Abstract
The xenobiotic-sensing transcription factors (xeno-sensors) AhR, CAR, and PXR upregulate the expression of many drug-processing genes (DPGs) in liver. Previous studies have unveiled profound changes in the basal expression of DPGs during development; however, knowledge on the ontogeny of the inducibility of DPGs in response to pharmacological activation of xeno-sensors is still limited. The goal of this study was to investigate the age-specific regulation of DPGs by prototypical xeno-sensor ligands: 2,3,7,8-tetrachlorodibenzodioxin (TCDD) for AhR; 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP) for CAR; and pregnane-16α-carbonitrile (PCN) for PXR during mouse liver development. The basal mRNAs of most DPGs were low during neonatal age, but gradually increased to adult levels, whereas some DPGs (Cyp1a2, Cyp2b10, Cyp3a11, Gstm2, Gstm3, Papss2, and Oatp1a4) exhibited an adolescent-predominant expression pattern. The inducibility of DPGs was age-specific: 1) during neonatal age, the highest fold increase in the mRNA expression was observed for Cyp1a2, Sult5a1, and Ugt1a9 by TCDD; Cyp3a11 and Mrp2 by TCPOBOP; as well as Gstm2 and Gstm3 by PCN; 2) during adolescent age, the highest fold increase in the mRNA expression was observed for Ugt1a6 and Mrp4 by TCDD, Cyp2b10, Ugt2b34, and Ugt2b35 by TCPOBOP, as well as Gsta1, Gsta4, Sult1e1, Ugt1a1, Mrp3, and Mrp4 by PCN; 3) in adults, the highest fold increase in the mRNA expression was observed for Aldh1a1, Aldh1a7, and Ugt2b36 by TCPOBOP, as well as Papss2 and Oatp1a4 by PCN. In conclusion, the inducibility of hepatic DPGs following the pharmacological activation of xeno-sensors is age specific.
Introduction
Drug-metabolizing enzymes and transporters play crucial roles in the absorption, metabolism, disposition, and elimination of various drugs and other xenobiotics. In the liver, which is the major organ for drug metabolism, the drug-metabolizing enzymes are categorized into phase I and II enzymes. Phase I enzymes are important for the oxidation, reduction, and hydrolysis of xenobiotics. The metabolites of phase I reactions can be further metabolized via conjugation reactions by phase II enzymes, resulting in increased water solubility of metabolites that are ready for elimination. The phase II reactions mainly include glucuronidation [which is catalyzed by UDP-glucuronosyltransferases (Ugts)], sulfation [which is catalyzed by sulfotransferases (Sults)], glutathione conjugation [which is catalyzed by glutathione S-transferases (Gsts)], as well as other conjugation reactions, such as methylation and amino acid conjugation (Handschin and Meyer, 2003; Wang et al., 2014). Either the parent compounds or their metabolites can be absorbed or eliminated from cells via transporter proteins (Xu et al., 2005; Aleksunes and Klaassen, 2012). The regulation of drug-metabolizing enzymes and transporters [together called drug-processing genes (DPGs)] is important for the pharmacokinetics of drugs, and they contribute to adverse drug-drug reactions.
In adults, it is well known that exposure to many xenobiotic chemicals can upregulate the expression of many phase I and II drug-metabolizing enzymes as well as transporters (Xu et al., 2005). Three major xenobiotic-sensing transcription factors (xeno-sensors), namely, AhR, CAR, and PXR, are known to be involved in the regulation of DPGs in adult mouse liver (Aleksunes and Klaassen, 2012).
The AhR is localized in the cytoplasm during its inactive state, but once it is activated by its prototypical ligand 2,3,7,8-tetrachlorodibenzodioxin (TCDD), AhR translocates into the nucleus and forms a heterodimer with the AhR nuclear translocator protein, which subsequently binds to the xenobiotic response element within the cytochrome P450 (P450) Cyp1a promoter region, activating the transcription of CYP1a genes (Nakajima et al., 2003; Klaassen and Slitt, 2005). TCDD does not induce Cyp1a mRNA in livers of AhR-null mice (Gonzalez and Fernandez-Salguero, 1998).
Following ligand activation, CAR, which is activated by the prototypical ligand 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), and PXR, which is activated by the prototypical ligand pregnane-16α-carbonitrile (PCN), both heterodimerize with the retinoid X receptor, and upregulate their prototypical target genes Cyp2b10 and Cyp3a11, respectively (Xu et al., 2005). TCPOBOP treatment does not increase Cyp2b10 mRNA in CAR-null mice (Wei et al., 2000), and similarly PXR-null mouse livers had no increase in Cyp3a11 mRNA following PCN treatment (Aleksunes and Klaassen 2012). Activation of CAR and PXR is important for the upregulation of many DPGs, and these nuclear receptors are implicated in many adverse drug reactions (Staudinger et al., 2001a,b; Hernandez et al., 2009).
Changes in the expression of DPGs during development are associated with differences in drug metabolism between children and adults. Fetal liver microsomes have extremely high CYP3A7 levels but low CYP3A4 levels, which is the major adult form (Stevens et al., 2003, 2008). A study of age-dependent CYP2B6 expression also found lower levels in infant liver when compared with adults (Croom et al., 2009). Because Cytochrome P450 enzymes are not mature at birth, and the mRNA of many hepatic transporters in all pediatric ages is lower than in adults (Anderson, 2002; Mooij et al., 2014), newborns and children are considered more vulnerable to xenobiotic insults (Kearns et al., 2003).
Although we and others have previously characterized the basal ontogenic expression patterns of various DPGs in liver (Hart et al., 2009; Cui et al., 2012a,b; Lu, 2013; Peng, 2013), the knowledge of the inducibility of DPGs in response to pharmacological activation of different xeno-sensors during development is still limited. It is critical to obtain this information because newborns and children are exposed to xenobiotics through their diet and other sources. Although it is known that DPGs are expressed at various levels during development, it is not known whether these DPGs at younger ages are more or less inducible. Therefore, the goal of this study was to investigate age-specific regulation of DPGs in mouse livers by ligands of xeno-sensors at various ages, namely, 2, 5, 25, and 60 days of age. The profiles of gene regulation by three different transcription factors (AhR, CAR, and PXR) can potentially provide insights into the understanding of the molecular basis of drug-drug interactions and adverse drug reactions in relation to therapy involving children.
Materials and Methods
Animals.
Eight-week-old male C57BL/6 mice were purchased from Charles River Laboratories, lnc. (Wilmington, MA) to be used as breeders. All mice were housed according to the animal care guidelines provided by the American Association for Animal Laboratory Sciences (https://www.aalas.org). and were bred under standard conditions at the University of Kansas Medical Center. Mice were allowed ad libitum access to food and water and were acclimated to the housing facility for at least 1 week before breeding. The offspring of mice were injected i.p. with a single dose of vehicle (corn oil), AhR ligand (TCDD at 40 μg/kg), CAR ligand (TCPOBOP at 300 μg/kg), or PXR ligand (PCN at 200 mg/kg) at 2, 5, 25, and 60 days of age, and tissues were collected 24 hours later. Livers were frozen immediately in liquid nitrogen and stored at −80°C. All studies were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
Total RNA Isolation.
Total RNA was isolated from frozen liver tissue using RNA Bee reagent (Tel-Test lnc., Friendswood, TX) according to the manufacturer’s protocol. The concentration of total RNA was quantified spectrophotometrically at 260 nm using a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA). The integrity of each RNA sample was evaluated by formaldehyde agarose gel electrophoresis before analysis and confirmed by visualization of 18S and 28S rRNA bands.
Quantification of mRNA by Quantitative Reverse Transcription Polymerase Chain Reaction Assay.
Total RNA was reverse transcribed to cDNA using the iScript cDNA Synthesis Kit from Bio-Rad (Hercules, CA) in a final volume of 10 μl containing 5X iScript reaction mix, 0.5 μl iScript reverse transcriptase, 1 μg total RNA, and nuclear-free water. The samples were incubated at 25°C for 5 minutes and 42°C for 30 minutes, and reverse transcriptase was inactivated by heating at 85°C for 5 minutes. The cDNA samples were subsequently amplified by polymerase chain reaction, using SsoAdvanced Universal SYBR Green Supermix in a CFX384 Real-Time Detection System (Bio-Rad). A 2 μl volume of appropriate diluted cDNA sample was added to 8 μl of the polymerase chain reaction master mix, containing SsoAdvanced Universal SYBR Green Supermix (2×), 200nM forward and reverse primers, and nuclear-free water. The thermal cycling conditions were comprised of an initial denature step at 95°C for 30 seconds and 40 cycles at 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. The primers for all real-time polymerase chain reactions were purchased from Integrated DNA Technologies (Coralville, IA), and the sequences are given in Supplemental Table 1. The dCq values of each target gene were calculated after subtracting the average Cq values of β-actin, and the ddCq values for each target gene were expressed as percentage of the housekeeping gene β-actin. The full name of each gene and its function are given in Supplemental Table 2.
Western Blotting.
Whole liver homogenates were prepared using sucrose tris (ST) buffer (250 mM sucrose, 10 mM Tris base, pH 7.5) with protease inhibitor, and protein concentrations were determined using the Qubit Protein Assay Kit (Thermo Fisher Scientific, Grand Island, NY) according to the manufacturer’s instructions. The crude membranes were prepared from these samples as described previously (Aleksunes et al., 2006). The samples were subjected to polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked in phosphate-buffered saline with 0.05% Tween 20 with 5% nonfat dry milk for 1 hour and incubated overnight with one of the following primary antibodies diluted in phosphate-buffered saline with 0.05% Tween 20 with 1% milk: 1) rabbit anti-mouse Cyp2b10 mAb (AB9916, 1:5000, EMD Millipore; Temecula, CA); 2) mouse anti-rat Cyp3a11 mAb (clone 2-12-1, 1:500), which was a generous gift from F. Gonzalez at the National Cancer Institute Rockville, MD; 3) rabbit anti-mouse Oatp1a4 (1:1000, University of Kansas Medical Center); and 4) rat anti-mouse Mrp4 (1:2000, Abcam, Cambridge, MA). After washing, the membranes were incubated for 1 hour with a species-appropriate secondary antibody (Sigma Aldrich, St. Louis, MO) diluted in phosphate-buffered saline with 0.05% Tween 20 with 1% milk. Horseradish peroxidase–linked secondary antibodies were applied at 1:2000 to detect the proteins. The membranes were washed and incubated with the Novex ECL Chemiluminescent Substrate Reagent Kit (Life Technologies, Carlsbad, CA). The membranes were stripped and reprobed with antibodies against β-actin (Abcam) as the loading control. The intensities of the protein bands were quantified using the Image J Software (National Institutes of Health, Bethesda, MD).
Enzyme Activity in Mouse Liver Microsomes.
Liver microsomes were isolated using differential centrifugation as described previously (Pelkonen et al., 1974). Frozen liver samples were weighed and transferred to a Teflon pestle/glass homogenizer with 5 w/v ST buffer (10 mM Tris base, 250 mM sucrose, pH 7.5) containing protease inhibitor cocktail (1:100). The homogenates were centrifuged at 10,000g for 10 minutes at 4°C. The supernatants were transferred to a clean centrifuge tube and centrifuged at 100,000g for 60 minutes at 4°C. The pellets were washed with 1 ml ST buffer, and dissolved in 100 μl of ST buffer with protease inhibitor. The P450 enzyme activity was measured using the P450-Glo Screening system (Promega, Madison, WI). P450 reactions (50 μl) were performed in white opaque 96-well plates. Briefly, a luminogenic P450-Glo substrate was incubated at 37°C with 10 μg of liver microsomal protein, control membrane, or positive P450 enzyme for 10 minutes (100 μM luciferin-ME for Cyp1a, 3 μM luciferin-2B6 for Cyp2b, or 3 μM luciferin-IPA for Cyp3a). The reactions were initiated by adding the NADPH-regeneration system and incubated for 10 minutes. At the end of the incubations, an equal volume of the luciferin detection reagent (50 μl) was added at room temperature, and luminescence was quantified 20 minutes later using a Glomax 96 Microplate Luminometer (Promega). The magnitude of the light signal is dependent on and directly proportional to the amount of luciferin product generated by the P450 reaction.
Statistical Analysis.
Data are presented as mean ± S.E.M. To test the effect of both age and chemicals on the gene expression of DPGs, data were analyzed using a generalized linear model followed by the Duncan’s post hoc test (P < 0.05) using the SPSS software (Armonk, NY). To test the effect of age on the basal mRNA expression of DPGs, only the data from the corn oil–treated group at various ages were analyzed. For the DPGs that displayed significant age versus chemical interactions, an individual generalized linear model was applied to identify the inducibility of DPGs by the xenobiotic-sensor ligands at each specific age. In Figs. 1–8, the asterisks (*) represent significant differences between vehicle- and chemical-treated mice at the same age.
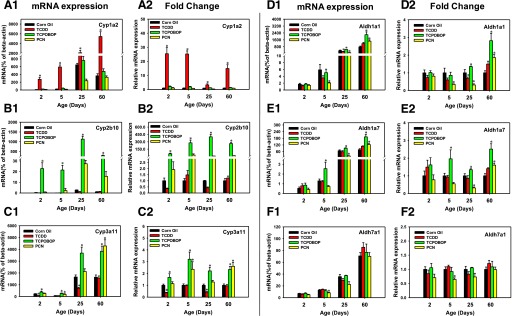
Fig. 1.
The mRNA expression of phase I drug-metabolizing enzymes in livers of male mice after treatment with corn oil (vehicle), TCDD (AhR ligand), TCPOBOP (CAR ligand), or PCN (PXR ligand) at 2, 5, 25, and 60 days of age (n = 4–6 per group). The expressions measured in triplicate are first normalized to the expression of the housekeeping gene β-actin, and then are expressed as fold changes compared with the vehicle-treated group at that particular age. Asterisks (*) represent statistical difference (P < 0.05) compared with the vehicle-treated group at the same age.
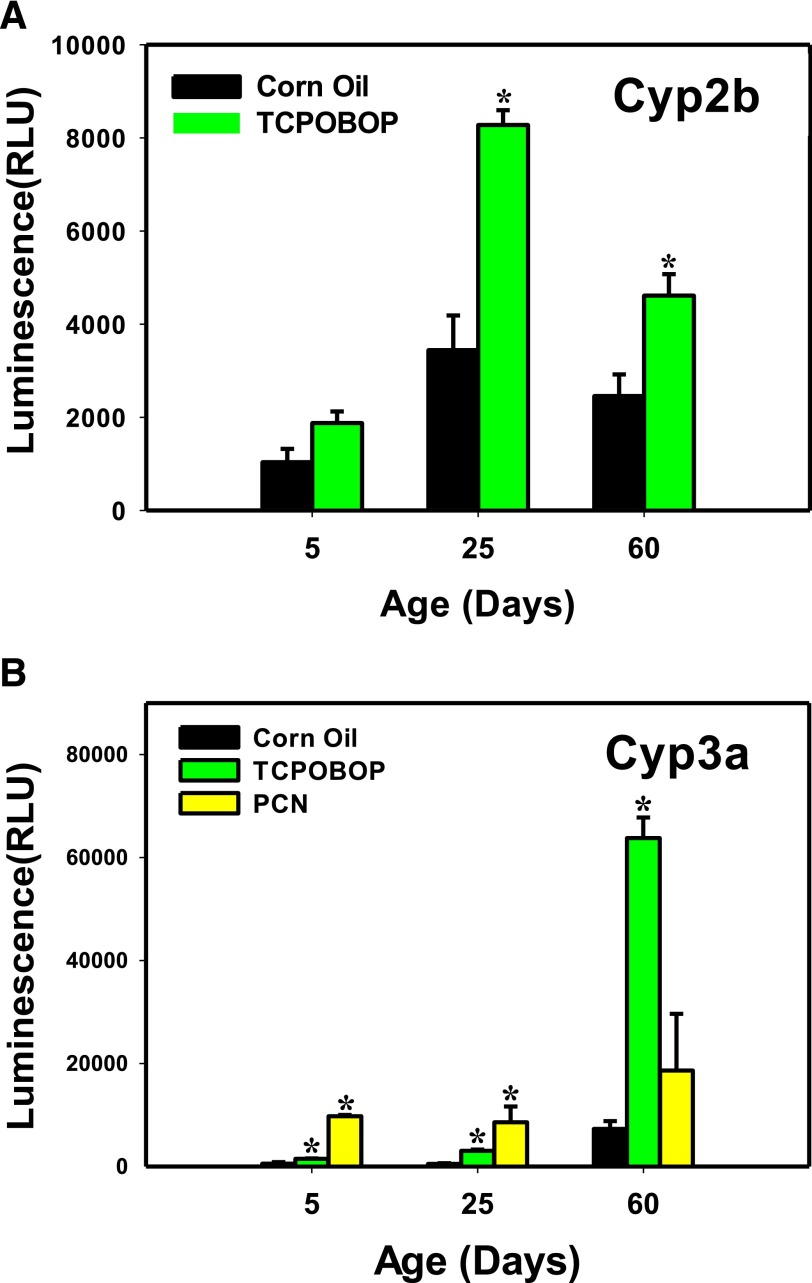
Fig. 8.
Enzyme activities of Cyp2b and Cyp3a in liver microsomes treated with corn oil, TCPOBOP, or PCN, at 5, 25, and 60 days of age (n = 4–6 per group). The magnitude of the luminescent signal is measured in triplicate per group and is proportional to the amount of luciferin product generated by the P450 reaction. Asterisks (*) represent values statistically different from the corn oil–treated mice at the same age.
Results
Effect of Age and Chemicals on the Gene Expression of DPGs.
According to the generalized linear model analysis, 24 out of 29 DPGs displayed a statistically significant interaction between age and chemicals, suggesting that the effect of chemical-mediated changes in the mRNA expression of the majority of DPGs was age specific (Supplement Table 3). Regarding the age effect, the basal mRNAs of most DPGs were less expressed during neonatal ages (days 2 and 5), but gradually increased to adult levels; whereas some DPGs (Cyp1a2, Cyp2b10, Cyp3a11, Gstm2, Gstm3, Papss2, and Oatp1a4) exhibited an adolescent-predominant expression pattern, with the highest constitutive expression observed at day 25. Sult1e1 appeared to be the only DPG in which the mRNA was not influenced by age. In summary, age not only impacts the constitutive expression but also the inducibility of DPGs in the liver. The DPGs in each category are described subsequently.
Developmental Regulation of mRNA for Phase I Drug-Metabolizing Enzymes by Xeno-Sensor Ligands.
To determine the effect of the ligands for the three xeno-sensors on the expression of their prototypical target genes in liver at various developmental ages, the mRNAs of phase I drug-metabolizing enzymes Cyp1a2 (AhR-target), Cyp2b10 (CAR-target), and Cyp3a11 (PXR-target) were quantified in vehicle- and ligand-treated groups (Fig. 1, A–C). Only the AhR-ligand TCDD markedly upregulated the mRNA of Cyp1a2 at all ages, and as anticipated neither the CAR-ligand TCPOBOP nor the PXR-ligand PCN altered the Cyp1a2 mRNA expression at any age (Fig. 1, A1). Regarding the abundance of Cyp1a2 mRNA, the basal mRNA expression of Cyp1a2 was low at 2 days of age (neonatal) but significantly increased 7.7-fold in 25-day-old mice and 19.7-fold in 60-day-old mice (Fig. 1, A1). Interestingly, compared with the vehicle-treated group of the same age, the fold induction of Cyp1a2 mRNA was most prominent at 2 and 5 days of age (25.4- and 25.2-fold, respectively), compared with 3.3-fold at 25 days of age and 14.9-fold at 60 days of age (Fig. 1, A2). Although the age-specific fold change mediated by TCDD was not the highest in the 60-day adult group, the mRNA abundance of Cyp1a2 in liver of TCDD-treated mice was the highest at this age (Fig. 1, A1 and A2).
Regarding the prototypical CAR-target gene Cyp2b10, only TCPOBOP produced a marked increase in mRNA at all four ages as anticipated, and the other two chemicals did not alter Cyp2b10 mRNA in liver during development (Fig. 1, B1). Regarding the abundance of Cyp2b10 mRNA, the basal mRNA of Cyp2b10 was low at all ages, but significantly increased at days 25 and 60, and the basal Cyp2b10 mRNA was highest at 25 days of age (Fig. 1, B1). Compared with the vehicle-treated group of the same age, the fold induction of Cyp2b10 mRNA was 55.0-fold at 2 days of age, followed by 279-fold at 5 days of age, which then reached 400-fold at 25 days of age and 263-fold at 60 days of age (Fig. 1, B2). Therefore, the fold increase of Cyp2b10 mRNA was upregulated the most at 25 days of age (adolescent).
The basal expression of Cyp3a11 mRNA was low in neonatal ages, but gradually increased at 25 days of age, and finally reached its adult level by 60 days of age (Fig. 1, C1). Compared with the vehicle-treated group of the same age, the AhR-ligand TCDD downregulated Cyp3a11 mRNA at 2 days (63.1%) and 25 days (57.4%) of age (Fig. 1, C2). In contrast, the CAR-ligand TCPOBOP increased Cyp3a11 mRNA at all four developmental ages: 70% at day 2, 220% at day 5 (highest fold increase), 120% at day 25, and 130% at day 60. The PXR-ligand PCN-treated groups increased Cyp3a11 mRNA at 5 and 60 days of age (130% and 160%, respectively), but not at the other two ages (Fig. 1, C2).
In summary, the expression of the three prototypical xeno-sensor target genes has demonstrated that 1) the fold induction of the xeno-sensor target gene expression is age specific and 2) there appear to be interactions between different pathways, that is, the AhR-ligand decreases Cyp3a11 and both the CAR and PXR ligands can activate the PXR-target gene Cyp3a11. To further investigate the age-specific effects of the three xeno-sensor ligands on the expression of drug metabolism and transport pathways in liver, the mRNA expression of other important DPGs was quantified at the four developmental ages. These genes were selected based on a previous publication showing that they are bona fide targets of AhR, CAR, and/or PXR in adult male livers (Aleksunes and Klaassen, 2012).
The aldehyde dehydrogenases (Aldhs) are important phase I enzymes that metabolize aldehydes, among which Aldh1a1 and Aldh1a7 are upregulated by TCPOBOP in adult mouse liver (Aleksunes and Klaassen, 2012). Regarding the mRNA abundance of these Aldhs, all three genes were minimally expressed at 2 and 5 days of age, followed by a gradual increase during development, and the highest mRNA levels in livers were observed in 60-day-old adult liver (Fig. 1, D1-F1). Compared with the vehicle-treated group of the same age, TCDD did not appear to alter the mRNAs of these Aldhs, except for a 31.8% decrease in the Aldh1a1 at 25 days of age (Fig. 1, D2-F2). In contrast, and consistent with the literature (Aleksunes and Klaassen, 2012), TCPOBOP increased the mRNAs of Aldh1a1 (180%) and Aldh1a7 (150%) in mice at 60 days of age (adult). In addition, TCPOBOP increased the mRNAs of Aldh1a1 (40%) and Aldh1a7 (30%) at 25 days of age, but to a lesser extent. TCPOBOP also increased Aldh1a7 mRNA (100%) in livers of mice at 5 days of age, but it did not alter Aldh1a1 at 5 days of age or any of the Aldhs in mice at 2 days of age. PCN increased the mRNAs of Aldh1a1 (90%) and Aldh1a7 (60%) at 60 days of age, but decreased Aldh1a1 (65% and 68.6% at days 5 and 25, respectively) and Aldh1a7 (68% at day 25) during neonatal and adolescent ages, as well as Aldh7a1 at 5 days of age (35.4%) (Fig. 1, D2-F2).
Developmental Regulation of Phase II Gsts and Sults by Xeno-Sensor Ligands.
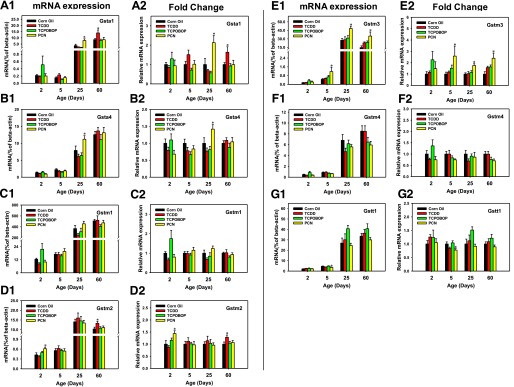
The mRNA expression of Gst isoforms was minimally expressed at 2 and 5 days of age (neonatal), but gradually increased to adult levels by 25 or 60 days of age (Fig. 2, A1-G1). Compared with the vehicle-treated group of the same age, the PXR-ligand PCN increased the mRNA expression of Gsta1 (110%) and Gsta4 (40%) at 25 days of age (Fig. 2, A2 and B2) and the AhR-ligand TCDD only increased the mRNA of Gsta1 at 60 days of age (63.5%), whereas none of the three chemicals significantly altered the mRNAs of Gstm1 and Gstm4 (Fig. 2, C2 and F2). Regarding the other Gstm isoforms, the PXR-ligand PCN upregulated Gstm2 mRNA (48%) at 2 days of age and Gstm3 mRNA at all ages (170%, 80%, and 2.4-fold at days 5, 25, and 60, respectively) (Fig. 2, D2 and E2). The AhR-ligand TCDD induced Gstm2 mRNA (23%) at 60 days of age (Fig. 2, D2 and E2). In contrast, PCN appeared to downregulate Gstt1 mRNA (23%) at 5 days of age (Fig. 2G, panel 2).
Fig. 2.
The mRNA expression of phase II Gst enzymes in livers of male mice after treatment with corn oil (vehicle), TCDD (AhR ligand), TCPOBOP (CAR ligand), or PCN (PXR ligand) at 2, 5, 25, and 60-days of age (n = 4–6 per group). The expressions measured in triplicate are first normalized to the expression of the housekeeping gene β-actin, and then are expressed as fold changes compared with the vehicle-treated group at that particular age. Asterisks (*) represent statistical difference (P < 0.05) compared with the vehicle-treated group at the same age.
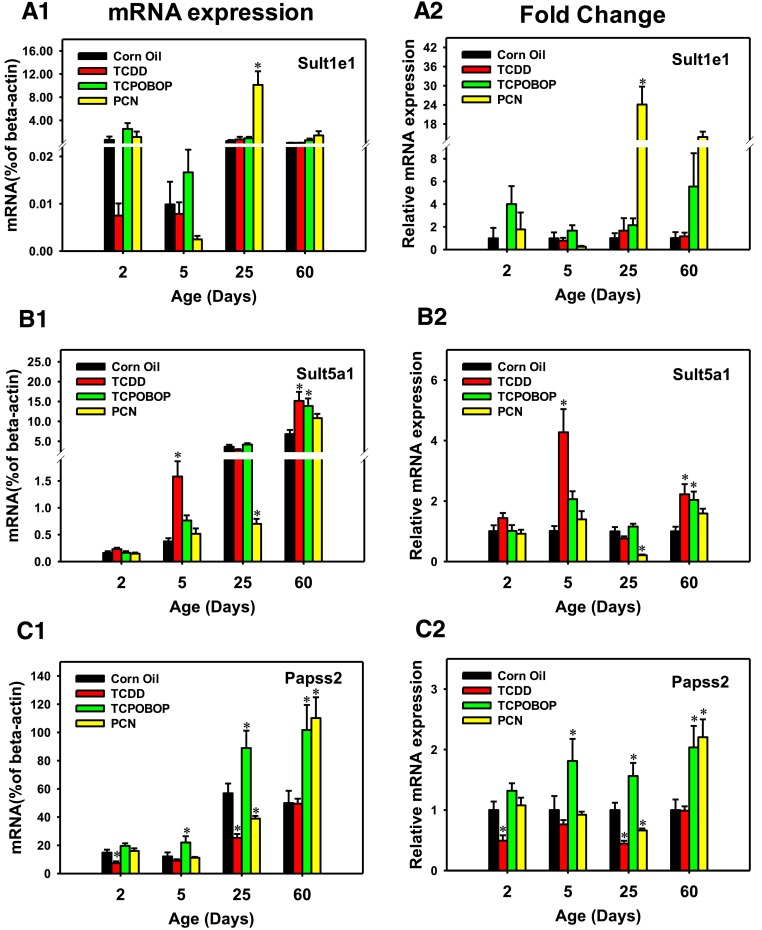
As for the expression of Sult isoforms in liver, Sult1e1 mRNA was lowly expressed at all ages (Fig. 3, A1) with no statistical difference. Compared with the vehicle-treated group of the same age, the mRNA expression of Sult1e1 was highly inducible by PCN at 25 days of age (10.1-fold) (Fig. 3, A2). Regarding the abundance of Sult5a1 mRNA, it gradually increased from the neonatal level and finally reached its adult level at 60 days of age. Compared with the vehicle-treated group of the same age, Sult5a1 mRNA was induced by TCDD at both 5 days (4.3-fold) and 60 days (120%) of age, and the highest fold increase of Sult5a1 was observed in 5-day-old mice. The Sult5a1 mRNA was also upregulated by TCPOBOP at 60 days of age (100%), but downregulated by PCN at 25 days of age (79% decrease) (Fig. 3, B2). With regard to the enzyme that synthesizes the cosubstrate for sulfation reactions, namely, Papss2, its basal mRNA was minimally expressed at neonatal ages but gradually increased to adult level, and the highest basal mRNA expression of Papss2 was observed in liver of 25-day-old mice (Fig. 3, C1). Compared with the vehicle-treated group of the same age, TCDD decreased the Papss2 mRNA at day 2 (50%) and day 25 (56%), but not at the other ages (Fig. 3, C2). TCPOBOP upregulated Papss2 mRNA at all ages except for 2 days of age (80%, 60%, and 100% at days 5, 25, and 60, respectively); PCN upregulated Papss2 mRNA only at 60 days of age (120%), but moderately decreased Papss2 mRNA at 25 days of age (34%) (Fig. 3, C2).
Fig. 3.
The mRNA expression of more phase II enzymes in livers of male mice after treatment with corn oil (vehicle), TCDD (AhR ligand), TCPOBOP (CAR ligand), or PCN (PXR ligand) at 2, 5, 25, and 60 days of age (n = 4–6 per group). The expressions measured in triplicate are first normalized to the expression of the housekeeping gene β-actin, and then are expressed as fold changes compared with the vehicle-treated group at that particular age. Asterisks (*) represent statistical difference (P < 0.05) compared with the vehicle-treated group at the same age.
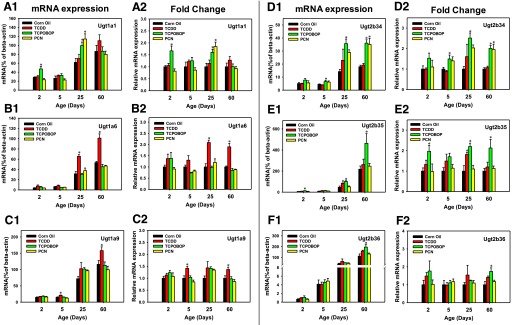
Developmental Regulation of Phase II Ugts by Xeno-Sensor Ligands.
To determine the effect of xenobiotic sensor ligand–mediated changes in the expression of Ugts that are known to be bona fide targets of at least one of the three xeno-sensors in adult mice (Aleksunes and Klaassen, 2012), the mRNAs of these Ugt isoforms, namely, Ugt1a1, 1a6, 1a9, 2b34, 2b35, and 2b36 were quantified at the four developmental ages (Fig. 4, A1-F1). Regarding the basal expression of Ugt1a1 mRNA, it gradually increased to adult levels by 60 days of age (Fig. 4, A1). Compared with the vehicle-treated group of the same age, Ugt1a1 mRNA was inducible by the CAR-ligand TCPOBOP at both 2 days (70%) and 25 days (60%) of age, as well as by the PXR-ligand PCN at 25 days of age (80%). The highest fold change was observed in livers from 25-day-old, PCN-treated mice (Fig. 4, A2). This is consistent with previous findings that hepatic Ugt1a1 expression was inducible by the CAR-ligand TCPOBOP or the PXR-ligand PCN, but to a lesser extent. The reason for this lower level of induction is probably due to the different dosage schedule—using only a single injection in this study compared with the previous study in which the mice were dosed once daily for four consecutive days (Aleksunes and Klaassen, 2012).
Fig. 4.
The mRNA expression of phase-II Ugt enzymes in livers of male mice after treatment with corn oil (vehicle), TCDD (AhR ligand), TCPOBOP (CAR ligand), or PCN (PXR ligand) at 2, 5, 25, and 60 days of age (n = 4–6 per group). The expressions measured in triplicate are first normalized to the expression of the housekeeping gene β-actin, and then are expressed as fold changes compared with the vehicle-treated group at that particular age. Asterisks (*) represent statistical difference (P < 0.05) compared with the vehicle-treated group at the same age.
The basal mRNA expression of Ugt1a6 and Ugt1a9 was low in neonatal ages, but gradually increased to adult levels by 60 days of age (Fig. 4, B1 and C1). Both Ugt1a6 and Ugt1a9 were upregulated by the AhR-ligand TCDD in an age-specific manner (Fig. 4, B1 and C1). Compared with the vehicle-treated group of the same age, Ugt1a6 mRNA was inducible by TCDD at 25 days (110%) and 60 days (70%) of age. Although the highest fold increase of Ugt1a6 was observed in the TCDD-treated group at 25 days of age (Fig. 4, B2), the highest mRNA values were seen in 60-day-old TCDD-treated mice (Fig. 4, B1). The fold induction of Ugt1a9 mRNA by TCDD was observed at both 5 days (40%) and 60 days (40%) of age (Fig. 4, C2). Consistently, the highest mRNA value of Ugt1a9 was also observed in 60-day-old TCDD-treated mice (Fig. 4, C1).
Ugt2b34 mRNA increased gradually to adult levels by 25 days of age (Fig. 4, D1), whereas Ugt2b35 mRNA was minimally expressed at 2 and 5 days of age, followed by a significant increase only at 60 days of age (Fig. 4, E1). Compared with the vehicle-treated group of the same age, TCDD had no effect on the mRNAs of Ugt2b34 or Ugt2b36 at any age (Fig. 4, D1 and F1), whereas it significantly induced the mRNA expression of Ugt2b35 at 25 days of age (81.2%). TCPOBOP increased the mRNAs of Ugt2b34 at all ages except 2 days of age (50%, 150%, and 100% at days 5, 25, and 60, respectively). In contrast, PCN increased Ugt2b34 only at day 60 (100%). The highest fold increase of Ugt2b34 mRNA was seen in 25-day-old TCPOBOP-treated mice (Fig. 4, D2). Similarly, TCPOBOP also induced the mRNA of Ugt2b35 at all ages except at 5 day of age (100%, 120%, and 110% at days 2, 25, and 60, respectively). The highest fold increase of Ugt2b35 was seen in 25-day-old TCPOBOP-treated mice (Fig. 4, E2). The fold increase of Ugt2b36 mRNA was only seen in 60-day-old TCPOBOP-treated mice (70%) (Fig. 4, F2).
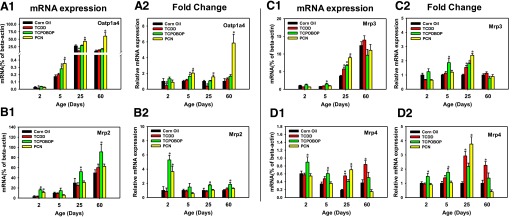
Developmental Regulation of Transporters by Xeno-Sensor Ligands.
The mRNA of the uptake transporter Oatp1a4 was minimally expressed at neonatal ages (2 and 5 days of age), but significantly increased after 25 days of age (Fig. 5, A1). Compared with the vehicle-treated group of the same age, the mRNA of Oatp1a4 was significantly downregulated by TCDD at both days 2 and 25 (61.6% and 62.8%, respectively), whereas it was upregulated by TCPOBOP (60%) and PCN (100%) at 5 days of age (Fig. 5, A2). At 25 days of age, PCN upregulated Oatp1a4 mRNA (60%). At 60 days of age, neither TCDD nor TCPOBOP altered the quantity of Oatp1a4 mRNA; however, PCN markedly induced the fold change of Oatp1a4 mRNA (5.8-fold) (Fig. 5, A2).
Fig. 5.
The mRNA expression of uptake (Oatp1a4) and efflux (Mrp2, Mrp3, Mrp4) transporters in livers of male mice after treatment with corn oil (vehicle), TCDD (AhR ligand), TCPOBOP (CAR ligand), or PCN (PXR ligand) at 2, 5, 25, and 60 days of age (n = 4–6 per group). The expressions measured in triplicate are first normalized to the expression of the housekeeping gene β-actin, and then are expressed as fold changes compared with the vehicle-treated group at that particular age. Asterisks (*) represent statistical difference (P < 0.05) compared with the vehicle-treated group at the same age.
The mRNA of efflux transporters Mrp2 and Mrp3 were gradually increased to adult levels at 60 days of age (Fig. 5, B1 and C1). Compared with the vehicle-treated group of the same age, the mRNA of Mrp2 was not altered by the AhR-ligand TCDD at any age; however, it was markedly upregulated by the CAR-ligand TCPOBOP (5.3-fold) and the PXR-ligand PCN (3.7-fold) at 2 days of age. It was also upregulated by TCPOBOP at day 25 (80%) and day 60 (80%), but to a lesser extent (Fig. 5, B2). Compared with the vehicle-treated group of the same age, the mRNA of Mrp3 was not altered by any of these three chemicals at 2 or 60 days of age, but there was minor upregulation by TCDD at day 25 (50%) (Fig. 5, C2). TCPOBOP also upregulated Mrp3 mRNA at 5 days (90%) and 25 days (80%) of age. The highest fold increase of Mrp3 mRNA was observed in 25-day-old PCN-treated mice (140%) (Fig. 5, C2). Compared to the vehicle-treated group of the same age, Mrp4 mRNA was inducible by all three chemicals at various ages. The CAR-ligand, TCPOBOP, upregulated Mrp4 mRNA at both 2 days (50%) and 5 days (80%) of age but not at the other two ages; the AhR-ligand, TCDD, induced a fold increase of Mrp4 mRNA at both 25 days (190%) and 60 days (130%) of age. The highest fold increase of Mrp4 mRNA was observed in 25-day-old PCN-treated mice (3.8-fold) (Fig. 5, D2).
In summary, as shown in Table 1, some of the DPGs were most induced at the neonatal ages (2 and 5 days of age), whereas others were induced most at adolescent or adult ages. For example, the DPGs were upregulated in livers of neonatal mice with the highest fold induction observed for the mRNAs of Cyp1a2, Sult5a1, and Ugt1a9 by TCDD; Cyp3a11 and Mrp2 by TCPOBOP; and Gstm2 and Gstm3 by PCN. Some DPGs were upregulated the most in livers of adolescent mice with the highest fold increase observed for the mRNAs of Ugt1a6 and Mrp4 by TCDD; Cyp2b10, Ugt2b34, and Ugt2b35 by TCPOBOP; as well as Gsta1, Gsta4, Sult1e1, Ugt1a1, Mrp3, and Mrp4 by PCN. Other DPGs were upregulated the most in adult livers: Aldh1a1, Aldh1a7, and Ugt2b36 by TCPOBOP, as well as Papss2 and Oatp1a4 by PCN. Therefore, a marked difference in the fold induction of the prototypical xeno-sensor target genes was observed during liver development, suggesting that age plays a major role in the sensitivity of the hepatic drug-processing machinery to xenobiotics.
TABLE 1.
Highest fold induction of DPGs among neonatal (days 2 and 5), adolescent (day 25) and adult (day 60) ages
| Age | Ligand (Xeno-Sensor) | DPG |
|---|---|---|
| Neonatal (days 2 and 5) | TCDD (AhR) | Cyp1a2, Sult5a1, Ugt1a9 |
| TCPOBOP (CAR) | Cyp3a11, Mrp2 | |
| PCN (PXR) | Gstm2, Gstm3 | |
| Adolescent (day 25) | TCDD (AhR) | Ugt1a6, Mrp4 |
| TCPOBOP (CAR) | Cyp2b10, Ugt2b34, Ugt2b35 | |
| PCN (PXR) | Gsta1, Gsta4, Sult1e1, Ugt1a1, Mrp3, Mrp4 | |
| Adult (day 60) | TCPOBOP (CAR) | Aldh1a1, Aldh1a7, Ugt2b36 |
| PCN (PXR) | Papss2, Oatp1a4 |
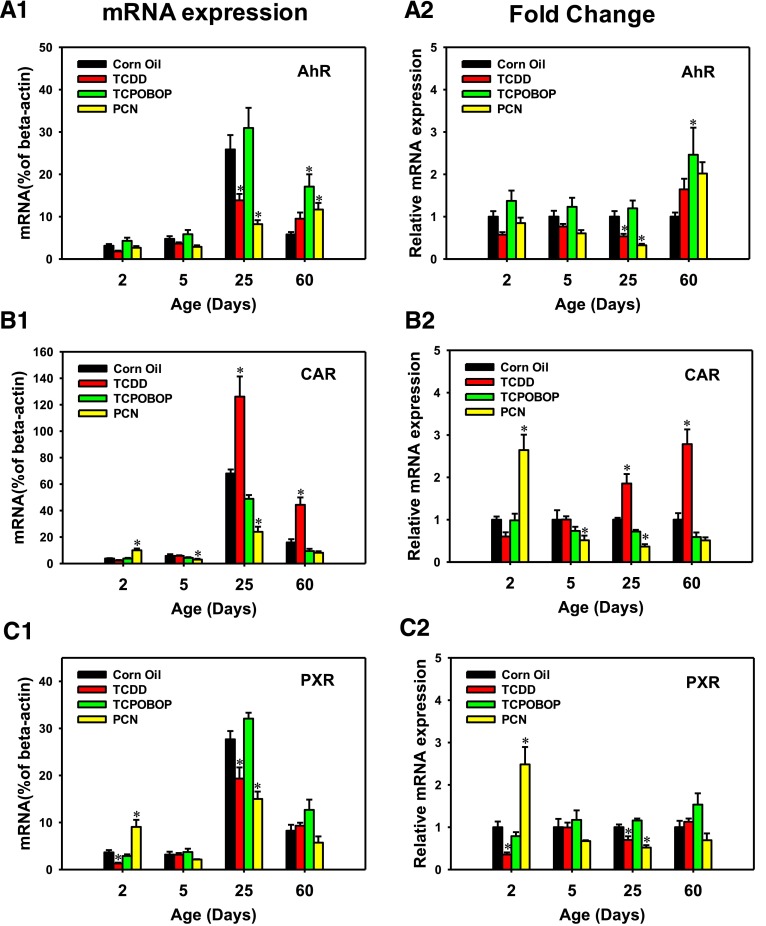
Expression of Xeno-Sensors.
To determine whether the chemicals that activate the three transcription factors also increase the mRNA of the transcription factors, the mRNAs of AhR, CAR, and PXR were quantified in control and the ligand-treated conditions. Regarding the abundance of AhR, CAR, and PXR mRNAs, they were minimally expressed at days 2 and 5 neonatal ages, but reached the highest mRNA level at the adolescent age of day 25 (Fig. 6, A1-C1). Compared with the vehicle-treated group, the AhR mRNA was not altered by any chemical at day 2 or 5 neonatal ages; however, at day 25, the AhR-ligand TCDD and the PXR-ligand PCN both decreased the AhR mRNA by 46.5% and 67.7%, respectively. At 60 days of age, both the CAR-ligand TCPOBOP and PXR-ligand PCN upregulated the AhR mRNA (150% and 200%, respectively) (Fig. 6, A2).
Fig. 6.
The mRNA expression of xeno-sensors in livers of male mice after treatment with corn oil (vehicle), TCDD (AhR ligand), TCPOBOP (CAR ligand), or PCN (PXR ligand) at 2, 5, 25, and 60 days of age (n = 4–6 per group). The expressions measured in triplicate are first normalized to the expression of the housekeeping gene β-actin, and then are expressed as fold changes compared with the vehicle-treated group at that particular age. Asterisks (*) represent statistical difference (P < 0.05) compared with the vehicle-treated group at the same age.
The basal mRNA expression of the transcription factor CAR was significantly increased in both 25- and 60-day-old mice compared with its neonatal level (Fig. 6, B1). Compared with the vehicle-treated group of the same age, the mRNA of CAR was not altered by its ligand TCPOBOP at any age. However, at days 25 and 60, the AhR-ligand TCDD induced the mRNA expression of CAR (90% and 180%, respectively), whereas this effect was not observed during neonatal ages. Interestingly, the PXR-ligand PCN also upregulated CAR mRNA at 2 days of age (100%); however, during development PCN downregulated CAR mRNA at both day 5 (48.6%) and day 25 (63.5%). The highest fold change of CAR mRNA was observed in 60-day-old TCDD-treated mice (Fig. 6, B2).
Similarly, the basal mRNA expression of the transcription factor PXR was also significantly expressed after 25 days of age (Fig. 6, C1). Compared with the vehicle-treated group of the same age, PXR mRNA was not altered by any of the three chemicals in 5- and 60-day-old mice (Fig. 6, C2). At 2 days of age, TCDD downregulated PXR mRNA (64.4%), whereas PCN markedly upregulated PXR mRNA (150%, highest fold change). At 25 days of age, both TCDD and PCN decreased PXR mRNA (30.1% and 48.1%, respectively) (Fig. 6, C2).
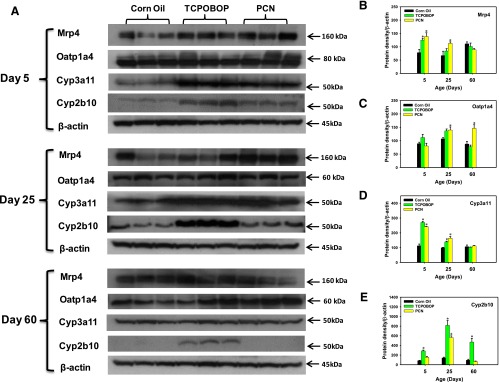
Protein Expression.
Western blot analysis was conducted for the phase I drug-metabolizing enzymes Cyp2b10 and Cyp3a11, uptake transporter Oatp1a4, and efflux transporter Mrp4 (Fig. 7A). Compared with the vehicle-treated group at the same age, Cyp2b10 protein was persistently upregulated by CAR-ligand TCPOBOP at all ages (3.5-, 5.9-, and 5.1-fold, respectively), whereas the PXR-ligand PCN induced a 4.1-fold increase of Cyp2b10 protein only at 25 days of age. At 5 and 60 days of age, PCN tended to increase 87% and 32%, respectively, of Cyp2b10 protein expression, although statistical significance was not achieved (Fig. 7E). Another drug-metabolizing enzyme Cyp3a11 was markedly increased in response to TCPOBOP (141%) and PCN (115%) at 5 days of age. Cyp3a11 protein was also induced by both TCPOBOP (40%) and PCN (65.3%) at 25 days of age but to a lesser extent. However, the protein expression of Cyp3a11 was not altered by any of these three chemicals in adult mice (Fig. 7D), suggesting the possibility of post-transcriptional control during development. The protein expression of Oatp1a4 was not altered by TCPOBOP at any age group; however, in PCN-treated mice Oatp1a4 was elevated at both 25 and 60 days of age (30.6% and 67%, respectively) (Fig. 7C). The protein expression of Mrp4 was increased by both TCPOBOP (60.9%) and PCN (78.5%) at 5 days of age. At 25 days of age, PCN also increased Mrp4 protein expression (69.1%) (Fig. 7B)
Fig. 7.
Western blotting analysis of Mrp4, Oatp1a4, Cyp3a11, and Cyp2b10 proteins in livers of mice treated with corn oil, TCPOBOP, or PCN, at 5, 25, and 60 days of age (n = 3 per group). The intensities of the protein bands were quantified using Image J software (National Institutes of Health), and data were normalized to the band intensity of β-actin protein. Asterisks (*) represent values statistically different from the corn oil–treated mice at the same age.
Enzyme Activities.
To determine the enzyme activities of target genes of xeno-sensors, two major DPGs, namely, Cyp2b and Cyp3a were quantified (Fig. 8, A and B). Because of the limited amount of mouse livers collected at 2 days age, the enzyme activities were only conducted at 5, 25, and 60 days of age. The activity of Cyp2b was significantly upregulated by TCPOBOP at 25 and 60 days of age compared with the activity in the vehicle-treated groups (140% and 90%, respectively), with the highest fold induction in 25-day-old adolescent mice (Fig. 8A). The Cyp3a activity was induced in TCPOBOP-treated mouse livers (2.8-, 6-, and 8.7-fold) at all ages, whereas this induction was also observed in the PCN-treated group only at 25 and 60 days of age (18.4- and 17-fold, respectively) (Fig. 8B).
Discussion
In conclusion, the current study has provided critical information regarding the age-specific regulation of drug-metabolizing enzymes and transporters in mouse liver by the prototypical ligands of three major xeno-sensors, including AhR-ligand TCDD, CAR-ligand TCPOBOP, and PXR-ligand PCN. This study has demonstrated that age not only influences the constitutive expression but also the inducibility of DPGs in liver by these chemicals.
Characterization of the developmental expression patterns of DPGs is of importance to predict drug clearance, efficacy, and risk of adverse drug reactions in neonates and children (Hines, 2007). In adults, it is well known that exposure to microsomal enzyme inducers can induce the expression of some phase I and II drug-metabolizing enzymes as well as transporters (Aleksunes and Klaassen, 2012). Earlier studies have characterized the ontogeny of many drug-metabolizing enzymes during human liver development (de Wildt et al., 1999), including hepatic cytochrome P450s (Hines, 2007), flavin-containing monooxygenases (Hines, 2006), Sults (Richard et al., 2001), Ugts (Strassburg et al., 2002), as well as transporters (Mooij et al., 2014). There are profound changes occurring in the basal ontogenic expression of drug-metabolizing enzymes in human liver during development.
Little is known about the inducibility of DPGs in pediatric patients, mainly due to ethical concerns for conducting chemical exposure experiments in humans during development. Considering the highly conserved nature of numerous DPGs between mouse and humans, the mouse model has become a common tool to recapitulate the regulation of DPGs in humans. Moreover, the mouse model has many technical advantages such as being easy to maintain and minimized influences from gene variation, environment, and dietary factors. Numerous studies using wild-type, knockout, and transgenic mice have reported gene expression profiles in mouse livers for many DPGs (Cui et al., 2009; Hart et al., 2009; Aleksunes and Klaassen, 2012), and these studies have provided mechanistic information about the regulation of DPGs in vivo.
In mouse livers, using RNA sequencing, we have characterized the ontogenic expression of many DPGs under basal conditions at various ages, including various phase I enzymes (Peng et al., 2012, 2013), phase II enzymes (Lu et al., 2013), and transporters (Cui et al., 2012a). However, there is a critical gap in the literature regarding whether xenobiotics that activate AhR, CAR, and PXR in adults are able to increase the expressions of DPGs in younger animals. Previous studies have illustrated various inducibilities of drug-metabolizing enzymes during development in different species. For example, the hepatic Gst exhibited functional heterogeneity in mice, rats, rabbits, and guinea pigs after pretreatment with Gst inducers (Gregus et al., 1985); The ontogenetic expression and localization of Cyp1a1 and Cyp1a2 in the livers of rabbits are differently regulated with increasing age (Rich et al., 1993); More studies used the rat model to identify the activities of P450 after maximal induction, such as Cyp1a1/1a2 and Cyp2b1/2b2 by phenobarbital (Horbach et al., 1992; Agrawal and Shapiro, 1996), Cyp3a by dexamethasone (Lee and Werlin, 1995; Wauthier et al., 2004), or Cyp1a, Cyp2b, and Cyp3a induction by inducer dimethylcyclosiloxanes in fetal liver of rats (Falany and Li, 2005). However, most of these studies are limited to one category of drug-metabolizing enzymes in response to a single inducer. Moreover, it appears that the effect of age cannot be generalized for the various P450s, and the influence of age on one specific P450 is dependent on the type of inducer used (Horbach et al., 1990). Therefore, our study has filled the critical knowledge gap regarding the inducibility of DPGs at various ages.
Studies have shown that activation of these transcription factors by specific inducers plays an important role in the induction of their associated target genes in adult mouse livers (Aleksunes and Klaassen, 2012). As expected, in the present study, the prototypical target genes for each transcription factor were markedly induced in adult mice in response to their corresponding ligands. For example, the AhR-ligand TCDD, CAR-ligand TCPOBOP, and PXR-ligand PCN markedly upregulated the mRNAs of Cyp1a2, Cyp2b10, and Cyp3a11, respectively, in adult mouse livers (Fig. 1). Similar to findings in the prior publication, these three xeno-sensor ligands were also involved in the upregulation of target genes of other transcription factors (Aleksunes and Klaassen, 2012). For example, previous studies using AhR-null mice reported that Sult5a1, Ugt1a6, and Ugt1a9 were all AhR-target genes, whereas in this study TCDD also induced the mRNA expression of some phase II DPGs (such as Sult5a1, Ugt1a6, and Ugt1a9) in liver of 60-day-old mice (Fig. 3, B1 and Fig. 4, B1, C1) (Petrick and Klaassen, 2007; Aleksunes and Klaassen, 2012). The activation of CAR signaling by TCPOBOP resulted in the upregulation of a series of genes as shown in Figs. 1–5, including phase I (Cyp3a11, Aldh1a1, and Aldh1a7), phase II (Sult5a1, Papss2, Ugt2b34, Ugt2b35, and Ugt2b36), and transporters (Mrp2). As illustrated in previous studies, these genes are known CAR-target genes, and are significantly induced by TCPOBOP to various degrees—for example, Cyp3a11 mRNA in adult male mice was induced 2- to 3-fold, Aldh1a1 was induced 2-fold, Aldh1a7 was induced 3-fold, and Mrp2 was induced 1.5-fold (Aleksunes and Klaassen, 2012). In addition, activation of PXR by its ligand PCN also significantly induced the expression of the Oatp1a4 transporter, as shown in the previous study (Aleksunes and Klaassen, 2012). The consistent findings of the present study in adult mice livers provide evidence for the involvement of transcription factors in the regulation of phase I, phase II, and transporter expression.
Some DPGs that have been shown previously to be induced by the transcription factor ligands (Aleksunes and Klaassen, 2012) were not significantly altered in the present study. For example, prior studies on adult male mice livers showed upregulation of the mRNA expressions by AhR-ligand TCDD (Cyp2b10 and Ugt2b35), CAR-ligand TCPOBOP (Cyp1a2, Gsta1, Gsta4, Gstm1-m4, Gstt1, all Sult enzymes, Ugt1a1, Ugt1a9, Mrp3, and Mrp4), and PXR-ligand PCN (Gsta1, Gstm1-m3, Ugt1a1, and Ugt1a9) (Aleksunes and Klaassen, 2012). This difference may be due to the differences in the dosing regimen; mice in the present study were treated only once with the same dose, whereas in previous studies mice received four consecutive days of treatment. However, even though these genes were not altered by ligands at 60 days of age in this study, there was remarkable upregulation occurring at younger ages, suggesting that younger ages are more sensitive to xenobiotic-mediated DPG upregulation.
The mRNA and protein upregulation of Cyp1a2 by TCDD was consistent at 5 days of age; however, we did not observe a significant upregulation of the Cyp1a2 protein at other developmental ages, whereas the Cyp1a2 mRNA continued to be upregulated. The mechanisms underlying such a discrepancy between the mRNA and the protein levels remain unknown, but it is possible that post-transcriptional modifications, such as mRNA degradation by miRNAs, may play a role. These mechanisms will be addressed in future studies. Another example is that at 25 days of age PCN tended to increase the Cyp3a11 mRNA, although statistical significance was not achieved; however, PCN significantly upregulated the Cyp3a11 protein and enzyme activity at 25 days of age. At 60 days of age, the Cyp3a11 mRNA was upregulated by both TCPOBOP and PCN, but there was no significant change in the protein level in these two treatment groups. Such a discrepancy is likely regulated by post-transcriptional mechanisms.
Although the regulation of many DPGs shares great similarities between mice and humans, there are certain species differences in the nuclear receptor–mediated gene expression (Gregus et al., 1985; Dickins, 2004; Lin, 2006; Hewitt et al., 2007; Mueller et al., 2010; Gonzalez et al., 2015). For example, TCPOBOP can only activate the mouse CAR resulting in an upregulation of Cyp2b, but not the human CAR target gene CYP2B6 (Dickins, 2004); PCN is a potent activator of the mouse PXR, resulting in an upregulation of Cyp3a, but it has little effect on the human PXR, which alternatively is known to be induced by rifampicin (Hewitt et al., 2007). Activation of the mouse CAR by phenobarbital results in increased cell proliferation and liver tumor formation; however, phenobarbital does not cause tumors in humans, and in fact it has long been used as a therapeutic drug to treat convulsions (Whysner et al., 1996). The species differences of the nuclear receptors are likely due to the differences in their ligand binding domains between human and rodents. Therefore, it is important to compare the mouse data with the pediatric patient data in future studies.
Taken together, the present data provide important insight into the age-specific xenobiotic regulation of drug-metabolizing and transporter genes during development. Understanding this gene regulation is critical to predict drug disposition, distribution, metabolism, and elimination across all ages, and can thus decrease the risk of an adverse reaction during drug treatment in pediatric patients.
Supplementary Material
Acknowledgments
The authors thank all of the laboratory members for the sample preparation, and rotation student Carly Strecker Wilder for assistance in the primer design.
Abbreviations
- Aldh
aldehyde dehydrogenase
- DPG
drug-processing gene
- Gst
glutathione S-transferase
- P450
cytochrome P450
- PCN
pregnane-16α-carbonitrile
- Sult
sulfotransferase
- Ugt
UDP-glucuronosyltransferase
- ST
sucrose tris
- TCDD
2,3,7,8-tetrachlorodibenzodioxin
- TCPOBOP
1,4-bis [2-(3,5-dichloropyridyloxy)] benzene
- xeno-sensor
xenobiotic-sensing transcription factor
Authorship Contributions
Participated in research design: Renaud, Klaassen, Cui.
Conducted experiments: Li, Renaud, Cui.
Performed data analysis: Li.
Wrote or contributed to the writing to the manuscript: Li, Renaud, Klaassen, Cui.
Footnotes
This work was supported by the National Institutes of Health [R01 Grants ES019487 and GM111381], as well as start-up funds from the University of Washington Center of Ecogenetics and Environmental Health [P30 ES0007033].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Agrawal AK, Shapiro BH. (1996) Phenobarbital induction of hepatic CYP2B1 and CYP2B2: pretranscriptional and post-transcriptional effects of gender, adult age, and phenobarbital dose. Mol Pharmacol 49:523–531. [PubMed] [Google Scholar]
- Aleksunes LM, Klaassen CD. (2012) Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab Dispos 40:1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Goedken M, Manautou JE. (2006) Up-regulation of NAD(P)H quinone oxidoreductase 1 during human liver injury. World J Gastroenterol 12:1937–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GD. (2002) Children versus adults: pharmacokinetic and adverse-effect differences. Epilepsia 43 (Suppl 3):53–59. [DOI] [PubMed] [Google Scholar]
- Croom EL, Stevens JC, Hines RN, Wallace AD, Hodgson E. (2009) Human hepatic CYP2B6 developmental expression: the impact of age and genotype. Biochem Pharmacol 78:184–190. [DOI] [PubMed] [Google Scholar]
- Cui JY, Gunewardena SS, Yoo B, Liu J, Renaud HJ, Lu H, Zhong XB, Klaassen CD. (2012a) RNA-Seq reveals different mRNA abundance of transporters and their alternative transcript isoforms during liver development. Toxicol Sci 127:592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JY, Renaud HJ, Klaassen CD. (2012b) Ontogeny of novel cytochrome P450 gene isoforms during postnatal liver maturation in mice. Drug Metab Dispos 40:1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YJ, Cheng X, Weaver YM, Klaassen CD. (2009) Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos 37:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. (1999) Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 37:485–505. [DOI] [PubMed] [Google Scholar]
- Dickins M. (2004) Induction of cytochromes P450. Curr Top Med Chem 4:1745–1766. [DOI] [PubMed] [Google Scholar]
- Falany CN, Li G. (2005) Effects of age and pregnancy on cytochrome P450 induction by octamethyltetracyclosiloxane in female Sprague-Dawley rats. J Biochem Mol Toxicol 19:129–138. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Fang ZZ, Ma X. (2015) Transgenic mice and metabolomics for study of hepatic xenobiotic metabolism and toxicity. Expert Opin Drug Metab Toxicol 11:869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FJ, Fernandez-Salguero P. (1998) The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metab Dispos 26:1194–1198. [PubMed] [Google Scholar]
- Gregus Z, Varga F, Schmelás A. (1985) Age-development and inducibility of hepatic glutathione S-transferase activities in mice, rats, rabbits and guinea-pigs. Comp Biochem Physiol C 80:85–90. [DOI] [PubMed] [Google Scholar]
- Handschin C, Meyer UA. (2003) Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev 55:649–673. [DOI] [PubMed] [Google Scholar]
- Hart SN, Cui Y, Klaassen CD, Zhong XB. (2009) Three patterns of cytochrome P450 gene expression during liver maturation in mice. Drug Metab Dispos 37:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Baldwin WS. (2009) Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Curr Pharmacogenomics Person Med 7:81–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt NJ, Lechón MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, et al. (2007) Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev 39:159–234. [DOI] [PubMed] [Google Scholar]
- Hines RN. (2006) Developmental and tissue-specific expression of human flavin-containing monooxygenases 1 and 3. Expert Opin Drug Metab Toxicol 2:41–49. [DOI] [PubMed] [Google Scholar]
- Hines RN. (2007) Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol 21:169–175. [DOI] [PubMed] [Google Scholar]
- Horbach GJ, Van Asten JG, Rietjens IM, Kremers P, Van Bezooijen CF. (1992) The effect of age on inducibility of various types of rat liver cytochrome P-450. Xenobiotica 22:515–522. [DOI] [PubMed] [Google Scholar]
- Horbach GJ, Van Asten JG, Van Bezooijen CF. (1990) The influence of age on the inducibility of rat liver cytochrome P450IA1 (CYPIA1) and P450IA2 (CYPIA2) mRNAs. Mutat Res 237:117–121. [DOI] [PubMed] [Google Scholar]
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. (2003) Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL. (2005) Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab 6:309–328. [DOI] [PubMed] [Google Scholar]
- Lee PC, Werlin SL. (1995) The induction of hepatic cytochrome P450 3A in rats: effects of age. Proc Soc Exp Biol Med 210:134–139. [DOI] [PubMed] [Google Scholar]
- Lin JH. (2006) CYP induction-mediated drug interactions: in vitro assessment and clinical implications. Pharm Res 23:1089–1116. [DOI] [PubMed] [Google Scholar]
- Lu H, Gunewardena S, Cui JY, Yoo B, Zhong XB, Klaassen CD. (2013) RNA-sequencing quantification of hepatic ontogeny and tissue distribution of mRNAs of phase II enzymes in mice. Drug Metab Dispos 41:844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooij MG, Schwarz UI, de Koning BAE, Leeder JS, Gaedigk R, Samsom JN, Spaans E, van Goudoever JB, Tibboel D, Kim RB, et al. (2014) Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab Dispos 42:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SO, Fery Y, Tuschl G, Schrenk D. (2010) Species-specific activation of nuclear receptors correlates with the response of liver drug metabolizing enzymes to EMD 392949 in vitro. Toxicol Lett 193:120–123. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Iwanari M, Yokoi T. (2003) Effects of histone deacetylation and DNA methylation on the constitutive and TCDD-inducible expressions of the human CYP1 family in MCF-7 and HeLa cells. Toxicol Lett 144:247–256. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Kaltiala EH, Larmi TKI, Kärki NT. (1974) Cytochrome P-450-linked monooxygenase system and drug-induced spectral interactions in human liver microsomes. Chem Biol Interact 9:205–216. [DOI] [PubMed] [Google Scholar]
- Peng L. (2013) RNA-sequencing quantification of hepatic ontogeny of phase-I enzymes in mice. Drug Metab Dispos 41:2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Cui JY, Yoo B, Gunewardena SS, Lu H, Klaassen CD, Zhong XB. (2013) RNA-sequencing quantification of hepatic ontogeny of phase-I enzymes in mice. Drug Metab Dispos 41:2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Yoo B, Gunewardena SS, Lu H, Klaassen CD, Zhong XB. (2012) RNA sequencing reveals dynamic changes of mRNA abundance of cytochromes P450 and their alternative transcripts during mouse liver development. Drug Metab Dispos 40:1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Klaassen CD. (2007) Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metab Dispos 35:1806–1815. [DOI] [PubMed] [Google Scholar]
- Rich KJ, Foster JR, Edwards RJ, Davies DS, Boobis AR. (1993) Ontogenetic development of the distribution of constitutive and 3-methylcholanthrene-induced CYP1A1 and CYP1A2 in rabbit liver. J Histochem Cytochem 41:915–925. [DOI] [PubMed] [Google Scholar]
- Richard K, Hume R, Kaptein E, Stanley EL, Visser TJ, Coughtrie MW. (2001) Sulfation of thyroid hormone and dopamine during human development: ontogeny of phenol sulfotransferases and arylsulfatase in liver, lung, and brain. J Clin Endocrinol Metab 86:2734–2742. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. (2001a) Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos 29:1467–1472. [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. (2001b) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98:3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, Zaya MJ. (2003) Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther 307:573–582. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Marsh SA, Zaya MJ, Regina KJ, Divakaran K, Le M, Hines RN. (2008) Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos 36:1587–1593. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, Manns MP. (2002) Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 50:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ren L, He Y, Wei Y, Chen Z, Yang W, Fu Y, Xu X, Fu W, Hu G, et al. (2014) Association between cytochrome P450 2C9 gene polymorphisms and colorectal cancer susceptibility: evidence from 16 case-control studies. Tumour Biol 35:4317–4322. [DOI] [PubMed] [Google Scholar]
- Wauthier V, Verbeeck RK, Buc Calderon P. (2004) The use of precision-cut liver slices from male Wistar rats as a tool to study age related changes in CYP3A induction and in formation of paracetamol conjugates. Toxicol In Vitro 18:879–885. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. (2000) The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature 407:920–923. [DOI] [PubMed] [Google Scholar]
- Whysner J, Ross PM, Williams GM. (1996) Phenobarbital mechanistic data and risk assessment: enzyme induction, enhanced cell proliferation, and tumor promotion. Pharmacol Ther 71:153–191. [DOI] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. (2005) Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res 28:249–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.