Abstract
Determining appropriate pharmacotherapy in young children can be challenging due to uncertainties in the development of drug disposition pathways. With knowledge of the ontogeny of drug-metabolizing enzymes and an emerging focus on drug transporters, the developmental pattern of the uptake transporters organic anion transporting polypeptide (OATP) 1B1 and 1B3 was assessed by relative protein quantification using Western blotting in 80 human pediatric liver specimens covering an age range from 9 days to 12 years. OATP1B3 exhibited high expression at birth, which declined over the first months of life, and then increased again in the preadolescent period. In comparison with children 6–12 years of age, the relative protein expression of highly glycosylated (total) OATP1B3 was 235% (357%) in children <3 months of age, 33% (64%) in the age group from 3 months to 2 years, and 50% (59%) in children 2–6 years of age. The fraction of highly glycosylated to total OATP1B3 increased with age, indicating ontogenic processes not only at the transcriptional level but also at the post-translational level. Similar to OATP1B3, OATP1B1 showed high interindividual variability in relative protein expression but no statistically significant difference among the studied age groups.
Introduction
Pharmacotherapy in young children is a very challenging proposition due to the intricate pattern of their physiologic development. This developmental pattern includes changes in every body system from the time of birth through adulthood. Many of these changes have an impact on drug disposition (Kearns et al., 2003; van den Anker et al., 2011). In the last 2 decades, much work has been focused on elucidating the ontogeny of drug-metabolizing enzymes, including phase I enzymes such as the cytochrome P450 enzyme subfamilies CYP3A, CYP2D, and CYP1A, as well as phase II enzymes, such as sulfotransferases, glutathione S-transferases, and UDP glucuronosyltransferases (Hines, 2008). One component of drug disposition that has so far received limited attention in terms of developmental expression is the area of drug transport, which has been highlighted and summarized in the recently published recommendations by the National Institutes of Health Pediatric Transporter Working Group (Brouwer et al., 2015).
Organic anion transporting polypeptide (OATP) is a family of transmembrane transport proteins that are responsible for the uptake of substances into the cells of a variety of organs. OATP1B1 (encoded by SLCO1B1) and OATP1B3 (encoded by SLCO1B3) are members of the OATP family that are located primarily on the basolateral surface of hepatocytes. These two transporters share approximately 80% sequence identity, resulting in an overlapping substrate profile (Hagenbuch and Gui, 2008). Endogenous substrates for OATP1B1 and OATP1B3 include bilirubin, bile salts, thyroid hormones, and steroid sex hormones and their conjugates, whereas exogenous substrates include 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, angiotensin II receptor antagonists, rifampicin, and methotrexate (Hagenbuch and Gui, 2008; Giacomini et al., 2010). The important role of OATP1B1 for systemic pharmacokinetics and treatment outcome has recently been highlighted for chemotherapy in pediatric patients with acute myeloid leukemia (Drenberg et al., 2015). In this study, patients homozygous for a SLCO1B1 genetic variant had significantly more favorable survival outcomes, likely because of reduced clearance and thus increased exposure to chemotherapeutic agents that are OATP1B1substrates.
Although it has been reported that the rodent ortholog of OATP1B1 and OATP1B3, Oatp1b2, exhibits a differential expression pattern based on the age of the rodent (Cheng et al., 2005), there is a paucity of similar information on the human transporters OATP1B1 and OATP1B3. Thus, the purpose of this article was to examine the ontogenic patterns of OATP1B1 and OATP1B3 protein expression in pediatric livers.
Materials and Methods
Pediatric Liver Specimens.
Post-mortem liver specimens (n = 48) from individuals 0.025–12 years of age were obtained from the Brain & Tissue Bank for Developmental Disorders, University of Maryland at Baltimore and University of Miami (Eunice Kennedy Shriver National Institute of Child Health and Human Development contracts N01-HD-8-3283 and N01-HD-8-3284) (Koukouritaki et al., 2002). Liver samples from living organ donors (n = 32), 0.25–12 years of age, were obtained from the Liver Tissue Procurement and Distribution System (Pittsburgh, PA; National Institutes of Health Contract N01-DK-9-2310). Basic demographic information, including age, sex, race, and post-mortem interval as appropriate, was available for most samples used in the study, and is summarized in Tables 1 and 2. Besides major diseases and cause of death, no other sample identifiers were available. Samples from individuals with disease conditions that potentially could involve liver damage were not included in the study. Tissue samples were stored at −70°C until analysis. The study was approved by the Institutional Review Board of the University of Tennessee Health Science Center.
TABLE 1.
Demographic information of study individuals
| Demographic Information | Values |
|---|---|
| Postnatal Age (N) | 80 |
| Age Range | 9 days – 12 yr |
| Mean Age (SD) | 3.53 yr (3.86 yr) |
| Sex (N) | |
| Male | 43 |
| Female | 27 |
| Unknown | 10 |
| Race (N) | |
| Caucasian | 43 |
| African-American | 20 |
| Other | 5 |
| Unknown | 12 |
| Source (N) | |
| Living Donor | 32 |
| Post Mortem | 48 |
TABLE 2.
Relative OATP1B protein expression in different age groups investigated in this study
| Age Groups |
||||
|---|---|---|---|---|
| A | B | C | D | |
| Postnatal Age Range | <3 mo | 3 mo to <2 yr | 2 to <6 yr | 6–12 yr |
| N | 20 | 21 | 17 | 22 |
| Mean age (yr) | 0.128 | 0.715 | 3.73 | 9.14 |
| S.D. (yr) | 0.067 | 0.412 | 1.13 | 1.67 |
| Highly Glycosylated OATP1B3 | ||||
| Geometric Mean | 1.49**B,*C | 0.210*D | 0.320 | 0.637 |
| 25th to 75th percentile | 0.801–2.79 | 0.028–1.05 | 0.267–0.763 | 0.223–3.31 |
| Relative to Group D | 235% | 33% | 50% | 100% |
| Core-Glycosylated OATP1B3 | ||||
| Geometric Mean | 3.59**B,**C,*D | 0.495 | 0.399 | 0.710 |
| 25th to 75th percentile | 2.41–5.11 | 0.240–2.17 | 0.319–1.11 | 0.471–2.08 |
| Relative to Group D | 505% | 70% | 56% | 100% |
| Total OATP1B3 | ||||
| Geometric Mean | 5.31**B,**C,*D | 0.956 | 0.873 | 1.49 |
| 25th to 75th percentile | 3.33–7.86 | 0.412–3.37 | 0.720–2.21 | 0.671–4.95 |
| Relative to Group D | 357% | 64% | 59% | 100% |
| OAPT1B1 | ||||
| Geometric Mean | 0.0535 | 0.0445 | 0.0277 | 0.139 |
| 25th to 75th percentile | 0.0045–0.296 | 0.0107–0.196 | 0.0155–0.0947 | 0.0275–0.871 |
| Relative to Group D | 38% | 32% | 20% | 100% |
Statistically significant differences are marked with asterisks (*P ≤ 0.05 and **P < 0.01); superscript letters denote the comparison group.
Relative Protein Quantification.
Membrane proteins from the post-mortem samples were isolated using the ProteoExtract Native Membrane Protein Extraction kit per manufacturer protocol (Calbiochem, La Jolla, CA). Membrane proteins from the living-donor samples were isolated using ultracentrifugation. Briefly, approximately 2 g of frozen liver tissue was quickly thawed and homogenized in 10 ml of homogenization buffer consisting of 0.1 M Tris, pH 7.4, 0.1 M potassium chloride, 0.02 mM butylated hydroxytoluene, and 1 mM EDTA. The homogenate was centrifuged at 12,000g for 15 minutes at 4°C. The supernatant was discarded, and the remaining pellet, consisting of membrane proteins, was resuspended in 5 ml of membrane storage buffer, consisting of 20% glycerol, 1 mM dithiothreitol, 0.02 mM butylated hydroxytoluene, and 0.1 potassium phosphate, pH 7.25. Aliquots of the membrane protein were prepared and stored at −70°C until further processing.
Total protein concentration was determined using a protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard (Bradford, 1976). Protein was separated using SDS-PAGE after loading 15 µg of total protein in each well. After separation, protein was transferred to an Invitrolon polyvinylidene difluoride membrane (ThermoFisher Scientific, Grand Island, NY) overnight at 30 V on ice. Membranes were incubated with Tris-buffered saline/0.5% Tween 20 (TBST) and 5% nonfat dried milk (TBST-M) for 3 hours. OATP1B1 and OATP1B3 were detected using a murine, monoclonal anti-OATP1B antibody, mMDQ (Novus Biologicals, Littleton, CO), incubated at a 1:33 dilution overnight at 4°C with fresh TBST-M. mMDQ had been raised against a synthetic peptide antigen that is common to both OATP1B1 and OATP1B3. It has been used widely and been characterized in detail with regard to its ability to detect both OATP1B1 and OATP1B3 (Cui et al., 2003; van de Steeg et al., 2012). Subsequently, the membrane was washed three times in TBST. The membrane was then incubated in fresh TBST-M with a horseradish peroxidase–conjugated horse anti-mouse IgG (Cell Signaling Technology, Danvers, MA) secondary antibody at 1:2500 dilution for 2 hours. After washing the membrane three times with TBST, enhanced chemiluminescence Amersham ECL Plus (GE Healthcare Life Sciences, Piscataway, NJ) was used for visualization of the proteins with exposure to Kodak BioMax XAR autoradiography film (Sigma-Aldrich, St. Louis, MO). The films were digitized using a CanoScan LiDE30 Scanner (Canon, Melville, NY). Digitized images were then analyzed in ImageJ (National Institutes of Health, Bethesda, MD).
Membranes were stripped using Restore Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL) and washed two times with phosphate-buffered saline/0.1% Tween 20 (PBST). Membranes were then incubated in PBST with 5% nonfat dried milk for 1 hour. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein was detected using a murine anti-GAPDH antibody (Sigma-Aldrich) incubated at 1:2500 for 2 hours with fresh PBST with 5% nonfat dried milk. The remaining steps of detecting GAPDH protein were the same as those for detecting OATP1B1 and OATP1B3.
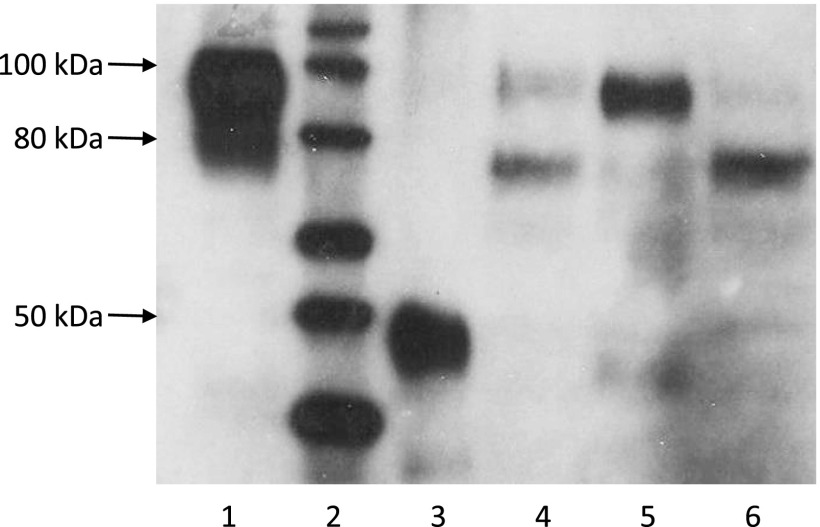
All OATP1B protein expression values were normalized to GAPDH expression within each sample. Each Western blot experiment included a protein ladder consisting of nine recombinant proteins of known molecular weight (MagicMark XP Western Protein Standard; Life Technologies, Carlsbad, CA), as well as two commercially available positive controls. For OATP1B3, the positive control was the lysate of OATP1B3 overexpressing HEK293 cells (Novus Biologicals). For OATP1B1, the positive control was an adult human liver membrane lysate (Abcam, Cambridge, MA). Comparison of the ratios between OATP1B1 or OATP1B3 and GAPDH for these positive controls across blots was used to control for consistency between blots. A representative Western blot, including these controls and several pediatric liver samples is displayed in Fig. 1.
Fig. 1.
Representative Western blot analysis of OATP1B1 and OAT1B3 in pediatric liver specimens. Lane 1, positive control for OATP1B3; lane 2, molecular ladder; lane 3, positive control for OATP1B1; lanes 4–6, pediatric liver specimens.
Statistical Analysis.
All statistical analyses were performed using SPSS version 23 (IBM, Armonk, NY). Relative protein expression among groups was compared by one-way analysis of variance with Bonferroni correction for post hoc comparisons after logarithmic transformation. Associations between select continuous variables were investigated by the Pearson product moment correlation. All P values ≤0.05 were considered to be significant.
Results
The ontogeny of OATP1B transporters was assessed with 80 pediatric liver specimens, with 32 obtained from living donors (biopsy specimen) and 48 obtained post mortem. The ages of sample donors ranged from 9 days to 12 years. General characteristics of the sample donors can be found in Table 1. For statistical analysis, samples were divided into the following four groups based on age: group A comprised samples from donors <3 months of age; group B comprised samples from donors 3 months to <2 years of age; group C comprised samples from donors 2 to <6 years of age; and group D comprised samples from donors 6-12 years of age (Table 2). As there was no difference in OATP1B expression detected between post-mortem and living donor samples, results are presented for the combined sample set. Similarly, no expression differences were detected based on sex, race, or the post-mortem sampling interval.
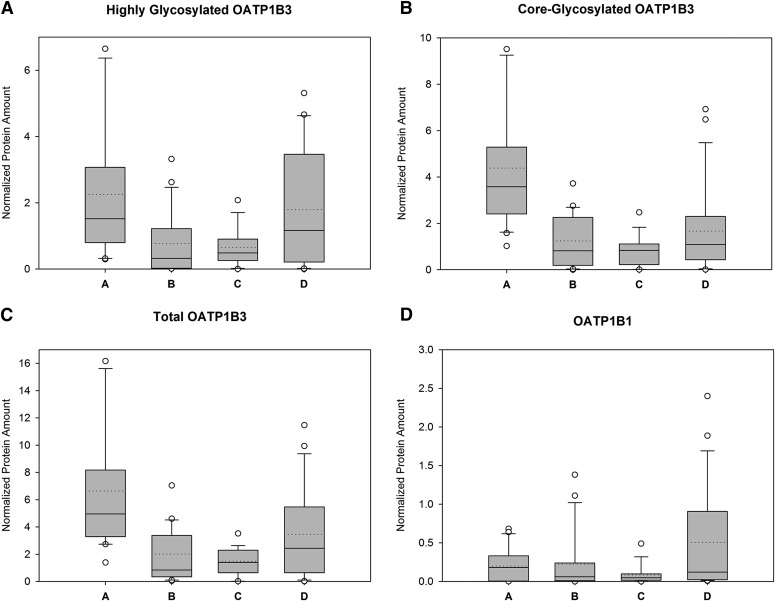
Western blot analysis with the OATP1B mMDQ antibody revealed one band representing OATP1B1 and two bands representing OATP1B3, as indicated in Fig. 1. Using the same primary mMDQ antibody and OATP1B3-overexpressing HEK293 cells as in our study as well as human liver membrane preparations, Cui et al. (2003) observed the same two bands for OATP1B3 and attributed them to a highly glycosylated and a core-glycosylated form of OATP1B3, which was confirmed by deglycosylation studies (Konig et al., 2000; Cui et al., 2001). The concurrent detection of highly glycosylated and core-glycosylated OATP1B3 is not surprising as OATP1B3 has been reported to be extensively glycosylated, and similar observations have been reported by others (Schwarz et al., 2011). Although one might expect that post-translational modifications such as glycosylation play an important role for the intracellular localization and/or activity of OATP1B3, information on its function have so far been scarce, and thus we report highly glycosylated, core-glycosylated, and total (highly glycosylated and core-glycosylated) OATP1B3, as summarized in Table 2 and Fig. 2.
Fig. 2.
Relative OATP1B protein expression (normalized to GAPDH) in pediatric liver for different age groups (group A, <3 months; group B, 3 months to <2 years; group C, 2 to <6 years; group D, 6–12 years), as follows: highly glycosylated OATP1B3 (A); core-glycosylated OATP1B3 (B); total (highly glycosylated and core-glycosylated) OATP1B3 (C); and OATP1B1 (D). Boxes represent the interquartile range, whiskers represent the 10th and 90th percentiles. The solid horizontal line in the box denotes the median, and the dotted line represents the mean of the data.
For OATP1B3, the relative expression of highly glycosylated protein in age group A was approximately seven times higher than that in age group B (P = 0.002) and four times higher than that in age group C (P = 0.036). The expression of highly glycosylated OATP1B3 protein in age group A was on average more than twice as high (235%) as that in age group D, whereas age groups B and C had only 33% and 50% of the expression in age group D, but only the difference between groups B and D reached statistical significance (P = 0.050). The expression of core-glycosylated OATP1B3 protein in age group A was 505% of that in group D (P = 0.013), and was also seven times higher than that in group B (P = 0.002) and nine times higher than that in group C (P = 0.001). Groups B and C had geometric mean expression levels for core-glycosylated OATP1B3 of 70% and 56% relative to group D, but those differences did not reach statistical significance. Total OATP1B3 protein expression relative to group D was 357% in group A, 64% in group B, and 59% in group C, with significant differences between group A and all other groups.
For OATP1B1, the average expressions in groups A, B, and C were 38%, 32%, and 20% of group D, but did not reach statistical significance (P = 0.12), likely due to large interindividual expression differences in each group, which are in line with previous reports (Nies et al., 2013).
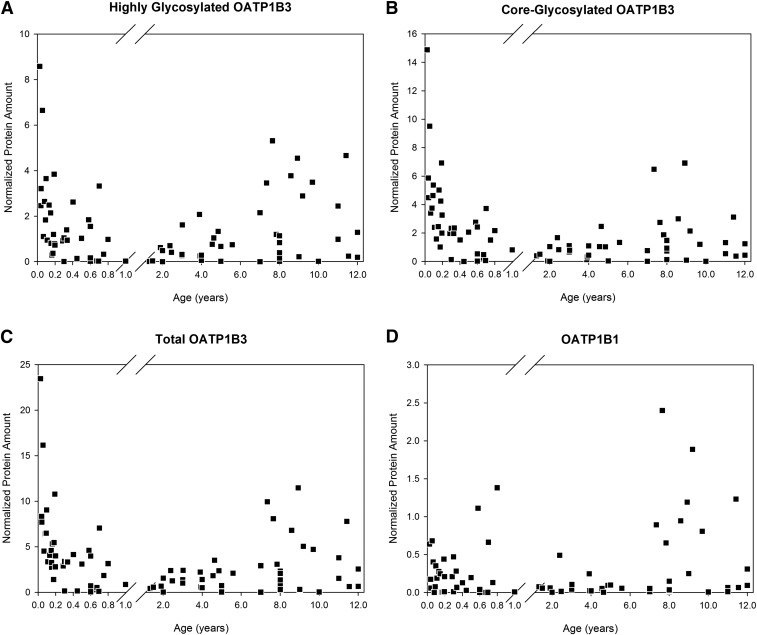
Figure 3 presents the individual expression versus age for highly glycosylated, core-glycoslated, and total OATP1B3, as well as for OATP1B1. Despite high variability, a clear developmental pattern emerges for OATP1B3, with the highest expression at the youngest ages early after birth, followed by a low but steady expression level until the prepubescent period, when OATP1B3 protein expression starts to increase again. In contrast, OATP1B1 expression was independent of age within the studied age range.
Fig. 3.
Relative OATP1B protein expression (normalized to GAPDH) in pediatric liver versus postnatal age. The x-axis is discontinuous, with ages less than 1 year shown as a fraction. Highly glycosylated OATP1B3 (A), core-glycosylated OATP1B3 (B), total (highly glycosylated and core-glycosylated) OATP1B3 (C), and OATP1B1 (D).
Discussion
Ontogeny patterns of drug-metabolizing enzymes fit into one of three categories, and as drug-metabolizing enzymes and transporters are coregulated, one might expect a similar behavior for transport proteins (Xu et al., 2005). The first category is characterized by high expression during gestation with low to no expression after 1 year of age. Examples of this pattern of expression include the phase I metabolizing enzymes CYP3A7 and flavin-containing monooxygenase 1, as well as, phase II metabolizing enzymes sulfotransferase (SULT) 1A3/4 and SULT1E1 (Koukouritaki et al., 2002; Hines, 2008). The second ontogeny pattern is characterized by relatively stable expression of the protein. Drug-metabolizing enzymes that exemplify this pattern include CYP3A5 and SULT1A1 (Hines, 2008). The current study failed to show a statistically significant correlation between OATP1B1 protein expression and age, indicating that the expression of the OATP1B1 transport protein likely fits into this second pattern of ontogeny. Finally, the third ontogeny pattern is characterized by no to low expression during gestation, with expression increasing postnatally (Hines, 2008). This third category appears to be the most vast, with many examples, including CYP1A2, CYP2C19, CYP2E1, CYP3A4, flavin-containing monooxygenase 3, and SULT2A1 (Koukouritaki et al., 2002, 2004; Hines, 2008). This third category also appears to be the most diverse in terms of the rate at which expression levels increase, with some of the drug-metabolizing enzymes achieving adult levels in the neonatal period and others not reaching that level of expression until after 1 year of age (Hines and McCarver, 2002). OATP1B1 might potentially also fit into this category as there was a trend toward lower expression at an earlier age, although this did not reach statistical significance, potentially due to the observed high interindividual variability.
Although the data for OATP1B1 can be ascribed to one of these described categories of ontogeny, the ontogeny pattern of the OATP1B3 protein is intriguing and unexpected. As can be seen in Fig. 3, the expression for OATP1B3 starts out high at birth with a fairly rapid decrease in expression during the first few months of life. This may be explained in part by looking at the normal physiology of a neonate. There are many endogenous substrates that are elevated in the neonate compared with the adult, including bilirubin and bile acids. The rate of bilirubin production is approximately two times greater in neonates than in adults and drops to roughly adult values within 2 weeks after birth (Porter and Dennis, 2002; Geaghan, 2011). The period in which the bilirubin production is high but starts to decrease, overlaps the same age range in which OATP1B3 expression is elevated in the current study. Bile acid production is known to start increasing during the end of gestation in the fetus (Nishiura et al., 2010). Since bilirubin and many bile salts are substrates for OATP1B3, it is possible that the increased presence of circulating bilirubin and bile salts induces SLCO1B3 expression.
Increased OAT1B3 expression could be modulated by one of the nuclear receptors, in particular the farnesoid X receptor (FXR). Bile acids are the endogenous substrate for FXR and modulate FXR expression to maintain bile acid homeostasis (Jung et al., 2002; Jung et al., 2007; Jonker et al., 2012). When bile acids do accumulate, the FXR pathway seems to up-regulate OATP1B3 expression and down-regulate OATP1B1 expression, although the latter seems to be limited to highly pathologic bile salt concentrations (Jung et al., 2007). In this study, OATP1B3 protein expression was elevated by approximately 200% in individuals less than 3 months of age compared with those 6–12 years of age, which is consistent with the FXR bile acid activation. OATP1B1 protein expression was not statistically lower in the samples from the youngest donors, although the average expression tended to be lower than that in individuals who were 6–12 years of age. Whether the bile acid insult has not been large enough to result in FXR-mediated OATP1B1 down-regulation or whether the tendency to lower the average expression in the youngest age group already indicates such a downregulation remains unclear. In rodent models, it has been suggested that the nuclear receptors, constitutive androstane receptor and pregnane X receptor, also are activated by bile acids and bilirubin, but that constitutive androstane receptor in particular may not be expressed in the neonate, showing the third ontogeny pattern previously described (Huang et al., 2003; Wagner et al., 2005). These regulatory pathways provide another possible explanation for the apparent activation of OATP1B3 and the lack thereof of OATP1B1 in the neonate.
The OATP1B3 expression pattern has a second increase during the preadolescent period, which may also be explained by substrate-mediated induction. Endogenous substrates for this transporter include sex steroids, including estrone-3-sulfate, estradiol 17-β-glucuronide, and dihydroepiandrosterone 3-sulfate (Meyer zu Schwabedissen and Kim, 2009). Endogenous levels of these substrates are expected to start increasing as a child approaches puberty, which tends to fall into age group D in our study in which increased OATP1B3 expression was observed (Sorensen et al., 2012).
Comparison between the highly glycosylated and core-glycosylated forms of OATP1B3 indicated a weak correlation between the fraction that is highly glycosylated and age (Pearson’s correlation coefficient = 0.377, P = 0.001), with average fractions of 31.2%, 32.7%, 43.3%, and 50.4%, respectively, in age groups A, B, C, and D. Differences of A versus D and B versus D were statistically significant (P < 0.05). Post-translational modification by glycosylation has been suggested to affect intracellular localization and the function of some transporters (Hardikar et al., 1995; Yao et al., 2012), including localization to the basolateral membrane and the transport function of OATP1B3 (Letschert et al., 2004; Schwarz et al., 2011). As the ratio of glycosylated to nonglycosylated protein changes, our data suggest that, in addition to protein expression, age-associated differences in post-translational modifications, such as glycosylation, may have an effect on the developmental pattern of OATP1B3 function. Future studies, however, will need to delineate the impact of differences in glycosylation on the functional activity of OATP1B3.
Two publications have recently addressed certain aspects of the age-associated expression of OATP1B1 and OATP1B3. Prasad et al. (2014) examined the protein expression of both transporters in 64 liver samples from donors 7–55 years of age. The investigators did not detect any age-associated expression, but only 6 of the investigated 64 individuals were below the age cutoff of 12 years of age used in this study. In addition, the investigators used a liquid chromatography–tandem mass spectrometry technique that removes all post-translational modifications such as glycosylation, and thus quantifies total rather than glycosylated OATP1B. Thus, our results are not inconsistent with the results reported by Prasad et al. (2014).
Mooij et al. (2014) investigated OATP1B1 and OATP1B3 mRNA expression in 45 liver specimens from fetal to adult age. Compared with the adult liver, OATP1B1 mRNA expression was 20-fold lower than that in fetal liver samples, 500-fold lower than that in neonates, and 90-fold lower than that in infants. The expression of hepatic OATP1B3 was 30-fold lower in fetuses, 600-fold lower in neonates, and 100-fold lower in infants than in adults. Given the fact that there is only a weak correlation, if there is any, between mRNA and protein expression for OATP1B1 and OATP1B3 (Nies et al., 2013), these findings are also not inconsistent with our results.
In summary, this study has shown that OATP1B3 protein expression shows an unusual pattern of development compared with those of other drug disposition proteins. Protein expression starts at a very high expression level throughout the neonatal phase, thereafter declining in the early childhood years. Then protein expression starts to increase again during the preadolescent period. OATP1B1 protein expression showed a pattern consistent with ontogeny category 2, with no association found between protein expressions across ages. Further understanding of the ontogeny of drug disposition pathways, including transport proteins, will contribute to the scientific basis for rational approaches to the improvement of pharmacotherapy in young children, including the currently widely used physiologically based modeling and simulation approaches (Laer et al., 2009; Barrett et al., 2012).
Abbreviations
- FXR
farnesoid X receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- OATP
organic anion transporting polypeptide
- PBST
phosphate-buffered saline/0.1% Tween 20
- SULT
sulfotransferase
- TBST
Tris-buffered saline/0.5% Tween 20
- TBST-M
Tris-buffered saline/0.5% Tween 20 and 5% nonfat dried milk
Authorship Contributions
Participated in research design: Thomson, Hines, Schuetz, Meibohm.
Conducted experiments: Thomson, Meibohm.
Performed data analysis: Thomson, Meibohm.
Wrote or contributed to the writing of the manuscript: Thomson, Hines, Schuetz, Meibohm.
Footnotes
This work was in part supported by the National Institutes of Health National Cancer Institute [Grant R01-CA-53106]; and the American Lebanese Syrian Associated Charities (ALSAC).
References
- Barrett JS, Della Casa Alberighi O, Läer S, Meibohm B. (2012) Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther 92:40–49. [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Brouwer KL, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, Meibohm B, Nigam SK, Rieder M, de Wildt SN, Pediatric Transporter Working Group (2015) Human ontogeny of drug transporters: review and recommendations of the Pediatric Transporter Working Group. Clin Pharmacol Ther 98:266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. (2005) Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (OATPs). Drug Metab Dispos 33:1062–1073. [DOI] [PubMed] [Google Scholar]
- Cui Y, König J, Keppler D. (2001) Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol Pharmacol 60:934–943. [DOI] [PubMed] [Google Scholar]
- Cui Y, König J, Nies AT, Pfannschmidt M, Hergt M, Franke WW, Alt W, Moll R, Keppler D. (2003) Detection of the human organic anion transporters SLC21A6 (OATP2) and SLC21A8 (OATP8) in liver and hepatocellular carcinoma. Lab Invest 83:527–538. [DOI] [PubMed] [Google Scholar]
- Drenberg CD, Paugh SW, Pounds SB, Shi L, Orwick SJ, Li L, Hu S, Gibson AA, Ribeiro RC, Rubnitz JE, et al. (2015) Inherited variation in OATP1B1 is associated with treatment outcome in acute myeloid leukemia. Clin Pharmacol Ther DOI: 10.1002/cpt.315 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geaghan SM. (2011) Critical values for the maternal-fetal unit, fetus, infant, child and adolescent: bilirubin reporting practice in North American Children’s Hospitals as a paradigm for critical value reporting assessment. Clin Biochem 44:483–484. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38:778–801. [DOI] [PubMed] [Google Scholar]
- Hardikar W, Ananthanarayanan M, Suchy FJ. (1995) Differential ontogenic regulation of basolateral and canalicular bile acid transport proteins in rat liver. J Biol Chem 270:20841–20846. [DOI] [PubMed] [Google Scholar]
- Hines RN. (2008) The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther 118:250–267. [DOI] [PubMed] [Google Scholar]
- Hines RN, McCarver DG. (2002) The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther 300:355–360. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, Moore DD. (2003) Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc Natl Acad Sci USA 100:4156–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Liddle C, Downes M. (2012) FXR and PXR: potential therapeutic targets in cholestasis. J Steroid Biochem Mol Biol 130:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Elferink MGL, Stellaard F, Groothuis GMM. (2007) Analysis of bile acid-induced regulation of FXR target genes in human liver slices. Liver Int 27:137–144. [DOI] [PubMed] [Google Scholar]
- Jung D, Podvinec M, Meyer UA, Mangelsdorf DJ, Fried M, Meier PJ, Kullak-Ublick GA. (2002) Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology 122:1954–1966. [DOI] [PubMed] [Google Scholar]
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. (2003) Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167. [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. (2000) Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275:23161–23168. [DOI] [PubMed] [Google Scholar]
- Koukouritaki SB, Manro JR, Marsh SA, Stevens JC, Rettie AE, McCarver DG, Hines RN. (2004) Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther 308:965–974. [DOI] [PubMed] [Google Scholar]
- Koukouritaki SB, Simpson P, Yeung CK, Rettie AE, Hines RN. (2002) Human hepatic flavin-containing monooxygenases 1 (FMO1) and 3 (FMO3) developmental expression. Pediatr Res 51:236–243. [DOI] [PubMed] [Google Scholar]
- Läer S, Barrett JS, Meibohm B. (2009) The in silico child: using simulation to guide pediatric drug development and manage pediatric pharmacotherapy. J Clin Pharmacol 49:889–904. [DOI] [PubMed] [Google Scholar]
- Letschert K, Keppler D, König J. (2004) Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8). Pharmacogenetics 14:441–452. [DOI] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Kim RB. (2009) Hepatic OATP1B transporters and nuclear receptors PXR and CAR: interplay, regulation of drug disposition genes, and single nucleotide polymorphisms. Mol Pharm 6:1644–1661. [DOI] [PubMed] [Google Scholar]
- Mooij MG, Schwarz UI, de Koning BA, Leeder JS, Gaedigk R, Samsom JN, Spaans E, van Goudoever JB, Tibboel D, Kim RB, de Wildt SN. (2014) Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab Dispos 42:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies AT, Niemi M, Burk O, Winter S, Zanger UM, Stieger B, Schwab M, Schaeffeler E. (2013) Genetics is a major determinant of expression of the human hepatic uptake transporter OATP1B1, but not of OATP1B3 and OATP2B1. Genome Med 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Kimura A, Yamato Y, Aoki K, Inokuchi T, Kurosawa T, Matsuishi T. (2010) Developmental pattern of urinary bile acid profile in preterm infants. Pediatr Int 52:44–50. [DOI] [PubMed] [Google Scholar]
- Porter ML, Dennis BL. (2002) Hyperbilirubinemia in the term newborn. Am Fam Physician 65:599–606. [PubMed] [Google Scholar]
- Prasad B, Evers R, Gupta A, Hop CE, Salphati L, Shukla S, Ambudkar SV, Unadkat JD. (2014) Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos 42:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz UI, Meyer zu Schwabedissen HE, Tirona RG, Suzuki A, Leake BF, Mokrab Y, Mizuguchi K, Ho RH, Kim RB. (2011) Identification of novel functional organic anion-transporting polypeptide 1B3 polymorphisms and assessment of substrate specificity. Pharmacogenet Genomics 21:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. (2012) Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr 77:137–145. [DOI] [PubMed] [Google Scholar]
- van den Anker JN, Schwab M, Kearns GL. (2011) Developmental pharmacokinetics. Handbook Exp Pharmacol 205:51–75. [DOI] [PubMed] [Google Scholar]
- van de Steeg E, Stránecký V, Hartmannová H, Nosková L, Hřebíček M, Wagenaar E, van Esch A, de Waart DR, Oude Elferink RP, Kenworthy KE, et al. (2012) Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest 122:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M. (2005) CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42:420–430. [DOI] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. (2005) Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res 28:249–268. [DOI] [PubMed] [Google Scholar]
- Yao J, Hong W, Huang J, Zhan K, Huang H, Hong M. (2012) N-Glycosylation dictates proper processing of organic anion transporting polypeptide 1B1. PLoS One 7:e52563. [DOI] [PMC free article] [PubMed] [Google Scholar]