Abstract
The hepatocyte nuclear factors, Hnf1a and Hnf4a, in addition to playing key roles in determining hepatocyte fate, have been implicated as candidate lineage-determining transcription factors in the kidney proximal tubule (PT) [Martovetsky et. al., (2012) Mol Pharmacol 84:808], implying an additional level of regulation that is potentially important in developmental and/or tissue-engineering contexts. Mouse embryonic fibroblasts (MEFs) transduced with Hnf1a and Hnf4a form tight junctions and express multiple PT drug transporters (e.g., Slc22a6/Oat1, Slc47a1/Mate1, Slc22a12/Urat1, Abcg2/Bcrp, Abcc2/Mrp2, Abcc4/Mrp4), nutrient transporters (e.g., Slc34a1/NaPi-2, Slco1a6), and tight junction proteins (occludin, claudin 6, ZO-1/Tjp1, ZO-2/Tjp2). In contrast, the coexpression (with Hnf1a and Hnf4a) of GATA binding protein 4 (Gata4), as well as the forkhead box transcription factors, Foxa2 and Foxa3, in MEFs not only downregulates PT markers but also leads to upregulation of several hepatocyte markers, including albumin, apolipoprotein, and transferrin. A similar result was obtained with primary mouse PT cells. Thus, the presence of Gata4 and Foxa2/Foxa3 appears to alter the effect of Hnf1a and Hnf4a by an as-yet unidentified mechanism, leading toward the generation of more hepatocyte-like cells as opposed to cells exhibiting PT characteristics. The different roles of Hnf4a in the kidney and liver was further supported by reanalysis of ChIP-seq data, which revealed Hnf4a colocalization in the kidney near PT-enriched genes compared with those genes enriched in the liver. These findings provide valuable insight, not only into the developmental, and perhaps organotypic, regulation of drug transporters, drug-metabolizing enzymes, and tight junctions, but also for regenerative medicine strategies aimed at restoring the function of the liver and/or kidney (acute kidney injury, AKI; chronic kidney disease, CKD).
Introduction
Because of the large number of pediatric and adult patients with kidney and liver disease, there is a great need to devise tissue engineering and regenerative medicine strategies to supplement and/or replace the function of both of these organs. Moreover, since neonates and preterm infants are routinely treated with a wide variety of drugs (i.e., antibiotics, nonsteroidal anti-inflammatory drugs, antivirals, and antihypertensives) whose absorption, disposition, and elimination (and therefore efficacy), as well as potential toxicity, are dependent upon functionally immature kidneys and livers (Kearns et al., 2003), it may be useful to devise strategies to enhance kidney and/or liver function, including transport capacity. For these purposes, it is important to establish cells with characteristics that can partially or fully attain the properties of mature cell types from a cultured cell source. This may require achieving the desired characteristics of the target cell type while silencing/excluding those of other cell types.
Since the advent of induced pluripotent stem cells, differentiating stem cells toward desired cell fates has become a promising strategy for future clinical application (Graf and Enver, 2009). However, due to the tumorigenic potential of stem cells (Lu and Zhao, 2013; Okano et al., 2013; Harding and Mirochnitchenko, 2014), there is an advantage to being able to generate desired cell fates without having to undergo a pluripotent state. There have recently been a number of advances in establishing hepatocyte-like cells from embryonic or mature fibroblasts. In these cases, ectopic expression of lineage-determining transcription factors was used to achieve transdifferentiation (Huang et al., 2011, 2014; Sekiya and Suzuki, 2011; Du et al., 2014; Simeonov and Uppal, 2014). Although the derivation of proximal tubule (PT)–like cells from stem cells has been reported (Narayanan et al., 2013), the establishment of PT cell characteristics starting with nonpluripotent cells, or by inducing a defined transcriptional program, is not well understood. Defining how sets of transcription factors guide the differentiation of cell types that may share some characteristics but ultimately perform very different essential functions in different organs (e.g., kidney, liver) is crucial for further refining regenerative medicine and tissue engineering strategies.
Here, we show that, although Hnf1a and Hnf4a alone are insufficient to completely transdifferentiate mouse embryonic fibroblasts (MEFs) toward a proximal tubule cell–like fate, they are capable of inducing the mRNA expression of a number of genes important for proximal tubule identity and function, including solute carrier (SLC) and ATP-binding cassette (ABC) drug transporters, as well as the establishment of tight junctions. Remarkably, coexpression of either Gata4, a combination of forkhead box protein A2 and A3 (Foxa2/3), or all three along with Hnf1a and Hnf4a in MEFs largely eliminated the induction of proximal tubule markers and, instead, strongly induced the expression of hepatocyte markers. In a similar manner, Gata4 and Foxa3 downregulated PT markers and induced expression of hepatocyte markers in primary PT cells (which endogenously express low levels of Hnf1a and Hnf4a). Together, these findings outline a foundation for transdifferentiation toward proximal tubule-like cells and help to clarify the involvement of Hnf1a and Hnf4a in transdifferentiation toward hepatocyte cellular identity. The results may be relevant to our understanding of proximal tubule and hepatocyte development and terminal differentiation as well as to regenerative medicine contexts.
Materials and Methods
Animals, MEF Isolation, and Cell Culture.
All animal procedures were approved by the University of California, San Diego (UCSD) Institutional Animal Care and Use Committee. E16.5 MEFs were prepared as previously described (Martovetsky et al., 2013), whereas E13.5 and E15.5 MEFs were made using a modified procedure. In brief, wild-type pregnant female mice on a 129-C57bl/6 mixed background that were housed under basal conditions with a 12-hour light/dark cycle with ad libitum access to food/water were sacrificed at 13.5, 15.5, and 16.5 days of gestation (day 0 of gestation corresponds to visualization of the vaginal plug). Uteri were removed. Unsexed embryos were isolated, and tissues (minus head and viscera) were minced in phosphate-buffered saline (PBS) in a culture dish. The PBS was then replaced with 0.25% Trypsin/EDTA containing DNase and incubated at 37°C for 10 minutes, triturated, and then incubated another 10 minutes. Dulbecco’s modified Eagle’s medium (DMEM)/F-12 medium with 10% fetal bovine serum (FBS), nonessential amino acid supplement (NEAA), and penicillin/streptomycin (media A) was then added to quench the reaction (this was the same medium later used for cell culture), and the suspension was transferred to a 50-ml conical tube and gravity pelleted to allow undigested pieces to settle. The suspension was then plated in tissue culture flasks with additional medium. In some cases, an aggregate composed of lysed cells and DNA formed, which was aspirated from the culture. The cells were then expanded, trypsinized, pelleted, and frozen in cryovials in 45% medium, 45% FBS, and 10% dimethylsulfoxide for future use.
Lentiviral Transduction.
Lentivirus was produced as previously described (Martovetsky et al., 2013), with slight modifications. In brief, the same plasmids were used as previously described, and the following plasmids were also used to make the corresponding lentiviral preparations: pWPI control plasmid, pWPI-Gata4, pWPI-Foxa2, and pWPI-Foxa3 (a gift from Dr. Lijian Hui, Shanghai Institutes for Biomedical Sciences, Shanghia, China). HEK 293T cells were cultured in “media A.” On the day of HEK 293T transfection, medium was replaced with DMEM/F-12 containing 10% FBS and NEAA, without antibiotics. The following day, the medium was replaced with media A, discarded, and replaced the next day. On the second day after transfection, the medium was collected, replaced with fresh medium, and collected again the next day. The supernatant from the first collection was kept at 4°C until the second collection, then pooled and filtered through a 0.45-μM syringe filter and then centrifuged at 23,000 × g and 4°C for 24 hours. One–one hundredth of initial volume of PBS containing magnesium and chloride was then added to the pellet and allowed to incubate at 4°C overnight. Then, the pellet was resuspended, aliquoted, and stored at −80C. Viral titer was then tested by infecting MEFs with serial dilutions of viral preps in the presence of 8 μg/ml polybrene (Sigma-Aldrich, St. Loius, MO) and measuring green fluorescent protein (GFP)–positive viable cell fractions using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA). After overnight infection, medium was switched to DMEM/F12 containing 1% FBS, penicillin/streptomycin, 1× NEAA, 1× Insulin-Transferrin-Selenium (Invitrogen, Carlsbad, CA), 20 ng/ml epidermal growth factor (R&D Systems, Minneapolis, MN), 4 ng/ml triiodothyronine (Sigma-Aldrich), and 20 ng/ml dexamethasone (Sigma-Aldrich) (medium B). For expression analysis, RNA was collected 1 week after transduction; immunohistochemistry was performed 3 weeks after transduction.
Primary Proximal Tubule Cell Culture.
Adult mouse kidneys were placed in ice-cold PBS and decapsulated. The cortex was then separated from the medulla, minced into small pieces, and transferred to a 50-ml conical tube. The PBS was aspirated and replaced with L-15 medium containing 1 mg/ml collagenase type IV, 10 U/ml DNase, and 1% penicillin/streptomycin, and placed in a shaker at 37°C for 15 minutes. The suspension was then triturated, placed back in the shaker for an additional 15 minutes, triturated again, and allowed to gravity pellet for 1 minute to allow the undigested pieces to settle. All of the following procedures were carried out in a sterile environment. The suspension was passed through a 100 μM mesh cell strainer. Tubules remaining on the mesh were washed with medium A, and then the cell strainer was inverted and the tubules were rinsed from the mesh with a 1:1 mix of medium A and medium B with the addition of 1× antibiotic/antimycotic into a tissue culture plate, and inspected under a microscope to confirm enrichment of proximal tubule segments and depletion of glomeruli. The resulting tubular suspension was then plated into collagen I–coated six-well plates. After 2 days, medium was replaced with medium B, cells were infected with lentivirus for 8 hours, and medium was replaced with fresh medium B. Because primary cells can be difficult to transduce, the number of different transducing factors was kept to a minimum; thus, since these primary PTs cells can produce some level of endogenous Hnf1a and Hnf4 (see Results), and since studies have shown that either Foxa2 or Foxa3 alone can induce hepatocyte-like characteristics in the presence of Hnf4a (with Foxa3 appears to be slightly more robust) (Huang et al., 2011; Sekiya and Suzuki, 2011; Yu et al., 2013), these cells were transduced with either Gata4, Foxa3, or a combination of these two factors. Medium was changed again 3 days after infection. RNA was collected for quantitative reverse-transcription polymerase chain reaction (PCR) analysis 6 days after infection.
Quantitative Reverse-Transcription PCR.
RNA extraction, cDNA preparation, and quantitative reverse-transcription PCR was carried out as previously described (Martovetsky et al., 2013). The list of primer sequences is included (Supplemental Table 1). Significance was determined using the raw values with GraphPad Prism 6 (GraphPad Software, La Jolla, CA) using a one-way analysis of variance with default settings (α value set to 0.05) followed by the Tukey post-hoc analysis.
Immunohistochemistry/Microscopy.
Phase microscopy was conducted using a Nikon Eclipse TE3000 microscope (Nikon Instruments, Melville, NY) with an attached Nikon D50 camera. Fluorescent microscopy of GFP-positive cells and immunostained cells was carried out using a Zeiss Axio Observer A1 microscope (Zeiss USA, Thornwood, NY). Immunostaining was carried out as previously described, with slight modifications (Martovetsky et al., 2013). Cells were fixed in 4% formaldehyde in PBS overnight at 4°C. Fixation was quenched with 50 mM glycine in PBS for 30 minutes at room temperature. Cells were then blocked with 10% bovine serum albumin in PBS containing 0.1% Tween 20 and 0.05% Triton X-100 for 1 hour at room temperature. Next, cells were incubated with a 1:250 dilution of anti–ZO-1 (TJP1) antibody (Invitrogen; 33-9100) overnight at 4°C in PBS containing 0.1% Tween 20 and 0.05% Triton X-100 with 2% bovine serum albumin [immunohistochemistry (IHC) buffer]. Cells were then washed with IHC buffer three times for at least 1 hour per wash at room temperature, and then incubated with secondary antibody (anti-mouse Alexa Fluor 594; Life Technologies, Carlsbad, CA) overnight at 4°C. Cells were then washed three times for at least 1 hour per wash with IHC buffer at room temperature, and then covered with a coverslip within the tissue culture plate using Fluoromount-G (Electron Microscopy Services, Hatfield, PA). ImageJ software (National Institutes of Health, Bethesda, MD) was used for image processing.
Microarray analysis.
For the analysis of expression in embryonic and adult isolated proximal tubules and liver tissue, publically available mRNA expression data were used (Supplemental Table 2): GSE6290–GSM144594-144595 (E15.5 CD1 mouse PT), GSE6589–GSM152247-152249 (E15.5 CD1 mouse PT), GSE10162–GSM256959-256961 (adult C57bl/6 mouse PT), GSE10162–GSM256959-256961 (adult C57bl/6 mouse PT), GSE7342–GSM177040-177042 (E15.5 C57bl/6 mouse liver), GSE11899–GSM300676-300680, GSE8969–GSM227410-227412, GSE32354–GSM801178-801182 (adult C57bl/6 mouse liver). To facilitate comparisons between samples generated by different laboratories, different mouse strains, and at different ages, the samples were normalized using the robust multi-array average algorithm. Probes that did not have a present flag in more than half of the samples in at least one of the four conditions as determined by the MAS5 algorithm were discarded. A moderated t test with a Benjamini-Hochberg multiple test correction was used to identify genes that are differentially expressed by at least 100-fold (P < 0.05) between either E15.5 PT and E15.5 liver, adult PT and adult liver, or in both E15.5 and adult tissues. The combined list of resulting genes was used to perform hierarchical clustering using default settings in GeneSpring (Agilent Technologies, Santa Clara, CA).
Chromatin Immunoprecipitation Sequencing.
Chromatin immunoprecipitation followed by high throughput sequencing (ChIP-seq) for Hnf4a and p300 (GSE50815) has been previously published (Martovetsky et al., 2013), and relevant reanalyses were performed here. In that study, the chromatin was prepared from adult Sprague-Dawley rat whole kidneys and kidney cortex, and the ChIP analyses were performed in duplicate using either 4 μg of anti-HNF4a antibody (sc-8987; Santa Cruz Biotechnology, Dallas, TX) or 10 μg of anti-p300 antibody (sc-585; Santa Cruz Biotechnology). The antibody-bound complexes were then recovered using a mix of preblocked protein A and protein G beads that were washed and eluted with SDS-containing buffer. DNA was purified and libraries were prepared with the ChIP-Seq DNA Sample Prep Kit (Illumina, Inc., San Diego, CA) using either pooled duplicates (the ChIP samples) or 50 ng for the inputs. The HiSEq. 2000 instrument (Illumina, Inc.) was used to sequence amplified DNA fragments (200–400 bp long) which were aligned to the rn4 genome by BIOGEM (Genomics Data Analysis Services, UCSD) according to the standard Illumina pipeline.
The HOMER v3.13 software package (UCSD; Heinz et al., 2010) was used for further analysis. Clonal reads were removed, and default settings designed for ChIP-seq analysis were used to define and annotate peaks and calculate measures for quality control. The UCSC genome browser was used to generate screenshots of Hnf4a and p300 binding at specific genes of interest. To quantify Hnf4a peaks in adult rat kidneys associated with either PT-enriched or hepatocyte-enriched genes, peaks were assigned to genes if their transcription start site was the nearest annotated transcription start site as opposed to any other protein-coding gene. Total number of peaks per gene were then quantified and graphed with a box-whisker plot in GraphPad Prism 6. Significance was calculated with a two-tailed t test.
Results
Using developmental transcriptomic and ChiP-seq data as a guide (discussed later), as well as the literature on stem cell differentiation, we sought to define the transcriptional program that leads to the expression of kidney proximal tubule versus hepatocyte SLC and ABC drug/solute transporters, tight and other junction molecules, as well as biomarkers considered selective for one cell type or the other.
Ectopic expression of Hnf1a and Hnf4a in MEFs leads to the mRNA expression of many PT-expressed SLC and ABC transporters and junctional component genes with differential contributions of the two transcription factors.
We have previously shown that hepatocyte nuclear factors Hnf1a and Hnf4a play a role in regulating drug-metabolizing enzymes and transporters in the kidney (Martovetsky et al., 2013). However, Hnf1a and Hnf4a, without additional factors, have also both been used in reprogramming fibroblasts toward a hepatocyte-like phenotype (Huang et al., 2011, 2014; Sekiya and Suzuki, 2011; Du et al., 2014; Simeonov and Uppal, 2014). With this in mind, we set out to further examine the potential of Hnf1a and Hnf4a to induce expression of proximal tubule–enriched versus liver-enriched genes (Fig. 1).
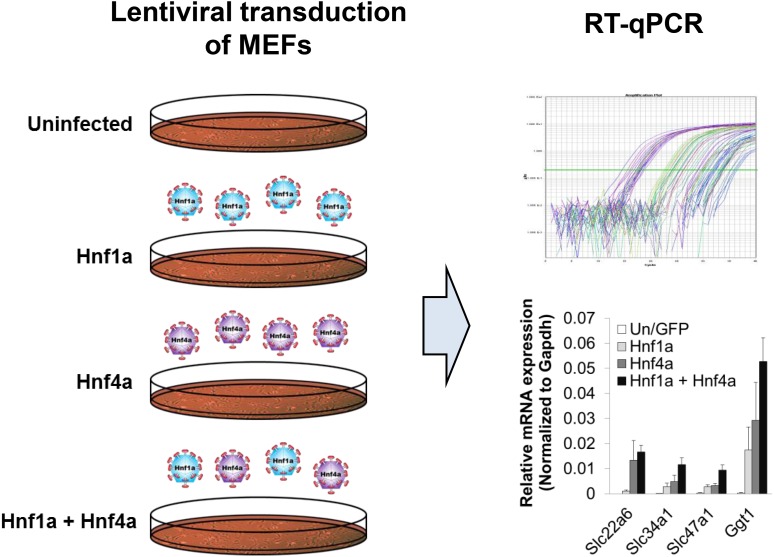
Fig. 1.
Lentiviral transduction of MEFs followed by quantitative reverse-transcription PCR (RT-qPCR) determination of changes in gene expression for selected PT markers. (Left panel) MEFs isolated from different days of gestation (i.e., E13.5, E15.5, and E16.5) were transduced without (uninfected) or with lentivirus expressing either GFP or the transcription factors Hnf1a and Hnf4a (either singly or in combination). (Right panel) The expression of several markers of the kidney proximal tubule [i.e., transmembrane transporters, Slc22a6 (Oat1), Slc34a1 (NaPi-2a), and Slc47a1 (Mate1), as well as the PT brush border marker Ggt1] was determined by quantitative reverse-transcription PCR. The graph on the bottom right shows the induction of several of these markers upon transduction of the MEFs with Hnf1a and Hnf4a; mean ± S.E.M. (n = 3) (Supplemental Fig. 1). Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
To better gauge the extent of transdifferentiation toward PT cell fate by Hnf1a and Hnf4a overexpression, a cohort of genes involved in influx/efflux transport of drugs/solutes and junction formation (Denker and Nigam, 1998), both defining characteristics of PT cells and relevant to their physiologic function, were examined. In all instances, when MEFs were transduced with both Hnf1a and Hnf4a, the transcription of several prominent proximal tubule markers [selected based on literature and various expression databases and later experimentally confirmed (discussed later)], including Slc22a6 (also known as Oat1 or NKT), Slc34a1, Slc47a1 (Mate1), and γ-glutamyltransferase 1 (Ggt1—a PT brush border marker), were markedly upregulated compared with MEFs that were either uninfected or transduced with GFP alone (Fig. 1). Interestingly, in two of the populations (E13.5 and E15.5, which were prepared slightly differently from the E16.5 MEFs, as described in Materials and Methods), ectopic expression of Hnf4a induced the expression of endogenous Hnf1a, albeit at lower levels than when transduced with Hnf1a (Supplemental Fig. 1). This level of Hnf1a appeared to be sufficient to complement exogenously expressed Hnf4a in inducing the expression of the PT-enriched markers. Nonetheless, the results are consistent with the finding that Hnf1a and Hnf4a act synergistically in the induction of several key proximal tubule genes upon transduction into MEFs (Martovetsky et al., 2013). Because of the opportunity to analyze the roles of Hnf1a and Hnf4a separately as well as together, we further characterized the response in the E16.5 MEFs, in which Hnf4a did not induce endogenous Hnf1a expression.
The transport of drugs, metabolites, and waste products by the postnatal, juvenile, and adult proximal tubule relies on a system of influx and efflux transporters on the basolateral (interacting with blood) and apical (inside of the lumen interacting with the glomerular filtrate) sides of the cell (Fig. 2A); these transporters fall into the ABC or SLC gene superfamilies. Thus, we selected a panel of representative apical and basolateral ABC and SLC transporters to test in our model system (Morrissey et al., 2012). We found that expression of a large fraction of the tested transporters from each category was induced in MEFs transduced with Hnf1a and Hnf4a (Fig. 2, B–E). Interestingly, we found that the individual contributions of Hnf1a and Hnf4a varied from gene to gene, and that Hnf1a alone was able to induce the expression of a substantial number of transporters, whereas this was rarely seen with Hnf4a alone. For example, of the tested transporters, the drug/metabolite transporter Abcg2 (also known as Bcrp, or breast cancer resistance protein) had the greatest PCR signal in MEFs transduced with Hnf1a and Hnf4a; however, its expression was almost as high when MEFs were transduced with only Hnf1a (Fig. 2B). However, genes such as Slc34a1 (Fig. 2D) and Slc22a6 (Fig. 2E), which are highly enriched in the PT in the kidney, required both Hnf1a and Hnf4a, suggesting that the more tissue-specific properties of the PT require both factors.
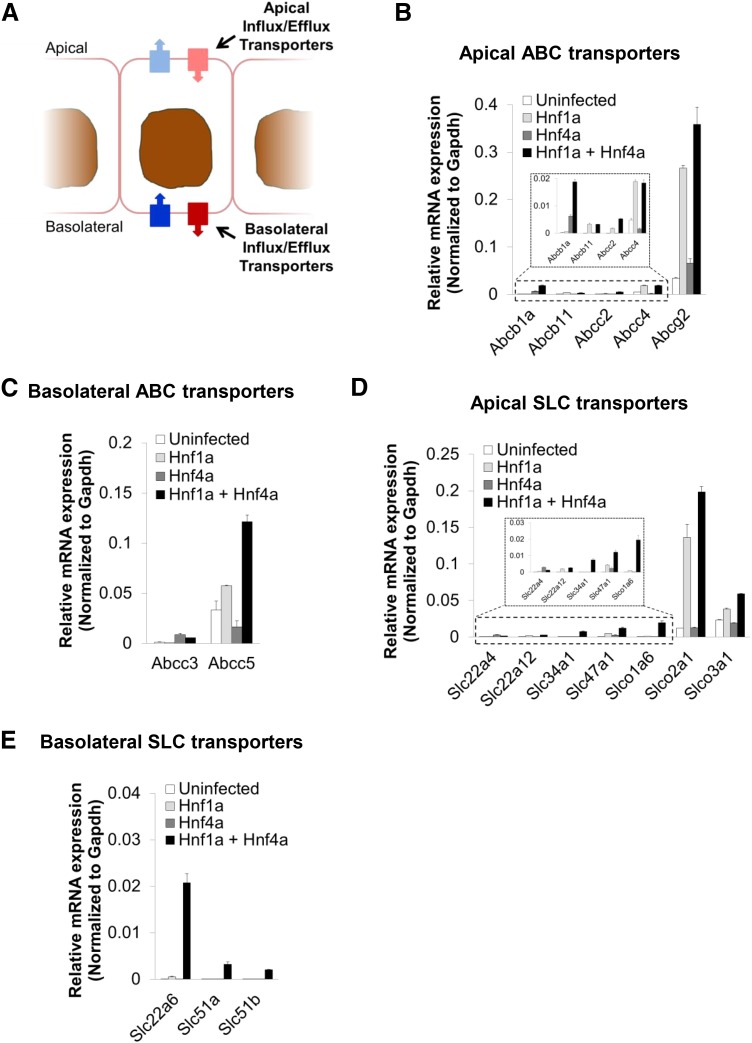
Fig. 2.
Differential induction of multiple apical and basolateral drug, toxin, and metabolite transporters in MEFs transduced with Hnf1a and Hnf4a. (A) The differential capacity of Hnf1a and Hnf4a, individually and together, to induce the transcription of a cohort of genes involved in transport was examined. (B) Expression of apical ABC transporters upon transduction. (C) Expression of basolateral ABC transporters upon transduction. (D) Expression of apical SLC transporters upon transduction. (E) Expression of basolateral SLC transporters upon transduction. The mean ± S.E.M. for each tested gene is depicted (n ≥ 3); significance of differential expression is summarized in Table 1. For convenience, results for genes within similar families are shown on a single graph. Gapdh, glyceraldehyde-3-phosphate dehydrogenase. Some localizations to apical or basolateral surfaces may be tenative, especially for SLCOs.
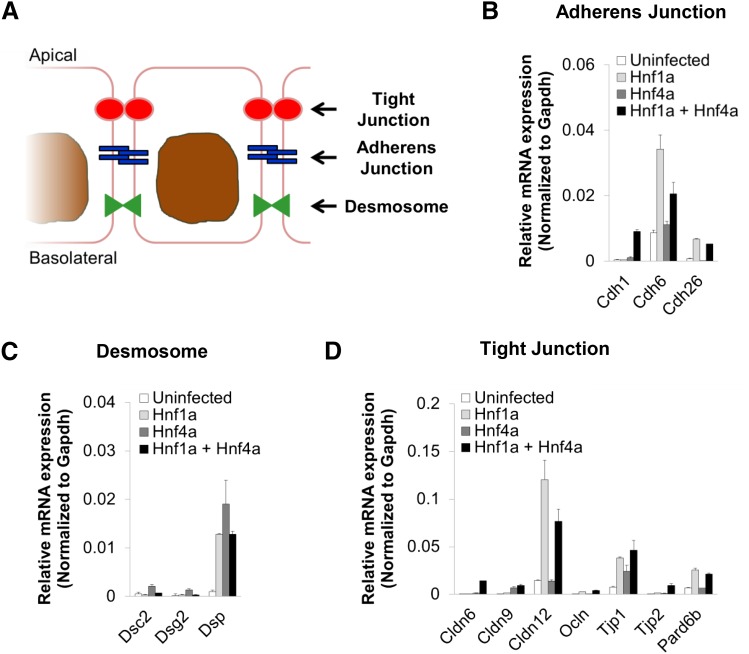
In addition to specific transporters, the selective transport properties of proximal tubule epithelia require the establishment of intercellular junctions (Lee et al., 2006; Balkovetz, 2009; Hou, 2014), thus we tested the induction of several key components of the three main types of junctions: tight junctions, adherens junctions, and desmosomes (Fig. 3A). As with transporters, the expression of many of the tested genes was induced in MEFs transduced with Hnf1a and Hnf4a (Fig. 3, B–D). Several genes, including E-cadherin (Cdh1), claudin 6 (Cldn6), and tight junction protein 2 (Tjp2, also known as ZO-2), required both Hnf1a and Hnf4a to be transcriptionally induced. However, multiple genes exhibited a response to Hnf1a alone, including cadherin 6 and 26 (Cdh6 and Cdh26), desmoplakin (Dsp), Cldn12, occludin (Ocln), Tjp1 (also known as Z0-1), and the Par-6 family cell polarity regulator, Pard6b; of those, Dsp and Tjp1 also exhibited some level of induction by Hnf4a alone. Interestingly, the desmosomal proteins, desmoglein 2 and desmocolin 2, were upregulated by Hnf4a alone, an effect that appeared to be repressed by coexpression of Hnf1a (Fig. 3C). The significance of the effect of ectopic expression induced by Hnf1a and Hnf4a in MEFs by themselves or together on transporter and junctional component gene expression is summarized in Table 1. In general, although we found that many genes respond to a single transcription factor, the transcriptional induction of several of these physiologically relevant genes appeared to be statistically more significant when both factors were coexpressed (Table 1). For example, there was no significant difference in the induction of Slc22a6 (Oat1) between uninfected MEFs and those expressing either Hnf1a and Hnf4a (Table 1). However, the difference in expression of Slc22a6 between MEFs expressing both transcription factors and either uninfected MEFS or those expressing either transcriptional factor singly was statistically much more significant (Table 1).
Fig. 3.
Induction of multiple intercellular junctional components in MEFs transduced with Hnf1a and Hnf4a. To test induction of junctional components, the same MEFs were used as in Fig. 2. (A) Simplified schematic shows which junctional features correspond to the tested genes. (B) Expression of three cadherin genes (Cdh1, Cdh6, and Cdh26) associated with adherens junctions upon transduction. (C) Expression levels of three desmosomal genes—desmocollin 2 (Dsc2), desmoglein 2, and desmoplakin (Dsp)—upon transduction. (D) Expression of several types of genes involved in tight junction formation—Cldn6, Cldn9, Cldn12, Ocln, tight junction proteins (Tjp1 and Tjp2, which are also known as ZO-1 and ZO-2), and epithelial polarity regulator Pard6b. The mean ± S.E.M. for each tested gene is depicted (n ≥ 3); significance of differential expression is summarized in Table 1. For convenience, results for genes within similar families are shown on a single graph. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
TABLE 1.
Significance (P value) of differential expression in Figures 1, 2 and 3 determined by one-way analysis of variance and Tukey's post-hoc analysis
| Gene Symbol | Uninfected versus |
Hnf1a + Hnf4a versus |
|||
|---|---|---|---|---|---|
| Hnf1 | Hnf4 | Hnf1 + Hnf4 | Hnf1 | Hnf4 | |
| Abcb1a (Mdr1a) | – | ** | *** | *** | *** |
| Abcb1 1 (Bsep) | *** | – | *** | – | *** |
| Abcc2 (Mrp2)a | *** | – | *** | *** | *** |
| Abcc4 (Mrp4) | *** | * | *** | – | *** |
| Abcg2 (Bcrp1) | *** | – | *** | * | *** |
| Cdh1 | – | – | *** | *** | *** |
| Cdh6 | ** | – | * | * | – |
| Cdh26 | *** | * | *** | ** | *** |
| Cldn12 | ** | – | * | – | * |
| Cldn6a | * | *** | *** | *** | *** |
| Cldn9 | – | ** | ** | ** | – |
| Dsc2 | – | ** | – | – | ** |
| Dsg2 | – | ** | – | – | ** |
| Dsp | * | ** | * | – | – |
| Ocln | ** | – | *** | ** | *** |
| Slc22a4 (Octn1) | – | *** | * | – | ** |
| Slc22a6 (Oat1)a | – | – | *** | *** | *** |
| Slc22a12 (Urat1) | *** | – | *** | ** | *** |
| Slc34a1a | – | – | *** | *** | *** |
| Slc47a1 (Mate1)a | * | – | *** | ** | *** |
| Slc51a (Osta)a | – | – | *** | *** | *** |
| Slc51b (Ostb)a | – | – | *** | *** | *** |
| Slco1a6 (Oatp1a6)a | – | – | *** | *** | *** |
| Slco2a1 (Oatp2a1) | *** | – | *** | * | *** |
| Tjp2a | – | – | ** | ** | ** |
| Pard6b | *** | – | *** | * | *** |
Expression was more robust in presence of both transcription factors.
The statistical significance of the differential expression as determined by one-way ANOVA and Tukey's post-hoc analysis of the various genes is shown (-No significant difference in expression; *P ≥ 0.05; **P ≥ 0.01; ***P ≥ 0.005).
Column 1 is uninfected MEFs versus those expressing Hnf1 alone; column 2 is uninfected MEFS versus those expressing Hnf4 alone; and column 3 is uninfected MEFs versus those expressing a combination of Hnf1 and Hnf4; column 4 is MEFs expressing both transcription factors versus those expressing Hnf1 alone;and column 5 is MEFs expressing both transcription factors versus those expressing Hnf4 alone.
Finally, we were interested in whether the genes that we found to be induced at the mRNA level by Hnf1a and Hnf4a showed a bias toward a PT-like or liver-like expression pattern. When the tested genes were sorted by their fold change of expression in MEFs transduced with Hnf1a and Hnf4a compared with control MEFs and compared with their relative expression in embryonic and adult proximal tubule versus liver, we found that the most upregulated genes (mainly transporters) had higher endogenous expression in proximal tubules than in liver tissue (Table 2). However, other induced genes, albeit to a much lower degree, did not reveal a bias toward either PTs or liver. The full list of tested genes, along with their fold change in expression in MEFs upon Hnf1a and Hnf4a transduction as well as their levels of endogenous expression in embryonic and adult proximal tubules and liver tissue, is included (Supplemental Table 3). In other words, whereas MEFs transduced with Hnf1a and Hnf4a began expressing PT-enriched genes, many of the other transcriptionally responsive genes are shared between PT cells and hepatocytes. Furthermore, some of the tested genes that are endogenously expressed at very high levels in proximal tubules exhibited a relatively weak transcriptional response to Hnf1a and Hnf4a transduction. This suggested that, although Hnf1a and Hnf4a induce the expression of several key PT-enriched genes, they were not sufficient to completely specify a PT-like transcriptome.
TABLE 2.
Most upregulated genes in MEFs expressing Hnf1a and Hnf4a are enriched in the proximal tubule compared with liver
| Gene Symbol | Category | Hnf1a + Hnf4a versus Uninfected (Fold Change) | E15.5 PT versus E 15.5 Liver (Ratio) | Adult PT versus Adult Liver (Ratio) |
|---|---|---|---|---|
| Slc22a6 (Oat1) | Transporter | 3439 | 10.3 | 19.4 |
| Slc34a1 (Npt2a) | Transporter | 1337 | 37.9 | 337.5 |
| Abcc2 (Mrp2) | Transporter | 1192 | 0.9 | 1.2 |
| Cldn6 (Claudin 6) | Junction | 895 | 7.2 | 1.0 |
| Slc51b (Ostb) | Transporter | 715 | 4.6 | 4.1 |
| Slc22a12 (Urat1) | Transporter | 576 | 4.8 | 16.3 |
| Slco1a6 (Oatp1a6) | Transporter | 347 | 115.4 | 118.3 |
| Slc47a1 (Mate1) | Transporter | 195 | 12.3 | 2.8 |
Transduction of Hnf1a and Hnf4a alters the morphology of MEFs and induces formation of tight junctions.
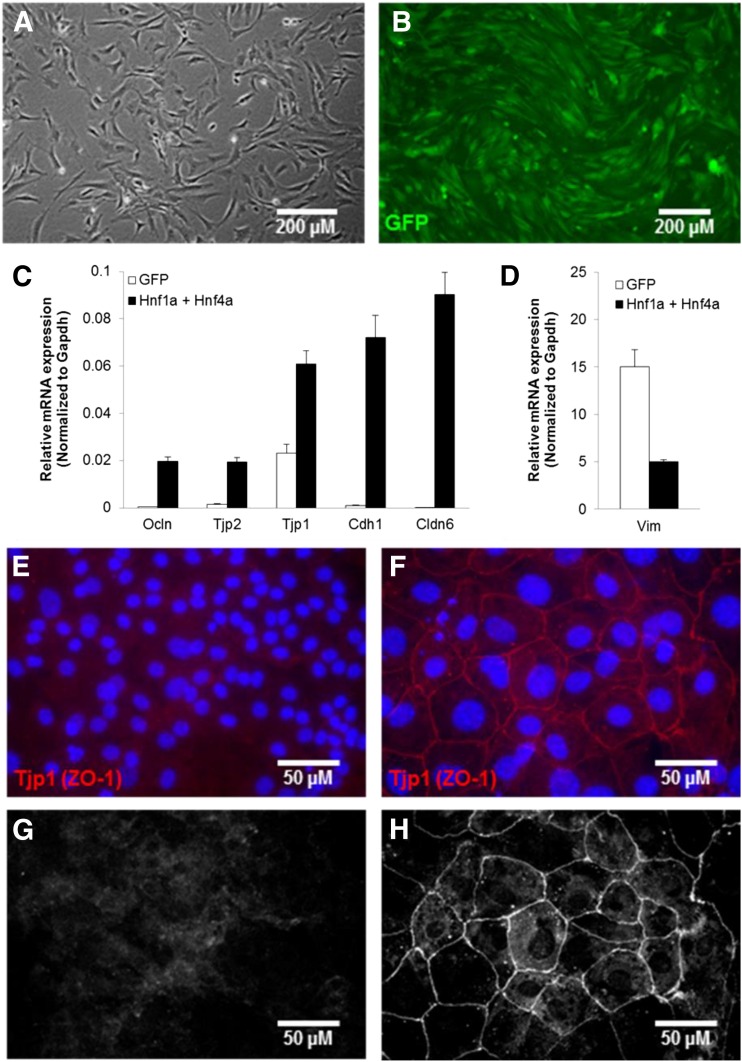
Whereas MEFs transduced with Hnf1a and Hnf4a began expressing proximal tubule markers, another distinct property of mature proximal tubule cells is their epithelial phenotype. We had previously shown that transduction of Hnf1a and Hnf4a leads to upregulation of mRNA expression for Cdh1, an adherens junction marker, and Tjp1 (also known as ZO-1 or zonula occludens-1), a tight junction marker, both of which are present in mature PT cells (Martovetsky et al., 2013). However, when the E13.5 or E15.5 MEFs were in culture for a week or more after transduction, we achieved substantially higher levels of expression (Fig. 4C). Furthermore, additional components of intercellular junctions were upregulated, including Ocln, Tjp2, and Cldn6 (Fig. 4C). Whereas other claudins tested also exhibited some transcriptional response, Cldn6 (which is endogenously expressed in developing and postnatal PTs but downregulated in mature PTs) was the most upregulated (Supplemental Table 3); this result suggests that, although MEFs transduced with Hnf1a and Hnf4a have not acquired mature PT-like cell properties, they do resemble immature PT-like cells to some extent. In addition to the upregulation of multiple junctional markers, the mesenchymal marker vimentin was downregulated in response to transduction (Fig. 4D). When an extracellular matrix was provided, the transduced MEFs that were cultured on collagen-coated plates for 3 weeks had extensive formation of epithelial sheets with Tjp1 localized to the cellular junctions, indicative of tight junction formation in MEFs transduced with Hnf1a and Hnf4a, but not control MEFs (Fig. 4, E–H).
Fig. 4.
Transduction of MEFs with Hnf1a and Hnf4a leads to tight junction formation. (A) E15.5 MEFs prior to infection. (B) MEFs 3 days after transduction with Hnf1a and Hnf4a were nearly all GFP-positive, indicating very efficient transgene expression. (C) Tight junction markers Ocln, Tjp1 (or ZO-1) and Tjp2 (or ZO-2), and Cldn6, as well as adherens junction marker Cdh1, were markedly upregulated at the mRNA level in MEFs transduced with Hnf1a and Hnf4a compared with a GFP control 1 week after transduction. Conversely, the mesenchymal marker vimentin (Vim) was downregulated (D). The mean ± S.E.M. for each tested gene is depicted (n ≥ 3). For convenience, results for genes within similar families are shown on a single graph. (E and F) Control MEFs transduced with only GFP showed no junctional formation after 3 weeks of culture on collagen I (E), whereas those transduced with Hnf1a and Hnf4a showed extensive tight junction formation (F) (blue, DAPI; red, Tjp1). A digitally enhanced view of Tjp1 staining from (E) and (F) is shown in grayscale in (G) and (H), respectively. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Liver lineage–determining factors Gata4 and Foxa2 and/or Foxa3 act as a transcriptional switch to redirect Hnf1a and Hnf4a activity from regulating PT-enriched genes to liver-enriched genes.
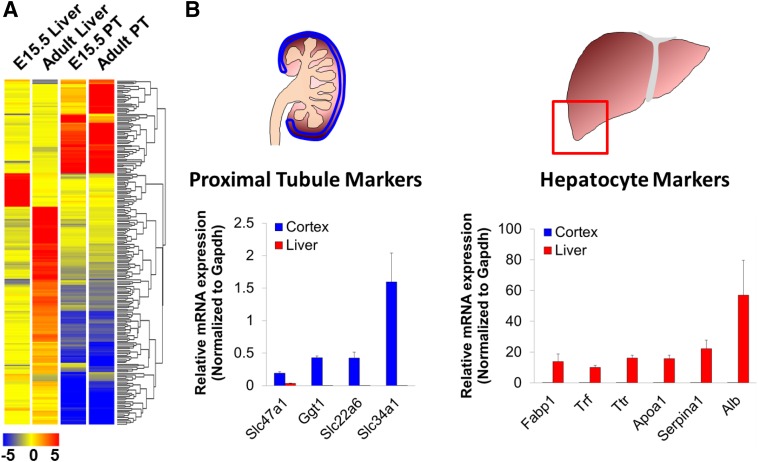
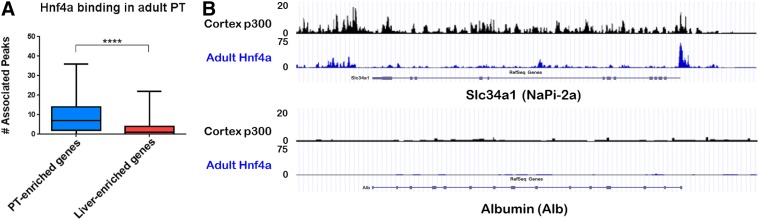
Although Hnf1a and Hnf4a might not be sufficient to fully transdifferentiate MEFs toward a proximal tubule fate, our results suggested that they are indeed “lineage-determining TFs” (Heinz et al., 2010) for proximal tubule cells, at least in the context of the SLC and ABC drug transporters and junctional molecules examined. To gain a deeper perspective of the shared and defining characteristics of PT cells and hepatocytes, the transcriptomes of isolated proximal tubules and liver tissue were examined at embryonic and adult time points. When we limited to genes that exhibit more than a 100-fold change in expression between either embryonic or adult PT and liver samples, or both, we derived a list of candidate markers that could be used to distinguish between the two tissues, which included multiple widely used markers for both PT cells and hepatocytes (Fig. 5A). We validated a number of selected markers by testing their expression in adult mouse kidney cortex and liver tissue (Fig. 5B). As predicted, Mate1, Oat1, NaPi-2a, and Ggt1 had a much higher expression in the kidney cortex compared with the liver, whereas markers such as transferrin (Trf), transthyretin (Ttr), apolipoprotein A (Apoa1), albumin (Alb), Fabp1, and Serpina1 had a much higher expression in liver compared with the kidney cortex. We also examined the genes deriving from the analysis shown in Fig. 5A in the context of existing Hnf4a ChIP-seq data (Martovetsky et al., 2013). Importantly, we found that in samples of adult rat kidney, the selected PT marker genes were highly bound by Hnf4a, whereas the selected hepatocyte markers were almost completely devoid of Hnf4a binding in this tissue (Fig. 6). In addition, comparison of p300 binding in adult rat kidney cortex was found to be highly similar to that seen for HNF4a in adult kidney for all of the selected kidney and liver marker genes (Fig. 6).
Fig. 5.
Systems-level analysis of PT and liver expression aid in selection of differential markers. (A) Hierarchical clustering of genes with at least a 100-fold change between either embryonic isolated proximal tubules compared with embryonic liver or adult isolated proximal tubules compared with adult liver (all mouse tissues). This analysis identified most (but not all) of the markers used for further experiments to differentiate between PT-like and hepatocyte-like cellular identity. (B) The PT markers used in this study—Slc47a1 (Mate1), Slc22a6 (Oat1), and Slc34a1 (NaPi-2a)—are highly enriched in the kidney cortex, where proximal tubule cells comprise more than half of the cellular content. Conversely, hepatocyte markers Fabp1, Trf, Ttr, Apoa1, Serpina1, and Alb are highly expressed in the liver and negligibly expressed in the PT. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Fig. 6.
Hnf4a binding events revealed by ChIP-seq colocalize near PT-enriched genes in the kidney while rarely binding near liver-enriched genes. (A) When Hnf4a peaks in adult rat kidney are assigned to genes based on the nearest transcription start site, on average there are more than 3 times as many peaks associated with highly PT-enriched genes compared with highly liver-enriched genes (derived from analysis depicted in Fig. 5A comparing embryonic and adult liver versus embryonic and adult isolated PTs). Whiskers set at 10th and 90th percentiles. (B) Previous p300 and HNF4a ChIP-seq data (Martovetsky et al., 2013) were re-examined for determining the binding of these transcription factors to selected kidney and liver marker genes in adult rat kidney cortex or proximal tubules. Shown are examples for the PT marker, Slc34a1 (NaPi-2a), and the liver marker, Alb. Patterns similar to Slc34a1 were also seen for the PT markers Slc22a6 (Oat1) and Slc47a1 (Mate1) (Martovetsky et al., 2013), whereas patterns similar to Alb were also seen for the liver markers fatty acid binding protein 1 (Fabp1), serpin peptidase inhibitor clade 1 (Serpina1), Trf, and Ttr.
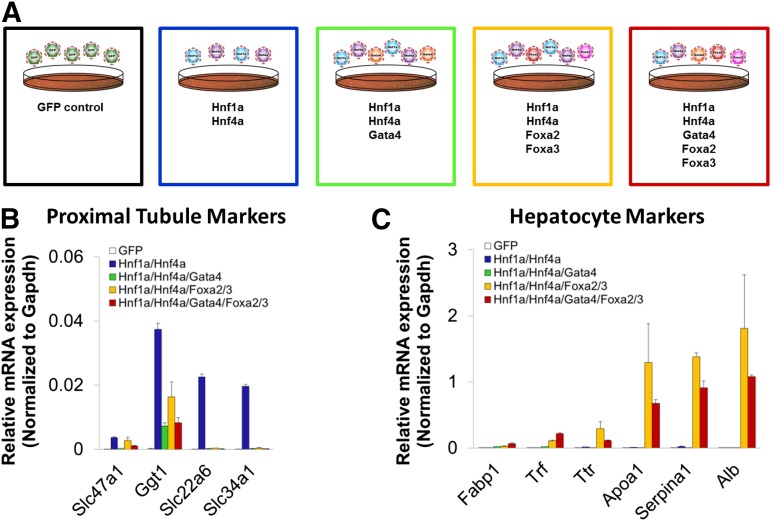
We then set out to determine what effect coexpression of Gata4, Foxa2, and Foxa3 with Hnf1a and Hnf4a would have on proximal tubule and hepatocyte transcriptional signatures. Because previous reports have shown that both Foxa2 and Foxa3 had the strongest effects in transdifferentiation protocols compared with Foxa1, we decided to use them in combination to activate transcription mediated by the Foxa family. We found that coexpression of either Gata4 or Foxa2/3 significantly downregulated the proximal tubule markers Slc22a6, Slc34a1, and Slc47a1, while dramatically upregulating hepatocyte markers Alb, Apoa1, Fabp1, Serpina1, Trf, and Ttr (Fig. 7, B and C; Table 3). While Gata4 coexpression had a strong inhibitory effect on proximal tubule marker expression, it was insufficient to upregulate liver markers regardless of Hnf4a and Hnf1a presence (Fig. 7, B and C; Table 3). In contrast, whereas Foxa2 and Foxa3 were capable of downregulating PT marker expression, they also appeared to be the main drivers of hepatocyte marker expression in the presence of Hnf1a and Hnf4a (Fig. 7, B and C; Table 3).
Fig. 7.
Expression of PT and hepatocyte markers in MEFs transduced with GFP control, Hnf1a and Hnf4a, Hnf1a and Hnf4a in combination with Gata4, Hnf1a and Hnf4a in combination with Foxa2/3, or all five factors. (A) Schematic of tested conditions. Color of outlining boxes around each condition corresponds to the color of columns for that condition in (B) and (C). (B and C) Expression of PT-enriched and liver-enriched markers upon transduction of E15.5 MEFs. Hnf1a and Hnf4a activate expression of PT markers, which is downregulated or silenced by Gata4 and Foxa2/3 (C). Conversely, liver marker expression is induced when Foxa2/3 is coexpressed with Hnf1a and Hnf4a, some of which is further upregulated by Gata4 (Fabp1, Trf). The mean ± S.E.M. for each tested gene is depicted (n ≥ 3); significance of differential expression is summarized in Table 3. For convenience, results for marker genes for the proximal tubule or hepatocytes are each shown on a single graph. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
TABLE 3.
Significance (P value) of differential expression in Fig. 7 determined by one-way analysis of variance and Tukey’s post-hoc analysis
| Gene Symbol |
Uninfected versus | Hnf1 + Hnf4 versus | Hnf1 +Hnf4 +Gata4 versus | Hnf1a Hnf4a Fox2/3 versus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hnf1a | Hnf1a | Hnf1a | Hnf1a | Hnf1a | Hnf1a | Hnf1a | Hnf1a | Hnf1a | Hnf1a | |
| Hnf4a | Hnf4a | Hnf4a | Hnf4a | Hnf4a | Hnf4a | Hnf4a | Hnf4a | Hnf4a | Hnf4a | |
| Gata4 | Foxa2/3 | Gata4 | Gata4 | Foxa2/3 | Gata4 | Foxa2/3 | Gata4 | Gata4 | ||
| Foxa2/3 |
Foxa2/3 |
Foxa2/3 |
Foxa2/3 |
|||||||
| Proximal tubule markers | ||||||||||
| Slc47a1 | *** | – | *** | * | *** | – | *** | *** | – | ** |
| Ggt1 | *** | * | *** | ** | *** | *** | *** | ** | – | * |
| Slc22a6 | *** | – | – | – | *** | *** | *** | – | – | – |
| Slc34a1 | *** | – | – | – | *** | *** | *** | – | – | – |
| Hepatocyte markers | ||||||||||
| Fabp1 | – | * | ** | *** | * | ** | *** | – | *** | *** |
| Trf | – | – | *** | *** | – | *** | *** | *** | *** | *** |
| Ttr | – | – | *** | * | – | *** | * | *** | * | * |
| Apoa1 | – | – | *** | * | – | *** | * | *** | * | * |
| Serpina1 | – | – | *** | *** | – | *** | *** | *** | *** | *** |
| Alb | – | – | *** | ** | – | *** | ** | *** | ** | – |
The statistical significance of the differential expression as determined by one-way ANOVA and Tukey's post-hoc analysis of the various genes is shown (-No significant difference in expression; *P ≥ 0.05; **P ≥ 0.01; ***P ≥ 0.005).
Column 1 is uninfected MEFs versus those expressing Hnf1 + Hnf4; column 2 is uninfected MEFs versus those expressing Hnf1 + Hnf4 + Gata4; column 3 is uninfected MEFs versus those expressing Hnf1 + Hnf4 + Foxa2/3; column 4 is uninfected MEFs versus those expressing Hnf1 + Hnf4 + Gata4 + Foxa2/3; column 5 is MEFs expressing Hnf1 + Hnf4 versus those expressing Hnf1 + Hnf4 + Gata4; column 6 is MEFs expressing Hnf1 + Hnf4 versus those expressing Hnf1 + Hnf4 + Foxa2/3; column 7 is MEFs expressing Hnf1 + Hnf4 versus those expressing Hnf1 + Hnf4 + Gata4 + Foxa2/3; column 8 is MEFs expressing Hnf1 + Hnf4 + Gata4 versus those expressing Hnf1 + Hnf4 + Foxa2/3; column 9 is MEFs expressing Hnf1 + Hnf4 + Gata4 versus those expressing Hnf1 + Hnf4 + Gata4 + Foxa2/3; and column 10 is MEFs expressing Hnf1 + Hnf4 + Foxa2/3 versus those expressing Hnf1 + Hnf4 + Gata4 + Foxa2/3.
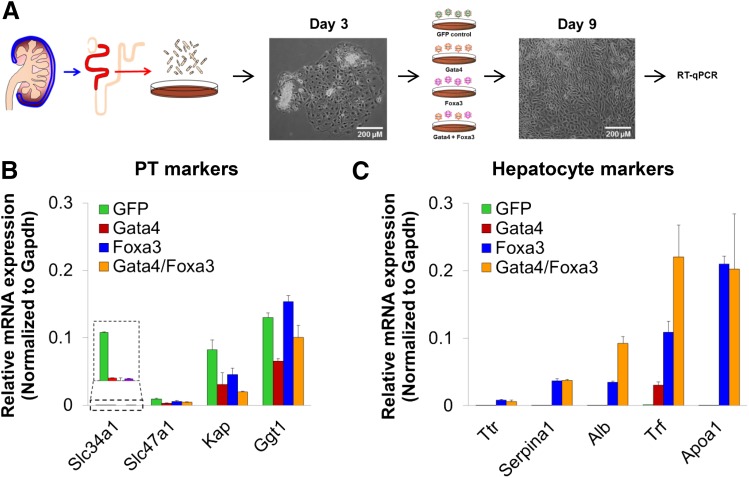
Finally, we tested the ability of Gata4 and Foxa3 to induce the expression of hepatocyte markers in primary mouse proximal tubule cells (Fig. 8; Table 4). These cells expressed some level of endogenous Hnf1a and Hnf4a, as well as multiple PT markers. Upon Gata4 transduction, several PT markers were downregulated (Slc34a1, Slc47a1, kidney androgen-regulated protein, and Ggt1) (Fig. 8B). Of the tested hepatocyte markers, only Trf expression was modestly induced (Fig. 8C). In contrast, Foxa3 overexpression not only had a repressive effect on kidney androgen-regulated protein but also strongly induced the expression of a number of hepatocyte markers (Alb, Apoa1, Trf, Serpina1, and Ttr), with Alb and Trf appearing to respond synergistically to Gata4 and Foxa3 coexpression (Fig. 8C). Although the transcriptional response in primary PT cells was more modest in scale compared with those observed in MEFs (Fig. 7, B and C), this might be due to the reduced plasticity of terminally differentiated cell types compared with embryonic fibroblasts.
Fig. 8.
Overexpression of Gata4 and Foxa3 in primary proximal tubule cells dowregulates PT marker expression and induces expression of liver marker genes. (A) Experimental design of primary proximal tubule cell transduction. (B and C) Transduction of primary PT cells with Gata4 results in downregulation of PT marker expression. Transduction of these cells with Foxa3 results in weaker downregulation of PT marker expression compared with Gata4, but leads to pronounced transcriptional induction of liver markers, an effect sometimes increased by coexpression of Gata4 (C). The mean ± S.E.M. for each tested gene is depicted (n ≥ 3); significance of differential expression is summarized in Table 4. For convenience, results for marker genes for the proximal tubule or hepatocytes are each shown on a single graph. Gapdh, glyceraldehyde-3-phosphate dehydrogenase. RT-qPCR, quantitative reverse-transcription PCR.
TABLE 4.
Significance (P value) of differential expression in Fig. 8 determined by one-way analysis of variance and Tukey’s post-hoc analysis
| Uninfected versus | Gata4 versus | Foxa3 versus | ||||
|---|---|---|---|---|---|---|
| Gene Symbol | Gata4 | Foxa3 | Gata4 Foxa3 | Foxa3 | Gata4 Foxa3 | Gata4 Foxa3 |
| Proximal tubule markers | ||||||
| Slc34a1 | *** | *** | *** | – | – | – |
| Slc47a1 | *** | * | ** | – | – | – |
| Kap | ** | * | ** | – | – | – |
| Ggt | *** | – | * | *** | * | ** |
| Hepatocyte markers | ||||||
| Ttr | – | *** | ** | *** | ** | – |
| Serpina1 | – | *** | *** | *** | *** | – |
| Alb | – | *** | *** | *** | *** | *** |
| Trf | – | ** | *** | * | *** | ** |
| Apoa1 | – | ** | ** | ** | ** | – |
The statistical significance of the differential expression as determined by one-way ANOVA and Tukey's post-hoc analysis of the various genes is shown (-No significant difference in expression; *P ≥ 0.05; **P ≥ 0.01; ***P ≥ 0.005).
Column 1 is uninfected primary proximal tubule (PE) cells versus those expressing Gata4; column 2 is uninfected primary PT cells versus those expressing Foxa3; column 3 is uninfected primary PT cells versus those expressing Gata4 + Foxa3; column 4 is primary PT cells expressing Gata4 versus those expressing Foxa3; and column 5 is primary PT cells expressing Gata4 versus those expressing Gata4 + Foxa3; column 6 is primary PT cells expressing Foxa3 versus those expressing Gata4 + Foxa3
Nevertheless, these findings further supported the idea that Hnf1a and Hnf4a serve as a foundation for proximal tubule and hepatocyte transcriptomes, but require additional inputs to establish tissue-specific expression. In the absence of additional hepatocyte lineage–determining factors, Hnf1a and Hnf4a induce expression of genes common to both PT cells and hepatocytes, with an apparent bias toward well described PT-specific genes. In cells expressing Hnf1a and Hnf4a, Gata4, Foxa2, and Foxa3 downregulate key PT genes (with Gata4 having the stronger repressive effect) and cooperate with Hnf1a and Hnf4a to induce hepatocyte-specific gene expression (with Foxa2/3 playing the major role in gene induction, synergized by Gata4 in some cases).
Discussion
Kidney proximal tubule cells and hepatocytes are both involved in the transport and metabolism of many drugs and toxins as well as metabolites. This requires establishment of a permeability barrier (mediated by tight junctions), expression of appropriate ABC and SLC drug and solute transporters, as well as drug-metabolizing enzymes. For example, the major transporter of many organic anion drugs, toxins, and metabolites, OAT1 (originally identified as NKT) must be expressed on the basolateral surface of the proximal tubule cell (Lopez-Nieto et al., 1997; Nigam et al., 2015b; Wu et al., 2015; Zhu et al., 2015); when this gene is deleted, there is considerable loss of renal transport of many organic anion drugs, toxins, and metabolites (Eraly et al., 2006; Truong et al., 2008; Nagle et al., 2011; Torres et al., 2011; Wikoff et al., 2011). Although there is some commonality of expressed genes, they are often differentially expressed. Other genes appear to be largely expressed in the kidney or in liver, and some of these are considered signature genes.
The transcriptional program regulating the development and differentiation of cells toward the proximal tubule as opposed to hepatocytes is only beginning to be defined; understanding this in detail is of relevance to organ development and maturation as well as regenerative medicine. Our experimental results seem generally consistent with developmental analyses of gene expression (Fig. 5) and ChIP-seq data (Fig. 6). To summarize, we have shown that, despite the use in the literature of Hnf1a and Hnf4a along with other factors in transdifferentiation toward hepatocyte-like cells, these two transcription factors are also at the core of proximal tubule gene expression. Overexpression of Hnf1a and Hnf4a in MEFs induced the expression of several key markers of proximal tubule cellular identity (Fig. 1). We also found that Hnf1a and Hnf4a induced the expression of a number of genes essential to intercellular junctions (tight, adherens, desmosomal), as well as apical and basolateral transporters of small solutes, such as drugs and metabolites (Figs. 2 and 3), which would be expected to be necessary for vectorial transport in the PT in vivo. MEFs transduced with these transcription factors and cultured on collagen I for 3 weeks revealed immunocytochemical evidence of tight junction formation around the full perimeter of cells in epithelial sheets (Fig. 4). Crucially, our studies indicate that, without the coexpression of additional hepatocyte lineage–determining transcription factors, such as Gata4, Foxa2, and Foxa3, the transactivation specificity of Hnf1a and Hnf4a is insufficiently defined toward hepatocytes and, indeed, may lean toward a proximal tubule cell expression signature (Fig. 7; Table 2). Thus, these additional transcription factors (Gata4, Foxa2/3) may be viewed as altering the direction of transdifferentiation from a cell expressing some PT markers to a more hepatocyte-like cell.
Apart from their potential relevance to kidney and liver development and maturation, our results may have translational importance, as the kidney and liver are major targets for a variety of cell-based tissue engineering and regenerative medicine approaches. These approaches often require the ability to generate large amounts of patient-specific cells in vitro. Although induced pluripotent stem cells have opened up a whole field of research focused on establishing various patient-specific cell types, it might be advantageous to circumvent the requirement for pluripotency and the threat of oncogenicity by using somatic cells as a cell source. If somatic cells can be utilized, it may be crucial that the cells be differentiated as specifically as possible toward a fate reflective of mature organ function. This study—which clarifies how Hnf4a/1a-expressing cells can be shifted from cells expressing some PT-specific genes to a hepatocyte-like fate—should be valuable in this regard. In light of the results in this study and others, it appears that, although Hnf4a has been called a “master regulator” and is necessary for the transcriptional regulation of a large number of genes, much of its function and specificity is dependent on the presence or absence of other coregulators. This could provide flexibility of transporter expression that may be physiologically important. Injury to the liver or the kidney (AKI, CKD) alters drug transporter expression in the injured organ and sometimes in the other tissue, which has been hypothesized to facilitate remote communication via small molecules to re-establish homeostasis–the “remote sensing and signaling hypothesis” (Ahn and Nigam, 2009; Nigam, 2015; Nigam et al., 2015a; Wu et al., 2011). Likewise, in the immediate postnatal period, kidney and liver gene expression—particularly those genes relevant for drug, toxin, and metabolite transport—must be coordinated. In light of our results, it is conceivable that Hnf4a/1a-centered regulation, modulated by coregulators, might provide the flexibility for the kidney or liver to temporarily take on a subset of functions of another injured organ or as development progresses.
The expression of SLC and ABC drug/solute transporters, junctional markers, and cell-type signature genes was analyzed in MEFs that were transduced with Hnf1a and Hnf4a. Whereas the transduced cells expressed a number of PT signature genes, as well as a number of other genes that are highly expressed in the PT (although also expressed in other tissues), some key PT genes were expressed at very low levels or not detected. Thus, it is likely that additional transcription factors are required to establish a full PT cell fate; these additional factors might help refine specificity and/or suppress differentiation toward non-PT cell fates. Based on our results showing that the presence of either Gata4 or Foxa2/3 alters the effects of Hnf1a and Hnf4a transduction, it is also conceivable that other transcription factors that are not endogenously expressed in the proximal tubule might be expressed to some extent in MEFs—thus interfering with the ability of Hnf1a and Hnf4a to further induce a PT cell–like transcriptome. All of these factors are further complicated by the pre-existing epigenetic landscape of starting cells prior to transdifferentiation, which may contain some features that may be difficult to remove or that may make it difficult to establish new regulatory elements (Mikkelsen et al., 2008; Sindhu et al., 2012). In the future, it will be important to consider various cell types that are available in the clinical setting, as some may require different/additional inputs for achieving transdifferentiation.
In addition to providing evidence that either proximal tubule signature genes or hepatocyte signature genes are induced depending on the absence or presence of Gata4 and Foxa2/3, we also demonstrated that Hnf4a binding in the kidney is enriched more than 3-fold at PT signature genes compared with hepatocyte signature genes (Fig. 6). This implies tissue-specific activity of Hnf4a directed at regulating PT-enriched genes in the kidney. Nevertheless, our lentiviral transduction data indicated that Hnf4a, even together with Hnf1a, was insufficient to fully specify a proximal tubule phenotype. For example, whereas certain PT transcripts were strongly increased (e.g., Oat1 or SLC22a6) and tight junctions were formed (Fig. 4), other genes that are considered to be indicators of the PT were not expressed or were induced at very low levels. This indicates that Hnf1a and Hnf4a likely require additional factors to fully specify PT cell fate. This is consistent with published studies that Foxa and Gata4 transcription factor binding often colocalizes with Hnf4a in hepatocytes, suggesting that their presence might partially determine Hnf4a binding sites. Indeed, it has been reported that both Gata and Foxa transcription factors are “pioneering transcription factors” (Zaret and Carroll, 2011), meaning that they can access condensed chromatin and establish binding sites de novo; thus, they may alter Hnf4a specificity not only by altering the functionality of enhancers established by Hnf4a through recruitment of coregulators and transcriptional machinery, but also by establishing new binding sites that are otherwise inaccessible to Hnf4a alone.
Thus, we have shown that, whereas Hnf1a and Hnf4a are lineage-determining factors for both proximal tubule cells and hepatocytes, the specificity toward either lineage appears to be affected by coexpression of Gata4 and Foxa2/3 (and possibly other factors that were not tested). Future studies should aim at identifying additional coregulators that may need to be added or silenced to achieve complete transdifferentiation toward one cell lineage or the other. Together, these findings advance the understanding of the transcriptional basis of proximal tubule cell differentiation and function, and clarify how two transcription factors central to both hepatocytes and PT cell fate can be guided toward divergent specificity by other coregulators. The results seem generally compatible with transcriptomic and ChIP-seq data during organ development (Figs. 5 and 6). Our results may also contribute to the future development of therapeutic strategies to enhance PT function and regenerative capacity as well as tissue engineering (reviewed in Nigam, 2013; Martovetsky and Nigam, 2014).
Supplementary Material
Acknowledgments
The authors thank Drs. Bing Ren, Chris Glass, Karl Willert, and Scott Thomson for valuable advice throughout this project. The authors also thank Dr. Lijian Hui for providing the lentiviral plasmids used in this study.
Abbreviations
- ABC
ATP-binding cassette
- AKI
acute kidney injury
- Alb
albumin
- Apoa1
apolipoprotein A
- Cdh
Cadherin
- ChIP-seq
chip immunoprecipitation followed by high throughput sequencing
- CKD
chronic kidney disease
- DMEM
Dulbecco’s modified Eagle’s medium
- Dsp
desmoplakin
- FBS
fetal bovine serum
- Fox
forkhead box protein
- GFP
green fluorescent protein
- IHC
immunohistochemistry
- MEF
mouse embryonic fibroblast
- NEAA
nonessential amino acid supplement
- Ocln
occludin
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PT
proximal tubule
- SLC
solute carrier
- Trf
transferrin
- Ttr
transthyretin
- UCSD
University of California, San Diego
Authorship Contributions
Participated in research design: Martovetsky, Nigam.
Conducted experiments: Martovetsky.
Performed data analysis: Martovetsky.
Wrote or contributed to the writing of the manuscript: Martovetsky, Bush, Nigam.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants GM098449 and GM104098], the National Institutes of Health National Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant U54 HD07160], and the Nancy Kaehr Chair in Research at the University of California, San Diego.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Ahn SY, Nigam SK. (2009) Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol 76:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkovetz DF. (2009) Tight junction claudins and the kidney in sickness and in health. Biochim Biophys Acta 1788:858–863. [DOI] [PubMed] [Google Scholar]
- Denker BM, Nigam SK. (1998) Molecular structure and assembly of the tight junction. Am J Physiol 274:F1–F9. [DOI] [PubMed] [Google Scholar]
- Du Y, Wang J, Jia J, Song N, Xiang C, Xu J, Hou Z, Su X, Liu B, Jiang T, et al. (2014) Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 14:394–403. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, et al. (2006) Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem 281:5072–5083. [DOI] [PubMed] [Google Scholar]
- Graf T, Enver T. (2009) Forcing cells to change lineages. Nature 462:587–594. [DOI] [PubMed] [Google Scholar]
- Harding J, Mirochnitchenko O. (2014) Preclinical studies for induced pluripotent stem cell-based therapeutics. J Biol Chem 289:4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38:576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J. (2014) The kidney tight junction (Review). Review Int J Mol Med 34:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. (2011) Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475:386–389. [DOI] [PubMed] [Google Scholar]
- Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, Cen J, Chen X, Liu C, Hu Y, et al. (2014) Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 14:370–384. [DOI] [PubMed] [Google Scholar]
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. (2003) Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167. [DOI] [PubMed] [Google Scholar]
- Lee DB, Huang E, Ward HJ. (2006) Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol 290:F20–F34. [DOI] [PubMed] [Google Scholar]
- Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. (1997) Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem 272:6471–6478. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhao T. (2013) Clinical therapy using iPSCs: hopes and challenges. Genomics Proteomics Bioinformatics 11:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martovetsky G, Nigam SK. (2014) Cellular and developmental strategies aimed at kidney tissue engineering. Nephron, Exp Nephrol 126:101. [DOI] [PubMed] [Google Scholar]
- Martovetsky G, Tee JB, Nigam SK. (2013) Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol Pharmacol 84:808–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A. (2008) Dissecting direct reprogramming through integrative genomic analysis. Nature 454:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey KM, Wen CC, Johns SJ, Zhang L, Huang SM, Giacomini KM. (2012) The UCSF-FDA TransPortal: a public drug transporter database. Clin Pharmacol Ther 92:545–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle MA, Truong DM, Dnyanmote AV, Ahn SY, Eraly SA, Wu W, Nigam SK. (2011) Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem 286:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K, Schumacher KM, Tasnim F, Kandasamy K, Schumacher A, Ni M, Gao S, Gopalan B, Zink D, Ying JY. (2013) Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int 83:593–603. [DOI] [PubMed] [Google Scholar]
- Nigam SK. (2013) Concise review: can the intrinsic power of branching morphogenesis be used for engineering epithelial tissues and organs? Stem Cells Transl Med 2:993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK. (2015) What do drug transporters really do? Nat Rev Drug Discov 14:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, Bhatnagar V, Wu W. (2015a) The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev 95:83–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V. (2015b) Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10:2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, Ikeda E, Yamanaka S, Miura K. (2013) Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res 112:523–533. [DOI] [PubMed] [Google Scholar]
- Sekiya S, Suzuki A. (2011) Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475:390–393. [DOI] [PubMed] [Google Scholar]
- Simeonov KP, Uppal H. (2014) Direct reprogramming of human fibroblasts to hepatocyte-like cells by synthetic modified mRNAs. PLoS One 9:e100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu C, Samavarchi-Tehrani P, Meissner A. (2012) Transcription factor-mediated epigenetic reprogramming. J Biol Chem 287:30922–30931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres AM, Dnyanmote AV, Bush KT, Wu W, Nigam SK. (2011) Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury. J Biol Chem 286:26391–26395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. (2008) Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J Biol Chem 283:8654–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Nagle MA, Kouznetsova VL, Tsigelny IF, Nigam SK. (2011) Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1). J Proteome Res 10:2842–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Bush KT, Liu HC, Zhu C, Abagyan R, Nigam SK. (2015) Shared ligands between organic anion transporters (OAT1 and OAT6) and odorant receptors. Drug Metab Dispos 43:1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Dnyanmote AV, Nigam SK. (2011) Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol Pharmacol 79:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, He ZY, You P, Han QW, Xiang D, Chen F, Wang MJ, Liu CC, Lin XW, Borjigin U, et al. (2013) Reprogramming fibroblasts into bipotential hepatic stem cells by defined factors. Cell Stem Cell 13:328–340. [DOI] [PubMed] [Google Scholar]
- Zaret KS, Carroll JS. (2011) Pioneer transcription factors: establishing competence for gene expression. Genes Dev 25:2227–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Nigam KB, Date RC, Bush KT, Springer SA, Saier MH, Jr, Wu W, Nigam SK. (2015) Evolutionary analysis and classification of OATs, OCTs, OCTNs, and other SLC22 transporters: Structure-function implications and analysis of sequence motifs. PLoS One 10:e0140569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.