BBB is fully developed.
Materials and Methods
Animals.
Mice of the C57BL/6NTac strain were produced in our breeding colony at The Ohio State University. Animals were housed in microisolator racks with positive airflow and 24 h access to food and water. They were kept on a 12-h light/dark cycle. All procedures were approved by The Ohio State University Institutional Animal Care and Use Committee and are in compliance with guidelines established by the National Institutes of Health published in the Guide for the Care and Use of Laboratory Animals (http://oacu.od.nih.gov/regs/guide/guide.pdf).
Drug Dosing and Tissue Collection for Pharmacokinetic Analysis.
6β-Naltrexol (https://pubchem.ncbi.nlm.nih.gov/compound/5486554) and naltrexone (https://pubchem.ncbi.nlm.nih.gov/compound/5360515) were provided by the National Institute for Drug Addiction, as previously reported (Wang et al., 2004). Drugs were dissolved in saline solution at concentrations between 10 and 20 mg/ml, and all dilutions were made in saline solution. Morphine (West-Ward Pharmaceuticals, Eatontown, PA) was purchased from the Ohio State Medical Center pharmacy as a 15 mg/ml solution in saline solution. Animals were injected subcutaneously in the region around the right hindquarters. Injection volume typically did not exceed 150 μl in adults and in juveniles ranged from 50 to 100 μl, and the drug dose in most cases was 10 mg/kg. Embryos and maternal tissues were collected for the pharmacokinetic (PK) study, and additional nonpregnant adult females were used to expand the adult sample size. After injection and variable survival times, adult female and pregnant mice were euthanized by cervical dislocation, and brain, liver, and plasma were collected, quickly frozen on dry ice and stored at −70°C until processing. After euthanizing the dam, embryos were collected into a large (150 × 15 mm) petri dish and kept on ice while maternal tissues were processed. Embryonic tissues were then dissected and collected in a cold room at 4°C to facilitate and preserve tissue integrity during dissection, pooled, then stored at −70°C. Thus, each embryonic brain or liver tissue sample in this study was actually a pool of tissue dissected from approximately seven fetuses (littermates) from a single pregnant female. Each maternal or adult tissue sample was one brain or liver. Tissues were thawed, resected, weighed, and quickly frozen again on dry ice in individual microcentrifuge tubes. Samples were later thawed, processed, and analyzed via liquid chromatography-tandem mass spectrometry to quantify levels of the drug. Detailed methods for tissue processing and liquid chromatography-tandem mass spectrometry analysis are located in the Supplemental Information.
Analysis of Dependence Behaviors in Early Postnatal Mice.
We used a standard dose-response analysis to determine the potency and efficacy of 6β-naltrexol for inhibition of morphine-induced withdrawal behavior in juvenile mice. Pups were injected for 5 days (chronic dosing) with saline, morphine, or morphine mixed with one of five concentrations of 6β-naltrexol, and withdrawal was induced on day 6. Morphine alone and saline alone determine the upper and lower limits of the dose-response curve, respectively, and the effect of 6β-naltrexol is determined by holding the morphine dose constant across animals and varying the 6β-naltrexol dose. We used three subcutaneous injections per day switching between the left and right hindquarters. Injections were started on postnatal day 12 (P12) and continued for 5 days (in mice, weaning typically occurs at P21). Morphine was dosed in a two-step ramp (see below) that was invariant across all animals receiving morphine, the only variable being the combination dose of 6β-naltrexol (0, 1/3000, 1/1000, 1/200, 1/67, and 1/20 the dose of morphine). The ramping procedure was used to ensure a robust withdrawal response (Kest et al., 2002). Each 6β-naltrexol dose was ramped proportionally to maintain a constant ratio with morphine, and, for purposes of plotting the dose-response curve, the higher of the two 6β-naltrexol concentration ramps was used. In the text, however, we report 6β-naltrexol dosage values as a two-dose range or, alternatively, as a ratio relative to morphine.
The morphine ramp is defined as follows.
On days 1–3, morphine was injected at 10 mg/kg, and on days 4 and 5 at 20 mg/kg. On day 6 (P18), a final injection of 20 mg/kg was made, and 3 hours later mice were injected with 30 mg/kg naloxone to induce withdrawal (Kest et al., 2002). Immediately upon injection with naloxone, mice were placed in a clear plastic container with a 6-inch square base and 10 inches tall, with a lid. Animals were videotaped, and from these tapes a blinded observer scored withdrawal jumping, a well-described withdrawal behavior (Kest et al., 2002). Jumps were counted in a 15-minute interval. Time zero is the time the animal is placed in the container.
The above-treated pups were weighed prior to the start of morphine treatment and again on the day of jump testing (equal to a 6-day growth period). The weight gain of the animals receiving saline or morphine combined with various doses of 6β-naltrexol was compared with that of animals that received morphine alone.
Statistical Analysis.
Summary data are presented as the means ± 1 S.E.M., unless otherwise indicated. Continuous data (tissue drug concentrations) were analyzed using one-way ANOVA and post hoc comparisons were made using either Dunnett’s procedure (for one-to-many comparisons) or t tests (for all-pairs contrasts). In the latter case, P values were adjusted for multiple comparisons using the method of Hommel (1988). Data were log transformed to account for heteroscedasticity. When ratio values such as the brain/plasma ratio were compared, these were log transformed before analysis to account for the constrained structure of ratio variables (e.g., Heslop, 2009; Schilling et al., 2012). The dependence of jumping behavior on 6β-naltrexol levels was modeled using a four-parametric log-normal curve taking into account that the response was a count variable. Areas under the curve (AUCs) were integrated over the time span analyzed (i.e., from 0 to 240 minutes; Wolfsegger and Jaki, 2009). Confidence intervals (CIs) for AUCs were obtained by bootstrapping, as recommended in the study by Jaki et al. (2009). Time-to-event data were plotted as Kaplan-Meier curves and compared using log-ratio testing (Harrington and Fleming, 1982). All statistical procedures were implemented in R (http://www.R-project.org/), using the packages multcomp (Hothorn et al., 2008) for multiple comparisons, PK (Jaki and Wolfsegger, 2011) for PK analyses, drc (Ritz and Streibig, 2005) for dose-response fitting, and survival (Therneau and Grambsch, 2000) for time-to-event analysis.
Results
6β-Naltrexol and Naltrexone Levels in Embryonic and Adult Tissues.
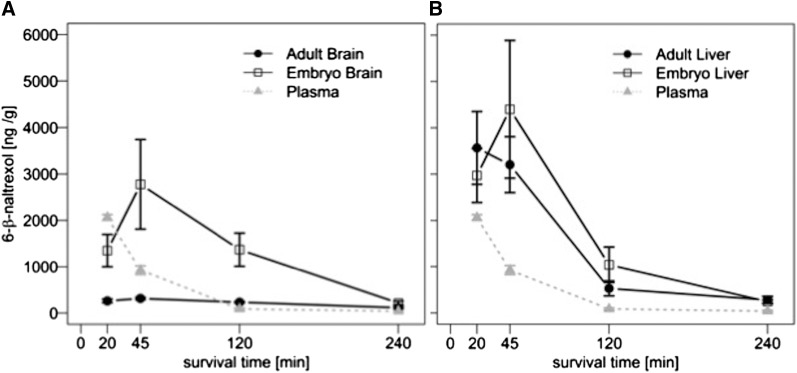
Previous studies using mass spectrometry showed that brain levels of 6β-naltrexol are ∼10-fold lower than plasma levels in adult mice 10 minutes after injection, whereas the levels of naltrexone, an FDA-approved opioid antagonist used to treat alcoholism, are roughly equivalent in plasma and brain (see Table 1 in Wang et al., 2004). This result indicates that 6β-naltrexol has limited access to the adult brain compared with naltrexone, which is due to the BBB. However, if 6β-naltrexol is able to pass through the placenta, it should readily enter the fetal brain because of an undeveloped BBB. To test this, we injected pregnant and nonpregnant adult female mice with 6β-naltrexol or naltrexone, and measured the drug levels in maternal and adult plasma, brain, and liver, and in embryonic brain and liver, using mass spectrometry. No difference was observed between pregnant and nonpregnant adult tissue levels (data not shown), and therefore we herein combine both groups of samples into a single group called “adult.” Four different survival times were examined postinjection. As shown in Fig. 1A, 6β-naltrexol levels are higher in embryonic brain than adult brain at 20, 45, and 120 minutes after injection, and drop to low residual levels at both ages after 4 hours. At peak, embryonic brain levels are approximately ninefold higher than in adult brain. To evaluate the statistical significance of this difference, we used a noncompartmental PK model. The AUC for embryonic brain was sixfold greater than that of adult brain (302 ± 60 × 103 ng/ml per minute with 95% CI of 146–1060 for embryonic brain versus 49 ± 2 × 103 with 95% CI of 44–54 for adult brain; significance was based on 95% CIs). In contrast, the AUC for adult versus embryonic liver was not significantly different (274 ± 36 × 103 ng/ml per minute with 95% CI of 208–372 for adult liver and 373 ± 84 with 95% CI of 46–1380 for embryonic liver; Fig. 1B). Moreover, 6β-naltrexol levels in adult and embryonic liver were not significantly different from embryonic brain levels (P > 0.05), supporting the finding that 6β-naltrexol diffuses unimpeded into fetal brain.
TABLE 1.
Comparison of single versus cumulative injections of 6β-naltrexol at 4-hour survival time point
One versus four injections of 10 mg/kg 6β-naltrexol with 4-hour survival time. Values are expressed as the mean ± S.E.M.
| 1 Injection | 4 Injections in 24 h | |
|---|---|---|
| Plasma | 40 ± 40 (n = 4) | 60 ± 20 (n = 2) |
| Adult brain | 110 ± 40 (n = 4) | 160 ± 20 (n = 2) |
| Embryo brain | 210 ± 60 (n = 2) | 290 ± 40 (n = 2) |
| Adult liver | 280 ± 160 (n = 4) | 360 ± 50 (n = 2) |
| Embryo liver | 250 ± 50 (n = 2) | 500 ± 190 (n = 2) |
Fig. 1.
6β-Naltrexol levels in embryonic and adult brain and liver at different survival times. (A) 6β-naltrexol levels in embryonic and adult brain at four different survival times after a single drug injection. (B) 6β-Naltrexol levels in embryonic and adult liver at four different survival times after drug injection. In (A) and (B), the curve for adult plasma is superimposed for comparison with solid tissues (note: units for plasma = ng/ml). The difference between embryonic and adult levels of drug was evaluated using a noncompartmental model (AUC; see Results) over all four survival time points (CIs were determined by the bootstrapping method; see Materials and Methods and Results). Secondarily, we note that the differences in brain were mathematically significant at the individual 20-, 45-, and 120-minute survival times using t tests (20 minutes, P < 0.05; 45 minutes, P < 0.01; 120 minutes, P < 0.01). n = 2 embryo brain pools (each independent embryonic sample consists of pooled brains from one litter; see Materials and Methods) and 3 adult brains at 20-minute survival, 3 embryo brain pools and 7 adult brains at 45 minutes, 2 embryo brain pools and 4 adult brains at 120 minutes, and 2 embryo brain pools and 4 adult brains at 240 minutes. n = the same for liver. Thus, in sum the AUC analysis incorporates 9 independent samples of fetal brains and 18 independent samples of adult brain (adult samples = 9 dams plus 9 additional nonpregnant females of matching age), and an equal number of liver samples.
We also examined the brain to plasma and liver to plasma concentration ratio (or Kp ratio = brain/plasma or liver/plasma) to get a measure of tissue exclusion or retention (Kalvass et al., 2007a; Liu et al., 2012). Consistent with previous results (Wang et al., 2004), the adult brain KP ratio for 6β-naltrexol was 0.13 (units = ml/g) at 20-minute survival (P < 0.0001 for difference from unity) and 0.34 at 45-minute survival (P < 0.0001 for difference from unity; Fig. 1 and Table 2), showing a barrier to drug entry into the brain. The brain KP ratio based on AUCs was 0.58 and was significantly different from unity based on 95% CIs, again supporting adult brain exclusion of drug (83 ± 6 × 103 ng/ml per minute with 95% CI of 70–98 × 103 for plasma and 49 ± 2 × 103 with 95% CI of 44–54 × 103 for adult brain). The greater KP ratio calculated based on AUCs integrated over 4 hours compared with single time points reflects the more rapid elimination of 6β-naltrexol from the circulation than from the brain in mice (Fig. 1); therefore, KP ratios measured under nonequilibrium conditions can vary with time as a function of rate of elimination and tissue distribution.
TABLE 2.
6β-Naltrexol brain and liver KP ratios during postnatal development
| Age | Survival Time (min) | Plasma (ng/ml) | Brain (ng/g) | Liver | Brain/Plasmaa Ratio | Liver/Plasma Ratio |
|---|---|---|---|---|---|---|
| min | ng/ml | ng/g | ng/g | |||
| E17 | 45 | 930 ± 220 (ad) | 2780 ± 1670* | 4400 ± 2580* | 2.9 | 4.7 |
| P7 | 45 | 3513 | 3030 ± 340 | 15,220 ± 1750 | 0.86 | 4.3 |
| P14 | 45 | 1410 ± 370 | 2860 ± 800 | 5050 ± 1260* | 2 | 3.6 |
| P20 | 20 | 1070 ± 100 | 350 ± 20* | 1830 ± 70* | 0.33 | 1.7 |
| P32 | 20 | 1160 ± 730 | 260 ± 80 | 1510 ± 640 | 0.22 | 1.3 |
| P50 | 45 | 430 ± 40 | 150 ± 10* | 820 ± 80* | 0.35 | 1.9 |
| Adult | 45 | 930 ± 220 | 310 ± 70* | 3200 ± 1600* | 0.34 | 3.4 |
ad, adult plasma.
Brain/Plasma units = ml/g.
P < 0.05 for comparison of brain or liver to plasma.
In contrast, the fetal brain/maternal plasma KP ratio was 2.9 at 45 minutes after drug injection (P < 0.05 for difference from unity; Figs. 1 and 2 and Table 2), and the ratio was 15.7 at 120 minutes after injection (P < 0.01 for difference from unity) (Figs. 1 and 2). At an earlier time point (20 minutes after injection), the fetal brain KP ratio is not significantly different from unity (P > 0.05), indicating rapid entry into fetal brain, and longer persistence than in maternal blood (Fig. 1). The fetal brain KP ratio based on noncompartmental AUCs is 3.6, which is significantly different from unity (83 ± 6 × 103 ng/ml per minute with 95% CI of 70–98 × 103 for maternal plasma and 302 ± 60 × 103 with 95% CI of 146–1060 × 103 for fetal brain). Similarly, the KP ratios based on AUCs is 4.3 for fetal liver and 2.6 for adult liver. Thus, in contrast to the relative exclusion of 6β-naltrexol from the adult brain, drug levels in fetal brain, and fetal and adult liver, are consistently higher than those in plasma. Owing to rapid elimination, 6β-naltrexol is largely depleted from all tissues between 2 and 4 hours after injection (Fig. 1).
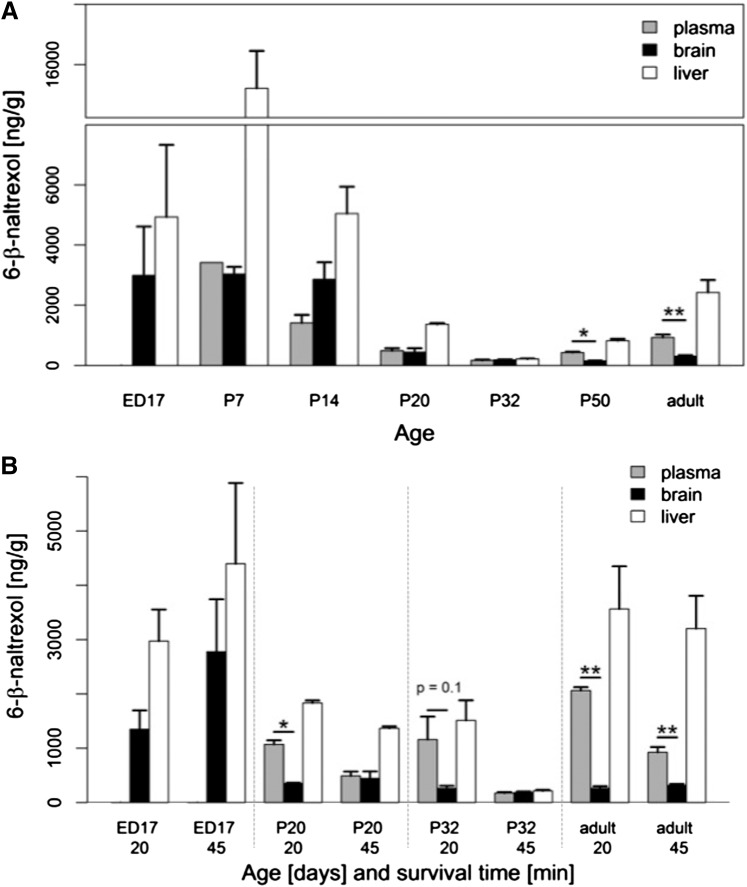
Fig. 2.
6β-Naltrexol levels across tissues and development. (A) Levels of drug in plasma, brain and liver after a single injection at P7, P14, P20, P32, and P50. E17 and adult (>2 months old) data from Fig. 1 were added for comparison purposes. There is a 45-minute survival time for all data. A break was introduced in the plot to accommodate the extremely high level of drug in P7 liver and to better illustrate the broad range of drug levels across all tissues. Drug levels in most tissues at E17, P7, and P14 are significantly higher than in the corresponding tissues at P20, P35, and P50 (P < 0.05 by t test; not indicated in the figure). Similarly, drug levels in all adult tissues are significantly higher than corresponding tissues at P32 (P < 0.05 by t test; not indicated in the figure). (B) A comparison of drug levels in plasma, brain, and liver at P20 and P32 with two survival times, 20 and 45 minutes. Data for embryonic and adult tissues from Fig. 1 have been added for comparison. The top number on the x-axis is age (days) and the bottom number is survival time (minutes). Asterisks in (A) and (B) indicate plasma vs. brain differences at a particular age; *P < 0.05; **P < 0.01. N = 2 for all samples at P7–P50 (except for plasma at P7, n = 1); for E17 and adults, n is indicated in the Fig. 1 legend.
In contrast to the relative exclusion of 6β-naltrexol from the adult brain, naltrexone (the parent compound) has free access to the brain of adults (Supplemental Table 1), confirming previous results (Kastin et al., 1991; Wang et al., 2004). The contrast of low 6β-naltrexol and high naltrexone levels in adult brain highlights the relative exclusion of 6β-naltrexol by the intact BBB.
Cumulative Injections of 6β-Naltrexol.
Although 6β-naltrexol is relatively excluded from adult brain, it might accumulate slowly upon multiple dosing because of slow exit from the brain. Therefore, we tested whether 6β-naltrexol accumulates in tissues over time after long-term delivery, and especially through retention in adult brain. After four injections of 6β-naltrexol over a 24-hour period (every 6 hours), we measured 6β-naltrexol levels in fetal and maternal tissues 4 hours after the last dose, and assessed accumulation in comparison with a single injection with a 4-hour survival. As shown in Table 1, a small increase of 6β-naltrexol occurred after cumulative injections with an average of ∼1.5-fold for all tissues. However, this was not significant (P > 0.2). Also, 6β-naltrexol trended toward greater accumulation in liver than brain (P = 0.084 for comparison of adult liver versus adult brain after 4 injections). Likewise, 6β-naltrexol trended toward greater accumulation in embryos than adults (P = 0.063 for comparison of fetal versus adult brain after four injections). These results indicate that although 6β-naltrexol gains some limited access to adult brain, it is not substantially retained upon multiple injections, a finding relevant to a potential therapy dosage regimen. Further studies with more samples would be needed to determine whether any of the small differences described above are significant; particularly any preferential buildup in embryos, which could benefit a proposed therapy.
Analysis of 6β-Naltrexol Levels in Brain during Postnatal Development.
Previous studies have been mixed in demonstrating robust withdrawal behaviors at birth in rodents, ranging from weak effects in rats (Enters et al., 1991; Robinson and Wallace, 2001) to no effects in mice (Richardson et al., 2006). Using the same opiate delivery paradigm in pregnant rats, more robust withdrawal behaviors are detectable 7 days after birth (Barr et al., 1998), and similarly, if morphine is delivered by direct injection in rat pups anytime after P7, robust preweaning withdrawal behaviors can be induced by naloxone (Jones and Barr, 1995). The refractoriness of the newborn rodent to easily observable dependence behaviors is likely due, at least in part, to the developmental state of the brain: mouse and rat brains at birth are developmentally equivalent to those of an early second trimester human (Clancy et al., 2001; Workman et al., 2013). This rodent-human developmental difference also extends to the BBB: in humans the BBB is widely thought to be nearly fully developed at or shortly after birth, whereas in mice, although controversial, it likely does not develop until P14 or later (Lossinsky et al., 1986; for review, see Ribatti et al., 2006). If this is indeed the case, then the early postnatal rodent could be a model to test BBB-dependent preventive effects of 6β-naltrexol on opioid dependence behaviors. Therefore, we determined the postnatal developmental time course of the exclusion of 6β-naltrexol from the brain in mice.
A single injection of 6β-naltrexol (10 mg/kg) was made in mice at P7, P14, P20, P32, and P50, and drug levels were measured after 45 minutes (data for embryos and adults were added for comparison). Shown in Fig. 2A, 6β-naltrexol levels in plasma, brain, and liver varied over a wide range during postnatal development as a result of drastically changing clearance rates. Plasma levels decreased progressively from 3660 ± 350 ng/ml at P7 to 170 ± 30 ng/ml at P32, then reversed to 930 ± 220 ng/ml in adults. Levels in the brain and liver roughly followed the same general pattern, with values in liver far exceeding those in all other tissues, especially during early postnatal development (P7 and P14). Most importantly, 6β-naltrexol levels in brain were stable from embryonic day 17 (E17) to P14, but dropped precipitously by P20, suggesting that the BBB develops in the time frame from P14 to P20. This interpretation is complicated, however, by dramatic changes in 6β-naltrexol clearance resulting in very low levels at P20 and P32 in all tissues. Clearly, at the 45-minute survival time point, ratios between tissues cannot be accurately assessed if 6β-naltrexol has been nearly completely cleared from the system. However, surveying the data at 20 minutes after administration reveals that effective brain exclusion of 6β-naltrexol was evident at P20 and P32 (Fig. 2B). Table 2 summarizes the brain/plasma and liver/plasma KP ratios for the indicated survival time points, showing that levels are lower in brain than plasma at P20 and older (with ratios that range from 0.22 to 0.35). In contrast, the levels in liver are higher than in plasma at all ages (with KP ratios that range from 1.3 to 4.2). Thus, the exclusion of 6β-naltrexol from brain starting at P20 is unique to that tissue. Also, in stark contrast to 6β-naltrexol, the brain/plasma ratio for naltrexone is greater than unity, or not significantly different from unity, at all ages including adults (Supplemental Fig. 1), which is consistent with its previously reported ability to cross the BBB (Kastin et al., 1991; Wang et al., 2004). Also, the developmental profile of naltrexone levels is similar to that observed for 6β-naltrexol (compare Fig. 2A and Supplemental Fig. 1), which is relevant to the known blood profile of these and other drugs in human development (see Discussion).
Suppression of Dependence Behavior by 6β-Naltrexol.
Considering that 6β-naltrexol continues to penetrate the brain at high levels prior to P20, we tested whether concomitant administration of 6β-naltrexol reduces withdrawal behaviors in preweaning age mice. We adapted a morphine dosing schedule based on studies in early postnatal rats and adult mice (Jones and Barr, 1995; Kest et al., 2002). Mice were injected with morphine alone, with morphine at a fixed dose in combination with one of five doses of 6β-naltrexol, or saline alone, starting at P12 and continuing for 5 days (see Materials and Methods). Withdrawal was then induced with an injection of naloxone. We observed robust jumping characteristic of adult withdrawal behavior (Kest et al., 2002), but not previously reported for preweaning mice or rats, to our knowledge (Supplemental Video 1). We also observed other features of withdrawal including wet-dog shakes, paw wringing, teeth chattering, and ptosis (Supplemental Video 2), but these behaviors are less suitable for quantification than jumping and have greater animal-to-animal variation. Shown in Fig. 3A, jumping behavior is suppressed by increasing concentrations of 6β-naltrexol with a dose eliciting 50% inhibition (ID50) of 0.022–0.044 mg/kg, which is ∼1/500 the morphine dose. At the highest dose tested, 0.5–1.0 mg/kg (equal to 1/20 that of morphine), withdrawal jumping is 94% reduced. The lowest 6β-naltrexol dose tested, 0.0033–0.0066 mg/kg (equal to 1/3000 that of morphine) caused a 20% decrease in jumping. There is also a time component to the effect of 6β-naltrexol on jumping behavior, which was quantified using time-to-event analysis for the first jump after the initiation of morphine withdrawal (Fig. 3B). Control mice receiving morphine with no 6β-naltrexol started jumping rapidly after withdrawal, and all (six) mice tested had done so within 74 seconds. With increasing doses of 6β-naltrexol, latencies to the first jump became increasingly larger, and frequency plots (Kaplan-Meier curves) for all doses were significantly different from those of the control group not receiving 6β-naltrexol (P < 0.025 by log-ratio test). During the treatment period, we also observed a significant inhibition of weight gain as a result of morphine presentation alone, which is alleviated by increasing concomitant doses of 6β-naltrexol (Fig. 3C). Survival was 100% for all conditions and for all animals tested by this procedure. In mice with low weight gain because of morphine, normal weight was usually restored 7–10 days after testing (data not shown).
Fig. 3.
Suppression of withdrawal behavior by 6β-naltrexol in juvenile mice. (A) 6β-Naltrexol prevents a dependence behavior, withdrawal jumping, when delivered in combination with morphine. Total jumps were counted over a period of 15 minutes starting immediately after the injection of naloxone to induce withdrawal. We used a two-concentration ramping procedure for the morphine injections with commensurate ramping of 6β-naltrexol (see Materials and Methods). Data are plotted using the higher of the two drug concentrations. Asterisks indicate a significant difference compared with morphine-treated animals with no 6β-naltrexol; *P < 0.05; **P < 0.01. Numbers below the data-points indicate n values. (B) Kaplan-Meier plots indicating a progressive delay in time to first jump with increasing 6β-naltrexol. Note that all concentrations of 6β-naltrexol result in a significant delay relative to morphine alone (P < 0.05 by log-ratio test). (C) Inhibition of weight gain by morphine is alleviated by 6β-naltrexol. The mass of each mouse was determined before and after the 6-day morphine dosing schedule and the percentage weight change was determined. Asterisk indicates significant difference from animals that received morphine but no (“0”) 6β-naltrexol (P < 0.05). n = the same as indicated in (A). In (C), drug concentration is reported as a ratio of 6β-naltrexol to morphine to emphasize the combination treatment.
Discussion
This study tests the feasibility of a new paradigm for preventing NAS in neonates born to mothers engaged in opioid maintenance therapy. We propose that an opioid antagonist that is relatively excluded from the maternal brain (enabling ongoing opioid therapy), but which is able to preferentially penetrate the placenta and immature BBB in the fetus could protect the fetus from opioid exposure and thereby prevent or reduce NAS. Previous studies had already indicated that the opioid antagonist, 6β-naltrexol, is relatively excluded from the brain while acting as a potent antagonist in the periphery (Wang et al., 2004; Yancey Wrona et al., 2009, 2011), limiting peripheral adverse opioid effects such as constipation. Here we provide evidence in mice that 6β-naltrexol readily enters the fetal circulation and fetal brain, resulting in substantially higher levels in fetal brain compared with adult brain.
We also tested the ability of 6β-naltrexol to prevent opioid dependence in preweaning juvenile mice when the BBB is still immature. When administered in combination with morphine for several days starting in the second postnatal week, 6β-naltrexol prevents withdrawal behavior with extreme potency (ID50 0.022–0.044 mg/kg), owing to ready access to the neonatal brain lacking full BBB protection. This 6β-naltrexol dose is ∼500-fold lower than the morphine dose used here for inducing dependence, and is 20–500-fold lower than the ID50 of 6β-naltrexol for the blockade of opiate antinociception in adults depending on the agonist used and the route (and timing) of administration (Wang et al., 2001; Porter et al., 2002; Sirohi et al., 2009; Yancey-Wrona et al., 2009). The extreme potency of 6β-naltrexol is further highlighted by the observation that even at a dosage that is 1/3000 that of morphine, there is a 20% reduction in quantifiable withdrawal behavior, and a significant inhibition of jump latency. Efficacy of 6β-naltrexol is also quite high, with nearly complete suppression of juvenile withdrawal (94%) at the highest dose tested in the current study. These results in mice suggest the potential therapeutic utility of 6β-naltrexol in pregnant women undergoing opioid maintenance treatments to selectively block fetal dependence without interfering with the mother’s pain and/or maintenance therapy.
The preweaning postnatal mouse model used here to test the behavioral efficacy and potency of 6β-naltrexol is not a perfect model of human NAS, which is characterized by withdrawal immediately at birth. Withdrawal behaviors resulting from fetal exposure are difficult to score in mice at birth (Richardson et al., 2006), likely owing, at least partially, to developmental delay in mice compared with humans (Workman et al., 2013). Nevertheless, the postnatal mouse model is relatively accessible with robust behavioral outcomes, and bears the one key feature that makes it relevant to NAS: lack of a mature BBB, which is an important novel finding of this study. Also, we do not claim that 6β-naltrexol is completely excluded from the brain in adults. Prior studies have demonstrated that 6β-naltrexol can induce withdrawal in adult mice; however, the required dose is ∼100-fold higher than that needed for either naltrexone or naloxone (Raehal et al., 2005). It is also 500-fold higher than the ID50 for suppression of juvenile withdrawal jumping, as we have shown here. Thus, we suggest that finding a dose that can prevent NAS while not interfering with the mother’s opioid maintenance therapy should, in principle, be feasible.
Most of the existing treatment regimens for NAS rely on reducing the severity of symptoms, but are not preventive. A 5-HT antagonist, ondansetron, recently entered clinical trials for the prevention of NAS symptoms when delivered maternally shortly before birth and continuing in the postnatal period (Elkomy et al., 2015; https://clinicaltrials.gov/ct2/show/NCT01965704). However, this treatment, although designed to reduce the length of stay in the intensive care unit, would not be expected to prevent the occurrence of dependence and any related developmental consequences. Our results suggest that prenatal therapy of the mother with 6β-naltrexol could be combined with other palliative therapies to reduce NAS substantially. Some uncertainty remains concerning the prenatal age at which the human fetal BBB matures, which could potentially exclude 6β-naltrexol from the brain already before birth, leading to some degree of dependence. Nevertheless, any reduction in fetal opioid dependence during pregnancy could yield considerable benefit in fetal development, term delivery, weight gain, and short- and long-term sequelae of NAS.
The current study further reveals substantial changes in both 6β-naltrexol and naltrexone clearance across developmental age in mice. A similar profile across early human development has been reported for half-lives of a broad panel of 45 drugs, including glucuronidated drugs such as morphine (Ginsberg et al., 2002). The studied drugs generally display a long half-life in premature and full-term human neonates, then the half-life decreases progressively over several months after birth, even below adult values, and then recovers to adult levels. This time course in humans was attributed at least partly to the immaturity of hepatic and renal systems in the early postnatal period. Our study shows a surprising stability of 6β-naltrexol from embryogenesis through the first 2 weeks after birth, after which levels drop precipitously as a result of increased clearance. This suggests that in addition to the known developmental delay in the brain of mice compared with humans at birth (Workman et al., 2013), there may also be a delay in developmental processes affecting drug metabolism and renal clearance in the postnatal period (6β-naltrexol is cleared both renally and by metabolism).
The developmental changes in 6β-naltrexol clearance during mouse postnatal development need to be considered with the analysis of BBB development. For each of the postnatal ages, a full analysis over different time periods after administration is needed to accurately determine the AUC, drug half-life, and clearance in target tissues to further develop the postnatal mouse as a model for testing BBB-dependent drug delivery mechanisms. Nevertheless, the results presented here support the conclusion that the BBB matures in mice between P14 and P20. This is consistent with the extreme potency of 6β-naltrexol to block withdrawal behaviors in juvenile mice. The mechanism by which 6β-naltrexol is largely excluded from the brain by a mature BBB remains to be determined, but likely involves the main efflux transporters Mdr1 and Bcrp1 genes (Liu et al., 2012).
The developmental delay renders the postnatal preweaning mouse a useful model for studying aspects of the late embryonic period in humans, and deserves more attention in the future. Specifically, the immature BBB that extends beyond 14 days after birth coupled with robust withdrawal behavior when opioid dependence is induced in this time period offers the opportunity to study preventive therapies that could be effective during pregnancy.
For the development of an effective NAS therapy, a sufficiently long half-life of the antagonist is desirable, as the half-life of methadone in humans is 8–59 hours. From the current study, we can estimate that the half-life of 6β-naltrexol in mice is in the range of 1–2 hours at most. In contrast, the half-life of 6β-naltrexol in humans is much longer than that in mice (>11 hours; Yancey-Wrona et al., 2011). Similarly, the half-life of methadone in rodents is only 1–3 hours, approximating the clearance and analgesic time profile of morphine (Pacifici et al., 1994; Kalvass et al., 2007b), highlighting species differences in opioid PK. Therefore, the metabolic properties of 6β-naltrexol are suitable for sustained alleviation of the fetal dependence-inducing effects of the common opiates used for the management of adult addiction in the clinic, methadone and buprenorphine. The key issues for the development of a NAS therapy based on 6β-naltrexol are, rather, the specifics of placental transfer and BBB development in humans. In this regard, future tests of the drug in a nonhuman primate may be most revealing.
In conclusion, we have laid the groundwork for the development of a novel preventive therapy for NAS. The following combined properties of 6β-naltrexol make it a candidate for this purpose: 1) it is a neutral antagonist of the μ-opioid receptor with low propensity to cause withdrawal compared with naloxone and naltrexone (Raehal et al., 2005; Sadee et al., 2005); 2) its relative exclusion from the adult CNS; and 3) its ability to enter the fetal circulation and brain. In addition to its potency and efficacy, 6β-naltrexol is the main metabolite of naltrexone in humans (but not in mice), which is approved by the FDA for the treatment of alcoholism (Pettinati et al., 2006). Therefore, the known safety profile of 6β-naltrexol may facilitate its eventual use in pregnant women.
Supplementary Material
Abbreviations
- AUC
area-under-the-curve
- BBB
blood-brain barrier
- CI
confidence interval
- CNS
central nervous system
- ID50
dose eliciting 50% inhibition
- NAS
neonatal abstinence syndrome
- PK
pharmacokinetic
Authorship Contributions
Participated in research design: Oberdick, Sadee, Phelps
Conducted experiments: Oberdick, Ling
Contributed new reagents or analytic tools: Yudovich
Performed data analysis: Oberdick, Schilling, Sadee
Wrote or contributed to writing the manuscript: Oberdick, Sadee, Phelps, Schilling
Footnotes
This work was supported by the National Institutes of Health, National Center for Advancing Translational Sciences [Grant UL1-TR-001070].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Barr GA, Zmitrovich A, Hamowy AS, Liu PY, Wang S, Hutchings DE. (1998) Neonatal withdrawal following pre- and postnatal exposure to methadone in the rat. Pharmacol Biochem Behav 60:97–104. [DOI] [PubMed] [Google Scholar]
- Chey WD, Webster L, Sostek M, Lappalainen J, Barker PN, Tack J. (2014) Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med 370:2387–2396. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. (2001) Translating developmental time across mammalian species. Neuroscience 105:7–17. [DOI] [PubMed] [Google Scholar]
- Dryden C, Young D, Hepburn M, Mactier H. (2009) Maternal methadone use in pregnancy: factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. BJOG 116:665–671. [DOI] [PubMed] [Google Scholar]
- Elkomy MH, Sultan P, Carvalho B, Peltz G, Wu M, Clavijo C, Galinkin JL, Drover DR. (2015) Ondansetron pharmacokinetics in pregnant women and neonates: towards a new treatment for neonatal abstinence syndrome. Clin Pharmacol Ther 97:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enters EK, Guo HZ, Pandey U, Ko DJ, Robinson SE. (1991) The effect of prenatal methadone exposure on development and nociception during the early postnatal period of the rat. Neurotoxicol Teratol 13:161–166. [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Hattis D, Sonawane B, Russ A, Banati P, Kozlak M, Smolenski S, Goble R. (2002) Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci 66:185–200. [DOI] [PubMed] [Google Scholar]
- Hall ES, Wexelblatt SL, Crowley M, Grow JL, Jasin LR, Klebanoff MA, McClead RE, Meinzen-Derr J, Mohan VK, Stein H, et al. OCHNAS Consortium (2014) A multicenter cohort study of treatments and hospital outcomes in neonatal abstinence syndrome. Pediatrics 134:e527–e534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DP, Fleming TR. (1982) A class of rank test procedures for censored survival data. Biometrika 69:553–566. [Google Scholar]
- Heslop D. (2009) On the statistical analysis of the rock magnetic S-ratio. Geophys J Int 178:159–161. [Google Scholar]
- Hommel G. (1988) A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 75:383–386. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. [DOI] [PubMed] [Google Scholar]
- Jaki T, Wolfsegger MJ. (2011) Estimation of pharmacokinetic parameters with the R package PK. Pharm Stat 10:284–288. [Google Scholar]
- Jaki T, Wolfsegger MJ, Ploner M. (2009) Confidence intervals for ratios of AUCs in the case of serial sampling: a comparison of seven methods. Pharm Stat 8:12–24. [DOI] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR, Fischer G. (2010) Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med 363:2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Barr GA. (1995) Ontogeny of morphine withdrawal in the rat. Behav Neurosci 109:1189–1198. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Maurer TS, Pollack GM. (2007a) Use of plasma and brain unbound fractions to assess the extent of brain distribution of 34 drugs: comparison of unbound concentration ratios to in vivo p-glycoprotein efflux ratios. Drug Metab Dispos 35:660–666. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. (2007b) Pharmacokinetics and pharmacodynamics of seven opioids in P-glycoprotein-competent mice: assessment of unbound brain EC50,u and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther 323:346–355. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pearson MA, Banks WA. (1991) EEG evidence that morphine and an enkephalin analog cross the blood-brain barrier. Pharmacol Biochem Behav 40:771–774. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. (2002) Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience 115:463–469. [DOI] [PubMed] [Google Scholar]
- Liu X, Ding X, Deshmukh G, Liederer BM, Hop CE. (2012) Use of the cassette-dosing approach to assess brain penetration in drug discovery. Drug Metab Dispos 40:963–969. [DOI] [PubMed] [Google Scholar]
- Lossinsky AS, Vorbrodt AW, Wisniewski HM. (1986) Characterization of endothelial cell transport in the developing mouse blood-brain barrier. Dev Neurosci 8:61–75. [DOI] [PubMed] [Google Scholar]
- Mainguet P, Fiasse R. (1977) Double-blind placebo-controlled study of loperamide (Imodium) in chronic diarrhoea caused by ileocolic disease or resection. Gut 18:575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W, Urso D. (2008) Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther 28:239–249. [DOI] [PubMed] [Google Scholar]
- Pacifici R, Patrini G, Venier I, Parolaro D, Zuccaro P, Gori E. (1994) Effect of morphine and methadone acute treatment on immunological activity in mice: pharmacokinetic and pharmacodynamic correlates. J Pharmacol Exp Ther 269:1112–1116. [PubMed] [Google Scholar]
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. (2012) Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA 307:1934–1940. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. (2006) The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol 26:610–625. [DOI] [PubMed] [Google Scholar]
- Porter SJ, Somogyi AA, White JM. (2002) In vivo and in vitro potency studies of 6beta-naltrexol, the major human metabolite of naltrexone. Addict Biol 7:219–225. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadée W, Bilsky EJ. (2005) In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther 313:1150–1162. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E, Artico M. (2006) Development of the blood-brain barrier: a historical point of view. Anat Rec B New Anat 289:3–8. [DOI] [PubMed] [Google Scholar]
- Richardson KA, Yohay AL, Gauda EB, McLemore GL. (2006) Neonatal animal models of opiate withdrawal. ILAR J 47:39–48. [DOI] [PubMed] [Google Scholar]
- Ritz C, Streibig JC. (2005) Bioassay analysis using R. J Stat Softw 12:1–22. [Google Scholar]
- Robinson SE, Wallace MJ. (2001) Effect of perinatal buprenorphine exposure on development in the rat. J Pharmacol Exp Ther 298:797–804. [PubMed] [Google Scholar]
- Sadée W, Wang D, Bilsky EJ. (2005) Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci 76:1427–1437. [DOI] [PubMed] [Google Scholar]
- Schilling K, Oberdick J, Schilling RL. (2012) Toward an efficient and integrative analysis of limited-choice behavioral experiments. J Neurosci 32:12651–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon HE, Lutz EA. (2002) Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology 42:253–261. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Madia PA, Yoburn BC. (2009) The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther 330:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. (2000) Modeling Survival Data: Extending the Cox Model, Springer, New York. [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadée W. (2001) Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem 77:1590–1600. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, Sadée W. (2004) Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. J Pharmacol Exp Ther 308:512–520. [DOI] [PubMed] [Google Scholar]
- Wolfsegger MJ, Jaki T. (2009) Non-compartmental estimation of pharmacokinetic parameters in serial sampling designs. J Pharmacokinet Pharmacodyn 36:479–494. [DOI] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. (2013) Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 33:7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Hammarlund-Udenaes M, de Boer AG, de Lange EC. (1999) The role of P-glycoprotein in blood-brain barrier transport of morphine: transcortical microdialysis studies in mdr1a (-/-) and mdr1a (+/+) mice. Br J Pharmacol 128:563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey-Wrona JE, Raymond TJ, Mercer HK, Sadée W, Bilsky EJ. (2009) 6beta-naltrexol preferentially antagonizes opioid effects on gastrointestinal transit compared to antinociception in mice. Life Sci 85:413–420. [DOI] [PubMed] [Google Scholar]
- Yancey-Wrona J, Dallaire B, Bilsky E, Bath B, Burkart J, Webster L, Magiera D, Yang X, Phelps M, Sadee W. (2011) 6β-naltrexol, a peripherally selective opioid antagonist that inhibits morphine-induced slowing of gastrointestinal transit: an exploratory study. Pain Med 12:1727–1737. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF. (2000) Oral methylnaltrexone for opioid-induced constipation. JAMA 284:1383–1384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.