Abstract
The identification of sigma receptor (σR) subtypes has been based on radioligand binding and, despite progress with σ1R cellular function, less is known about σR subtype functions in vivo. Recent findings that cocaine self administration experience will trigger σR agonist self administration was used in this study to assess the in vivo receptor subtype specificity of the agonists (+)-pentazocine, PRE-084 [2-(4-morpholinethyl) 1-phenylcyclohexanecarboxylate hydrochloride], and 1,3-di-o-tolylguanidine (DTG) and several novel putative σR antagonists. Radioligand binding studies determined in vitro σR selectivity of the novel compounds, which were subsequently studied for self administration and antagonism of cocaine, (+)-pentazocine, PRE-084, or DTG self administration. Across the dose ranges studied, none of the novel compounds were self administered, nor did they alter cocaine self administration. All compounds blocked DTG self administration, with a subset also blocking (+)-pentazocine and PRE-084 self administration. The most selective of the compounds in binding σ1Rs blocked cocaine self administration when combined with a dopamine transport inhibitor, either methylphenidate or nomifensine. These drug combinations did not decrease rates of responding maintained by food reinforcement. In contrast, the most selective of the compounds in binding σ2Rs had no effect on cocaine self administration in combination with either dopamine transport inhibitor. Thus, these results identify subtype-specific in vivo antagonists, and the utility of σR agonist substitution for cocaine self administration as an assay capable of distinguishing σR subtype selectivity in vivo. These results further suggest that effectiveness of dual σR antagonism and dopamine transport inhibition in blocking cocaine self administration is specific for σ1Rs and further support this dual targeting approach to development of cocaine antagonists.
Introduction
Two subtypes of σ receptors (σRs), σ1 and σ2, have been identified based largely on radioligand binding assays. These assays use [3H](+)-pentazocine for σ1Rs or [3H]1,3-di-o-tolylguanidine (DTG), with a σ1R-selective cold ligand added to mask σ1Rs, for σ2Rs (Hellewell et al., 1994). Although evidence supports the selectivity of (+)-pentazocine (Hellewell et al., 1994; Hiranita et al., 2013b), as well as PRE-084 [2-(4-morpholinethyl) 1-phenylcyclohexanecarboxylate hydrochloride] (Garcés-Ramírez et al., 2011), for σ1Rs, there are few identified σ2R-selective ligands (Matsumoto, 2007). Moreover, identification of in vivo functional agonists and antagonists at either σR subtype has historically been problematic.
Recent studies indicated that the σ1R is a unique intracellular chaperone protein that, with cellular stress, dissociates from binding immunoglobulin protein (BiP or Grp78) at the endoplasmic reticulum and translocates to other cellular compartments (Hayashi and Su, 2001, 2007). In addition, dissociation of the σ1R from BiP can be induced by ligand binding. For example, the σ1R ligands, PRE-084 and (+)-pentazocine, produced dose-dependent dissociation of σ1R from BiP, whereas other σ1R ligands (NE-100 [4-methoxy-3-(2-phenylethoxy)-N,N-dipropylbenzeneethanamine monohydrochloride], haloperidol) were inactive but inhibited the dissociation produced by (+)-pentazocine (Hayashi and Su, 2001, 2007). These results suggest an in vitro basis for distinguishing between σ1R agonists and antagonists.
A more recent suggestion (Xu et al., 2011) that the σ2R was actually the progesterone receptor membrane component-1 was promising as a potential advance in σ2R pharmacology, although the most current evidence suggests that the two proteins are distinct and derived from different genes (Abate et al., 2015; Chu et al., 2015). Consequently, the identification of a σ2 target protein, which would facilitate advances in the in vivo pharmacology of σ2Rs, remains elusive.
The novel σ2R selective compound, CB-64D [(+)-1R,5R-(E)-8-benzylidene-5-(3-hydroxyphenyl)-2-methylmorphan-7-one], released calcium in human neuroblastoma cells (Vilner and Bowen, 2000) and produced cell death in tumor cell lines (Crawford and Bowen, 2002), suggesting that it was an agonist. These effects were well characterized pharmacologically showing stereoselectivity and antagonism by BD 1047 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide) and BD 1063 (1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride) (Vilner and Bowen, 2000). The selectivity in radioligand binding studies suggested that CB-64D has utility for characterizing agonist–antagonist interactions at σ2Rs. However, its high affinity for µ-opioid receptors (Bertha et al., 1994) renders it poorly suited for in vivo studies. A variety of novel σ2R-selective ligands were recently characterized in vitro using cell-viability and caspase-3 activation assays (Zeng et al., 2014). The compounds were classified as agonists, partial agonists, or antagonists based on their effectiveness compared with that of the putative σ2R-selective ligand, siramesine, although agonist–antagonist interactions were not reported (Zeng et al., 2014).
Studies of in vivo antagonism and in particular the ability to differentiate agonist from antagonist activities at either σR subtype have encountered difficulties due in large part to the absence of validated functional assays. Dystonia in rats produced by microinjections of σR ligands into the red nucleus, a motor area with a high density of σRs, was considered an in vivo σR agonist effect. Two σR antagonists, BD 1047 and BD 1063, had little effect of their own but attenuated the dystonia produced by microinjections of DTG (Matsumoto et al., 1995). Complicating the picture is haloperidol. The in vitro evidence suggests that haloperidol is a σR antagonist (Hayashi and Su, 2007); however, it produced dystonia in rats comparable to that of DTG on microinjection into the red nucleus (Walker et al., 1988). Studies of several acute toxic effects of cocaine also show promise for differentiating agonist or antagonist effects of σR ligands (Matsumoto et al., 2014). Although the acute toxicity produced by cocaine may in part be mediated by σRs (Lever et al., 2016), the complex pharmacology of cocaine and the cocaine-antagonist effects of both σ1R and σ2R ligands renders these outcomes less than definitive. Thus, an in vivo procedure for definitively characterizing subtype-selective σR agonist and antagonist activity has not yet been widely accepted.
The present study exploited a recent finding that subjects with cocaine self administration experience will also self administer the σR ligands (+)-pentazocine, PRE-084, and DTG (Katz et al., 2016) to examine in vivo potential antagonist effects and selectivity of several novel ligands (Fig. 1). Rats trained to self administer cocaine under fixed-ratio (FR) schedules continued to respond unabated when either (+)-pentazocine, PRE-084, or DTG was substituted for cocaine, although responding decreased with vehicle substitution. Further, pretreatment with several known nonselective σR antagonists blocked σR ligand self administration (Katz et al., 2016). This study used radioligand binding to assess subtype selectivity, and self administration in an attempt to distinguish subtype-selective agonist or antagonist effects of the novel σR ligands. Using these results, a pattern for σR subtype-selective effects became evident.
Fig. 1.
Chemical structures of the novel σR ligands used in these studies.
Materials and Methods
σ1R and σ2R Binding Assays.
Guinea pig brain tissue was thawed on ice, homogenized (with a glass and Teflon apparatus) in buffer, and subsequently centrifuged at 800g for 10 minutes at 4°C. The supernatant was collected into a clean centrifuge tube and the remaining pellet was resuspended by vortex in 10 ml buffer (tissue) and centrifuged at 800g for 10 minutes at 4°C. The supernatants were pooled and centrifuged at 50,000g for 15 minutes at 4°C. The remaining pellet was resuspended 80 mg/ml, original wet weight (OWW), in buffer and vortexed. The tissue suspension was incubated at 25°C for 15 minutes and then centrifuged at 50,000g for 15 minutes. The supernatant was decanted and the pellet was gently resuspended in buffer to 80 mg/ml OWW. Incubations were conducted in polypropylene assay tubes containing 0.50 ml buffer, 1.4 nM radioligand [and 200 nM (+)-pentazocine for σ2 binding], tissue, and various concentrations of inhibitors. See Table 1 for details.
TABLE 1.
Specific conditions used for studies of displacement of radioligands by the compounds under study
| Assay | Radiolabel | Tissue | Incubation Buffer | Incubation | Nonspecific Binding |
|---|---|---|---|---|---|
| σ1R | 3.0 nM [3H](+)-pentazocine (Perkin-Elmer, Boston, MA) | 8.0 mg/tube, frozen guinea pig brains excluding the cerebella (Pel Freez Biologicals, Rogers, AR) | 10 mM Tris-HCl with 0.32 M sucrose, pH 7.4 | 120 min at room temperature | 10 μM haloperidol |
| σ2R | 3.0 nM [3H]DTG (Perkin-Elmer) with 200 nM (+)-pentazocine | 8.0 mg/tube, frozen guinea pig brains excluding the cerebella (Pel Freez Biologicals) | 10 mM Tris-HCl with 0.32 M sucrose, pH 7.4 | 120 min at room temperature | 100 μM haloperidol |
| DAT | 0.50 nM [3H]WIN 35,428 (Perkin-Elmer) | 1.0 mg/tube, frozen striatum (from male Sprague-Dawley rats brains supplied on ice; Bioreclamation, Hicksville, NY) | Modified sucrose phosphate buffer (0.320 M sucrose, 7.74 mM Na2HPO4, 2.26 mM NaH2PO4, pH adjusted to 7.4) | 120 min on ice | 100 µM cocaine HCl |
WIN 35,428, (−)-2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane-1,5-napthalenedisulfonate.
Dopamine Transporter Binding Assay.
Tissue was dissected and homogenized in buffer using a Brinkmann Polytron (Brinkmann Instruments, Westbury, NY) at setting 6 for 20 seconds, and subsequently centrifuged at 20,000g for 10 minutes at 4°C. The resulting pellet was resuspended in buffer, recentrifuged and suspended in buffer again to a concentration of 10 mg/ml OWW. Incubations were conducted in assay tubes containing 0.50 ml buffer, 0.50 nM radioligand, tissue, and various concentrations of inhibitors. See Table 1 for details.
The reactions in all binding assays were started with the addition of tissue and terminated by rapid filtration through Whatman GF/B filters (presoaked in 0.050% polyethylenimine) using a Brandel Cell Harvester (Brandel Instruments, Gaithersburg, MD). The filters were washed twice with 5.0 ml cold buffer and transferred to scintillation vials, to which Beckman Ready Safe scintillation cocktail (3.0 ml; Beckman Coulter Instruments, Fullerton, CA) was added. The vials were assessed for radioactivity the next day using a Beckman LS6000 liquid scintillation counter (Beckman Coulter Instruments) at 50% efficiency. Assays were typically conducted as three or more independent experiments, each performed with triplicate tubes.
The IC50 values for the displacement of radioligands were computed using a nonlinear, least-squares regression analysis for competitive binding (GraphPad Prism Software Inc., San Diego, CA). Inhibition constants (Ki values) were calculated using the Cheng–Prusoff equation (Cheng and Prusoff, 1973), with the IC50 value of inhibitors used in the assay and the Kd value of the radioligand previously determined in this laboratory.
Behavioral Procedures.
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) served as subjects. The subjects were acclimated to the housing facility, which was temperature- and humidity-controlled and maintained on a 12-hour/12-hour light/dark cycle (lights on at 07:00 hours). Subjects were fed Scored Bacon-Lover Treats (Bio-Serv, Frenchtown, NJ), with water continuously available in home cages. Weights of subjects were maintained at approximately 320 g by adjusting daily food rations. Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program, Animal Care and Use Program, which is fully accredited by AAALAC International.
Experimental sessions were conducted with subjects placed in operant-conditioning chambers (25.5 × 32.0 × 25.0 cm; modified ENV-008CT; Med Associates, St. Albans, VT) that were enclosed within ventilated sound-attenuating cubicles, supplied with white noise to mask extraneous sounds. On the front wall of each chamber were two response levers (5.0 cm from the midline and 4.0 cm above the grid floor) with a row of three light-emitting diodes above each. A downward displacement of either lever with a force exceeding approximately 20 g defined a response and produced an audible “feedback” click. Food pellets (20-mg food pellets; Bio-Serv) were delivered by a dispenser (ENV-203; Med Associates) to a receptacle behind an opening (5.0 × 5.0 cm) in the front wall midway between the two levers and 2.0 cm above the floor. A syringe driver (model 22; Harvard Apparatus, Holliston, MA) containing a 10-ml syringe delivered injections. The syringe was connected by tubing to a fluid swivel (375 Series Single Channel Swivels; Instech Laboratories, Inc., Plymouth Meeting, PA) mounted on a balance arm above the chamber. Tubing, protected by a metal surrounding spring, connected the swivel to the subject’s catheter.
Subjects were placed in chambers during daily experimental sessions in which subjects were initially trained with food reinforcement to press the right lever under a FR 5–response schedule of reinforcement (each fifth response produced a food pellet). During these sessions, the lights above the right lever were illuminated when food presentations were available. Completion of each FR requirement turned off lights, delivered food, and was followed by a 20-second timeout (TO) period during which all lights were off and responses had no scheduled consequences other than the feedback click. After the TO, the lights were illuminated and the FR schedule was again in effect. Sessions lasted 20 minutes or until 30 food pellets were delivered.
After subjects were responding reliably, they were divided into two groups. One group (n = 19) continued with food reinforcement; subjects in the other group (n = 30) were surgically implanted under anesthesia (ketamine/xylazine, 60.0/12.0 mg/kg, i.p.) with chronic indwelling catheters in the right or left external jugular vein. Catheters were externalized in the midscapular region. Catheters were infused daily with a heparin (30.0 IU/ml) and penicillin G potassium (250,000 IU/ml) solution in 0.1 ml sterile saline to minimize the likelihood of infection and clot or fibroid formation. All animals were allowed to recover from surgery for approximately 1 week before cocaine self administration studies were initiated.
Cocaine self administration sessions lasted 2 hours during which lights above the right lever were illuminated when cocaine injections were available. Completion of the FR 5 turned off lights and delivered 1.0 mg/kg cocaine HCl. A 20-second TO, during which lights were off and responses produced only feedback clicks, started with the injection. After the TO, the lights were illuminated and the FR schedule was again in effect. With stable responding, the session was divided into five 20-minute components, each preceded by a 2-minute TO, allowing the assessment of a different cocaine dose within each component (Schenk, 2002; Barrett et al., 2004; Hiranita et al., 2009). The cocaine dose per injection was incremented in the five sequential components in an ascending order by adjusting infusion volumes and durations, as follows: no injection (also referred to as extinction, or EXT, because responses had no scheduled consequences other than the feedback click and turning off the lights for 20 seconds), 0.03, 0.10, 0.32, and 1.0 mg/kg per injection. Infusion volumes (and durations) producing those doses were, respectively, 0 μl (0 seconds), 5.6 μl (0.32 seconds), 18.0 μl (1.0 seconds), 56.0 μl (3.2 seconds), and 180 μl (10 seconds), based on a body weight of 0.32 kg. A response-independent “sample” injection of cocaine at the corresponding dose was administered at the start of each component.
Training continued until 1) at least 5.0 mg/kg cocaine was self administered within a session with less than 20% variation in the total number of cocaine injections (or food presentations) compared with the previous session, 2) the dose of cocaine (or amount of food) that maintained maximal response rates varied by no more than one-half log unit (or number of food pellets) over two consecutive sessions, and 3) maximum response rates were at least 2-fold higher than response rates maintained during EXT.
The schedule of food reinforcement was also modified to resemble that for cocaine self administration, with five sequential 20-minute components, each preceded by a 2-minute TO. In the first of the five components, each fifth response produced only the stimulus change that accompanied food presentation and a 20-second TO. In the subsequent four components, each completion of the FR 5 requirement produced one, two, three, and four 20-mg food pellets. Subjects were fed a daily ration of food (approximately 15 g, Harlan Rodent Chow; Harlan Laboratories, Indianapolis, IN) 60 minutes before sessions, so that their response rates approached those maintained by cocaine. The goal was to have the food-reinforcement comparator procedurally and functionally as similar as possible to the drug self administration procedure. As shown previously with this procedure, the rates of food-reinforced responding were an inverted U–shaped function of food amount (Hiranita et al., 2013b; Li et al., 2013).
Once performances were stable across successive sessions, the effects of substitutions for cocaine of saline or σR ligands were assessed, with a minimum of at least 72 hours between test sessions. That interval was based on in vivo metabolism studies available for several of the present compounds (Kaushal et al., 2011; James et al., 2012; Seminerio et al., 2012). The σR agonists studied were DTG, PRE-084, and (+)-pentazocine at doses specified in the figures. The novel or less studied σR ligands examined were AZ 66 [3-[4-(4-cyclohexylpiperazine-1-yl)pentyl]-6-fluorobenzo[d]thiazole-2(3H)-one hydrochloride], CM 304 [3-(2-(azepan-1-yl)ethyl)-6-(3-fluoropropyl)benzo[d]thiazol-2(3H)-one hydrochloride], CM 353 [1-(4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-3-(4-fluorophenyl)-1H-benzo[d]imidazol-2(3H)-one hydrochloride], CM 398 [1-(4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-3-methyl-1H-benzo[d]imidazol-2(3H)-one hydrochloride], (±)-SM 21 [(±)-tropanyl 2-(4-chlorophenoxy)butanoate maleate], SN 79 [6-acetyl-3-(4-(4-(4-fluorophenylpiperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one) hydrochloride], and SN 167 [3-(4-(4-(4-fluorophenyl)piperazin-1yl)butyl)-6-propionylbenzo[d]oxazol-2(3H)-one dihydrochloride], also at doses specified in the figures. Subsequently, the effects of presession intraperitoneal injections of the novel σR ligands were assessed on the self administration of cocaine and the σR agonists when substituted for cocaine. The effects of these pretreatments were compared with those of the standard σR antagonist, BD 1063. Effects of the novel σR ligands on food-maintained responding were also assessed to determine the specificity of the observed effects on drug self administration.
A previous study demonstrated that combinations of several standard σR antagonists with dopamine transporter (DAT) inhibitors produced an insurmountable antagonism of cocaine self administration (Hiranita et al., 2011). Our results with binding assays and with self administration of σR ligands indicated that CM 304 and CM 398 had potential to determine whether the effects of dual DAT/σR inhibition were specific to one subtype of σR. To assess this, the effects of pretreatments with CM 304 or CM 398 were each assessed in combination with methylphenidate or nomifensine. These two DAT inhibitors were used previously to assess the effects of dual DAT/σR inhibition on cocaine self administration and were used at doses that were inactive against cocaine self administration when administered alone (Hiranita et al., 2011).
Response rates were determined by dividing responses by elapsed time in each component, excluding the TOs that followed reinforcement, and mean values and standard errors were calculated for all subjects at each treatment. The control response rates were an average of all pretest response rates. Various analyses of variance (ANOVA) were used to assess statistical significance with post hoc Bonferroni t tests for pairwise comparisons as detailed in the following tables. Effects on responding during the fourth component (in which maximal response rates were maintained by cocaine injection or food presentation) were analyzed as described above, with ED50 values calculated to determine selectivity of drug effects. To provide a more complete profile for BD 1063, data from a previous study using identical methods (Hiranita et al., 2010) were borrowed to supplement data gathered exclusively for this study.
Drugs.
The drugs used in this study and their sources were as follows: (−)-cocaine HCl (Sigma-Aldrich, St. Louis, MO), DTG (Sigma-Aldrich), PRE-084 (Tocris, Ballwin, MO), (+)-pentazocine (National Institute on Drug Abuse, Drug Supply Program), BD 1063 (Tocris), and (±)-SM 21 (Tocris; Mach et al., 1999). AZ 66 (Seminerio et al., 2012), SN 79 (Kaushal et al., 2011), SN 167, CM 304 (James et al., 2012), CM 353, and CM 398 (Chu et al., 2015) were synthesized in the Division of Medicinal Chemistry, Department of BioMolecular Sciences, University of Mississippi School of Pharmacy (University, MS). Structures are shown in Fig. 1. Self administration of the test drugs was assessed with intravenous delivery of injections, whereas drug pretreatments were administered intraperitoneally. All drug pretreatments were administered 5 minutes before experimental sessions, with solutions prepared fresh daily in 0.9% NaCl. The exception was DTG, which was initially dissolved in 1 N HCl, neutralized with 1 N NaOH, and diluted to the necessary concentration with water. Pretreatment times and doses of drugs used in this study were chosen based on published (Matsumoto, 2007; Kaushal et al., 2011) or preliminary data obtained in this laboratory.
Results
Radioligand Binding Assays.
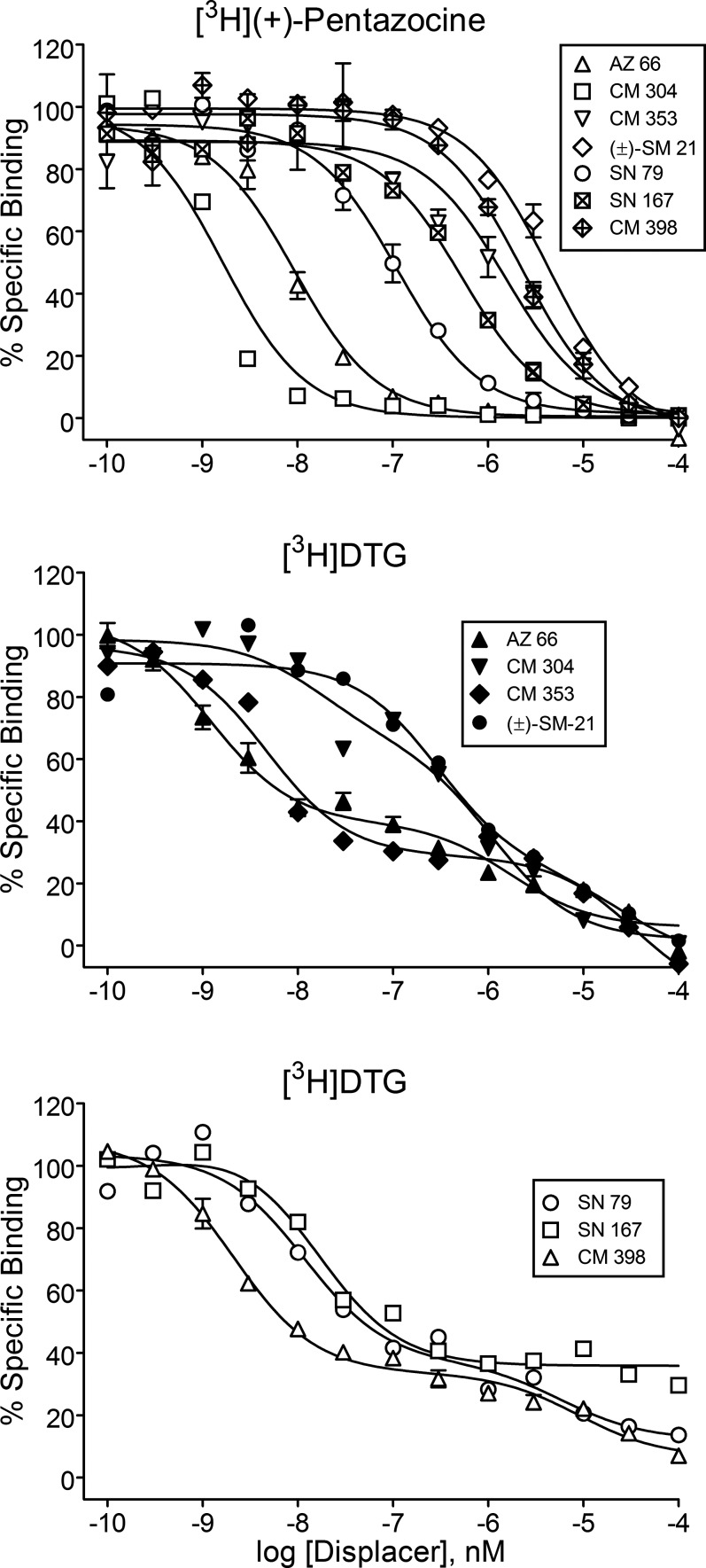
All of the novel σR ligands displaced [3H](+)-pentazocine (Fig. 2, top panel) from σ1Rs, with CM 304 having affinity in the subnanomolar range (Table 2). Representative curves for displacement of [3H](+)-pentazocine by CM 304 and the other compounds show the variations in affinity as per Table 2, as well as displacement over a 100-fold range of concentrations suggesting a single binding site.
Fig. 2.
Displacement of radioligands for σRs by the novel compounds studied. Ordinates show the percentage of specific radiotracer bound to membrane preparations as described in the Materials and Methods. Abscissae show the concentration of each competing compound. The top panel shows displacement of [3H](+)-pentazocine from binding to σ1Rs. The middle and lower panels show displacement of [3H]DTG. The curves represent the results of a single experiment with vertical bars representing S.E.M. values from averages of results from three samples. The results were selected from at least three replications as representative of the binding parameters resulting from a global modeling of all of the data.
TABLE 2.
Inhibition by various compounds of specific binding to the DAT, σ1, or σ2 receptors
Ki values are presented with 95% confidence limits in parentheses.
| Compound |
σ1
Ki Value |
σ2
Ki Value |
σ1/σ2 |
DAT Ki Value |
|---|---|---|---|---|
| CM 304 | 0.684 (0.552–0.847) | 388 (215–702) | 0.002 | 6840 (5270–8880) |
| BD 1063a | 8.81 (7.15–10.9) | 626 (447–876) | 0.014 | 8020 (7100–9060) |
| AZ 66 | 4.70 (4.06–5.45) | 1.35 (0.911–2.00) | 3.48 | 1950 (1740–2190) |
| SN 79 | 78.6 (65.2–94.7) | 11.3 (7.91–16.3) | 6.96 | 1820 (1590–2070) |
| SN 167 | 392 (317–485) | 16.1 (10.4–24.9) | 24.3 | 949 (821–1100) |
| CM 398 | 1490 (1200–1860) | 4.50 (2.78–7.27) | 331 | 11,100 (9940–12,400) |
| CM 353 | 1120 (905–1380) | 4.48 (2.66–7.55) | 250 | 206 (184–229) |
| (±)-SM 21 | 2760 (1700–4470) | 263 (166–409) | 10.5 | 293 (264–326) |
Low-affinity site Ki values for compounds for which σ2 binding modeled better for two sites than one site are as follows: CM 304, 12,800 (87.7–1,860,000); (±)-SM 21, 30,800 (4730–200,000); SN 79, 9130 (2860–29,100); SN 167, 373,000 (89,500–1,560,000); and CM 398, 180,000 (2230–14,500,000).
The values for these compounds are those reported in the literature by Garcés-Ramírez et al. (2011) using methods identical to those in our study.
All of the compounds displaced [3H]DTG binding from σ2Rs (Fig. 2, middle and bottom panels). As in a previous studies using the same methods (e.g., Garcés-Ramírez et al., 2011), displacement curves were better fit by a two- than a one-site model. Because Ki values for known compounds at the high-affinity DTG binding site correspond more closely with published values for the σ2R (Garcés-Ramírez et al., 2011), the values for that site are included in Table 2 proper as σ2R affinities. The calculated affinities at the DTG low-affinity site are listed in the footnote for Table 2. Those compounds with nanomolar affinity for σ2Rs included AZ 66, CM 353, and CM 398. Interestingly, several compounds appeared to have a substantial difference in affinities for the two DTG binding sites. For SN 167, the separation was greater than 23,000-fold. Those with the lowest affinity for σ2Rs included PRE-084, BD 1063, CM 304, (±)-SM 21, and (+)-pentazocine. Among these, CM 304 and PRE-084 had greater than 100-fold selectivity for σ1Rs. A comparable magnitude of selectivity for σ2Rs coupled with nanomolar affinity was obtained with CM 398 and CM 353 (Table 2).
Cocaine Self Administration and Substitution of σR Ligands.
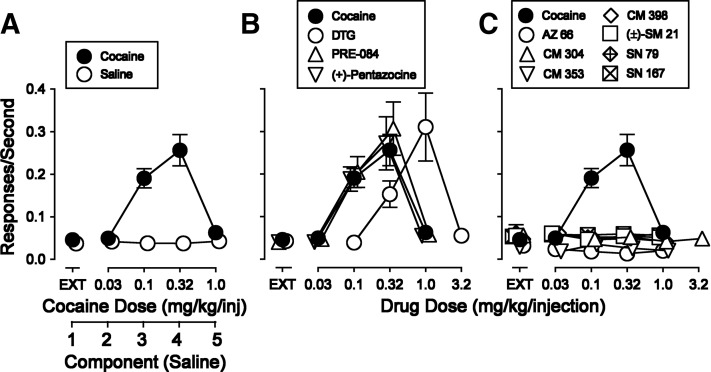
The average response rates maintained by cocaine injections were a bell-shaped function of dose, with a maximum of 0.256 ± 0.034 responses per second at 0.32 mg/kg per injection, which was approximately 6-fold, and significantly, greater than the 0.046 responses per second during EXT in the first component (Fig. 3A). Low response rates were obtained in the first component in which responding had no consequences other than stimulus change and feedback click (extinction, EXT), with higher response rates at doses of 0.10 and 0.32 mg/kg per injection. Further, the temporal patterns of responding were characteristic of FR schedules of reinforcement (Fig. 4A). A two-way, repeated-measures ANOVA indicated a significant effect of treatment (cocaine versus saline), dose of cocaine, and a significant interaction of the two (Table 3).
Fig. 3.
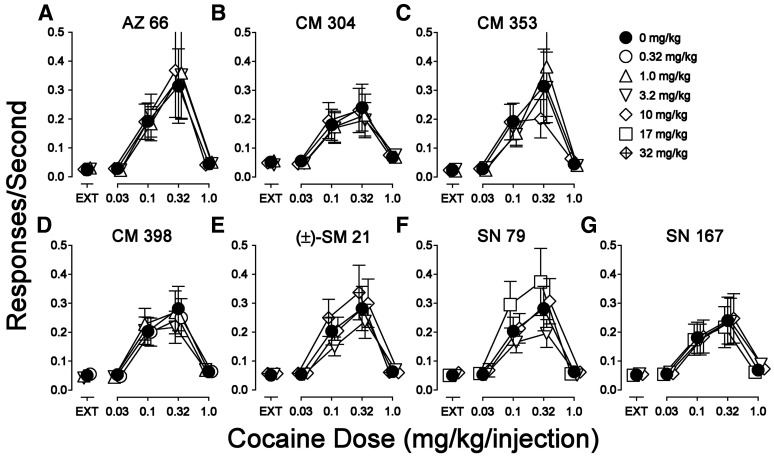
Substitution of saline or σR ligands for cocaine in rats trained to self administer cocaine. Ordinates show the responses per second. Abscissae are the dose of each substituted drug in milligrams per kilogram per injection. (A) Cocaine (filled circles) and saline (open circles). (B) Cocaine repeated from (A) (filled circles), and σR agonists (open symbols). (C) Cocaine repeated from (A) (filled circles) and the novel σR ligands (open symbols). Each point represents the mean ± S.E.M. (n = 6–30)
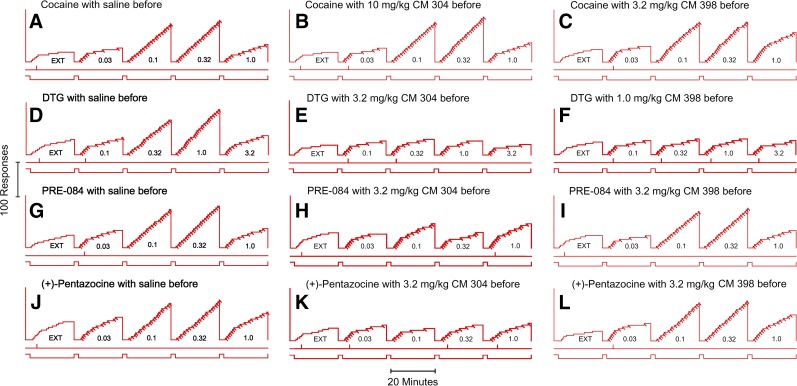
Fig. 4.
Representative cumulative records showing patterns of self administration in real time maintained by intravenous cocaine or σR agonist injection under the FR 5-response schedule. Ordinates show cumulative responses. Abscissae show time. The five 20-minute self administration components of each session are indicated by the lower event line displaced down. The preceding 2-minute TO periods are indicated by the lower event line displaced up. In the first component, each fifth response turned off the light-emitting diodes for 20 seconds but did not activate the infusion pump (extinction, EXT); in subsequent components, injections were also delivered with each fifth response (diagonal marks on the cumulative record) with doses (in milligrams per kilogram per injection) indicated. Vertical marks on the line below the cumulative curve indicate responses on the left (inactive) lever. The cumulative curve reset to the baseline at the end of the 20-minute component. Note that CM 304 decreased the self administration of DTG, PRE-084, and (+)-pentazocine, but not cocaine, whereas CM 398 only decreased responding maintained by DTG.
TABLE 3.
Statistical analyses of dose-effect curves of various compounds compared with saline availability as shown Fig. 3
Δ values were calculated as a subtraction of response rates maintained by test compounds from those of saline substitution for cocaine.
| Treatment | Drug | Dose | Interaction | Post Hoc Test |
|---|---|---|---|---|
| Cocaine versus saline substitution (n = 30, Fig. 3, A–C) | F1,116 = 48.6, P < 0.001 | F4,116 = 39.2, P < 0.001 | F4,166 = 42.3, P < 0.001 | 0.10 mg/kg per injection, t = 8.55, P < 0.001 |
| 0.32 mg/kg per injection, t = 12.3, P < 0.001 | ||||
| DTG versus saline substitution (n = 12, Fig. 3B) | F1,44 = 15.7, P = 0.002 | F4,44 = 13.5, P < 0.001 | F4,44 = 13.1, P < 0.001 | 0.32 mg/kg per injection, t = 3.33, P = 0.002 |
| 1.0 mg/kg per injection, t = 7.68, P < 0.001 | ||||
| PRE-084 versus saline substitution (n = 18, Fig. 3B) | F1,68 = 23.6, P < 0.001 | F4,68 = 20.0, P < 0.001 | F4,68 = 20.9, P < 0.001 | 0.10 mg/kg per injection, t = 5.49, P < 0.001 |
| 0.32 mg/kg per injection, t = 8.91, P < 0.001 | ||||
| (+)-Pentazocine versus saline substitution (n = 18, Fig. 3B) | F1,68 = 21.5, P < 0.001 | F4,68 = 15.7, P < 0.001 | F4,68 = 16.4, P < 0.001 | 0.10 mg/kg per injection, t = 5.14, P < 0.001 |
| 0.32 mg/kg per injection, t = 8.03, P < 0.001 | ||||
| AZ 66 versus saline substitution (n = 6, Fig. 3C) | F1,20 = 5.28, P = 0.007 | F4,20 = 5.81, P = 0.003 | F4,20 = 5.60, P = 0.003 | 0.10 mg/kg per injection, t = 4.07, P < 0.001 (Δ = 0.014) |
| 0.32 mg/kg per injection, t = 2.86, P = 0.008 (Δ = 0.010) | ||||
| CM 304 versus saline substitution (n = 6, Fig. 3C) | F1,20 = 13.2, P = 0.015 | F4,20 = 0.451, P = 0.770 | F4,20 = 7.57, P < 0.001 | EXT, t = 5.28, P < 0.001 (Δ = −0.029) |
| 0.32 mg/kg per injection, t = 4.18, P = 0.002 (Δ = −0.023) | ||||
| 3.2 mg/kg per injection, t = 2.61, P = 0.027 (Δ = −0.014) | ||||
| CM 353 versus saline substitution (n = 6, Fig. 3C) | F1,20 = 26.4, P = 0.070 | F4,20 = 22.0, P < 0.001 | F4,20 = 22.1, P = 0.501 | N.S. |
| CM 398 versus saline substitution (n = 6, Fig. 3C) | F1,20 = 2.09, P = 0.208 | F4,20 = 4.73, P = 0.008 | F4,20 = 2.52, P = 0.074 | N.S. |
| (±)-SM 21 versus saline substitution (n = 6, Fig. 3C) | F1,20 = 8.67, P = 0.032 | F4,20 = 4.32, P = 0.011 | F4,20 = 1.89, P = 0.152 | EXT, t = 3.33, P = 0.014 (Δ = -0.028) |
| 0.10 mg/kg per injection, t = 3.36, P = 0.013 (Δ = −0.018), | ||||
| 1.0 mg/kg per injection, t = 2.48, P = 0.044 (Δ = −0.020) | ||||
| SN 79 versus saline substitution (n = 6, Fig. 3C) | F1,20 = 6.99, P = 0.046 | F4,20 = 2.35, P = 0.089 | F4,20 = 2.76, P = 0.056 | EXT, t = 3.53, P = 0.011 (Δ = −0.008) |
| 1.0 mg/kg per injection, t = 2.52, P = 0.042 (Δ = 0.011) | ||||
| SN 167 versus saline substitution (n = 6, Fig. 3C) | F1,20 = 6.18, P = 0.055 | F4,20 = 3.15, P = 0.037 | F4,20 = 0.621, P = 0.652 | EXT, t = 2.54, P = 0.044 (Δ = 0.008) |
| 0.10 mg/kg per injection, t = 2.73, P = 0.034 (Δ = 0.108) |
N.S., nonsignificant.
Three σR ligands [PRE-084, (+)-pentazocine, and DTG] maintained rates of responding that resembled those maintained by cocaine in all important aspects such as maximal rates maintained (0.307, 0.272, and 0.311, respectively), the FR characteristic temporal patterns of responding (Fig. 4, left column), and the shapes of the dose-effect curves (Fig. 3B, open symbols). DTG was about 3-fold less potent than cocaine, whereas PRE-084 and (+)-pentazocine were equipotent to cocaine (Fig. 3B, compare filled symbols with open symbols). Two-way, repeated-measures ANOVA (Table 3) indicated that self administration of each σR ligand was significantly different from saline (P values ≤ 0.002).
In contrast, response rates maintained by injections of the novel σR ligands were comparable to those obtained with saline (Fig. 3C). The maximal rates of responding maintained were less than 0.061 responses per second, which approximates the rates obtained with saline or during extinction and is approximately 5-fold lower than rates maintained by cocaine. Two-way repeated-measures ANOVA indicated significant effects of drug versus saline for CM 304, (±)-SM 21, and SN 79 (Table 3), although the maximal response rates maintained were uniformly < 0.045 responses per second.
Pretreatment Studies.
None of the novel σR ligands tested appreciably altered the dose-effect curve for cocaine self administration (Fig. 5) nor did they change the temporal patterns of responding (Fig. 4, top row). Rates of responding maintained by cocaine were most frequently similar with and without pretreatment; however, statistical analyses occasionally identified points after pretreatments that differed from those after cocaine with saline pretreatment (Table 4). Those significant differences were typically increases in response rates less than 0.055 responses per second [CM 398, (±)-SM 21], or decreases less than 0.092 responses per second [CM 304, CM 398, (±)-SM 21, SN 79, and SN 167].
Fig. 5.
Effects of presession treatments with novel σR ligands on responding maintained by cocaine injection. Each point represents the mean ± S.E.M. (n = 6). Ordinates are responses per sec. Abscissae show the cocaine injection dose in milligrams per kilogram. Closed circles represent rates of responding maintained by cocaine when vehicle was injected 5 minutes before the experimental session. Open symbols represent rates of responding maintained by cocaine when various σR ligands at doses specified in the key were injected 5 minutes before the experimental session.
TABLE 4.
Statistical analyses of effects of various compounds on self administration of cocaine as shown in Fig. 5
Δ values were calculated as a subtraction of response rates maintained by cocaine pretreated with test compounds from those with saline vehicle.
| Treatment | Self Administered Drug Dose | Pretreatment Dose (i.p.) | Interaction | Post Hoc Test |
|---|---|---|---|---|
| AZ 66 (i.p.) before cocaine self administration | F4,60 = 5.22, P = 0.005 | F3,60 = 2.41, P = 0.107 | F12,60 = 2.67, P = 0.006 | 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 4.24, P < 0.001 |
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.54, P = 0.004 | ||||
| 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.05, P < 0.001 | ||||
| CM 304 (i.p.) before cocaine self administration | F4,60 = 7.44, P < 0.001 | F3,60 = 4.52, P = 0.019 | F12,60 = 3.25, P = 0.001 | 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.53, P = 0.010 (Δ = 0.026) |
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.28, P < 0.001 (Δ = 0.043) | ||||
| CM 353 (i.p.) before cocaine self administration | F4,60 = 5.77, P = 0.003 | F3,60 = 2.37, P = 0.112 | F12,60 = 3.04, P = 0.002 | 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.88, P = 0.001 |
| CM 398 (i.p.) before cocaine self administration | F4,60 = 4.45, P < 0.001 | F3,60 = 12.7, P = 0.020 | F12,60 = 6.22, P < 0.001 | 1.0 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 2.91, P = 0.030 (Δ = 0.031) |
| 0.32 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.72, P = 0.002 (Δ = −0.024) | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 7.65, P < 0.001 (Δ = 0.065) | ||||
| (±)-SM 21 (i.p.) before cocaine self administration | F4,60 = 13.1, P < 0.001 | F3,60 = 6.57, P = 0.005 | F12,60 = 8.19, P < 0.001 | 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.92, P = 0.001 (Δ = −0.053) |
| 32 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.44, P = 0.007 (Δ = −0.046) | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 4.09, P < 0.001 (Δ = 0.045) | ||||
| 32 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.30, P = 0.010 (Δ = −0.055) | ||||
| SN 79 (i.p.) before cocaine self administration | F4,60 = 13.0, P < 0.001 | F3,60 = 7.91, P = 0.002 | F12,60 = 6.43, P < 0.001 | 17 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 4.16, P < 0.001 (Δ = 0.092) |
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.89, P = 0.001 (Δ = 0.086) | ||||
| 17 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 4.15, P < 0.001 (Δ = 0.092) | ||||
| SN 167 (i.p.) before cocaine self administration | F4,60 = 7.16, P < 0.001 | F3,60 = 4.87, P = 0.015 | F12,60 = 3.17, P = 0.001 | 17 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.77, P = 0.002 (Δ = 0.023) |
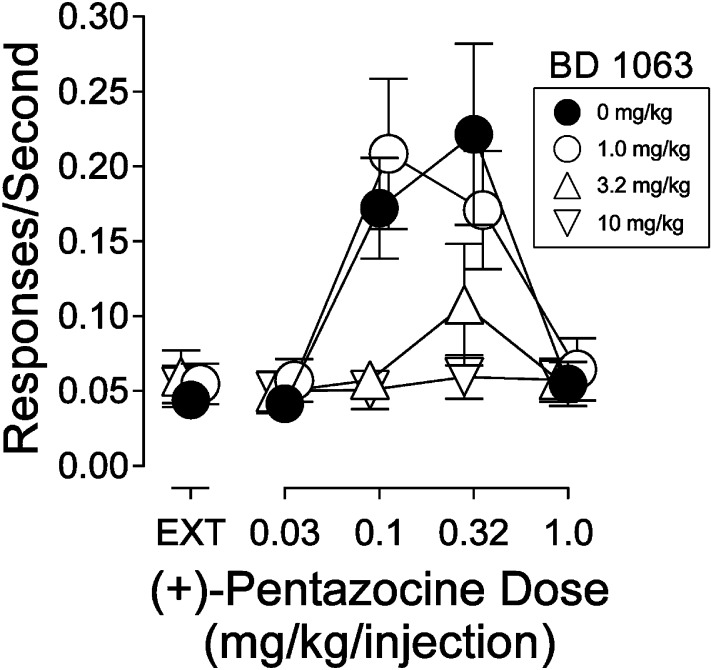
At doses that had no effect on cocaine self administration (Hiranita et al., 2010, 2013a), BD 1063 produced dose-related decreases in maximal self administration of (+)-pentazocine. At the highest dose, response rates were virtually eliminated (Fig. 6). A two-way, repeated-measures ANOVA indicated a significant effect of (+)-pentazocine dose, presession treatment dose of BD 1063, and interaction of the two (Table 5). In a previous study BD 1063 produced similar effects on the self administration of DTG and PRE-084 (Hiranita et al., 2010, 2013a).
Fig. 6.
Effects of presession treatment with the σR antagonist, BD 1063, on responding maintained by (+)-pentazocine injection when substituted for cocaine. Each point represents the mean ± S.E.M. (n = 6). Ordinates are responses per second. Abscissae are the (+)-pentazocine injection dose in milligrams per kilogram (i.p.). Closed circles represent rates of responding maintained by (+)-pentazocine when vehicle was injected 5 minutes before the experimental session. Open symbols represent rates of responding maintained by cocaine when BD 1063 at doses specified in the key was injected 5 minutes before the experimental session.
TABLE 5.
Statistical analyses of effects of various compounds on self administration of DTG, PRE-084, or (+)-pentazocine when substituted for cocaine as shown in Figs. 6–8 Delta values were calculated as a subtraction of response rates maintained by test compounds from those of saline substitution for cocaine.
| Treatment | Self Administered Drug Dose | Pretreatment Dose (i.p.) | Interaction | Post Hoc Test |
|---|---|---|---|---|
| BD 1063 (i.p.) before (+)-pentazocine self administration | F4,60 = 14.2, P < 0.001 | F3,60 = 14.0, P < 0.001 | F12,60 = 11.9, P < 0.001 | 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 5.98, P < 0.001 |
| 10 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 6.32, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.93, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 8.44, P < 0.001 | ||||
| AZ 66 (i.p.) before PRE-084 self administration | F3,60 = 6.20, P = 0.002 | F3,60 = 5.35, P = 0.010 | F12,60 = 4.52, P < 0.001 | 0.32 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 2.74, P = 0.048 |
| 1.0 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.29, P = 0.010 | ||||
| 0.32 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 4.47, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.69, P < 0.001 | ||||
| AZ 66 (i.p.) before DTG self administration | F4,60 = 6.06, P = 0.002 | F3,60 = 5.81, P = 0.008 | F12,60 = 4.99, P < 0.001 | 0.32 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 2.77, P = 0.044 |
| 0.32 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 4.47, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 6.14, P < 0.001 | ||||
| AZ 66 (i.p.) before (+)-pentazocine self administration | F3,60 = 5.41, P = 0.004 | F3,60 = 5.28, P = 0.011 | F12,60 = 4.46, P < 0.001 | 1.0 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.24, P = 0.011 |
| 0.32 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.06, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 6.15, P < 0.001 | ||||
| CM 304 (i.p.) before PRE-084 self administration | F4,60 = 11.8, P < 0.001 | F3,60 = 10.6, P < 0.001 | F12,60 = 10.3, P < 0.001 | 1.0 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 4.26, P < 0.001 |
| 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 4.45, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 7.33, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 9.16, P < 0.001 | ||||
| CM 304 (i.p.) before DTG self administration | F4,60 = 7.33, P < 0.001 | F3,60 = 6.92, P = 0.004 | F12,60 = 6.80, P < 0.001 | 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.21, P = 0.013 |
| 1.0 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 5.60, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 7.81, P < 0.001 | ||||
| CM 304 (i.p.) before (+)-pentazocine self administration | F4,40 = 13.7, P < 0.001 | F2,40 = 12.9, P = 0.002 | F8,40 = 10.4, P < 0.001 | 1.0 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 4.76, P < 0.001 |
| 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 6.33, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.11, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 8.16, P < 0.001 | ||||
| SN 79 (i.p.) before PRE-084 self administration | F4,60 = 12.5, P < 0.001 | F3,60 = 14.7, P < 0.001 | F12,60 = 9.11, P < 0.001 | 17 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 4.00, P < 0.001 |
| 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 2.93, P = 0.027 | ||||
| 17 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 8.05, P < 0.001 | ||||
| SN 79 (i.p.) before DTG self administration | F4,60 = 5.12, P = 0.005 | F3,60 = 6.01, P = 0.007 | F12,60 = 5.04, P < 0.001 | 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 2.85, P = 0.034 |
| 1.0 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 3.53, P = 0.004 | ||||
| 3.2 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 7.61, P < 0.001 | ||||
| SN 79 (i.p.) before (+)-pentazocine self administration | F4,40 = 14.7, P < 0.001 | F2,40 = 10.8, P = 0.003 | F8,40 = 6.63, P < 0.001 | 17 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 4.33, P < 0.001 |
| 17 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.07, P < 0.001 | ||||
| SN 167 (i.p.) before PRE-084 self administration | F4,60 = 11.3, P < 0.001 | F3,60 = 6.57, P = 0.005 | F12,60 = 5.67, P < 0.001 | 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 6.03, P < 0.001 |
| 17 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 6.10, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.86, P = 0.001 | ||||
| 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 4.95, P < 0.001 | ||||
| SN 167 (i.p.) before DTG self administration | F4,60 = 7.64, P < 0.001 | F3,60 = 7.09, P = 0.003 | F12,60 = 6.93, P < 0.001 | 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.29, P = 0.010 |
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.42, P = 0.006 | ||||
| 0.32 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 4.55, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 6.45, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 8.43, P < 0.001 | ||||
| SN 167 (i.p.) before (+)-pentazocine self administration | F4,20 = 15.6, P < 0.001 | F1,20 = 0.236, P = 0.648 | F4,20 = 2.00, P = 0.133 | 17 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 2.56, P = 0.018 |
| CM 353 (i.p.) before PRE-084 self administration | F4,60 = 5.00, P = 0.006 | F3,60 = 3.22, P = 0.053 | F12,60 = 2.56, P = 0.008 | 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 4.05, P < 0.001 |
| CM 353 (i.p.) before DTG self administration | F4,60 = 6.67, P = 0.001 | F3,60 = 6.22, P = 0.006 | F12,60 = 5.47, P < 0.001 | 0.32 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 2.77, P = 0.042 |
| 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 2.75, P = 0.046 | ||||
| 0.32 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 5.80, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 6.75, P < 0.001 | ||||
| CM 353 (i.p.) before (+)-pentazocine self administration | F4,20 = 5.04, P = 0.006 | F1,20 = 1.09, P = 0.344 | F4,20 = 3.17, P = 0.036 | 10 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 2.62, P = 0.016 (Δ = -0.018) |
| 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 2.71, P = 0.013 (Δ = 0.019) | ||||
| CM 398 (i.p.) before PRE-084 self administration | F4,60 = 6.83, P = 0.001 | F3,60 = 3.79, P = 0.033 | F12,60 = 2.98, P = 0.003 | 1.0 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.22, P = 0.012 |
| 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.47, P = 0.005 | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.37, P < 0.001 | ||||
| CM 398 (i.p.) before DTG self administration | F4,60 = 6.76, P = 0.001 | F3,60 = 7.04, P = 0.004 | F12,60 = 6.77, P < 0.001 | 0.32 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 2.91, P = 0.031 |
| 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.06, P = 0.020 | ||||
| 0.32 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 5.81, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 7.48, P < 0.001 | ||||
| CM 398 (i.p.) before (+)-pentazocine self administration | F4,20 = 14.0, P < 0.001 | F1,20 = 0.334, P = 0.588 | F4,20 = 4.42, P = 0.010 | 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.61, P = 0.001 |
| (±)-SM 21 (i.p.) before PRE-084 self administration | F4,60 = 12.0, P < 0.001 | F3,60 = 8.03, P = 0.002 | F12,60 = 6.07, P < 0.001 | 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.87, P = 0.001 |
| 32 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.29, P = 0.009 | ||||
| 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 5.56, P < 0.001 | ||||
| (±)-SM 21 (i.p.) before DTG self administration | F4,60 = 8.65, P < 0.001 | F3,60 = 6.72, P = 0.004 | F12,60 = 6.22, P < 0.001 | 5.6 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.12, P = 0.016 |
| 10 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 3.13, P = 0.016 | ||||
| 3.2 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 3.71, P = 0.003 | ||||
| 5.6 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 6.94, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 1.0 mg/kg per injection, t = 8.32, P < 0.001 | ||||
| (±)-SM 21 (i.p.) before (+)-pentazocine self administration | F4,20 = 13.3, P < 0.001 | F1,20 = 11.9, P = 0.018 | F4,20 = 5.15, P = 0.005 | 32 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 3.55, P = 0.002 |
| 32 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 4.96, P < 0.001 |
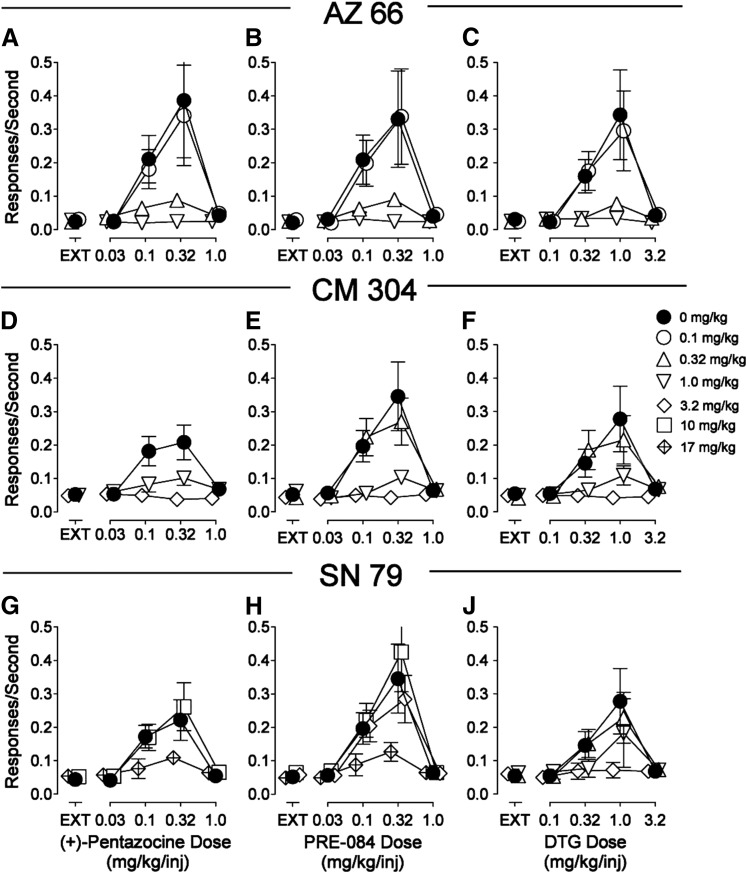
The novel σR ligands AZ 66, CM 304, and SN 79 produced dose-related decreases in the maximal self administration of (+)-pentazocine, PRE-084, or DTG. At the highest doses, response rates were comparable to those obtained during extinction (Fig. 7) or with saline self administration. The temporal patterns of responding maintained by the σR agonists after treatment with 3.2 mg/kg CM 304 were similar to those obtained during extinction in the first component (Fig. 4, E, H, and L). The doses of AZ 66 or CM 304 that decreased the self administration of DTG, PRE-084, and (+)-pentazocine were similar (Fig. 7, top and middle rows). In contrast, SN 79 decreased self administration of (+)-pentazocine, PRE-084, and DTG, although the doses necessary were lower for DTG self administration than for the other two σR ligands (Fig. 7, bottom row). Table 5 gives results of the statistical analyses of these effects, indicating significant effects for dose of the self administered drug (P values ≤ 0.005), pretreatment dose (P values ≤ 0.011), and the interaction of the two (P values < 0.001) for all of the compounds.
Fig. 7.
Effects of presession treatments with novel σR ligands (AZ 66, CM 304, and SN 79) on responding maintained by (+)-pentazocine, PRE-084, or DTG injection. Each point represents the mean ± S.E.M. (n = 6). Ordinates are responses per second. Abscissae are the injection dose in milligrams per kilogram (i.p.). Closed circles represent rates of responding maintained by the σR agonists when vehicle was injected 5 minutes before the experimental session. Open symbols represent rates of responding maintained by cocaine when AZ 66, CM 304, or SN 79 at doses specified in the key were injected 5 minutes before the experimental session.
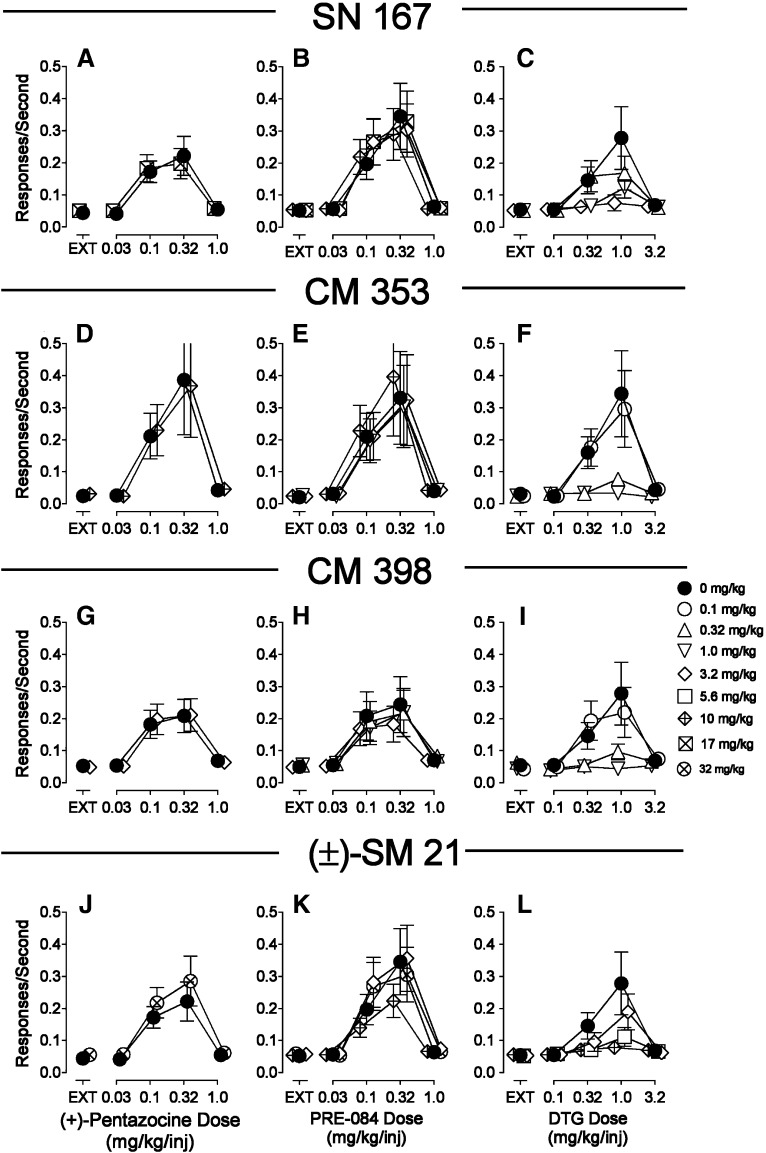
Across the range of SN 167, CM 353, CM 398, and (±)-SM 21 doses examined, there were no substantial effects on (+)-pentazocine or PRE-084 self administration (Fig. 8, left and middle columns). In contrast, each of these drugs across the same range of doses decreased the maximal self administration of DTG in a dose-related manner. At the highest doses of each of these compounds, response rates maintained by DTG were comparable to those obtained during extinction (Fig. 8, right column). Further, the patterns of responding maintained by DTG after treatment with 1.0 mg/kg CM 398 (Fig. 8J, open triangles down) were similar to those obtained with saline substitution for cocaine (Fig. 3A, open symbols) or during extinction in the first component (Fig. 4F), however those maintained by PRE-084 or (+)-pentazocine (Fig. 8, G and H, downward open triangles and diamonds) were comparable to those obtained with saline pretreatment (Fig. 4, G, H, K, and L). Table 5 shows statistical analyses of pretreatment dose on (+)-pentazocine, PRE-084, or DTG self administration with each of these four compounds. These analyses indicate significant pretreatment dose effects (P values ≤ 0.004) for each of the compounds with DTG self administration. In contrast, the pretreatments did not significantly decrease (+)-pentazocine self administration with any of the compounds. A small (less than 0.014 responses per second) but significant increase in self administration of two doses of (+)-pentazocine was obtained with (±)-SM 21. Small but significant effects of pretreatment dose on PRE-084 self administration were also obtained (Table 5).
Fig. 8.
Effects of presession treatments with novel σR ligands (SN 167, CM 353, CM 398, and (±)-SM 21) on responding maintained by (+)-pentazocine, PRE-084, or DTG injection. Each point represents the mean ± S.E.M. (n = 6). Ordinates are responses per second. Abscissae are the injection dose in milligrams per kilogram (i.p.). Closed circles represent rates of responding maintained by the σR agonists when vehicle was injected 5 minutes before the experimental session. Open symbols represent rates of responding maintained by cocaine when SN 167, CM 353, CM 398, or (±)-SM 21 at doses specified in the key were injected 5 minutes before the experimental session.
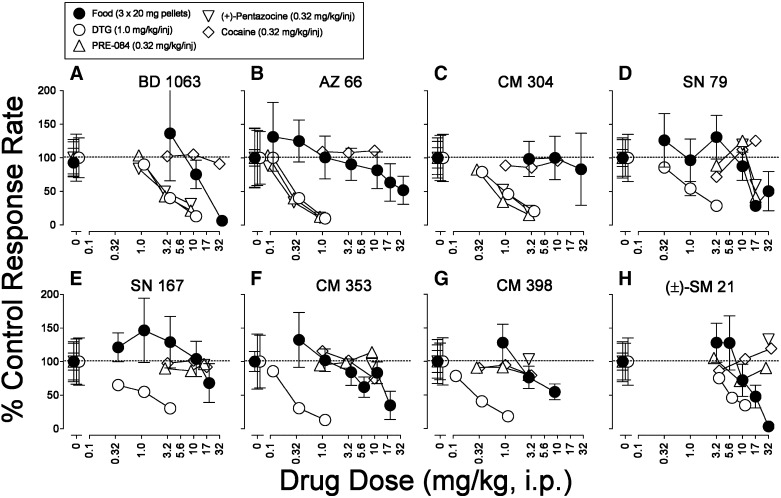
Each of the σR ligands across the range of doses studied produced dose-related decreases in food-maintained responding, although some to a greater extent than others (Fig. 9, filled symbols). The potencies with which BD 1063, AZ 66, and CM 304 decreased rates of responding maintained by food were less than those for decreasing responding maintained by DTG, PRE-084, and (+)-pentazocine (Fig. 9, A–C). Further, the potencies for decreasing responding maintained by the three σR agonists were comparable (Table 6). In contrast, for SN 79, SN 167, CM 353, CM 398, and (±)-SM 21, the potencies for decreasing rates of responding maintained by DTG were greater than those for decreasing rates of responding maintained by food presentation or PRE-084 and (+)-pentazocine, which were equivalent (Fig. 9, D–H; Table 6).
Fig. 9.
Effects of presession treatments with σR ligands on responding maintained by cocaine, PRE-084, (+)-pentazocine, or DTG injection or food presentation. Ordinates are response rates as percentage of control response rates (sessions prior to drug tests). Abscissae are the dose in milligrams per kilogram of test compounds administered intraperitoneally, log scale (5 minutes before the experimental session). Rates of responding were from the fourth 20-minute component of the session (see the Materials and Methods) with responding maintained by injections of cocaine (open diamonds; 0.32 mg/kg per injection), DTG (open circles; 1.0 mg/kg per injection), PRE-084 (triangles pointing up; 0.32 mg/kg per injection), (+)-pentazocine (downward triangles; 0.32 mg/kg per injection), or food presentation (closed circles; 3 × 20-mg pellets). Each point represents the mean ± S.E.M. (n = 6). Rates of responding maintained by food reinforcement averaged 0.557 ± 0.053 responses per second, whereas those maintained by cocaine, DTG, PRE-084, and (+)-pentazocine averaged 0.256 ± 0.037, 0.311 ± 0.080, 0.307 ± 0.063, and 0.272 ± 0.062 responses per second, respectively. None of the control response rates maintained by drugs were significantly different (t values ≤ 0.699, P values = 1.00, Bonferroni t test). Further, the control response rates maintained by DTG were not significantly different from those maintained by food (t = 0.659, P = 0.070, Bonferroni t test). However, the control response rates maintained by food were significantly greater than those maintained by cocaine, PRE-084, and (+)-pentazocine (t values ≥ 3.15, P values ≤ 0.022, Bonferroni t test).
TABLE 6.
ED50 values for σR ligands in decreasing rates of responding maintained by injections of DTG (1.0 mg/kg per injection), PRE-084 (0.32 mg/kg per injection), or (+)-pentazocine (0.32 mg/kg per injection) or food presentation
ED50 values are presented with 95% confidence limits. Response rates were calculated as percentage of control response rates (sessions before drug tests), which averaged 0.311 ± 0.080 (n = 12), 0.307 ± 0.063 (n = 18), 0.272 ± 0.062 (n = 18), and 0.557 ± 0.053 (n = 19) responses per second, respectively, for DTG-, PRE-084-, (+)-pentazocine-, and food-maintained responding. The data were shown in Fig. 9.
| Compound | DTG | PRE-084 | (+)-Pentazocine | Food |
|---|---|---|---|---|
| μmol/kg | ||||
| BD 1063a | 8.52 (7.11–10.2) | 10.8 (8.99–13.2) | 11.2 (8.60–15.1) | 43.3 (15.1–10,400) |
| AZ 66 | 0.664 (0.574–0.767) | 0.700 (0.637–0.771) | 0.562 (0.503–0.627) | Approximately 108 |
| CM 304 | 2.56 (2.20–2.97) | 2.21 (2.09–2.34) | 2.91 (2.34–3.41) | >85.9 |
| SN 79 | 2.68 (1.92–4.02) | 33.3 (31.9–35.0) | 38.1 (34.2–45.4) | >66.1 |
| SN 167 | 2.03 (1.46–2.82) | >34.1 | >34.1 | 44.5 (34.0–182) |
| CM 353 | 0.498 (0.449–0.549) | >19.5 | >19.5 | 32.4 (20.3–77.1) |
| CM 398 | 0.623 (0.495–0.771) | 7.05 × 103 (389–9.71 × 107) | >7.41 | 24.8 (13.5–124) |
| (±)-SM 21 | 13.2 (12.1–14.5) | >70.5 | >70.5 | 33.4 (6.22–58.5) |
The values for food, DTG, and PRE-084 were calculated with data from Hiranita et al. (2011).
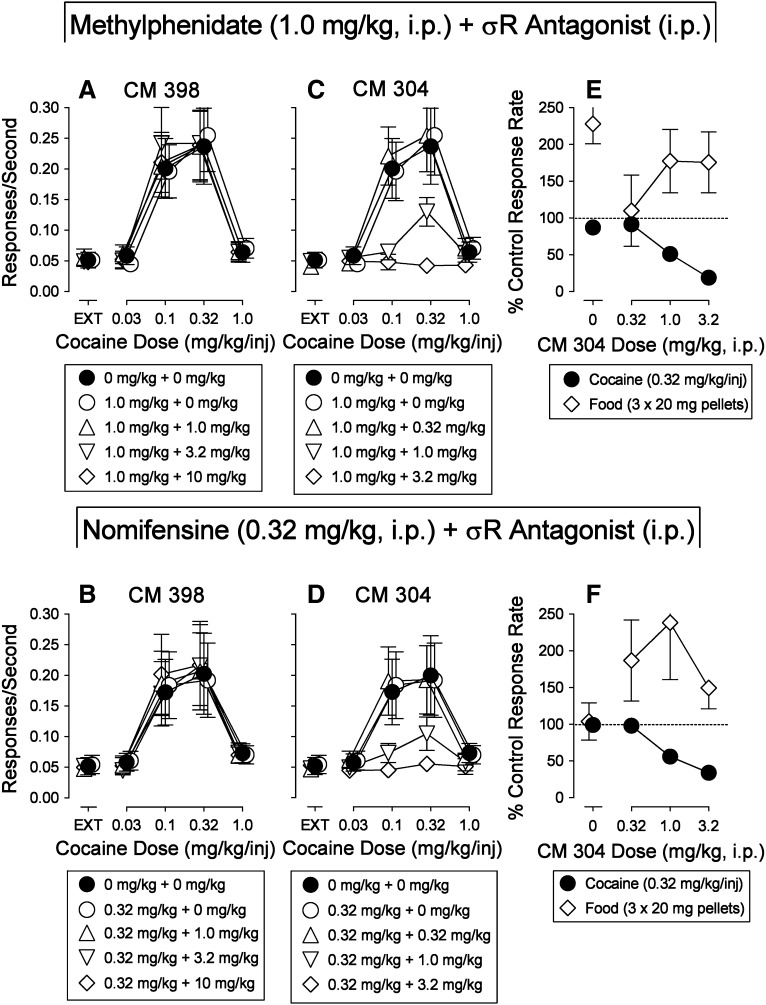
Combinations of the selective σ2R ligand CM 398 with either methylphenidate (1.0 mg/kg) or nomifensine (0.32 mg/kg) were without effects on cocaine self administration (Fig. 10, A and B; Table 7). In contrast, the selective σ1R ligand CM 304 dose-dependently decreased maximal cocaine self administration when combined with either methylphenidate or nomifensine (Fig. 10, C and D; Table 7). These effects of CM 304 in combination with DAT inhibitors markedly contrast with the inaction of CM 304 on cocaine self administration when administered alone (Fig. 5B). The doses of the two DAT inhibitors when administered alone were without effects on cocaine self administration (Fig. 10, A–D, open circles). In addition, the combination with CM 304 and either dopamine uptake inhibitor decreased rates of responding maintained by cocaine injections at dose combinations that did not decrease rates of food-maintained responding (Fig. 10, E and F; Table 8).
Fig. 10.
Effects of presession treatments with dopamine uptake inhibitors combined with CM 304 or CM 398 on responding maintained by cocaine injection or food presentation. (A–D) Ordinates are response rates in responses per second. Abscissae are the dose in milligrams per kilogram of cocaine (i.p.). (E and F) Ordinates are response rates as percentage of control response rates (sessions prior to drug tests). Abscissae are the dose in milligrams per kilogram of CM 304 administered intraperitoneally, log scale. Rates of responding were from the fourth 20-minute component of the session (see the Materials and Methods) with responding maintained by injections of cocaine (closed circles; 0.32 mg/kg per injection) or food presentation (diamonds; 3 × 20-mg pellets). Each point represents the mean ± S.E.M. (n = 6).
TABLE 7.
Statistical analyses of effects of various compounds on self administration of cocaine as shown in Fig. 10, A–D
Δ values were calculated as a subtraction of response rates maintained by cocaine pretreated with combined test compounds from those with saline vehicle.
| Treatment | Self Administered Drug Dose | Pretreatment Dose (i.p.) | Interaction | Post Hoc Test |
|---|---|---|---|---|
| CM 398 (i.p.) and 1.0 mg/kg methylphenidate (i.p.) before cocaine self administration | F4,80 = 12.8, P < 0.001 | F4,80 = 1.55, P = 0.226 | F16,80 = 4.39, P < 0.001 | 3.2 mg/kg CM 398 (i.p.) at 0.10 mg/kg per injection, t = 5.94, P < 0.001 (Δ = −0.041) |
| CM 398 (i.p.) and 0.32 mg/kg nomifensine (i.p.) before cocaine self administration | F4,80 = 7.33, P < 0.001 | F4,80 = 1.08, P = 0.394 | F16,80 = 2.59, P = 0.003 | 10 mg/kg CM 398 (i.p.) at 0.10 mg/kg per injection, t = 3.59, P = 0.005 (Δ = −0.026) |
| CM 304 (i.p.) and 1.0 mg/kg methylphenidate (i.p.) before cocaine self administration | F4,80 = 13.9, P < 0.001 | F4,80 = 13.3, P < 0.001 | F16,80 = 12.3, P < 0.001 | 3.2 mg/kg CM 304 (i.p.) at 0.10 mg/kg per injection, t = 6.07, P < 0.001 |
| 10 mg/kg CM 304 (i.p.) at 0.10 mg/kg per injection, t = 6.81, P < 0.001 | ||||
| 1.0 mg/kg CM 304 (i.p.) at 0.32 mg/kg per injection, t = 4.75, P < 0.001 | ||||
| 3.2 mg/kg CM 304 (i.p.) at 0.32 mg/kg per injection, t = 8.66, P < 0.001 | ||||
| CM 304 (i.p.) and 0.32 mg/kg nomifensine (i.p.) before cocaine self administration | F4,80 = 8.02, P < 0.001 | F4,80 = 7.75, P < 0.001 | F16,80 = 6.4, P < 0.001 | 1.0 mg/kg CM 304 (i.p.) at 0.10 mg/kg per injection, t = 4.44, P < 0.001 |
| 3.2 mg/kg CM 304 (i.p.) at 0.10 mg/kg per injection, t = 5.65, P < 0.001 | ||||
| 1.0 mg/kg CM 304 (i.p.) at 0.32 mg/kg per injection, t = 4.31, P < 0.001 | ||||
| 3.2 mg/kg CM 304 (i.p.) at 0.32 mg/kg per injection, t = 6.44, P < 0.001 |
TABLE 8.
Statistical analyses of effects of various compounds on rates of responding maintained by cocaine injection or food presentation as shown in Fig. 10, E and F
| Treatment | Reinforcer | CM 304 Dose (i.p.) | Interaction | Post Hoc Test |
|---|---|---|---|---|
| CM 304 (i.p.) and 1.0 mg/kg methylphenidate (i.p.) before cocaine self administration or food-maintained behavior | F1,30 = 7.38, P = 0.022 | F3,30 = 7.59, P < 0.001 | F3,30 = 8.82, P < 0.001 | 0 mg/kg CM 304 (i.p.), t = 2.92, P = 0.010 |
| 1.0 mg/kg (i.p.), t = 2.87, P = 0.011 | ||||
| 3.2 mg/kg (i.p.), t = 3.75, P = 0.002 | ||||
| CM 304 (i.p.) and 0.32 mg/kg nomifensine (i.p.) before cocaine self administration or food-maintained behavior | F1,30 = 13.9, P = 0.059 | F3,30 = 13.3, P = 0.381 | F3,30 = 12.3, P = 0.179 | 1.0 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 4.44, P < 0.001 |
| 3.2 mg/kg (i.p.) at 0.10 mg/kg per injection, t = 5.65, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 4.31, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg per injection, t = 6.44, P < 0.001 |
Discussion
Subjects with cocaine self administration experience, as in previous studies (Hiranita et al., 2010, 2013a), self administered the σR agonists, PRE-084, (+)-pentazocine, and DTG, and that effect was used to characterize in vivo antagonist effects of several novel σR ligands. As discussed above, in vitro assay of σ1R agonist or antagonist activity has advanced with the identification of the intracellular chaperone function of σ1Rs (Hayashi and Su, 2001, 2007). However, there is little clear information on cellular function of σ2Rs comparable to that available for σ1Rs, and its molecular response to agonists or antagonists may be dissimilar to that of σ1Rs. A clear definition of agonists and antagonists at σ2Rs has the potential to substantially further the understanding of σ2R function.
Among the compounds studied, CM 304 had the highest affinity for σ1Rs and was over 500-fold selective for that site compared with σ2Rs as determined by displacement of radioligands. In contrast, BD 1063, previously suggested to be σ1R preferential over σ2R (Matsumoto et al., 1995), was only about 70-fold selective in radioligand binding. Thus, CM 304 has potential as an in vivo tool for distinguishing σ1R- versus σ2R-mediated effects. Several of the other novel σR ligands studied had selectivity in binding to σ2Rs over σ1Rs, with CM 398 having over 300-fold selectivity. Greater σ2R over σ1R selectivity has been reported (Xu et al., 2011); however, pilot studies indicated that CM 398 was active in vivo, and our in vitro study indicated its selectivity for σ2 over σ1Rs for our in vivo studies.
Both PRE-084 and (+)-pentazocine were previously identified as selective σ1R agonists (Hayashi and Su, 2007; Garcés-Ramírez et al., 2011) and maintained responding when substituted for cocaine. In addition, DTG, previously identified as a nonselective σR agonist (Bergeron et al., 1999), also maintained responding when substituted for cocaine. In contrast, none of the novel σR ligands tested in our study maintained responding above vehicle levels. Further, several compounds recognized as σR antagonists, although not as selective for σR subtypes as the present compounds, blocked the self administration of the three σR agonists in this study and previous studies (Hiranita et al., 2010, 2013a,b; see Katz et al., 2016 for a review). The results together indicate that self administration, when substituted for cocaine, is a characteristic σR agonist action. In addition, the novel compounds tested in our study had affinity for σRs, were not self administered, and effectively blocked the self administration of at least one of the σR agonists, indicating that these ligands were σR antagonists.
That both PRE-084 and (+)-pentazocine maintained responding above vehicle levels when substituted for cocaine along with their selective σ1R radioligand displacement data suggests that σ1R agonist activity is sufficient for the maintenance of self administration under the current conditions. Further supporting that conclusion is the finding that the novel ligand, CM 304, was selective in binding σ1Rs over σ2Rs and blocked the self administration of each of the σ1R agonists. Moreover, the preferential σ2R antagonists, with the exception of SN 79, were inactive in blocking the self administration of the selective σ1R agonists. These findings together support the conclusion that σ1R agonist activity is sufficient condition for self administration in cocaine-experienced subjects. For SN 79 the radioligand binding, conditions of the present study indicated an approximate 80 nM σ1R binding affinity, which was sufficient for antagonism of σ1R agonist self administration, albeit with lower potency than its antagonism of DTG self administration. The affinity of SN 167 for σ1Rs was approximately 5-fold lower than that for SN 79. That difference in σ1R affinity, with σ2R affinity approximately unchanged, rendered the latter compound inactive against σ1R agonist self administration.
DTG, in contrast with PRE-084 and (+)-pentazocine, has affinity for both subtypes of σR and its self administration was blocked by all of the antagonists studied. That the self administration of DTG was blocked by either σ1- or σ2-selective antagonists, suggests that both subtypes are necessary for the self administration of the nonselective σR agonist. Further, action at either subtype of σR alone was not sufficient for DTG self administration, despite the findings suggesting that σ1R agonist actions alone were sufficient for self administration of PRE-084 and (+)-pentazocine. These findings suggest a previously unidentified interaction between subtypes of σRs in the self administration of DTG. It remains possible that actions at another site contribute to the self administration of DTG, although the antagonism by the selective σR antagonists argues otherwise. However, if such an unidentified site contributes to DTG self administration, that site is not likely the DAT, as the affinity of DTG for the DAT was more than 4000-fold lower than its affinity for the σ2R (Garcés-Ramírez et al., 2011). Previous reports of the discovery and pharmacological characterization of DTG indicate that its reinforcing effects are unlikely to be due to either activity at opioid sites or the phencyclidine-binding site on the N-methyl-d-aspartate receptor (Weber et al., 1986; Scherz et al., 1990).
A recent study reported increased dopamine levels in the nucleus accumbens of rats treated with the σR agonists, DTG or PRE-084. Although increased dopamine in the nucleus accumbens is reportedly involved in the reinforcing effects of abused drugs (Pontieri et al., 1995; Tanda et al., 1997), it is unlikely that the dopamine effect underlies the self administration of these drugs as doses of DTG or PRE-084 effect on dopamine levels were 10- or 100-fold greater than their minimally effective doses/injection in the self administration procedure. Further, self administration of σR agonists was not altered by dopamine antagonists (Hiranita et al., 2013a,b). Moreover, although self administration of PRE-084 was antagonized by σR antagonists, the elevated dopamine levels produced by this compound were unaltered by σR antagonists, indicating that the effect of PRE-084 on dopamine was mediated by a mechanism in which σRs were uninvolved (Garcés-Ramírez et al., 2011). On the other hand, SN 79 was equipotent in antagonizing the increases in nucleus accumbens dopamine and self administration of DTG. However, a more than 5-fold higher dose of SN 79 was required to antagonize the selective σ1 agonists PRE-084 and (+)-pentazocine in our study. These findings suggest that dopamine increases after DTG administration may reasonably be used in conjunction with the present procedures to further characterize σR activity in vivo.
Previous studies have indicated that, whereas σR antagonism is insufficient for blockade of cocaine self administration (Martin-Fardon et al., 2007; Hiranita et al., 2010, 2011), combinations of σR antagonists and dopamine uptake inhibitors are effective and selective for cocaine or d-methamphetamine self administration (Hiranita et al., 2011, 2014). Our radioligand displacement results combined with in vivo actions of the selective σ1R and σ2R antagonists CM 304 and CM 398, respectively, indicate that these compounds could serve to identify which σR subtype subserves the dual DAT/σR inhibitory effects. The effectiveness of CM 304 and lack of effectiveness of CM 398 each in combination with two DAT inhibitors suggests an exclusive role of the σ1R in the effect of dual DAT/σR inhibition on cocaine self administration. As with previous findings, combinations of CM 304 with the dopamine uptake inhibitors produced a dose-dependent insurmountable antagonism at doses that did not decrease rates of responding maintained by food presentation. The absence of effects on cocaine self administration of CM 353 and (±)-SM 21, two preferential σ2R antagonists with moderate affinity for the DAT, is consistent with this conclusion.
In summary, this study demonstrates the utility of σR agonist self administration as an in vivo assay for assessing subtype-selective σR agonist and antagonist actions. Among compounds with affinity for σRs, those that are self administered in cocaine-experienced subjects are σR agonists, whereas antagonists are those that fail to maintain responding above vehicle levels in cocaine-experienced subjects but block the self administration by σR agonists. Further radioligand binding data along with antagonism of σR agonist self administration can identify σ1- or σ2-selective antagonists. Our results identified CM 304 and CM 398 as selective in vivo antagonists at σ1Rs and σ2Rs, respectively. In addition, a potential role of joint σ1R and σ2R function was identified in the self administration of DTG, as it was blocked by both selective σ2R and σ1R antagonists. These findings should be of further use in confirming σR subtype-selective in vivo effects of the various drugs available for studying σR function. Further, combinations of the selective σ1R antagonist with DAT inhibitors selectively blocked the self administration of cocaine, refining earlier suggestions of dual DAT/σR inhibition to the σ1R subtype as a novel approach to medical treatment of cocaine abuse.
Acknowledgments
The authors thank Lekyla Whitaker and Maryann Carrigan for administrative assistance.
Abbreviations
- σR
σ receptor
- ANOVA
analysis of variance
- AZ 66
3-[4-(4-cyclohexylpiperazine-1-yl)pentyl]-6-fluorobenzo[d]thiazole-2(3H)-one hydrochloride
- BD 1008
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide
- BD 1047
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide
- BD 1063
1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride
- BiP
binding immunoglobulin protein [glucose-regulated protein (GRP-78) or heat shock 70-kDa protein 5 (HSPA5)]
- CB-64D
(+)-1R,5R-(E)-8-benzylidene-5-(3-hydroxyphenyl)-2-methylmorphan-7-one
- CM 304
3-(2-(azepan-1-yl)ethyl)-6-(3-fluoropropyl)benzo[d]thiazol-2(3H)-one hydrochloride
- CM 353
1-(4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-3-(4-fluorophenyl)-1H-benzo[d]imidazol-2(3H)-one hydrochloride
- CM 398
1-(4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-3-methyl-1H-benzo[d]imidazol-2(3H)-one hydrochloride
- DAT
dopamine transporter
- DTG
1,3-di-o-tolylguanidine
- EXT
extinction
- FR
fixed ratio
- NE-100
4-methoxy-3-(2-phenylethoxy)-N,N-dipropylbenzeneethanamine monohydrochloride
- OWW
original wet weight
- PRE-084
2-(4-morpholinethyl) 1-phenylcyclohexanecarboxylate hydrochloride
- (±)-SM 21
(±)-tropanyl 2-(4-chlorophenoxy)butanoate maleate
- SN 79
6-acetyl-3-(4-(4-(4-fluorophenylpiperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one) hydrochloride
- SN 167
3-(4-(4-(4-fluorophenyl)piperazin-1yl)butyl)-6-propionylbenzo[d]oxazol-2(3H)-one dihydrochloride
- TO
timeout
Authorship Contributions
Participated in research design: Katz, Hiranita, Kopajtic, Rice, Mesangeau, Narayanan, Abdelazeem, McCurdy.
Conducted experiments: Hiranita, Kopajtic, Mesangeau, Narayanan, Abdelazeem.
Contributed new reagents or analytic tools: Rice, Mesangeau, Narayanan, Abdelazeem, McCurdy.
Performed data analysis: Katz, Hiranita, Kopajtic, Rice, Mesangeau, Narayanan, Abdelazeem, McCurdy.
Wrote or contributed to the writing of the manuscript: Katz, Hiranita, Kopajtic, Rice, Mesangeau, Narayanan, Abdelazeem, McCurdy.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA023205 (to C.R.M.)] and the National Institutes of Health National Institute of General Medical Sciences [Grant P20-GM104932 (to C.R.M.)]. This research was supported [in part] by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Some of the data reported herein were presented at the following meetings: Hiranita T, Mereu M, Tanda G, Kopajtic TA, Mesangeau C, McCurdy CR and Katz JL (2012) Combined dopamine transporter and σ receptor actions: effects of σ receptor subtype. Annual Meeting at Society for Neuroscience; 2012 Oct 13-17; New Orleans, LA. Katz JL, McCurdy CR, and Hiranita T (2013) Validation of σ-Receptor Agonist Self Administration as a Method for in vivo Characterization of σ-Receptor Agonist or Antagonist Activity. 56th Annual Meeting at Behavioral Pharmacology Society; 2013 Apr 20-24; Boston, MA.
References
- Abate C, Niso M, Infantino V, Menga A, Berardi F. (2015) Elements in support of the ‘non-identity’ of the PGRMC1 protein with the σ2 receptor. Eur J Pharmacol 758:16–23. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. (2004) Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 47 (Suppl 1):256–273. [DOI] [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. (1999) Pregnancy reduces brain sigma receptor function. Br J Pharmacol 127:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertha CM, Mattson MV, Flippen-Anderson JL, Rothman RB, Xu H, Cha XY, Becketts K, Rice KC. (1994) A marked change of receptor affinity of the 2-methyl-5-(3-hydroxyphenyl)morphans upon attachment of an (E)-8-benzylidene moiety: synthesis and evaluation of a new class of sigma receptor ligands. J Med Chem 37:3163–3170. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108. [DOI] [PubMed] [Google Scholar]
- Chu UB, Mavlyutov TA, Chu ML, Yang H, Schulman A, Mesangeau C, McCurdy CR, Guo LW, Ruoho AE. (2015) The sigma-2 receptor and progesterone receptor membrane component 1 are different binding sites derived from independent genes. EBioMedicine 2:1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford KW, Bowen WD. (2002) Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res 62:313–322. [PubMed] [Google Scholar]
- Garcés-Ramírez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, Mesangeau C, Narayanan S, McCurdy CR, Katz JL, et al. (2011) Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biol Psychiatry 69:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2001) Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci USA 98:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. (1994) Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol 268:9–18. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL. (2014) Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. J Pharmacol Exp Ther 348:174–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Mereu M, Soto PL, Tanda G, Katz JL. (2013a) Self-administration of cocaine induces dopamine-independent self-administration of sigma agonists. Neuropsychopharmacology 38:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH, Tanda G, Katz JL. (2011) Decreases in cocaine self-administration with dual inhibition of the dopamine transporter and σ receptors. J Pharmacol Exp Ther 339:662–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. (2009) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. (2010) Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther 332:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL. (2013b) Stimulants as specific inducers of dopamine-independent σ agonist self-administration in rats. J Pharmacol Exp Ther 347:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ML, Shen B, Zavaleta CL, Nielsen CH, Mesangeau C, Vuppala PK, Chan C, Avery BA, Fishback JA, Matsumoto RR, et al. (2012) New positron emission tomography (PET) radioligand for imaging σ-1 receptors in living subjects. J Med Chem 55:8272–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Hong WC, Hiranita T, Su TP. (2016) A role for sigma receptors in stimulant self-administration and addiction. Behav Pharmacol 27:100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Robson MJ, Vinnakota H, Narayanan S, Avery BA, McCurdy CR, Matsumoto RR. (2011) Synthesis and pharmacological evaluation of 6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (SN79), a cocaine antagonist, in rodents. AAPS J 13:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JR, Fergason-Cantrell EA, Watkinson LD, Carmack TL, Lord SA, Xu R, Miller DK, Lever SZ. (2016) Cocaine occupancy of sigma1 receptors and dopamine transporters in mice. Synapse 70:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hiranita T, Hayashi S, Newman AH, Katz JL. (2013) The stereotypy-inducing effects of N-substituted benztropine analogs alone and in combination with cocaine do not account for their blockade of cocaine self-administration. Psychopharmacology (Berl) 225:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach RH, Wu L, West T, Whirrett BR, Childers SR. (1999) The analgesic tropane analogue (+/-)-SM 21 has a high affinity for sigma2 receptors. Life Sci 64:PL131–PL137. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. (2007) Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology 32:1967–1973. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. (2007) σ Receptors: historical perspective and background, in Sigma Receptors (Su TP, Matsumoto RR, Bowen WD. eds) pp 1–23, Springer, New York. [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. (1995) Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol 280:301–310. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Nguyen L, Kaushal N, Robson MJ. (2014) Sigma (σ) receptors as potential therapeutic targets to mitigate psychostimulant effects. Adv Pharmacol 69:323–386. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA 92:12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S. (2002) Effects of GBR 12909, WIN 35,428 and indatraline on cocaine self-administration and cocaine seeking in rats. Psychopharmacology (Berl) 160:263–270. [DOI] [PubMed] [Google Scholar]
- Scherz MW, Fialeix M, Fischer JB, Reddy NL, Server AC, Sonders MS, Tester BC, Weber E, Wong ST, Keana JF. (1990) Synthesis and structure-activity relationships of N,N′-di-o-tolylguanidine analogues, high-affinity ligands for the haloperidol-sensitive sigma receptor. J Med Chem 33:2421–2429. [DOI] [PubMed] [Google Scholar]
- Seminerio MJ, Robson MJ, Abdelazeem AH, Mesangeau C, Jamalapuram S, Avery BA, McCurdy CR, Matsumoto RR. (2012) Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J 14:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, Bowen WD. (2000) Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. J Pharmacol Exp Ther 292:900–911. [PubMed] [Google Scholar]
- Walker JM, Matsumoto RR, Bowen WD, Gans DL, Jones KD, Walker FO. (1988) Evidence for a role of haloperidol-sensitive sigma-‘opiate’ receptors in the motor effects of antipsychotic drugs. Neurology 38:961–965. [DOI] [PubMed] [Google Scholar]
- Weber E, Sonders M, Quarum M, McLean S, Pou S, Keana JF. (1986) 1,3-Di(2-[5-3H]tolyl)guanidine: a selective ligand that labels sigma-type receptors for psychotomimetic opiates and antipsychotic drugs. Proc Natl Acad Sci USA 83:8784–8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S, et al. (2011) Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat Commun 2:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Rothfuss JM, Zhang J, Vangveravong S, Chu W, Li S, Tu Z, Xu J, Mach RH. (2014) Functional assays to define agonists and antagonists of the sigma-2 receptor. Anal Biochem 448:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]