Abstract
For infants and very young children with brain tumors, chemotherapy after surgical resection is the main treatment due to neurologic and neuroendocrine adverse effects from whole brain irradiation. Topotecan, an anticancer drug with antitumor activity against pediatric brain tumors, can be given intravenous or orally. However, high interpatient variability in oral drug bioavailability is common in children less than 3 years old. Therefore, this study aimed to determine the population pharmacokinetics of oral topotecan in infants and very young children, specifically evaluating the effects of age and ABCG2 and ABCB1 on the absorption rate constant (Ka), as well as other covariate effects on all pharmacokinetic parameters. A nonlinear mixed effects model was implemented in Monolix 4.3.2 (Lixoft, Orsay, France). A one-compartment model with first-order input and first-order elimination was found to adequately characterize topotecan lactone concentrations with population estimates as [mean (S.E.)]; Ka = 0.61 (0.11) h−1, apparent volume of distribution (V/F) = 40.2 (7.0) l, and apparent clearance (CL/F) = 40.0 (2.9) l/h. After including the body surface area in the V/F and CL/F as a power model centered on the population median, the ABCG2 rs4148157 allele was found to play a significant role in the value of Ka. Patients homozygous or heterozygous for G>A demonstrated a Ka value 2-fold higher than their GG counterparts, complemented with a 2-fold higher maximal concentration as well. These results demonstrate a possible role for the ABCG2 rs4148157 allele in the pharmacokinetics of oral topotecan in infants and very young children, and warrants further investigation.
Introduction
The current standard of care that has the improved overall survival for children with brain tumors consists of surgical resection of the tumor, radiotherapy, and chemotherapy (Karajannis et al., 2008). However, infants and very young children less than 3 years old with primary central nervous system tumors have a poor prognosis due to the increased likelihood of disseminated disease at diagnosis and the morbidity of whole brain irradiation, among other factors (Duffner et al., 1983; Gajjar et al., 1994; Walter et al., 1999; Mulhern et al., 2004; Laughton et al., 2008). Little is known about the disposition of many anticancer drugs in infants and young children, which often leads to increased risk of morbidity, poor tumor control, and increased risk of late effects. Thus, it is essential that thorough pharmacokinetic studies be performed in this patient population to provide the data upon which rational dosing schedules can be developed that will increase patient survival and decrease toxicity.
One promising anticancer drug, topotecan (structure as in Beijnen et al., 1990), is a topoisomerase I inhibitor and camptothecin analog that has shown effective cytotoxic activity in previous pediatric solid tumor trials (Pratt et al., 1994; Stewart and Ratain, 1997; Takimoto et al., 1998; Santana et al., 2003). Topoisomerase I is an intracellular enzyme that binds to DNA, introducing single strand DNA breaks. Topotecan binds to and stabilizes the topoisomerase I/DNA complex, preventing DNA replication and causing apoptosis (Hsiang and Liu, 1988; Kingsbury et al., 1991; Taudou et al., 1993). At physiologic pH topotecan exists in equilibrium between the active lactone (closed form) and the inactive hydroxy acid (open-ring form) (Creemers et al., 1994). Topotecan is effective in mouse xenograft models of various types of pediatric tumors, and preclinical studies have shown that the protracted dosing schedules were associated with greater antitumor effect (Houghton et al., 1992, 1995; Tubergen et al., 1996; Nitschke et al., 1998; Guichard et al., 2001; Kretschmar et al., 2004; Minturn et al., 2011). It is most commonly administered intravenously but clinical studies have shown evidence of efficacy with low-dose oral topotecan given once or twice daily on a protracted schedule in adults (Schellens et al., 1996; Creemers et al., 1997). In infants and very young children especially, oral administration provides an attractive alternative to intravenous administration due to the decreased discomfort to the patient and the overall convenience for the long-term care of the patient during maintenance therapy.
However, a confounding factor of oral chemotherapy is the extensive inter- and intrapatient variability in oral bioavailability (Hande et al., 1993; Zhou et al., 1994; Zamboni et al., 1999). Medications with low bioavailability, like topotecan, can have significantly higher inter-patient variability compared with compounds with high bioavailability (DeMario and Ratain, 1998; Daw et al., 2004). Infants demonstrate even greater variability in the bioavailability of orally administered agents due to the physiologic maturation of factors affecting drug absorption, such as higher gastric pH and slower gastric motility (Berseth, 1996; Alcorn and McNamara, 2003). Furthermore, topotecan is a substrate for the drug efflux ATP-binding cassette transporters ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein) both in vitro and in vivo (Chen et al., 1991; Hendricks et al., 1992; Brangi et al., 1999; Maliepaard et al., 1999, 2001; Erlichman et al., 2001; de Vries et al., 2007), suggesting that polymorphisms in these transporters could also affect the oral absorption of topotecan from the gut. Therefore, several factors could contribute to interpatient variability with oral topotecan in infants and very young children. As a result, this study aimed to describe the population pharmacokinetics of oral topotecan in infants and very young children, specifically focusing on the influence of age and/or efflux transporters (ABCB1 and ABCG2) on the absorption rate constant (Ka) of oral topotecan. In addition, other common patient characteristics (i.e., weight, height, etc.) were evaluated on all parameters describing the oral topotecan pharmacokinetics to determine if other covariate effects could explain interindividual variability in oral topotecan for infants and very young children.
Patients and Methods
Patients and Study Design.
Risk-Adapted Therapy for Infants and Young Children with Embryonal Brain Tumors, High Grade Glioma, Choroid Plexus Carcinoma or Ependymoma (SJYC07; NCT00602667; https://clinicaltrials.gov/ct2/show/NCT00602667?term=NCT00602667&rank=1) is a multicenter protocol that is carried out in accordance with the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/17c.pdf) and approved by our institutional review board. Parental permission and written informed consent was obtained before conducting any study procedures. This study is currently ongoing. Inclusion criteria included newly diagnosed tumor of the central nervous system, age <5 at the time of diagnosis, and no previous radiotherapy or chemotherapy. Additional criteria included adequate organ function, performance status with a Lansky score ≥30, and patients beginning their treatment within 31 days of definitive surgery. Patients were stratified into one of three risk arms (low, intermediate, and high) based on their stage of metastasis, diagnosis, extent of resection, histologic subtype, and age at diagnosis. Each treatment arm consists of an induction, consolidation, and final optional maintenance phase. During the maintenance phase, patients on all treatment arms receive oral topotecan (0.8 mg/m2) for 10 days and oral cyclophosphamide (30 mg/m2) for 21 days on a 28-day cycle for three cycles. Oral topotecan was administered as a liquid mixed in a flavored vehicle of the patient’s choosing. Additional parental consent had to be obtained before inclusion into the oral topotecan pharmacokinetic analysis, which only included patients from St. Jude Children’s Research Hospital. Additionally, patients who were simultaneously enrolled in and consented to the ongoing Pharmacogenetic Determinants of Treatment Response in Children study (PGEN5; NCT00730678; https://clinicaltrials.gov/ct2/show/NCT00730678?term=NCT00730678&rank=1) were used in the genotyping analysis for genetic polymorphisms in ABCG2 and ABCB1.
Genotyping.
Genome-wide genotyping was completed in germline DNA using the Illumina Infinium Omni2.5Exome-8 BeadChip (illumina Inc., Sand Diego, CA). Variants were filtered on the basis of Hardy–Weinberg disequilibrium, minor allele frequency [(MAF) 0.1] and call rate (<95%). Within variants that passed these quality control criteria, a candidate gene approach was implemented to specifically evaluate ABCG2 and ABCB1 single nucleotide polymorphism genotypes following those reported in PharmGKB (www.pharmgkb.org). The alleles reported in PharmGKB that met with the aforementioned criteria in this analysis included rs4148157 (intron 11, G>A, population MAF 0.1006), rs2622628 (intron 9, C>A, population MAF 0.2362), rs2725252 (intron 1, C>A, population MAF 0.4145), rs1045642 (exon 27, A>G, Ile>Ile, population MAF 0.3952), rs2032582 (exon 22, A>C, Ser>Thr, population MAF 0.3343), and rs1128503 (exon 13, A>G, Gly>Gly, population MAF 0.4161) (dbSNP; http://www.ncbi.nlm.nih.gov/SNP/).
Pharmacokinetic Sample Collection and Analysis.
Whole blood (2 ml) was collected before the dose was administered and at 15 minutes, 90 minutes, and 6 hours after treatment, as indicated in a limited sampling model (Turner et al., 2006). Samples were placed in a heparinized tube, immediately centrifuged at 10,000 rpm for 2 minutes to separate plasma and 0.2 ml of plasma was added to 0.8 ml of ice-cold methanol. The methanolic mixture was vortexed for 10 seconds and centrifuged for 2 minutes at 10,000 rpm. The supernatant was retained and assayed for topotecan lactone by an isocratic high-performance liquid chromatography assay with fluorescence detection, as previously described (Beijnen et al., 1990). Briefly, a Shimadzu RF 10AXL detector (Candby, OR) was used at 370 nm excitation and 520 nm emission wavelengths with a lower limit of quantitation of 1 ng/ml.
Population Pharmacokinetic Analysis.
Topotecan lactone concentrations were estimated using a nonlinear mixed-effects model implemented in Monolix 4.3.2 (Lixoft, Orsay, France) using the stochastic approximation expectation maximization algorithm (Kuhn and Lavielle, 2005) combined with a Markov chain Monte Carlo procedure. The minimum sample size was set to 50, resulting in the number of chains set to one. Concentration measurements that were below the limit of quantitation were marked as left censored for the analysis, meaning actual concentrations were set to the lower limit of quantitation (1 ng/ml); the extended stochastic approximation expectation maximization approach simulates concentrations below the limit of quantitation according to a right-truncated normal distribution (Samson et al., 2006). A Bayesian prior of 0.69 h−1 with a deviation of 0.50 was applied to the Ka value as previously described (Daw et al., 2004). One- and two-compartment models were evaluated and the final model selection was based on their goodness of fit. Models that did not converge and those with erroneous results (e.g., negative parameter values) were disregarded. Diagnostic plots were used to assess the model’s fit. Population weighted residuals (PWRES) and individual weighted residuals were plotted versus time and population or individual predictions, respectively. Models were compared using statistical criteria [−2 × log likelihood or the objective function value (OFV)], variability of the parameter estimates (e.g., <50%), diagnostic plots (e.g., graphical representation of the goodness of fit), and assessment of the physiologic relevance of the parameter estimate.
Model variability and random effects were classified as between-subject variability or residual unexplained variability. Between-subject variability was assumed to be log-normally distributed according to the following exponential equation:
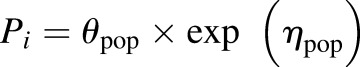 |
(1) |
where Pi is the pharmacokinetic parameter of the ith individual; θpop is the population mean for P; and η represents the normally distributed between-subject random effect with a mean of zero and a variance of ω2. Additive, proportional, combined additive and proportional, and exponential equations were evaluated for the residual unexplained variability. The final model used the following additive error model:
| (2) |
where Yij is the observed concentration for the ith individual at time j; Ŷij is the individual predicted concentration; and ε1 represents the additive error, which is a normally distributed error term with a mean of zero and variance of σ2.
Several patient characteristics were tested for their influence on the topotecan lactone pharmacokinetic parameters. The covariates tested included sex, age, estimated glomerular filtration rate (eGFR) calculated using the Schwartz equation (Schwartz et al., 1976), body surface area (BSA), weight, height, ABCG2 alleles (rs4148157, rs2622628, and rs2725252), and ABCB1 alleles (rs1045642, rs2032582, and rs1128503). Only 42 patients had relevant serum creatinine measurements (serum creatinine measured within 24 hours of the pharmacokinetic analysis); therefore, the average of those measured was imputed for those missing a serum creatinine to calculate the eGFR value. Additionally, only 52 patients had gene data; therefore, those with missing gene information were handled as a missing covariate within Monolix (Lixoft). Power and linear models were evaluated for the continuous covariates (i.e., weight, height, age, eGFR, BSA) and exponential models were evaluated for categorical covariates (i.e., sex and genotype). In addition, each continuous covariate was evaluated centered on the median population value. Forward addition was used to determine significant covariates. A decrease in the OFV ≥3.84 was considered significant for one degree of freedom at P = 0.05 based on the χ2 distribution. Backward elimination was used to remove covariates from the model with an increase in the OFV ≥6.63 corresponding to one degree of freedom at P = 0.01.
The final model was evaluated using diagnostic plots to assess the fit of the model. Observed drug concentrations were evaluated against their predicted concentrations to evaluate their correlation. PWRES as well as the individual weighted residuals were plotted versus time and population or individual predictions. Models were compared by assessing the variability of the parameter estimates in addition to the OFV. Normalized prediction distribution errors were plotted against time and population-predicted topotecan lactone concentrations to assess for model misspecification.
Results
Patient Characteristics.
The initial data set included 69 patients with 207 concentration measurements enrolled in the trial at the time of analysis. Eight patients with 24 concentration measurements were eliminated from the analysis because they did not have at least one concentration measurement above the lower limit of quantitation. Another concentration measurement from an individual was eliminated because the PWRES was >6. Thus, the final data set for the population analysis contained 61 patients with 182 concentration measurements. Seventy-one concentration measurements (39%) were below the limit of quantitation and were marked as left censored for the pharmacokinetic analysis. The demographics for the study subjects at the time of the pharmacokinetic study are depicted in Table 1. The age range for those enrolled spanned from about 6 months to ∼4.5 years, with 20 of the 61 patients being less than 2 years of age (33%) at the time of study. In addition, about two-thirds of the patients enrolled were male. Pharmacogenomic data were available from 52 patients of the population and the frequencies of germline mutations examined were all in Hardy–Weinberg equilibrium. Alleles specifically evaluated in the model for ABCG2 were rs4148157, rs2622628, and rs2725252, and for ABCB1 were rs1045642, rs2032582, and rs1128503 with the frequencies described in Table 2.
TABLE 1.
Patient demographics
| Patient Characteristic | n | Mean | Median (Range) |
|---|---|---|---|
| Age (year) | 61 | 2.4 | 2.37 (0.48–4.59) |
| Weight (kg) | 61 | 12.6 | 12.60 (5.20–17.50) |
| Height (cm) | 61 | 87.1 | 89.10 (59.50–102) |
| BSA (m2) | 61 | 0.57 | 0.57 (0.31–0.72) |
| eGFR (ml/min/1.73 m2) | 42 | 148.1 | 139.79 (100.67–200.01) |
| SCr (mg/dl) | 42 | 0.25 | 0.24 (0.14–0.40) |
| Male | 38 | ||
| Female | 23 |
SCr, serum creatinine.
TABLE 2.
Pharmacogenomic analysis
|
ABCG2 |
ABCB1 |
||
|---|---|---|---|
| Allele | n | Allele | n |
| rs4148157 (G>A) | rs1045642 (A>G) | ||
| GG | 42 | AA | 8 |
| AG | 9 | AG | 22 |
| AA | 1 | GG | 22 |
| Not available | 9 | Not available | 9 |
| rs2622628 (C>A) | rs2032582 (A>C) | ||
| CC | 43 | AA | 10 |
| AC | 7 | AC | 20 |
| AA | 2 | CC | 22 |
| Not available | 9 | Not available | 9 |
| rs2725252 (C>A) | rs1128503 (A>G) | ||
| CC | 14 | AA | 9 |
| AC | 26 | AG | 18 |
| AA | 12 | GG | 25 |
| Not available | 9 | Not available | 9 |
Population Pharmacokinetic Analysis.
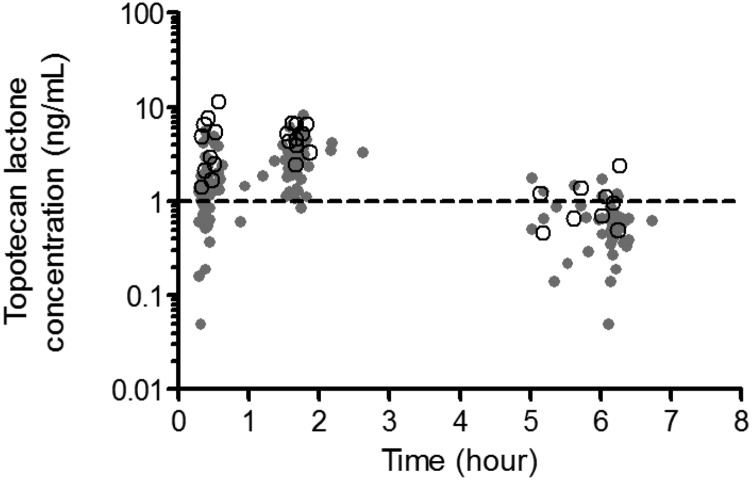
A concentration-time plot of the data is represented in Fig. 1. The data were well described by a one-compartment model with first-order input and first-order elimination as assessed by visual inspection of diagnostic plots, physiologic relevance of the estimates, and a significant reduction of the OFV.
Fig. 1.
Concentration-time data for the patients included in the analysis represented as topotecan lactone concentrations (ng/ml) versus time (hour). Patients with wild-type rs4148157 and variant rs4148157 are represented by gray circles and black open circles, respectively. The dashed line at 1 ng/ml represents the lower limit of quantitation for the assay.
The covariate analysis revealed a significant influence of BSA (centered on the population median) on the apparent clearance (CL/F). Additionally, the inclusion of the ABCG2 allele rs4148157 dichotomously as either GG homozygous or AG heterozygous and AA homozygous for the variant allele (represented as a 0 or 1, respectively) resulted in a significant effect on the Ka value. Heterozygous or homozygous G>A patients were grouped together because there was only one homozygous G>A patient in the analysis. Including this covariate in the model resulted in a Ka value of 0.610 (S.E. 0.11) h−1 for GG and 1.76 (S.E. 0.49) h−1 for AG or AA patients. Further analysis of the effect of the ABCG2 allele on the Ka value demonstrated a significant difference in observed maximal concentrations (Cmax) between the two groups as assessed by the 1.5-hour concentration (P = 0.0001; Student’s t test). An almost 2-fold difference in the Cmax was observed in patients with the GG genotype compared with those with the AG or AA genotype (3.10 and 5.34 ng/ml, respectively), supporting the inclusion of the Ka value. No ABCB1 alleles significantly affected the Ka value. Additionally, after including these covariates, no other covariates significantly affected the oral topotecan lactone pharmacokinetics. However, the BSA on the apparent volume of distribution (V/F) was included in the model for biologic plausibility (Anderson et al., 2006). The final model estimates are presented in Table 3 and were derived from the following final model:
| (3) |
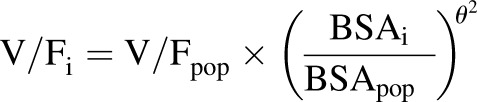 |
(4) |
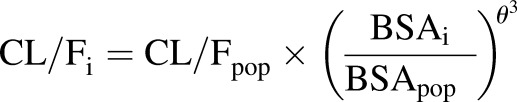 |
(5) |
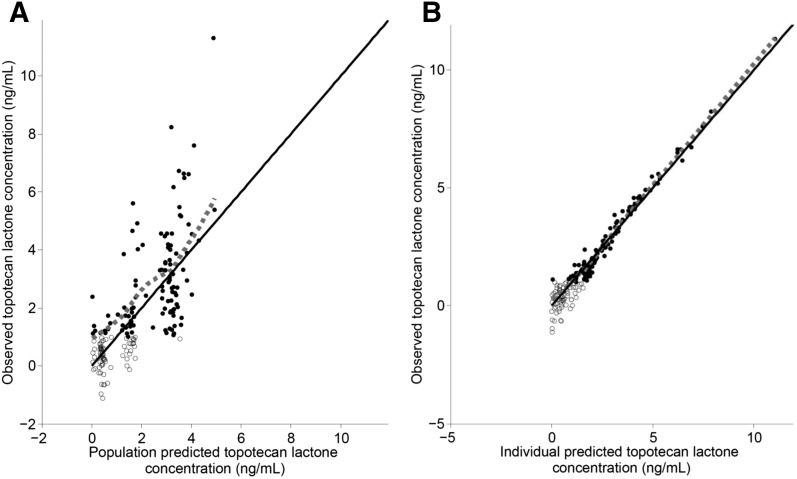
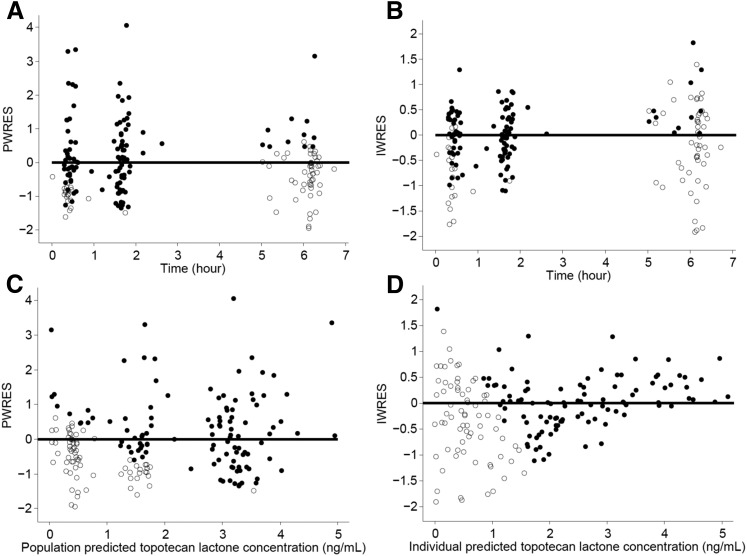
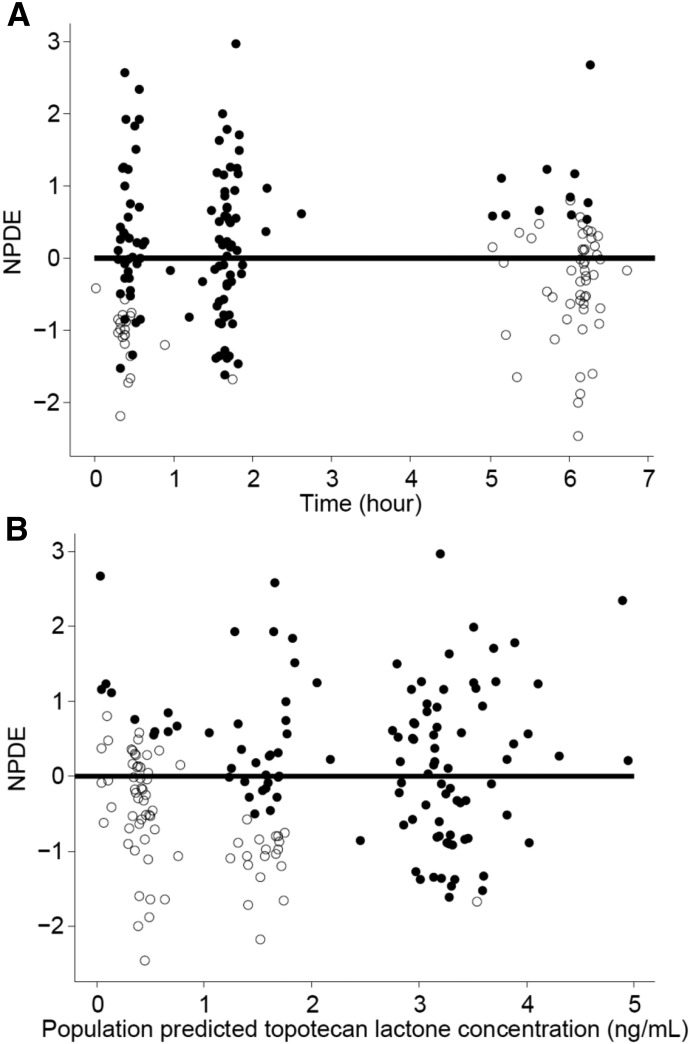
where Kai is the individual absorption rate constant; Kapop is the estimated population absorption rate constant; θ1 is the estimated coefficient of the effect of the ABCG2 allele rs4148157 on Ka; GENECAT is the categorical covariate of the GG allele compared with the AG and AA alleles (dichotomously defined as 0 or 1, respectively); V/Fi is the individual apparent volume of distribution; V/Fpop is the estimated population apparent volume of distribution; BSAi is the individual body surface area; BSApop is the median population body surface area; and θ2 is the estimated exponent for the effect of the BSA on the topotecan lactone apparent volume of distribution. Similarly, CL/Fi is the individual apparent clearance, CL/Fpop is the estimated population apparent clearance, and θ3 is the estimated exponent for the effect of the BSA on the topotecan lactone apparent clearance. Diagnostic plots were visually inspected to confirm the selection of the final model (Fig. 2, A and B). To further evaluate the model the PWRES, individual weighted residuals, and normalized prediction distribution error plots were examined (Fig. 3, A–D; Fig. 4, A and B). The data were equally distributed around zero and the majority of the data were in the −2 to +2 range, indicating acceptable agreement between observed and predicted concentrations.
TABLE 3.
Final population model estimates
| Parameter | Mean Parameter Estimate | S.E. |
|---|---|---|
| Ka (1/h; wild-type rs4148157) | 0.61 | 0.11 |
| θ1 | 1.06 | 0.25 |
| Ka (1/h; variant rs4148157) | 1.76 | 0.49 |
| V/F (l) | 40.2 | 7.0 |
| θ2 | 0.78 | 0.48 |
| CL/F (l/h) | 40.0 | 2.9 |
| θ3 | 1.25 | 0.39 |
| ω -BSV | ||
| Ka | 0.517 | 0.12 |
| V/F | 0.338 | 0.17 |
| CL/F | 0.465 | 0.11 |
| σ -RUV | ||
| Additive (ng/ml) | 0.592 | 0.44 |
BSV, between-subject variability; RUV, residual unexplained variability; θ1, estimated coefficient of the effect of ABCG2 rs4148157 G>A heterozygotes and homozygotes on the absorption rate constant; θ2, estimated exponent of the effect of the BSA on apparent volume of distribution; θ3, estimated exponent of the effect of the BSA on apparent clearance.
Fig. 2.
Observed topotecan lactone concentrations versus population predicted (A) and individual predicted (B) topotecan lactone concentrations for the final topotecan lactone model. The closed circles represent data above the lower limit of quantitation, the open circles represent data below the limit of quantitation or simulated data, the solid line represents the line of identity, and the dashed gray line represents the spline of the model.
Fig. 3.
(A–D) PWRES and the individual weighted residuals (IWRES) versus time and predicted topotecan lactone concentrations. The closed circles represent data above the lower limit of quantitation, the open circles represent data below the limit of quantitation or simulated data, and the solid line is the reference line at zero.
Fig. 4.
The normalized prediction distribution errors (NPDE) plotted against time (A) and population predicted topotecan lactone concentrations (B). The closed circles represent data above the lower limit of quantitation, the open circles represent data below the limit of quantitation or simulated data, and the solid line is the reference line at zero.
Discussion
This study is the first to specifically evaluate the population pharmacokinetics of oral topotecan in infants and very young children with primary central nervous system tumors. Furthermore, it is the first study to implicate the ABCG2 rs4148157 allele as a possible contributor to variations in the pharmacokinetics of oral topotecan. This study demonstrated that the oral topotecan pharmacokinetics in this population of infants and very young children was well described by a one-compartment model with a population Ka value equal to 0.61 h−1, a V/F value equal to 40.2 l (70.5 l/m2), and a CL/F value equal to 40.0 l/h (70.2 l/h/m2). Covariate analysis indicated that after inclusion of the BSA in the CL/F and V/F, the ABCG2 rs4148157 allele also played a significant role in the Ka value, with patients either heterozygous or homozygous with the G>A allele resulting in a Ka value 2-fold higher than their homozygous GG counterparts. Through the forward addition covariate analysis, age, weight, and height were also significant covariates in CL/F. However, after taking into account the BSA, the weight and height were no longer significant as assessed by the change in the OFV due to the high correlation between these covariates, supporting the use of a single covariate to capture the effect of body size on the CL/F. In addition, weight was no more significant than BSA in univariate analysis, supporting the current approach of normalizing the oral topotecan dosage to the BSA. Furthermore, after taking into account the BSA, age was no longer significant in relation to CL/F. However, these results may be confounded with the sparse sampling strategy due to small blood volume in infants and very young children, which could decrease the ability of finding multiple covariates in a small population size. Renal function has been shown in other studies to play a significant role in topotecan CL (Léger et al., 2004; Devriese et al., 2015); however, all patients included in this study demonstrated normal renal function, possibly explaining why eGFR was not a significant covariate in CL/F. Surprisingly, age was not a significant covariate in the Ka value, although having concentrations below the limit of quantitation for some of the patients at 15 minutes could have decreased the ability of the model to find age as a significant covariate in the Ka value.
Further covariate analysis evaluating ABCG2 and ABCB1 in relation to the Ka value showed that only one single nucleotide polymorphism, the ABCG2 rs4148157 allele, had any significant effect on the Ka value in this patient population. A literature review, including information found in PharmGKB (www.pharmgkb.org), described this allele as intronic, and to date this allele has not been demonstrated to have clinical significance. The only publication specifically discussing the rs4148157 G>A allele was a study in patients with epilepsy, which showed no significant resistance to antiepileptic drugs in 441 Asian patients with the A allele, although no alleles addressed in this study demonstrated any significance (Kwan et al., 2011). Further analysis indicated strong linkage disequilibrium between rs4148157 and the well-known ABCG2 rs2231142 allele (exon 6; MAF 0.119). It is possible that the intronic allele (i.e., rs4148157) is serving as a surrogate for rs2231142. Gene imputation was performed to identify the genotype for the rs2231142 allele for our patient cohort and was included in the model in the Ka value, CL/F, and V/F as a categorical covariate. For CL/F, including rs2231142 was not a significant covariate, and including it in the Ka value and V/F resulted in a model where the Fisher information matrix could not be estimated. Although the ABCG2 rs4148157 allele was a significant covariate in the Ka value, when we included it as a categorical covariate in the CL/F or V/F it resulted in a model in which the Fisher information matrix could not be estimated. Therefore, these covariates were not included in further model development. Several explanations could explain this observation, including our limited sampling of three plasma samples per patient, the relatively small population sample size in the study, or the extent of data that were below the limit of quantitation. Therefore, further in vitro and in vivo research is required to determine if the ABCG2 rs4148157 G>A allele does play a significant role in the decreased efflux of topotecan into the gut—which would therefore impact the clinical use of oral topotecan in infants and very young children who expressed the G>A rs4148157 allele—or if it is truly serving as a surrogate for the ABCG2 rs2231142 allele.
It is possible that the expression of ABCG2 differs between adults and very young children, which could contribute to the pharmacokinetic differences in oral topotecan. While the majority of the research has focused on central nervous system and hepatic ABCG2 expression, no difference between adults and term neonates was observed (Daood et al., 2008; Yanni et al., 2011). Therefore, it is unlikely that ontogeny of ABCG2 is playing a role.
In the analysis, it was noted that a number of concentrations that were marked as censored, and therefore simulated in Monolix (Lixoft) using a right-truncated normal distribution, resulted in negative concentrations (Fig. 2, A and B). Simulations resulting in negative data likely mean that these concentrations were probably zero. However, instead of treating these concentrations as zero (as the simulations suggested) and removing them from the analysis, we believed it important to leave these in the model to not overly bias the estimation of the pharmacokinetic parameters.
Other population pharmacokinetic models in adults and children have published oral topotecan as a two-compartment model. However, the majority of these models also included intravenous data as part of their analysis, which was not included in our analysis. A study of adults estimated the Ka value as 1.7 h−1 and the CL/F value as about 63 l/h, with creatinine clearance and World Health Organization performance status included as significant covariates (Léger et al., 2004). Compared to the population means in this study, the Ka value was about two times higher than those for wild-type rs4148147, but the CL/F was very similar. However, patients who did have the G>A rs4148147 allele did have a Ka value closer to that of adults (1.76 h−1). In a pediatric study, the CL/F was estimated to be about 20.82 l/h in older pediatric patients using a two-compartment model (Wagner et al., 2004), which was about half that found in this study. In another pediatric study completed the same year, Daw et al. (2004) found the CL/F to be about 79.7 l/h/m2, which is almost identical to the CL/F found in our population. In addition, the Ka value of 0.69 h−1 was similar to our population mean of 0.61 h−1, which is not surprising since the Ka value reported by Daw et al. (2004) was used as a Bayesian prior in our model. Finally, comparing our CL/F to a study completed in 1999 showed very similar determinations, with the CL/F reported as about 64.4 L/h/m2, which was also determined using a one-compartment model (Zamboni et al., 1999). Comparing all the studies together, it seems that the patients from this study with the GG rs4148157 allele had an estimated Ka value similar to older pediatric patients, but those expressing the AG or AA allele had a Ka value closer to adult values. In addition, CL/F in infants and very young children is similar to other pediatric patients as well as adults based on the results from this study.
This is the first study to evaluate the oral topotecan pharmacokinetics in infants and very young children, and our results have implicated a possible role for the ABCG2 rs4148157 allele in the Ka value. BSA was also a significant covariate in CL/F, with BSA also included in V/F for biologic plausibility. Future analyses investigating the clinical implications of the ABCG2 rs4148157 allele on oral topotecan chemotherapy in infants and very young children are warranted.
Acknowledgments
The authors thank the clinical nursing team, including Sheri Ring, Paula Condy, and Terri Kuehner, for their assistance in this study; the Stewart Laboratory for bedside collection and processing of the samples; the Hartwell Center for performing the Illumina Chip; Wenjian Yang for expertise in creating the database that housed the genetic information for the patients; and Jun J. Yang for valuable expertise in genetic analyses.
Abbreviations
- BSA
body surface area
- CL/F
apparent clearance
- eGFR
estimated glomerular filtration rate
- Ka
absorption rate constant
- MAF
minor allele frequency
- OFV
objective function value
- PWRES
population weighted residuals
- V/F
apparent volume of distribution
Authorship Contributions
Participated in research design: Broniscer, Robinson, Gajjar, Stewart.
Conducted experiments: Roberts, Birg, Lin.
Performed data analysis: Roberts, Birg, Lin.
Wrote or contributed to the writing of the manuscript: Roberts, Birg, Lin, Daryani, Panetta, Broniscer, Robinson, Gajjar, Stewart.
Footnotes
This work was supported by the National Cancer Institute [Grants CA154619 and CA21765] and the American Lebanese Syrian Associated Charities at St. Jude Children’s Hospital.
References
- Alcorn J, McNamara PJ. (2003) Pharmacokinetics in the newborn. Adv Drug Deliv Rev 55:667–686. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Allegaert K, Holford NH. (2006) Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 165:819–829. [DOI] [PubMed] [Google Scholar]
- Beijnen JH, Smith BR, Keijer WJ, van Gijn R, ten Bokkel Huinink WW, Vlasveld LT, Rodenhuis S, Underberg WJM. (1990) High-performance liquid chromatographic analysis of the new antitumour drug SK&F 104864-A (NSC 609699) in plasma. J Pharm Biomed Anal 8:789–794. [DOI] [PubMed] [Google Scholar]
- Berseth CL. (1996) Gastrointestinal motility in the neonate. Clin Perinatol 23:179–190. [PubMed] [Google Scholar]
- Brangi M, Litman T, Ciotti M, Nishiyama K, Kohlhagen G, Takimoto C, Robey R, Pommier Y, Fojo T, Bates SE. (1999) Camptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res 59:5938–5946. [PubMed] [Google Scholar]
- Chen AY, Yu C, Potmesil M, Wall ME, Wani MC, Liu LF. (1991) Camptothecin overcomes MDR1-mediated resistance in human KB carcinoma cells. Cancer Res 51:6039–6044. [PubMed] [Google Scholar]
- Creemers GJ, Gerrits CJ, Eckardt JR, Schellens JH, Burris HA, Planting AS, Rodriguez GI, Loos WJ, Hudson I, Broom C, et al. (1997) Phase I and pharmacologic study of oral topotecan administered twice daily for 21 days to adult patients with solid tumors. J Clin Oncol 15:1087–1093. [DOI] [PubMed] [Google Scholar]
- Creemers GJ, Lund B, Verweij J. (1994) Topoisomerase I inhibitors: topotecan and irenotecan. Cancer Treat Rev 20:73–96. [DOI] [PubMed] [Google Scholar]
- Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. (2008) ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 39:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NC, Santana VM, Iacono LC, Furman WL, Hawkins DR, Houghton PJ, Panetta JC, Gajjar AJ, Stewart CF. (2004) Phase I and pharmacokinetic study of topotecan administered orally once daily for 5 days for 2 consecutive weeks to pediatric patients with refractory solid tumors. J Clin Oncol 22:829–837. [DOI] [PubMed] [Google Scholar]
- DeMario MD, Ratain MJ. (1998) Oral chemotherapy: rationale and future directions. J Clin Oncol 16:2557–2567. [DOI] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. (2007) P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res 13:6440–6449. [DOI] [PubMed] [Google Scholar]
- Devriese LA, Witteveen PE, Mergui-Roelvink M, Smith DA, Lewis LD, Mendelson DS, Bang YJ, Chung HC, Dar MM, Huitema AD, et al. (2015) Pharmacodynamics and pharmacokinetics of oral topotecan in patients with advanced solid tumours and impaired renal function. Br J Clin Pharmacol 80:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffner PK, Cohen ME, Anderson SW, Voorhess ML, MacGillivray MH, Panahon A, Brecher ML. (1983) Long-term effects of treatment on endocrine function in children with brain tumors. Ann Neurol 14:528–532. [DOI] [PubMed] [Google Scholar]
- Erlichman C, Boerner SA, Hallgren CG, Spieker R, Wang XY, James CD, Scheffer GL, Maliepaard M, Ross DD, Bible KC, et al. (2001) The HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7- ethyl-10-hydroxycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug efflux. Cancer Res 61:739–748. [PubMed] [Google Scholar]
- Gajjar A, Mulhern RK, Heideman RL, Sanford RA, Douglass EC, Kovnar EH, Langston JA, Jenkins JJ, Kun LE. (1994) Medulloblastoma in very young children: outcome of definitive craniospinal irradiation following incomplete response to chemotherapy. J Clin Oncol 12:1212–1216. [DOI] [PubMed] [Google Scholar]
- Guichard S, Montazeri A, Chatelut E, Hennebelle I, Bugat R, Canal P. (2001) Schedule-dependent activity of topotecan in OVCAR-3 ovarian carcinoma xenograft: pharmacokinetic and pharmacodynamic evaluation. Clin Cancer Res 7:3222–3228. [PubMed] [Google Scholar]
- Hande KR, Krozely MG, Greco FA, Hainsworth JD, Johnson DH. (1993) Bioavailability of low-dose oral etoposide. J Clin Oncol 11:374–377. [DOI] [PubMed] [Google Scholar]
- Hendricks CB, Rowinsky EK, Grochow LB, Donehower RC, Kaufmann SH. (1992) Effect of P-glycoprotein expression on the accumulation and cytotoxicity of topotecan (SK&F 104864), a new camptothecin analogue. Cancer Res 52:2268–2278. [PubMed] [Google Scholar]
- Houghton PJ, Cheshire PJ, Hallman JD, 2nd, Lutz L, Friedman HS, Danks MK, Houghton JA. (1995) Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol 36:393–403. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Cheshire PJ, Myers L, Stewart CF, Synold TW, Houghton JA. (1992) Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharmacol 31:229–239. [DOI] [PubMed] [Google Scholar]
- Hsiang YH, Liu LF. (1988) Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 48:1722–1726. [PubMed] [Google Scholar]
- Karajannis M, Allen JC, Newcomb EW. (2008) Treatment of pediatric brain tumors. J Cell Physiol 217:584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury WD, Boehm JC, Jakas DR, Holden KG, Hecht SM, Gallagher G, Caranfa MJ, McCabe FL, Faucette LF, Johnson RK, et al. (1991) Synthesis of water-soluble (aminoalkyl)camptothecin analogues: inhibition of topoisomerase I and antitumor activity. J Med Chem 34:98–107. [DOI] [PubMed] [Google Scholar]
- Kretschmar CS, Kletzel M, Murray K, Thorner P, Joshi V, Marcus R, Smith EI, London WB, Castleberry R. (2004) Response to paclitaxel, topotecan, and topotecan-cyclophosphamide in children with untreated disseminated neuroblastoma treated in an upfront phase II investigational window: a pediatric oncology group study. J Clin Oncol 22:4119–4126. [DOI] [PubMed] [Google Scholar]
- Kuhn E, Lavielle M. (2005) Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 49:1020–1038. [Google Scholar]
- Kwan P, Wong V, Ng PW, Lui CH, Sin NC, Wong KS, Baum L. (2011) Gene-wide tagging study of the association between ABCC2, ABCC5 and ABCG2 genetic polymorphisms and multidrug resistance in epilepsy. Pharmacogenomics 12:319–325. [DOI] [PubMed] [Google Scholar]
- Laughton SJ, Merchant TE, Sklar CA, Kun LE, Fouladi M, Broniscer A, Morris EB, Sanders RP, Krasin MJ, Shelso J, et al. (2008) Endocrine outcomes for children with embryonal brain tumors after risk-adapted craniospinal and conformal primary-site irradiation and high-dose chemotherapy with stem-cell rescue on the SJMB-96 trial. J Clin Oncol 26:1112–1118. [DOI] [PubMed] [Google Scholar]
- Léger F, Loos WJ, Fourcade J, Bugat R, Goffinet M, Mathijssen RH, Verweij J, Sparreboom A, Chatelut E. (2004) Factors affecting pharmacokinetic variability of oral topotecan: a population analysis. Br J Cancer 90:343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, Floot BG, Schellens JH. (1999) Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res 59:4559–4563. [PubMed] [Google Scholar]
- Maliepaard M, van Gastelen MA, Tohgo A, Hausheer FH, van Waardenburg RC, de Jong LA, Pluim D, Beijnen JH, Schellens JH. (2001) Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res 7:935–941. [PubMed] [Google Scholar]
- Minturn JE, Janss AJ, Fisher PG, Allen JC, Patti R, Phillips PC, Belasco JB. (2011) A phase II study of metronomic oral topotecan for recurrent childhood brain tumors. Pediatr Blood Cancer 56:39–44. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. (2004) Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol 5:399–408. [DOI] [PubMed] [Google Scholar]
- Nitschke R, Parkhurst J, Sullivan J, Harris MB, Bernstein M, Pratt C. (1998) Topotecan in pediatric patients with recurrent and progressive solid tumors: a Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol 20:315–318. [DOI] [PubMed] [Google Scholar]
- Pratt CB, Stewart C, Santana VM, Bowman L, Furman W, Ochs J, Marina N, Kuttesch JF, Heideman R, Sandlund JT, et al. (1994) Phase I study of topotecan for pediatric patients with malignant solid tumors. J Clin Oncol 12:539–543. [DOI] [PubMed] [Google Scholar]
- Samson A, Lavielle M, Mentre F. (2006) Extension of the SAEM algorithm to left-censored data in nonlinear mixed-effects model: Application to HIV dynamics model. Comput Stat Data Anal 51:1562–1574. [Google Scholar]
- Santana VM, Zamboni WC, Kirstein MN, Tan M, Liu T, Gajjar A, Houghton PJ, Stewart CF. (2003) A pilot study of protracted topotecan dosing using a pharmacokinetically guided dosing approach in children with solid tumors. Clin Cancer Res 9:633–640. [PubMed] [Google Scholar]
- Schellens JH, Creemers GJ, Beijnen JH, Rosing H, de Boer-Dennert M, McDonald M, Davies B, Verweij J. (1996) Bioavailability and pharmacokinetics of oral topotecan: a new topoisomerase I inhibitor. Br J Cancer 73:1268–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263. [PubMed] [Google Scholar]
- Stewart CF, Ratain MJ. (1997) Topoisomerase interactive agents, in Cancer: Principles and Practice of Oncology (DeVita VT, Jr, Hellman S, Rosenberg SA, eds) pp 452–467, Lippincott-Raven, Philadelphia. [Google Scholar]
- Takimoto CH, Wright J, Arbuck SG. (1998) Clinical applications of the camptothecins. Biochim Biophys Acta 1400:107–119. [DOI] [PubMed] [Google Scholar]
- Taudou G, Portemer C, Jaxel C, Duguet M. (1993) Inhibition of DNA synthesis and DNA fragmentation in stimulated splenocytes by the concerted action of topoisomerase I and II poisons. Biochem Pharmacol 45:331–337. [DOI] [PubMed] [Google Scholar]
- Tubergen DG, Stewart CF, Pratt CB, Zamboni WC, Winick N, Santana VM, Dryer ZA, Kurtzberg J, Bell B, Grier H, et al. (1996) Phase I trial and pharmacokinetic (PK) and pharmacodynamics (PD) study of topotecan using a five-day course in children with refractory solid tumors: a pediatric oncology group study. J Pediatr Hematol Oncol 18:352–361. [DOI] [PubMed] [Google Scholar]
- Turner PK, Iacono LC, Panetta JC, Santana VM, Daw NC, Gajjar A, Stewart CF. (2006) Development and validation of limited sampling models for topotecan lactone pharmacokinetic studies in children. Cancer Chemother Pharmacol 57:475–482. [DOI] [PubMed] [Google Scholar]
- Wagner S, Erdlenbruch B, Längler A, Gnekow A, Kühl J, Albani M, Völpel S, Bucsky P, Emser A, Peters O, et al. (2004) Oral topotecan in children with recurrent or progressive high-grade glioma: a Phase I/II study by the German Society for Pediatric Oncology and Hematology. Cancer 100:1750–1757. [DOI] [PubMed] [Google Scholar]
- Walter AW, Mulhern RK, Gajjar A, Heideman RL, Reardon D, Sanford RA, Xiong X, Kun LE. (1999) Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children’s Research Hospital. J Clin Oncol 17:3720–3728. [DOI] [PubMed] [Google Scholar]
- Yanni SB, Smith PB, Benjamin DK, Jr, Augustijns PF, Thakker DR, Annaert PP. (2011) Higher clearance of micafungin in neonates compared with adults: role of age-dependent micafungin serum binding. Biopharm Drug Dispos 32:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni WC, Bowman LC, Tan M, Santana VM, Houghton PJ, Meyer WH, Pratt CB, Heideman RL, Gajjar AJ, Pappo AS, et al. (1999) Interpatient variability in bioavailability of the intravenous formulation of topotecan given orally to children with recurrent solid tumors. Cancer Chemother Pharmacol 43:454–460. [DOI] [PubMed] [Google Scholar]
- Zhou XJ, Zhou-Pan XR, Favre R, Rahmani R. (1994) Relative bioavailability of two oral formulations of navelbine in cancer patients. Biopharm Drug Dispos 15:577–586. [DOI] [PubMed] [Google Scholar]