Abstract
The pharmacokinetics, pharmacodynamics, safety, and tolerability of BMS-932481, a γ-secretase modulator (GSM), were tested in healthy young and elderly volunteers after single and multiple doses. BMS-932481 was orally absorbed, showed dose proportionality after a single dose administration, and had approximately 3-fold accumulation after multiple dosing. High-fat/caloric meals doubled the Cmax and area under the curve and prolonged Tmax by 1.5 hours. Consistent with the preclinical pharmacology of GSMs, BMS-932481 decreased cerebrospinal fluid (CSF) Aβ39, Aβ40, and Aβ42 while increasing Aβ37 and Aβ38, thereby providing evidence of γ-secretase enzyme modulation rather than inhibition. In plasma, reductions in Aβ40 and Aβ42 were observed with no change in total Aβ; in CSF, modest decreases in total Aβ were observed at higher dose levels. Increases in liver enzymes were observed at exposures associated with greater than 70% CSF Aβ42 lowering after multiple dosing. Although further development was halted due to an insufficient safety margin to test the hypothesis for efficacy of Aβ lowering in Alzheimer’s disease, this study demonstrates that γ-secretase modulation is achievable in healthy human volunteers and supports further efforts to discover well tolerated GSMs for testing in Alzheimer’s disease and other indications.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by the deposition of amyloid plaques and neurofibrillary tangles. Approximately 5.2 million people suffer from AD in the United States alone and the socioeconomic burden on caregivers and health care systems is extremely high (Alzheimer’s Association, 2014). The importance of γ-secretase complex modulation to AD is highlighted by genetic studies describing associations of presenilin 1 or 2 (PS1 or PS2) and amyloid precursor protein (APP) mutations in early-onset familial forms of AD (Barber, 2012). In addition, some APP mutations that reduce amyloid peptide formation (Jonsson et al., 2012) are protective. The prevalence of the amyloidogenic peptides Aβ40 and Aβ42 is high in brain amyloid plaques in both familial and late-onset forms of the disease (Iwatsubo et al., 1994; Ishii et al., 1997), suggesting a common pathway for plaque formation in both early- and late-onset AD. The genetics suggest that decreasing amyloidogenic peptide generation through frank inhibition or by modulation of γ-secretase complex cleavage preference could, in conjunction with improved amyloid clearance, lead to amelioration of amyloid plaque formation (Haass and Selkoe, 1993), thereby stabilizing disease progression or prevention of symptoms associated with AD dementia.
The γ-secretase complex produces the final cleavage of APP and works in conjunction with the β-APP cleaving enzyme to generate the amyloidogenic peptides Aβ40 and Aβ42 (Dries and Yu, 2008). The enzyme complex has been reported to yield cleavage products ranging from 37 to 43 amino acids (Beher et al., 2002; Qi-Takahara et al., 2005; Takami et al., 2009) and is composed of the primary intramembrane aspartyl protease PS1 or PS2 along with nicastrin, Pen-2, and Aph-1a or Aph-1b (De Strooper, 2003; De Strooper et al., 2012). The γ-secretase complex is promiscuous and cleaves a number of targets, including Notch. Therapeutic inhibition of Notch can be beneficial in targeting oncology, angiogenic, and/or immune-specific indications (Golde et al., 2013; Olsauskas-Kuprys et al., 2013). However, inhibition of Notch activity is also known to be associated with several related toxicities (Barten et al., 2006), complicating the drug development path for γ-secretase inhibitors in AD. More recently, γ-secretase inhibition has been associated with increase in the β C-terminal fragment of APP hypothesized to interfere with synaptic function (Mitani et al., 2012; Tamayev and D'Adamio, 2012). Indeed, mild-moderate AD clinical trials with the γ-secretase inhibitors LY450139 (Semagacestat; Eli Lilly & Co., Indianapolis, IN) [(2S)-2-hydroxy-3-methyl-N-((1S)-1-methyl-2-{[(1S)-3-methyl-2-oxo-2,3,4,5-tetrahydro-1H-3-benzazepin-1-yl]amino}-2-oxoethyl)butanamide)] and BMS-708163 (Avagacestat; Bristol-Myers Squibb, New York, NY) [(2R)-2-[(4-chlorophenyl)sulfonyl-[[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide] resulted in Notch-related nonmelanoma skin cancers and/or progression and worsening of cognitive measures (Fleisher et al., 2008; Coric et al., 2012; Doody et al., 2013). Although there were hints of Aβ42 lowering in both studies, the effects were not robust in cerebrospinal fluid (CSF) and one could argue the hypothesis for Aβ42 peptide lowering in AD had not been sufficiently tested in these studies (Toyn and Ahlijanian, 2014; Toyn, 2015).
Early studies using nonsteroidal anti-inflammatory drugs (NSAIDs) provided evidence that γ-secretase cleavage preferences could be shifted away from longer amyloidogenic peptide products to shorter nonamyloidogenic peptides without completely inhibiting the complex and thereby avoiding Notch-related toxicities (Weggen et al., 2001; Golde et al., 2013; Pettersson et al., 2013). As a class, these types of compounds were categorized as γ-secretase modulators (GSMs). In general, the GSMs fall into three groupings: 1) NSAID-derived carboxylic acids, 2) non-NSAID heterocyclics, and 3) natural product–derived GSMs. Different GSMs produce different shifts in cleavage preference, with some showing increases in Aβ38 and others showing increases in Aβ37 peptide generation with concomitant reductions in Aβ42 and Aβ40 (Caldwell et al., 2010; Kounnas et al., 2010; Wan et al., 2011a,b; Borgegard et al., 2012; Tate et al., 2012).
BMS-932481, a non-NSAID bicyclic pyrimidine, was developed as a selective GSM that showed selectivity for Aβ40 and Aβ42 reduction while sparing total Aβ levels both in vitro and in preclinical models and after single doses in CSF of normal healthy volunteers (see companion paper, Toyn et al., 2016). Our studies examined the effects of BMS-932481 on the safety, tolerability, pharmacokinetics, and pharmacodynamics in young and elderly healthy volunteers. Food effect and pH substudies were included to further characterize bioavailability. Pharmacokinetic differences were also explored in healthy versus elderly subjects. Changes in CSF and plasma Aβ fragments were used to confirm mechanistic activity of the compound. Pharmacogenomic endpoints were included to examine potential effects on Aβ and bilirubin levels, respectively. Results confirmed BMS-932481 as an active GSM in both peripheral and central compartments in humans with good oral bioavailability.
Materials and Methods
Study Participants
A total of 83 subjects were randomized and 80 subjects completed the single ascending dose (SAD) study. A total of 97 subjects were enrolled and 24 were treated in the multiple ascending dose (MAD) study. Healthy young (SAD, aged 18–45 years; MAD, aged 18–55 years) and elderly (aged ≥70 years) individuals were recruited for participation. Men and women of nonchild-bearing potential were deemed eligible based on no clinically significant deviation from normal on the following criteria: medical history, physical examination, electrocardiogram (ECG) results, clinical laboratory evaluations, and a body mass index of 18–32 kg/m2 inclusive. Elderly participants with hypertension, diabetes, dyslipidemia, and other common non-neurologic age-related disorders were permitted to participate as long as they had been taking an approved disease-controlling stable dose of medication for at least 3 months before screening and had no significant organ dysfunction. Men and women with a history of recent gastrointestinal disease and/or a positive test result for Gilbert’s syndrome were excluded. Every subject gave written informed consent to participate. All protocols were approved by ethics review boards of the respective study sites. Studies were conducted in accordance with the guidelines on good clinical practice and with ethical standards for human experimentation established by the Declaration of Helsinki.
Study Design
Both studies were designed as placebo-controlled, double-blinded, ascending dose studies.
SAD Study.
The SAD study was composed of nine panels split into parts A (panels 1–8) and B (panel 9). Part A focused on the safety, tolerability, pharmacokinetics, pharmacodynamics, and food/pH effects of BMS-932481. Part B focused on the exposure-response relationship of BMS-932481 and CSF Aβ peptides. For panels 1–7, eight healthy men and women were randomized in a 3:1 ratio to receive 10, 30, 100, 300, 600, 900, and 1200 mg, respectively (BMS-932481, n = 6; placebo, n = 2) as an oral solution or a capsule formulation. Twelve healthy elderly men and women were assigned to panel 8 and received a single 900-mg dose (BMS-932481, n = 9; placebo, n = 3). Panel 4 was designed with three periods with dosing administered either during a fasted state (period 1), after a high-fat meal (period 2), or 2 hours after administration of 40 mg famotidine. Subjects who met enrollment criteria in panels 1–3 were confined to the clinical facility for 7 days, whereas those who met criteria in panels 5–7 were confined for 12 days. All subjects were discharged on day 14 except for panel 4 subjects, who were discharged on day 14 of period 3. For panel B, 15 healthy subjects received a single 900-mg dose, which was projected to reduce CSF Aβ42 by ≥50% from baseline. Subjects were admitted to the clinical facility on day −2 and remained in the clinic until they were furloughed on day 10. Before dosing, all subjects underwent a lumbar puncture (day −1/1) and then received a single dose (2:1 ratio, 900 mg BMS-932481 or placebo) in the fasted state. Lumbar opening pressure was measured prior to cannulation. All predose CSF sample collections occurred between 7 and 10 AM on day 1. Subjects were discharged from part B (panel 9) on day 14.
MAD Study.
The MAD study was designed as an 11-panel study with panels 1–5 and 11 (optional) in healthy young subjects, panels 6 and 7 in elderly participants, and panels 8–10 in healthy young Japanese individuals. Subjects meeting eligibility requirements upon screening were admitted on day −3 to undergo a baseline lumbar puncture on day −2, followed by baseline assessments on day −1. For the first five panels, the original plan was to dose eight subjects per panel (3:1 ratio; BMS-932481, n = 6; placebo, n = 2) once daily for 28 days in each of five sequential dose panels (50, 100, 200, 400, and 800 mg) and furlough participants on day 35 (9 days after the last dose). Subjects returned for follow-up visits on day 37, followed by a follow-up visit on day 42 and a second follow-up visit and discharge on day 56. Safety findings halted dosing after the third panel (see below).
Pharmacokinetic/Pharmacodynamic Plasma and CSF Collection
SAD Study.
EDTA whole blood was collected at screening for genotype analysis. In addition, EDTA plasma samples from panels 1–9 were collected for measurement of BMS-932481 at predose and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 18, 24, 36, 48, 60, and 72 hours after dosing. Parallel aliquots for the measurement of plasma Aβ40, Aβ42, and total Aβ were collected at −1, 0 (predose), 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 18, 24, 30, 36, 48, and 60 hours postdose. Within 30 minutes of collection and after tubes were gently inverted and placed on ice, samples were centrifuged at room temperature for 15 minutes at approximately 1000g. Plasma was transferred to polypropylene transfer tubes and stored at −80°C until analysis.
CSF samples were collected via lumbar cannulation at −1, 0, 2, 4, 6, 8, 12 15, 18, and 24 hours postdose using best practices for CSF collection. A total of 3 ml from each time point was collected and aliquoted into 0.5-ml aliquots in polypropylene tubes and was stored at −80°C until analysis. Only polypropylene materials were used during the collection; a pump was used to aid in the CSF draws and no filter placed between the cannula tubing and the collection tubes. Care was taken to standardize collection times across subjects, minimize volume collected, sampling frequency and polypropylene quality based on prior reports that such factors can influence Aβ levels (Teunissen et al., 2009; Pica-Mendez et al., 2010; del Campo et al., 2012; Li et al., 2012; Vanderstichele et al., 2012; Mattsson et al., 2013) .
MAD Study.
EDTA whole blood was collected at screening for genotype analysis. For pharmacokinetic analysis, EDTA plasma samples were collected intensively on days 1, 14, and 28 and on days 5, 7, 11, 18, 21, and 25 at trough. On days 1 and 14, plasma was collected at 0 (predose), 0.5, 1, 2, 3, 4, 5, 6, 8, 12, and 24 hours postdose. On day 28, plasma was collected at 0 (predose), 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 18, 24, 48, 72, 96, 120, 144, 168 (day 35), and 216 hours. For pharmacodynamic analysis, EDTA blood was collected on days 1 and 28 at 0 (predose), 2, 4, 8, 12, and 24 hours after dosing and also at trough on days 7, 14, and 35.
CSF was collected by a single lumbar puncture in all patients predose (day −2) and then on day 28 for the 50- and 100-mg dose cohorts. For the 200-mg cohort, dosing was shortened to 24 days as a result of safety findings and a third lumbar puncture was added at either 48 or 72 hours after the last dose (group A, n = 3) or 72 or 96 hours after the last dose (group B, n = 3) via a protocol amendment. All CSF was collected between 2 and 18 hours postdose.
Pharmacokinetic and Pharmacodynamic Assays
The MSD Triplex kit for Aβ38, Aβ40, and Aβ42 and the MSD Total Aβ assays were used to measure these Aβ peptide fragments in CSF (both from Meso Scale Diagnostics, Rockville, MD). Plasma Aβ40 and Aβ42 were measured using Luminex Innogenetics plasma duplex kits (EMD Millipore, Billerica, MA). Total plasma was measured using an MSD kit for total Aβ. All of these assays were validated at Quest Pharmaceutical Services and run per the manufacturer’s instructions on either an MSD Sector Imager 6000 or a Luminex 200 analyzer. The CSF Aβ37 assay was a sandwich immunoassay with an antibody from Dr. Pankaj Mehta and 6E10. All samples were analyzed in duplicate. Pooled CSF was used to generate low and high human quality control samples for each analyte. All samples were run in batches with calibration curves and quality control materials that had to meet pre-established acceptance criteria. The lower limit of quantitation for CSF Aβ38, Aβ40, Aβ42, and total Aβ was 9.77 pg/ml, 14.6 pg/ml, 1.42 pg/ml, and 20 mg/ml, respectively. The lower limit of quantitation for plasma Aβ40, Aβ42, and total Aβ was 30 pg/ml, 3 pg/ml, and 50 pg/ml, respectively.
BMS-932481 Assay.
Quantitative liquid chromatography (LC)–tandem mass spectrometry (MS/MS) assays were developed to measure BMS-932481 drug levels. Results were generated in batch runs using validated assays with calibration curves and quality control materials that had to meet pre-established acceptance criteria. The pharmacokinetic parameters were expressed in nanograms per milliliter and were used to calculate the area under the plasma concentration-time curve (AUC0–∞, AUC0–τ, and AUCτ),Cmax, C24h, Cτ, Tmax, Vss, accumulation index for AUC and Cmax, and apparent terminal half-life.
LC-MS/MS of Aβ Peptides.
Measurement of Aβ peptides was performed according to assays provided by Pharmaceutical Product Development (see the Supplemental Methods for details). The lower limit of quantitation for all CSF peptides was 30 pg/ml.
4β- and 4α-Hydroxycholesterol.
The plasma 4β- and 4α-hydroxycholesterol (4βHC and 4αHC, respectively) measurements were performed using a validated LC-MS/MS assay provided by PPD and as referenced in Goodenough et al. (2011). The lower limit of quantitation for both analytes was 2 ng/ml for 50 μl plasma.
Pharmacogenomic Assays.
Quantitative polymerase chain reaction assays for UDP glucuronosyltransferase 1 family, polypeptide A1 (UGT1A1) *6 and *28 and apolipoprotein ApoE were performed by Gentris (Raleigh Durham, NC), according to standard procedures.
Safety Assessments for the SAD/MAD Studies
Safety and tolerability were assessed using vital sign measures, physical examinations, 12-lead ECGs, and laboratory safety studies. Adverse events (AEs) were spontaneously reported or elicited during open-ended questioning, examination, or evaluation. The onset, duration, intensity, seriousness, relationship to the investigational product, action taken, and treatment required were all recorded and tabulated according to the Medical Dictionary for Regulatory Activities (version 12.1) system organ class, preferred term, and treatment. Columbia suicidality ratings were obtained for patients enrolled in the MAD study.
Statistical Analysis
Pharmacokinetic parameters were derived from total plasma concentration versus time using noncompartmental methods. The AUC during a specified interval was calculated using trapezoidal and log-trapezoidal approaches. In the MAD study, the terminal log-linear phase of the plasma concentration versus time curve (on days 14 and 28) was derived using least-squares linear regression that yielded a minimum mean square error. Slope (k) of the terminal log-linear phase was used to calculate elimination half-life and was estimated as ln2/k. Analysis of covariance was applied to analysis of Aβ peptides.
Results
Starting Dose Rationale for the SAD Study
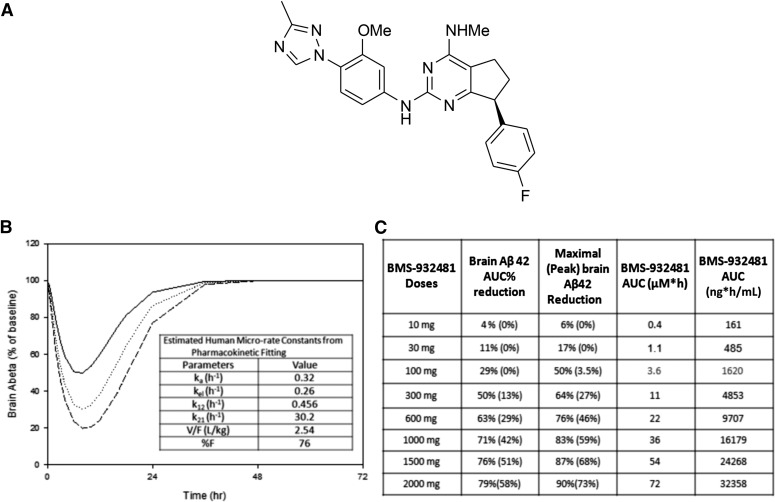
In vivo, BMS-932481 exhibited dose-dependent Aβ42 and Aβ40 lowering in both the brain and CSF in rats and dogs (see Toyn et al., 2016 for a detailed discussion on preclinical pharmacology). The chemical structure of BMS-932481 is depicted in Fig. 1A. Based on an indirect-response model using preclinical data and using allometric scaling of the pharmacokinetic parameters, brain Aβ42 lowering was predicted over time and at differential exposures (Fig. 1B). The preclinical pharmacodynamic and in vivo preclinical toxicity testing data were then used to project the initial starting dose in humans. In brief, a 10-fold safety factor was applied to exposures associated with the no observed adverse effect level in rats and dogs and was then applied to the human equivalent doses. On the basis of these projections, the maximum recommended starting dose was estimated to be 10 mg, a dose predicted by the model to elicit minimal to no change in brain Aβ reduction. Doses expected to elicit 50% and 70% brain Aβ42 lowering were predicted to be 300 mg and 1000 mg, respectively (Fig. 1C). CSF Aβ peptides were used as a surrogate for brain Aβ lowering and preclinical data demonstrated excellent correlation between the degree of brain and CSF Aβ lowering (see Toyn et al., 2016 for a detailed description of the CSF and brain Aβ-lowering relationship).
Fig. 1.
(A) Graphical representation of BMS-932481 structure. (B) Indirect-response modeling for brain Aβ lowering based on preclinical rat data. (C) Projected estimated doses with associated estimated brain Aβ42 lowering and exposures predicted from the model.
Demographics
Table 1 summarizes subject demographics for both the SAD and MAD studies. In the SAD study, 83 subjects were enrolled and 80 subjects completed the study. Twelve healthy subjects aged ≥70 years were enrolled in the elderly cohort (panel 8). Three subjects withdrew consent from the SAD study. A total of 97 subjects were enrolled in the MAD study, in which 24 subjects were randomized for treatment and 13 subjects completed the study. Eight subjects discontinued for other reasons, 2 discontinued due to AEs (see below), and 1 subject withdrew consent. In the MAD study, a total of 70 healthy young participants and 9 elderly individuals received BMS-932481 and 23 received placebo (Table 1). Baseline age, body mass index, and race distribution were similar across the dose groups and across the studies in the healthy young cohorts (Table 1).
TABLE 1.
Demographics of the SAD and MAD studies
Data are given as means (S.D.).
| Study | Participants | Age | Men/Women | Weight (kg) | Body Mass Index | Race (C, B, A, O) |
|---|---|---|---|---|---|---|
| n | yr | kg | kg/m2 | |||
| SAD | ||||||
| Placebo (panels 1–7) | 14 | 29 (7) | 14/0 | 80 (16) | 26.3 (0.8) | 7/3/4/0 |
| Panel 1 (10 mg) | 6 | 27 (5) | 6/0 | 82 (16) | 27.1 (4.0) | 5/1/0/0 |
| Panel 2 (30 mg) | 6 | 39 (5) | 6/0 | 84 (14) | 26.6 (1.9) | 5/1/0/0 |
| Panel 3 (100 mg) | 6 | 32 (7) | 6/0 | 84 (16) | 27.3 (3.7) | 3/0/3/0 |
| Panel 4 (300 mg) | 6 | 32 (7) | 6/0 | 78 (9) | 26.0 (2.6) | 4/1/0/1 |
| Panel 5 (600 mg) | 6 | 31 (9) | 6/0 | 79 (10) | 26.2 (3.3) | 3/3/0/0 |
| Panel 6 (900 mg) | 6 | 30 (5) | 6/0 | 78 (1) | 25.3 (3.3) | 6/0/0/0 |
| Panel 7 (1200 mg) | 6 | 28 (4) | 6/0 | 75 (9) | 24.3 (1.8) | 5/0/0/1 |
| Panels 1–7 | 56 | 31 (7) | 56/0 | 80 (13) | 26.1 (3.0) | 38/9/7/2 |
| Panel 8 (elderly) | ||||||
| 900 mg | 9 | 73 (3) | 6/3 | 75 (16) | 27.4 (3.3) | 6/0/3/0 |
| Placebo | 3 | 75 (4) | 1/2 | 68 (19) | 25.3 (4.1) | 2/0/1/0 |
| Panel 9 | ||||||
| 900 mg | 10 | 30 (6) | 10/0 | 85 (15) | 26.9 (3.4) | 9/0/1/0 |
| Placebo | 5 | 35 (9) | 4/1 | 74 (10) | 25.0 (3.6) | 3/2/0/0 |
| MAD | ||||||
| Placebo | 6 | 38 (12) | 6/0 | 77 (13) | 25.6 (2.7) | 4/1/1/0 |
| Panel 1 (50 mg) | 6 | 44 (8) | 4/2 | 77 (12) | 26.2 (2.9) | 4/1/0/1 |
| Panel 2 (100 mg) | 6 | 41 (10) | 5/1 | 84 (8) | 27.1 (3.0) | 3/1/0/2 |
| Panel 3 (200 mg) | 6 | 48 (4) | 5/1 | 80 (15) | 25.6 (3.7) | 4/2/0/0 |
| Total | 24 | 43 (9) | 20 (4) | 80 (12) | 26.1 (3.0) | 15/5/1/3 |
A, Asian; B, black/African American; C, Caucasian; O, other.
Pharmacokinetic Parameters for Both Studies
Table 2 summarizes the pharmacokinetic properties after single ascending dosing. BMS-932481 was orally absorbed with a median Cmax of 3–5 hours and a mean terminal half-life ranging from 16 to 32 hours in healthy young participants. Between-subjects variability was moderate to high (range, 27%–83%). Panel 4 was designed as a fixed-sequence, crossover, three-period panel to examine food effect (period 2) and gastric pH (panel 3) on exposure. High-fat/high-caloric meals significantly increased Cmax by approximately 2.2-fold, increased AUC by 2.3-fold, and prolonged Tmax by 1.5 hours (Table 2; Supplemental Fig. 1). The change in gastric pH elicited by administration of famotidine produced only modest effects and increased Cmax by 60% with minimal increases in AUC of approximately 15% (Table 2; Supplemental Fig. 1). Elderly subjects who received 900 mg experienced a 3.8-fold (range, 3.4–4.3) higher Cmax and 4.5-fold (2.8- to 5.1-fold) higher AUC than normal healthy young participants (Table 2; Supplemental Fig. 2). CSF exposure was less than 1% of plasma and CSF Tmax occurred at 6 hours after a single 900-mg dose administration (Table 2; Supplemental Fig. 3). CSF and plasma exposure demonstrated good correlation, with r2 of 0.612 and a slope of 114.5 (Supplemental Fig. 3)
TABLE 2.
Summary statistics pharmacokinetic parameters from the SAD study
| SAD Study Panel | Participants |
Cmax |
Tmax
|
AUC0–t |
AUC0–∞ |
t1/2a |
CLT/F |
|---|---|---|---|---|---|---|---|
| GM (CV%) | Median (Minimum–Maximum) | GM (CV%) | GM (CV%) | Mean (S.D.) | GM (CV%) | ||
| n | ng/ml | h | ng⋅h/ml | h | l/h | ||
| Panel 1 (10 mg) | 6 | 11 (50) | 3 (1.5–5) | 128 (76) | 153 (89) | 16 (15) | 66 (60) |
| Panel 2 (30 mg) | 6 | 49 (52) | 4 (3–5) | 895 (45) | 982 (5) | 19 (9) | 31 (65) |
| Panel 3 (100 mg) | 6 | 280 (42) | 4 (2–5) | 4586 (39) | 5143 (52) | 23 (9) | 19 (40) |
| Panel 4 (300 mg, fasted) | 6 | 325 (52) | 3.5 (2–5) | 8574 (46) | 8749 (48) | 20 (10) | 34 (54) |
| Panel 4 (300 mg, high-fat meal) | 6 | 721 (45) | 5 (4–12) | 19,426 (34) | 20,352 (41) | 28 (14) | 15 (31) |
| Panel 5 (600 mg famotidine) | 6 | 512 (57) | 3 (2–5) | 9750 (59) | 10,073 (63) | 23 (14) | 30 (47) |
| Panel 6 (900 mg) | 6 | 748 (32) | 4 (2–5) | 21,878 (33) | 21,440 (37) | 20 (8) | 28 (31) |
| Panel 7 (1200 mg) | 6 | 1597 (29) | 4 (3–6) | 50,104 (58) | 52,245 (56) | 22 (7) | 23 (51) |
| Panel 8 (900 mg; elderly) | 9 | 2870 (24) | 5 (3–12) | 134,138 (38) | 155,797 (43) | 63 (20) | 6 (38) |
| Panel 9 (900 mg; plasma) | 10 | 876 (42) | 4.5 (4–18) | 42,576 (26) | 439,701 (26) | 32 (21) | 20 (24) |
| Panel 9 (900 mg; CSF) | 10 | 6.5 (40) | 6 (4–18) | 97 (41) |
AUC0–t, area under the concentration-time curve from zero to the last quantifiable time; CLT/F, apparent total body clearance; GM (CV%), geometric mean and coefficient of variation.
Calculated at day 25 for 200 mg in the MAD study.
In the MAD study, steady state was achieved by day 14 in the 50- and 100-mg dose groups and maximal concentrations occurred between 5 and 8.5 hours (Table 3). Dosing was terminated on day 24 before steady state was achieved in the 200-mg panel. The accumulation index AUC ranged between 3.1 and 3.3 (Table 3). Day 1 plasma drug concentrations in the MAD study were dose proportional and only slightly more than dose proportional on day 14 (Fig. 2; Table 3).
TABLE 3.
Summary statistics pharmacokinetic parameters from the MAD study
| MAD Study Panel | Participants |
Cmax |
Tmax
|
AUC0–t |
C24 |
t1/2 |
AI AUCτ |
|---|---|---|---|---|---|---|---|
| GM (CV%) | Median (Minimum–Maximum) | GM (CV%) | GM (CV%) | Mean (S.D.) | Mean(S.D.) | ||
| n | ng/ml | h | ng⋅h/ml | ng/ml | |||
| Panel 1 (50 mg) | |||||||
| Day 1 | 6 | 134 (29) | 6 (5–12) | 1521 (25) | 47 (29) | ||
| Day 14 | 5 | 323 (32) | 5 (4–5) | 4216 (57) | 204 (46) | 3.1 (1.2) | |
| Day 28 | 5 | 293 (37) | 5 (2–8) | 4770 (49) | 165 (54) | 24 (10) | 3.3 (1.0) |
| Panel 2 100mg | |||||||
| Day 1 | 6 | 247 (28) | 6 (3–12) | 2912 (16) | 87 (36) | ||
| Day 14 | 6 | 539 (18) | 8.5 (5–12) | 9085 (8) | 308 (12) | 3.1 (0.6) | |
| Day 28 | 6 | 670 (20) | 5 (5–8) | 9080 (10) | 297 (16) | 29 (11) | 3.1 (0.7) |
| Panel 3 (200 mg) | |||||||
| Day 1 | 6 | 707 (19) | 5 (4–6) | 8023 (8.6) | 220 (5.6) | ||
| Day 14 | 6 | 1975 (10) | 5 (4–8) | 31,376 (11) | 1151 (22) | 37 (7)a | 3.9 (0.4) |
AI, accumulation index; AUC0–t, area under the concentration-time curve from zero to the last quantifiable time; C24, concentrations at trough, 24 hours after previous dose.
Predicted.
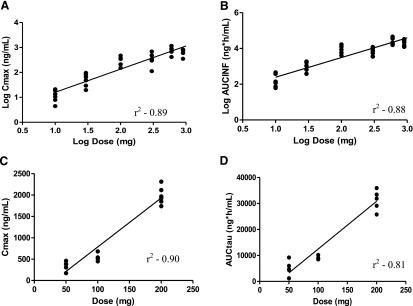
Fig. 2.
Relationship between dose and human pharmacokinetic parameters. (A) Log of Cmax versus log dose on day 1 of the SAD study. (B) Log of AUC0–∞ (AUCINF) versus log dose on day 1 of the SAD study. (C) Cmax versus dose on day 14 of the MAD study. (D) AUCτ (AUCtau) versus dose on day 14 of the MAD study.
Figure 2 highlights the dose proportionality of BMS-932481 in both the SAD and MAD studies. In brief, plasma exposure was dose proportional from 10 to 1200 mg in healthy young volunteers after a single-dose administration and from 50 to 200 mg after the first dose of multiple-dose administrations. In the SAD study, the 95% confidence interval on the slope of log Cmax versus log dose ranged from 0.82 to 1.03, with an r2 of 0.89 (Fig. 2A); the 95% CI for log AUC0–∞ versus log dose ranged from 0.97 to 1.23, with an r2 of 0.88 (Fig. 2B). Figure 2, C and D, summarizes greater than dose proportionality effects from the MAD study observed at day 14. After multiple dosing, the slope was 1.36 (95% CI, 1.06–1.67; r2 = 0.9) for Cmax versus dose and 1.46 (95% CI, 1.03–1.88; r2 = 0.81) for AUCτ. It is acknowledged, however, that steady state was not achieved for the 200-mg daily dose by day 14 and further deviation from dose proportionality may be expected if steady-state exposures were obtained for that dose.
Pharmacodynamic Effects on CSF Aβ Peptides
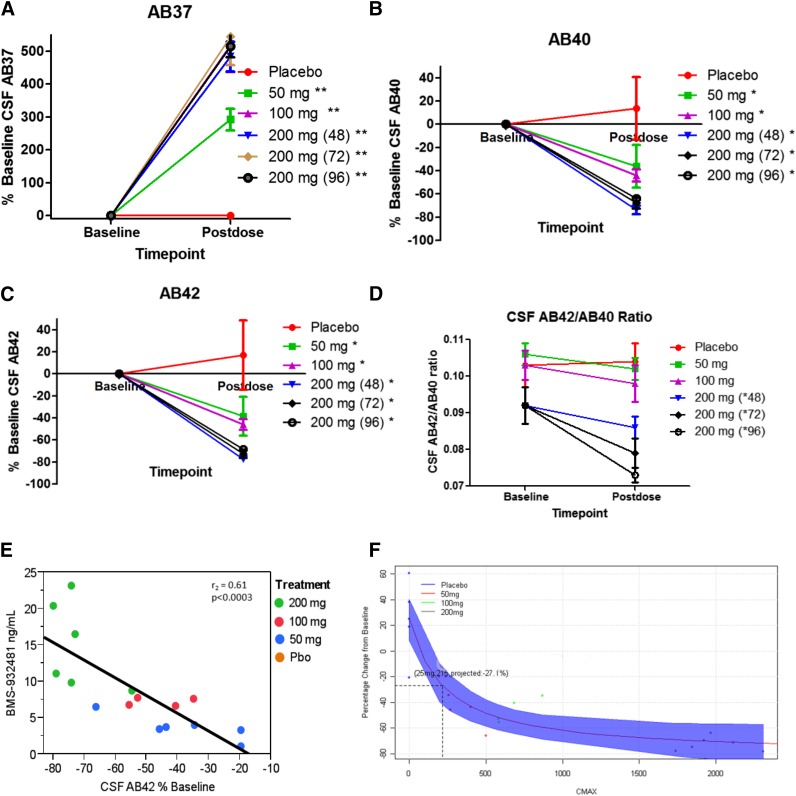
Pharmacodynamic effects on CSF Aβ peptides after the single-dose administration of BMS-932481 are detailed in Toyn et al. (2016). The drug appeared to lower CSF Aβ39 while having no effect on the shorter fragments Aβ14, Aβ15, and Aβ34 in the SAD study (Supplemental Fig. 4). Pharmacodynamic effects were also measured after multiple-dose administration on day 28 in the 50- and 100-mg cohort and also on day 24 at 48, 72, or 96 hours after dosing in the 200-mg cohort. Artifactual placebo increases in hydrophobic Aβ peptides observed with cannulated SAD CSF collection methodology were minimal using single lumbar puncture collection applied in the MAD study. As a result, the percentage change from baseline was calculated with no corrections for placebo rise. BMS-932481 dose-dependently increased CSF Aβ37 by 292%, 518%, and 484%–545% of baseline at 50-mg, 100-mg, and 200-mg, respectively (Fig. 3A). Mean decreases were −36%, −44%, and −64% to 74% in Aβ40 (Fig. 3B) and −38%, −46%, and −69% to 77% in Aβ42 (Fig. 3C) at 50, 100, and 200 mg, respectively. Aβ38 was increased by 20%, 69%, and 68% at the 50-, 100-, and 200-mg doses, respectively (Supplemental Fig. 5A). No effects were noted on Aβ14 or APL1β28 fragments (Supplemental Fig. 5, C and E). However, slight increases in AB17 were noted (Supplemental Figs. 4C and 5F). Four days after the last day of dosing (e.g., 96 hours), CSF Aβ40 and Aβ42 levels were still significantly reduced (Fig. 3, B and C). Trends in lowering of the CSF Aβ42/CSF Aβ40 ratio were noted, especially at the 200-mg dose (Fig. 3D). The correlation between BMS-932481 CSF drug levels and percentage of baseline CSF Aβ42 lowering was significant, with an r2 of 0.61 (Fig. 3E). Using an indirect-response model, CSF Aβ42 lowering was simulated based on exposure-response results from the MAD study (Fig. 3F). Liver toxicity findings (see below) limited further dose escalations and the MAD study was halted after the 200-mg cohort. Dotted lines in Fig. 3F represent what was considered the maximal safely tolerated exposure as a result of the emergent liver toxicity findings and associated degree of Aβ42 lowering. CSF Aβ42 levels were decreased by ≤25% within exposures that were free of liver toxicity findings (Fig. 3F).
Fig. 3.
Pharmacodynamic effects on CSF Aβ peptides after multiple-dose administration of BMS-932481 young healthy volunteers. Pharmacodynamics are expressed as percentage change from baseline. (A) Aβ37. (B) Aβ40. (C) Aβ42. (D) Aβ42/Aβ40 ratio. Values are means ± S.E.M. Analysis of covariance methods were applied to examine significance across groups (n = 6 per group for placebo and 50, 100, and 200 mg for 72 hours; n = 3 per group for 200 mg for 48 and 96 hours). *P < 0.05; **P < 0.01 (versus placebo). Postdose time was 28 days for the placebo and 50- and 100-mg groups. For 200 mg, postdose was 28 (or 24 days for those with emergent liver AEs) plus 48, 72, or 96 hours. (E) Exposure-response relationship of BMS-932481 (in nanograms per milliliter) versus CSF Aβ42% change baseline. (F) Percentage change baseline versus Cmax exposure modeling based on MAD results. The shaded area represents 95% CIs. The dotted box highlights the exposure region associated with no liver toxicity and associated percentage changes in Aβ42.
Pharmacodynamic Effects on Plasma Aβ42, Aβ40, and Total Aβ
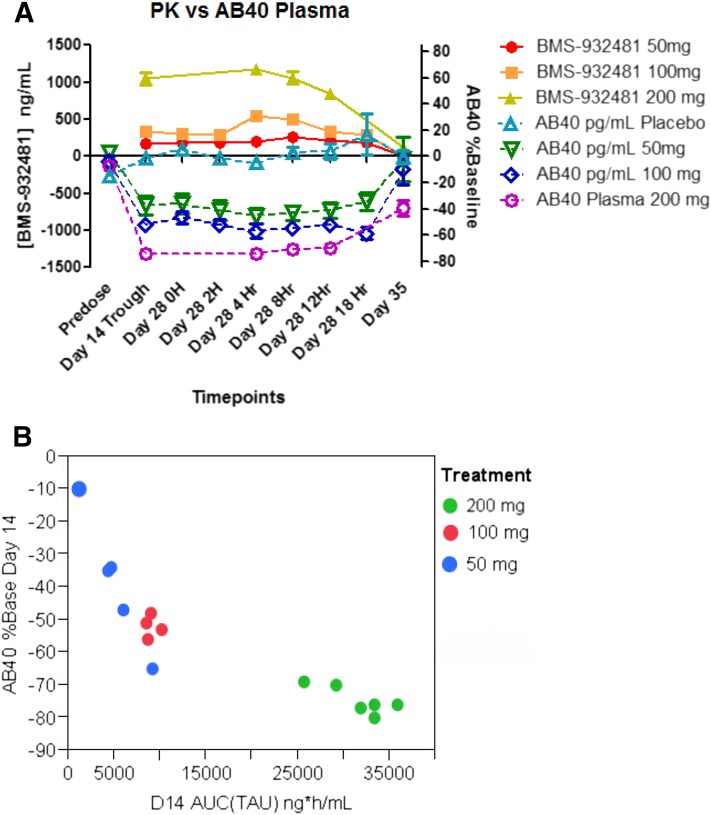
BMS-932481 dose-dependently decreased plasma Aβ42 and Aβ40 after single-dose (Supplemental Fig. 6) and multiple-dose administrations (Fig. 4A). Figure 4A shows the time course of BMS-932481 drug levels (in nanograms per milliliter) versus the percentage of baseline in Aβ40 peptide on day 14 at trough and day 28 over an 18-hour dosing period. Plasma Aβ40 levels decreased up to 40%–50% at the 50-mg dose, the lowest dose tested. No changes in total Aβ were observed across the single or multiple dose cohorts (Supplemental Figs. 6 and 7). Baseline levels of CSF Aβ42 levels were higher in elderly persons than in young participants in the SAD study and the degree of CSFAβ42 lowering was greater in elderly than in young, largely due to higher exposures in elderly participants (Supplemental Fig. 6). Although baseline levels of Aβ42 were measurable in the SAD study, reductions in Aβ42 dropped below the limit of quantitation in the MAD 100- and 200-mg cohorts during the dosing period and the degree of Aβ42 lowering in blood could not be reliably measured at higher doses. However, plasma Aβ42 levels were still reduced on day 35 (11 days after the last dose) by 46% in the 200-mg cohort. Figure 4B shows the relationship between BMS-932481 AUCτ on day 14 and % of baseline Aβ40 at steady state (just prior to dosing on day 14). The exposure-response relationship between total plasma drug and plasma Aβ40 was significant (P < 0.0001), with an r2 of 0.74.
Fig. 4.
BMS-932481 exposure versus plasma Aβ levels in the MAD study. (A) Time course of drug levels and plasma Aβ40 after daily dosing of 50-mg (n = 6 per group), 100-mg (n = 4), and 200-mg (n = 6 per group) doses (n = 6 per group for placebo). Data are presented as means ± S.E.M. (repeated-measures analysis of covariance). P < 0.0001 for decreases in plasma Aβ40 at day 14 all time points through day 28. (B) Correlation between BMS-932481 AUCτ at steady state (day 14) versus percentage change of plasma Aβ40 on day 14.
Pharmacogenomic Effect of ApoE4 and UGT1A1
Supplemental Table 1 summarizes ApoE genotype and baseline CSF and Aβ42 plasma levels across both studies. There were 34 subjects with ApoE genotype and baseline CSF Aβ42 data and 99 subjects with ApoE genotype and baseline plasma Aβ42 from both studies. There was only one subject who was homozygous for ε4ε4. Those subjects with ε3ε4 or ε4ε4 genotypes did not show significant differences in baseline CSF or plasma Aβ42 levels across the studies. However, subjects with one ε2 allele showed trends in higher baseline plasma Aβ42 levels compared with ε3ε3 subjects or ε3ε4 subjects. ApoE genotype had no effect on Aβ pharmacodynamic effects in either study.
Based on nonclinical data, BMS-932481 was considered to be a weak inhibitor of UGT1A1, with an IC50 of 1.34 μM. Some mutations within UGT1A1 result in reduced enzyme function and alterations in bilirubin levels. To better understand the effects of BMS-932481 on bilirubin, subjects homozygous for the UGT1A1 *6 and *28 mutations were excluded from the study. In addition, patients with Gilbert’s syndrome, a condition known to be associated with mutations in UGT1A1 and associated reduced function, were also excluded from both studies. UGT1A1 *6 and *28 heterozygotes were allowed to enroll in both studies. There were 2 UGT1A1 *6 heterozygotes and 64 UGT1A1 *28 heterozygotes in the SAD and MAD studies combined (data not shown). UGT1A1 *28 heterozygotes showed slightly elevated levels in baseline direct, indirect (data not shown) and total bilirubin compared with the wild type (Supplemental Fig. 8). These baseline elevations were minor and not clinically meaningful. There were four patients in the 200-mg cohort who experienced elevations in bilirubin. Only one of the four was heterozygous for UGT1A1 *28. UGT1A1 *6 or *28 alleles were not associated with bilirubin or elevated liver enzyme findings in the MAD study.
4βHC Biomarkers of CYP3A4
BMS-932481 is primarily metabolized by CYP3A4. Preclinical results showed the drug is a time-dependent inhibitor (KI = 82.5 μM, concentration required for half maximal rate of inactivation; kinact = 0.47 min−1, the maximal rate of inactivation) and a weak inducer of CYP3A4 enzyme activity. As a result, intensive pharmacokinetic measurements were conducted and 4βHC, a CYP3A4 generated cholesterol metabolite, was measured in the SAD and MAD studies. In addition, total cholesterol and 4αHC levels were also determined as normalization factors for 4βHC concentration. There were no significant changes observed in 4βHC levels in any of the treatment cohorts in the MAD study, suggesting a minimal effect on CYP3A4 enzyme activity (Supplemental Fig. 8).
Safety/Tolerability
Table 4 summarizes the safety findings from the SAD study. BMS-932481 was generally well tolerated from 10 to 1200 mg and was not associated with apparent safety issues in healthy subjects after a single dose. There were no deaths, SAEs, or discontinuations due to AEs in the SAD study. All AEs were of mild or moderate intensity across both BMS-932481 and placebo groups and the proportion of subjects with any AEs was similar between treated and placebo groups. Drug-related AEs were reported in two participants (3.3%) in the BMS-932418 groups and in one participant (4.5%) in the placebo group. AEs considered to be drug related included sensory disturbance (one subject), headache (one subject), and elevated plasma total bilirubin (one subject). The elevations in total bilirubin (2.5 times the upper limit of normal at 3 days postdose) were attributable to an increase in indirect bilirubin and were asymptomatic and transient (below the upper limit of normal by the follow-up day 13 postdose visit). In the one patient with elevated bilirubin, there were no associated increases in alanine aminotransferase (ALT), aspartate aminotransferase, alkaline phosphatase levels, or any other marked abnormalities. Finally, there were no clinical relevant findings on ECG parameters.
TABLE 4.
Safety summaries in the SAD study
Data are given as n (%).
| All |
Placebo |
BMS-932481 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Treated | NHY | Elderly | CSF | 10 mg | 30 mg | 100 mg | 300 mg P | 300 mg P2 | 300 mg P3 | 600 mg | 900 mg | 1200 mg | 900 mg El | 900 mg C | |
| Participants, n | 22 | 61 | 14 | 3 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Discontinuation due to AEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs | 7 (31.8) | 17 (27.9) | 24 (28.6) | 1 (33.3) | 2 (40.0) | 3 (50.0) | 2 (33.3) | 1 (16.7) | 0 | 0 | 1 (16.7) | 0 | 3 (50.0) | 0 | 0 | 7 (70.0) |
| Drug-related AEs | 1 (4.5) | 2 (3.3) | 1 (7.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (10.0) |
E1, Elderly; NHY, Normal healthy young; P1, P2, P3, cross-over phase 1, 2 or 3.
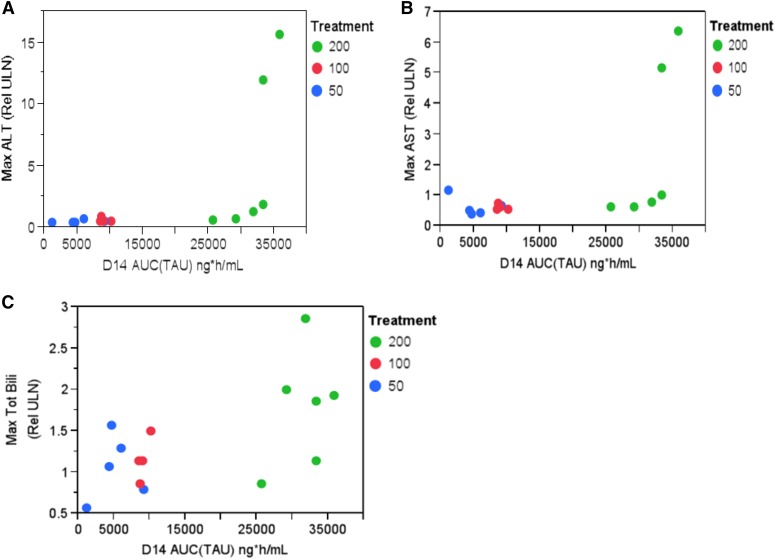
Table 5 summarizes the safety findings from the MAD study. There were no deaths. AEs were reported in 15 subjects (83.3%) and the proportion of subjects with any AEs was similar between the BMS-932481 and placebo groups. Treatment-related AEs were reported in eight subjects (44.4%) in the BMS-932481 group and one (16.7%) in the placebo group. Three of the six subjects (50%) receiving 50 and 200 mg showed low leukocyte counts. Three of six subjects (50%) in the 200-mg group experienced low estimated creatinine clearance. There were no ECG- or vital sign–related AEs. Of note were liver safety findings. Increases in total bilirubin were observed in three of six subjects (50%) receiving 50- and 100-mg doses versus none in the placebo group. Elevations of bilirubin in the 50- and 100-mg dose groups were not associated with elevations in ALT or aspartate aminotransferase, and the relationship between bilirubin and exposure was less clear (Fig. 5C). In the 200-mg cohort, the most frequent liver-related abnormalities were increases in bilirubin (reported in five of six subjects; 83%) and increased ALT (reported in four of six subjects; 66%). Two of the four from the 200-mg cohort were SAEs with grade 3 ALT elevations that met protocol-specified stopping criteria with subsequent study discontinuation. Exposures in these two patients were high (Fig. 5, A and B).
TABLE 5.
Safety summary for all treated subjects in the MAD study
Data are given as n (%).
| Placebo | BMS-932481 |
||||
|---|---|---|---|---|---|
| 50 mg | 100 mg | 200 mg | All | ||
| Participants, n | 6 | 6 | 6 | 6 | 18 |
| Deaths | 0 | 0 | 0 | 0 | 0 |
| SAEs | 0 | 0 | 0 | 2 (33.3) | 2 (11.1) |
| Discontinuation due to AEs | 0 | 0 | 0 | 2 (33.3) | 2 (11.1) |
| AEs | 4 (66.7) | 5 (83.3) | 4 (66.7) | 6 (100.0) | 15 (83.3) |
| Treatment-related AEs | 1 (16.7) | 3 (50.0) | 1 (16.7) | 4 (66.7) | 8 (44.4) |
| Selected marked laboratory abnormalities | |||||
| Hematology | |||||
| Low hemoglobin | 0 | 2 (33.3) | 1 (16.7) | 1 (16.7) | 4 (22.2) |
| Low hematocrit | 0 | 1 (16.7) | 1 (16.7) | 0 | 2 (11.1) |
| Low leukocytes | 1 (16.7) | 3 (50.0) | 1 (16.7) | 3 (50.0) | 7 (38.9) |
| Low lymphocytes | 0 | 1 (16.7) | 0 | 0 | 1 (5.6) |
| Low neutrophils | 0 | 0 | 1 (16.7) | 2 (33.3) | 3 (16.7) |
| Liver and kidney | |||||
| Elevated ALT | 0 | 0 | 0 | 4 (66.7) | 4 (22.2) |
| Elevates AST | 0 | 0 | 0 | 2 (33.3) | 2 (11.1) |
| Elevated bilirubin | 0 | 3 (50.0) | 3 (50.0) | 5 (83.3) | 11 (61.1) |
| Low estimated creatinine clearance | 0 | 0 | 0 | 3 (50.0) | 3 (16.7) |
AST, aspartate aminotransferase.
Fig. 5.
BMS-932481 day 14 AUCτ exposure versus maximum ALT relative to upper limit of normal (A), maximum AST (relative to ULN) (B), and maximal total bilirubin (relative to ULN) (C). AST, aspartate aminotransferase; ULN, upper limit of normal.
Discussion
This study demonstrates that BMS-932481 is an orally absorbed pharmacodynamically active GSM in healthy young and elderly volunteers. The compound showed good dose proportionality after single-dose administration with greater than dose proportionality after multiple dosing. Gastric pH had minimal effect on exposure, whereas food effects were significant. Exposures in elderly patients were higher compared with healthy young adults. CSF and plasma Aβ40 and Aβ42 were significantly decreased after multiple dosing, whereas total Aβ remained relatively unchanged, supporting pharmacodynamic activity in both the central and peripheral compartments. Furthermore, BMS-932481 dose-dependently increased CSF Aβ37 and AB38 while concurrently decreasing CSF Aβ39, confirming γ-modulatory mechanistic activity in the central compartments. After daily 200-mg dosing, two cases of grade 3 elevations in ALT (which resolved after dosing ceased) occurred, resulting in the termination of the MAD study. Although BMS-932481 is clearly an active GSM in humans, it was determined the compound was not an optimal tool to test the hypothesis of Aβ lowering in AD due to an inability to achieve >25% CSF Aβ lowering at safe and tolerated exposures. Other indications might be considered if efficacy for the indication can be achieved at lower doses or if alternative formulations not associated with liver enzyme elevations could be applied.
Consistent with other GSMs within the class, our study showed that BMS-932481 shifts toward a preferential cleavage of Aβ37 with a more modest effect on Aβ38, resulting in an increased ratio of Aβ37/Aβ38 peptides while showing less of a preference for Aβ40 and Aβ42 cleavage with concurrent decreases in these two peptides (reviewed in Tate et al., 2012). In addition, the drug appeared to lower CSF Aβ39 while having no effect on the shorter fragments (Aβ14, Aβ15, APL1B28, and Aβ34). Some increases were noted in Aβ17, which were likely a consequence of α-secretase cleavage of the more predominant CSF Aβ37 and CSF Aβ38 peptides. Although APL1β28 has been described as a surrogate for CSF Aβ42 in AD and GSM modulation has been described for this fragment (Yanagida et al., 2009; Okochi et al., 2010), BMS-932481 had no effect on CSF APL1β28 levels. BMS-932481 activity is in contrast with typical NSAID-derived GSM activity, which tends to favor increases in CSF Aβ38 with concomitant decreases in CSF Aβ42. BMS-932481 pharmacodynamic activity is also in contrast with natural-derived triterpene GSMs, which favor increases in Aβ37 and Aβ39 with decreases in Aβ38 and Aβ42 (Pettersson et al., 2013). BMS-932481 clinical and nonclinical activity is consistent with non-NSAID–derived heterocyclic GSMs that can increase Aβ37 and Aβ38 to varying degrees while lowering Aβ40 and Aβ42 (Pettersson et al., 2013).
It is possible that large increases in the shorter peptides could aberrantly affect Aβ clearance; however, the levels of total Aβ remained unchanged in both the peripheral and central compartments even at the highest doses in both nonclinical and in our human studies (see Toyn et al., 2016). In both preclinical species and humans, the compound reduced Aβ42 to a modestly greater degree compared with Aβ40. However, these differences were not robust in vivo. In patients with early-onset familial AD carrying PSEN1 or PSEN2 mutations, it has been hypothesized that the ratio of Aβ42/Aβ40 may be elevated (Borchelt et al., 1996; Jankowsky et al., 2004), albeit the findings have been controversial (Moonis et al., 2005; Ringman et al., 2008). More recent studies have suggested that fractional turnover rates of soluble Aβ42 relative to Aβ40 may be higher in mutation carriers (Potter et al., 2013). Hypothetically, this could lead to increased deposition in plaques, an apparent increase in Aβ42/Aβ40 ratio in the brain, and reduced recovery in CSF Aβ42, as evidenced by a decreased CSF Aβ42/Aβ40 ratio. Thus, as a class, non-NSAID–derived heterocyclic GSMs could prove useful in patients in which the cleavage preference has shifted toward a pathologic overproduction of Aβ42 relative to shorter Aβ fragments as observed in autosomal dominant AD.
Pharmacogenomic effects on both exposure and pharmacodynamics were also investigated in this study and included genotyping for ApoE4 alleles and for UGT1A1 *6 and *28 alleles. Previous studies examining the relationship between ApoE genotype and Aβ42 levels have been reported reduced levels in patients with AD with one or more ε4 alleles and increased levels with one or more ε2 alleles (Morris et al., 2010). In our study, there were too few patients to see any clear trends in CSF Aβ42 levels. However, carriers of an ε2 allele did show significantly higher levels of plasma Aβ42 compared with ε3ε3 or ε3ε4 carriers. In addition, elderly patients showed higher plasma Aβ42 levels compared with healthy young participants. Thus, age and ApoE genotype appear to affect baseline plasma Aβ42 levels. The ApoE genotype did not affect the Aβ pharmacodynamic response to drug.
BMS-932481 is a weak inhibitor of UGT1A1 (IC50 = 1.34 μM), an enzyme responsible for transforming small lipophilic molecules such as bilirubin into water-soluble excretable metabolites. Subjects with mutations in the gene can experience problems with bilirubin metabolism because UGT1A1 is the sole enzyme responsible for bilirubin metabolism. In addition, patients with mutations in UGT1A1 can be susceptible to drug toxicity as a result of glucuronidation inhibition. Because BMS-932481 was a weak inhibitor of UGT1A1 and did elevate bilirubin at very high doses in nonclinical studies, patients with Gilbert’s syndrome or those who are nonsymptomatic homozygous for either of the *6 or *28 polymorphisms were excluded from the current phase I studies. Although levels of bilirubin levels were slightly higher in UGT1A1 *28 heterozygotes in our studies, the elevations were not clinically significant and there were no clear associations between patients who developed elevated bilirubin levels as a result of BMS-932481 administration and UGT1A1 *28 genotype. Thus, UGT1A1 *28 may have limited predictive power in identifying those at risk of developing elevations in bilirubin as a result of BMS-932481 treatment.
Previous preclinical reports showed that use of the cholesterol metabolite 4βHC might be a good indicator of alterations in CYP3A4 function in vivo (Diczfalusy et al., 2011). BMS-932481 is primarily metabolized by CYP3A4. Nonclinical studies suggested that BMS-932481 was both a time-dependent inhibitor and a potential weak inducer of CYP3A4. Plasma αHC and 4βHC along with cholesterol were examined in the study to test whether drug levels may be indirectly altering CYP3A4 activity. Preliminary data showed no changes in 4βHC levels over time, suggesting that CYP3A4 activity was not greatly affected by BMS-932481 administration.
Although BMS-932481 was relatively safe and well tolerated after single-dose administrations, liver-related AEs after multiple-dose testing resulted in cessation of the study. In brief, two subjects receiving daily 200-mg doses showed grade 3 elevations in ALT, meeting stopping criteria for the study. In addition, the incidence of increases in bilirubin was evident in both the single- and multiple-dose studies. After we unblinded the study, we observed that the frequency of minor elevations in liver enzymes in the 100- and 200-mg cohorts of the multiple-dose study was higher compared with placebo-treated subjects. As a result, further clinical development was halted due to an insufficient safety and tolerability margin to test the hypothesis for efficacy of Aβ modulation in AD. There did not appear to be a clear relationship between elevations in bilirubin and elevated enzyme levels, as elevations in bilirubin were not always accompanied by increases in liver enzymes. In the two cases that showed concurrent increases in liver enzymes and bilirubin, the increases did not occur at the same time. There did appear to be a clearer relationship between exposure and elevations in liver enzymes because patients with the highest exposures also showed increases in liver enzymes. The discrepancy between increased bilirubin and elevated liver enzymes suggests that these two events may be the result of two discrete mechanisms. The off-target but pharmacologic inhibition of UGT1A1 is likely to be the cause of elevated bilirubin, whereas exposure-related hepatic xenobiotic burden may be the cause of the elevated liver enzymes. However, exposures associated with >50% CSF Aβ42 reduction were associated with an increased rate of bilirubin elevations and abnormal liver enzyme elevations. In all cases, liver abnormalities resolved once subjects stopped taking the drug. Nevertheless, there was low confidence that BMS-932481 could be a suitable tool to test the hypothesis for the efficacy of Aβ lowering in AD.
In summary, these studies show pharmacodynamic activity of a GSM after multiple-dose administration in healthy volunteers and highlight the importance of mechanistic biomarkers to inform dose selection and clinical development decisions. Although ≥50% CSF Aβ42 lowering could not be achieved at safe and tolerated exposures with BMS-932481, these results support the further development of well tolerated GSMs for testing as therapeutics for AD or other diseases in which γ modulation may be a component of the disease etiology. In addition, alternative formulations may mitigate some of the safety issues identified in our studies using oral dose administration.
Supplementary Material
Acknowledgments
The authors thank many additional colleagues who contributed to this work, including Terrye Delmonte, Lester Hui for pharmacogenomic contributions; Adela Buzescu, Shenita Basdeo, and Hao Jiang for pharmacokinetic assay support; Madhushree Gokhale for critical formulation support; Dr. Pankaj Mehta for rabbit polyclonal Aβ1–Aβ37 antibodies; and Michael Belcourt, Wai Chan, and James Hazel for operational support.
Abbreviations
- 4αHC
4α-hydroxycholesterol
- 4βHC
4β-hydroxycholesterol
- 95% CI
95% confidence interval
- AD
Alzheimer’s disease
- AE
adverse event
- ALT
alanine aminotransferase
- Apo
apolipoprotein
- APP
amyloid-β precursor protein
- AUC
area under the concentration-time curve
- BMS-708163
(2R)-2-[(4-chlorophenyl)sulfonyl-[[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide
- BMS-932481
(S)-7-(4-fluorophenyl)-N2-(3-methoxy-4-(3-methyl-1H-1,2,4-triazol-1-yl)phenyl)-N4-methyl-6, 7-dihydro-5H-cyclopenta[d]pyrimidine-2,4-diamine
- CSF
cerebrospinal fluid
- ECG
electrocardiogram
- GSM
γ-secretase modulator
- LC
liquid chromatography
- LY450139
(2S)-2-hydroxy-3-methyl-N-((1S)-1-methyl-2-{[(1S)-3-methyl-2-oxo-2,3,4,5-tetrahydro-1H-3-benzazepin-1-yl]amino}-2-oxoethyl)butanamide)
- MS/MS
tandem mass spectrometry
- NSAID
nonsteroidal anti-inflammatory drug
- PS
presenilin
- SAE
serious adverse event
Authorship Contributions
Participated in research design: Soares, Gasior, Wang, Hong, Berman, Ahlijanian, AbuTarif.
Conducted experiments: Soares, Toyn, Wang, Hong, Berisha, Furlong, Raybon, Sweeney, Zheng, Akinsanya, Morrison, Drexler.
Contributed new reagents or analytic tools: Thompson, Olson, Macor.
Performed data analysis: Soares, Gasior, Toyn, Wang, Hong, Berisha, Raybon, Lentz, Berman, Thompson, Olson, Morrison, Drexler, Macor, Albright, Ahlijanian, AbuTarif.
Wrote or contributed to the writing of the manuscript: Soares, Gasior, Hong.
Footnotes
All Bristol-Myers Squibb authors were, at the time of the study, full-time employees and holders of Bristol-Myers Squibb stock. Drs. Gasior, Berisha, Berman, Hong, Wang, and Furlong have transitioned to other companies since study completion.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Alzheimer’s Association (2014) 2014 Alzheimer’s disease facts and figures. Alzheimers Dement 10:e47–e92. [DOI] [PubMed] [Google Scholar]
- Barber RC. (2012) The genetics of Alzheimer’s disease. Scientifica (Cairo) 2012:246210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten DM, Meredith JE, Jr, Zaczek R, Houston JG, Albright CF. (2006) Gamma-secretase inhibitors for Alzheimer’s disease: balancing efficacy and toxicity. Drugs R D 7:87–97. [DOI] [PubMed] [Google Scholar]
- Beher D, Wrigley JD, Owens AP, Shearman MS. (2002) Generation of C-terminally truncated amyloid-beta peptides is dependent on gamma-secretase activity. J Neurochem 82:563–575. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, et al. (1996) Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 17:1005–1013. [DOI] [PubMed] [Google Scholar]
- Borgegard T, Juréus A, Olsson F, Rosqvist S, Sabirsh A, Rotticci D, Paulsen K, Klintenberg R, Yan H, Waldman M, et al. (2012) First and second generation γ-secretase modulators (GSMs) modulate amyloid-β (Aβ) peptide production through different mechanisms. J Biol Chem 287:11810–11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JP, Bennett CE, McCracken TM, Mazzola RD, Bara T, Buevich A, Burnett DA, Chu I, Cohen-Williams M, Josein H, et al. (2010) Iminoheterocycles as gamma-secretase modulators. Bioorg Med Chem Lett 20:5380–5384. [DOI] [PubMed] [Google Scholar]
- Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, Soininen H, Thein S, Shiovitz T, Pilcher G, et al. (2012) Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol 69:1430–1440. [DOI] [PubMed] [Google Scholar]
- De Strooper B. (2003) Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron 38:9–12. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Iwatsubo T, Wolfe MS. (2012) Presenilins and γ-secretase: structure, function, and role in Alzheimer disease. Cold Spring Harb Perspect Med 2:a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo M, Mollenhauer B, Bertolotto A, Engelborghs S, Hampel H, Simonsen AH, Kapaki E, Kruse N, Le Bastard N, Lehmann S, et al. (2012) Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: an update. Biomarkers Med 6:419–430. [DOI] [PubMed] [Google Scholar]
- Diczfalusy U, Nylén H, Elander P, Bertilsson L. (2011) 4β-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol 71:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, et al. Alzheimer’s Disease Cooperative Study Steering Committee. Semagacestat Study Group (2013) A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med 369:341–350. [DOI] [PubMed] [Google Scholar]
- Dries DR, Yu G. (2008) Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer’s disease. Curr Alzheimer Res 5:132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, Farlow MR, Galvin JE, Peskind ER, Quinn JF, et al. (2008) Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol 65:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Koo EH, Felsenstein KM, Osborne BA, Miele L. (2013) γ-Secretase inhibitors and modulators. Biochim Biophys Acta 1828:2898–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough AK, Onorato JM, Ouyang Z, Chang S, Rodrigues AD, Kasichayanula S, Huang SP, Turley W, Burrell R, Bifano M, et al. (2011) Quantification of 4-beta-hydroxycholesterol in human plasma using automated sample preparation and LC-ESI-MS/MS analysis. Chem Res Toxicol 24:1575–1585. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. (1993) Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 75:1039–1042. [DOI] [PubMed] [Google Scholar]
- Ishii K, Ii K, Hasegawa T, Shoji S, Doi A, Mori H. (1997) Increased A beta 42(43)-plaque deposition in early-onset familial Alzheimer’s disease brains with the deletion of exon 9 and the missense point mutation (H163R) in the PS-1 gene. Neurosci Lett 228:17–20. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. (1994) Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron 13:45–53. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, et al. (2004) Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13:159–170. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, et al. (2012) A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488:96–99. [DOI] [PubMed] [Google Scholar]
- Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, et al. (2010) Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron 67:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Llano DA, Ellis T, LeBlond D, Bhathena A, Jhee SS, Ereshefsky L, Lenz R, Waring JF. (2012) Effect of human cerebrospinal fluid sampling frequency on amyloid-β levels. Alzheimers Dement 8:295–303. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, Cutler N, Dufour-Rainfray D, Fagan AM, Heegaard NH, et al. Alzheimer’s Association QC Program Work Group (2013) CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement 9:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani Y, Yarimizu J, Saita K, Uchino H, Akashiba H, Shitaka Y, Ni K, Matsuoka N. (2012) Differential effects between γ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J Neurosci 32:2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonis M, Swearer JM, Dayaw MP, St George-Hyslop P, Rogaeva E, Kawarai T, Pollen DA. (2005) Familial Alzheimer disease: decreases in CSF Abeta42 levels precede cognitive decline. Neurology 65:323–325. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. (2010) APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M, Tagami S, Takeda M. (2010) Analysis of APL1beta28, a surrogate marker for Alzheimer Abeta42, indicates altered precision of gamma-cleavage in the brains of Alzheimer disease patients. Neurodegener Dis 7:42–45. [DOI] [PubMed] [Google Scholar]
- Olsauskas-Kuprys R, Zlobin A, Osipo C. (2013) Gamma secretase inhibitors of Notch signaling. Onco Targets Ther 6:943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson M, Stepan AF, Kauffman GW, Johnson DS. (2013) Novel γ-secretase modulators for the treatment of Alzheimer’s disease: a review focusing on patents from 2010 to 2012. Expert Opin Ther Pat 23:1349–1366. [DOI] [PubMed] [Google Scholar]
- Pica-Mendez AM, Tanen M, Dallob A, Tanaka W, Laterza OF. (2010) Nonspecific binding of Aβ42 to polypropylene tubes and the effect of Tween-20. Clin Chim Acta 411:1833. [DOI] [PubMed] [Google Scholar]
- Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W, Mawuenyega K, Blazey T, Goate A, Chott R, et al. (2013) Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med 5:189ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, et al. (2005) Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci 25:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Younkin SG, Pratico D, Seltzer W, Cole GM, Geschwind DH, Rodriguez-Agudelo Y, Schaffer B, Fein J, Sokolow S, et al. (2008) Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology 71:85–92. [DOI] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. (2009) gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 29:13042–13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayev R, D’Adamio L. (2012) Inhibition of γ-secretase worsens memory deficits in a genetically congruous mouse model of Danish dementia. Mol Neurodegener 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate B, McKee TD, Loureiro RM, Dumin JA, Xia W, Pojasek K, Austin WF, Fuller NO, Hubbs JL, Shen R, et al. (2012) Modulation of gamma-secretase for the treatment of Alzheimer’s disease. Int J Alzheimers Dis 2012:210756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen CE, Petzold A, Bennett JL, Berven FS, Brundin L, Comabella M, Franciotta D, Frederiksen JL, Fleming JO, Furlan R, et al. (2009) A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73:1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyn J. (2015) What lessons can be learned from failed Alzheimer’s disease trials? Expert Rev Clin Pharmacol 8:267–269. [DOI] [PubMed] [Google Scholar]
- Toyn JH, Ahlijanian MK. (2014) Interpreting Alzheimer’s disease clinical trials in light of the effects on amyloid-β. Alzheimers Res Ther 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyn JH, Raybon J, Meredith JE, Robertson AS, Guss V, Hoque N, Sweeney F, Zhuo X, Clarke W, Snow K, et al. (2016) Robust translation of GSM pharmacology across preclinical species and human subjects. J Pharmacol Exp Ther 358:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, Parnetti L, Perret-Liaudet A, Shaw LM, Teunissen C, et al. (2012) Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 8:65–73. [DOI] [PubMed] [Google Scholar]
- Wan Z, Hall A, Jin Y, Xiang JN, Yang E, Eatherton A, Smith B, Yang G, Yu H, Wang J, et al. (2011a) Pyridazine-derived γ-secretase modulators. Bioorg Med Chem Lett 21:4016–4019. [DOI] [PubMed] [Google Scholar]
- Wan Z, Hall A, Sang Y, Xiang JN, Yang E, Smith B, Harrison DC, Yang G, Yu H, Price HS, et al. (2011b) Pyridine-derived γ-secretase modulators. Bioorg Med Chem Lett 21:4832–4835. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, et al. (2001) A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature 414:212–216. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Okochi M, Tagami S, Nakayama T, Kodama TS, Nishitomi K, Jiang J, Mori K, Tatsumi S, Arai T, et al. (2009) The 28-amino acid form of an APLP1-derived Abeta-like peptide is a surrogate marker for Abeta42 production in the central nervous system. EMBO Mol Med 1:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.