Abstract
The lipodystrophies represent a class of diseases characterized by leptin deficiency. Leptin deficiency is associated with a severe form of the metabolic syndrome characterized by dyslipidemia, insulin resistance, diabetes, and ovarian dysfunction. Metreleptin is the pharmaceutical derived product that has been approved by the Food and Drug Administration (FDA) to treat the severe metabolic abnormalities of the generalized forms of lipodystrophy. Herein we describe the properties of metreleptin, its use in patients, which includes the administration of the drug and how it may be acquired by medical professionals as well as its safety, tolerability, and properties. Finally, we speculate on future uses and development of metreleptin.
Keywords: Lipodystrophy, leptin, metreleptin, metabolic syndrome, insulin resistance
Lipodystrophy
The lipodystrophies are a group of conditions characterized by the loss of adipose tissue and, usually, metabolic disturbance. They are subdivided into generalized and partial forms with further subdivision into congenital (gene mutations) or acquired (autoimmune); forms.
The generalized lipodystrophies are characterized by near-total loss of subcutaneous body fat. Some patients with generalized lipodystrophy have preservation of mechanical fat in the face, hands, and feet, while in others mechanical fat depots are preserved. In the partial forms of lipodystrophy there is typically preservation of fat particularly in the head, neck, and torso, and loss of fat in the extremities [1, 2]. The reason for this particular distribution is unknown. In both generalized and partial lipodystrophy there may be a marked increase in abdominal girth due to fatty infiltration of the liver. Most known gene mutations leading to lipodystrophy code for proteins involved in lipid droplet formation, but how this leads to the loss of fat cells is unknown. Due to our interest in insulin resistant syndromes, we have studied these patients for many years at the National Institutes of Health (NIH). Initially the studies focused on insulin receptor regulation [3], but more recently we have participated in collaborative studies identifying mutations [4–6].
Regardless of the genotype or phenotype of patients with lipodystrophy, they share common features of extreme insulin resistance, dyslipidemia, diabetes, and reproductive disorders related to hypothalamic dysfunction and/or polycystic ovarian syndrome (PCOS). These different complications can be seen in Table 1. Patients with both generalized and partial lipodystrophy are highly susceptible to pancreatitis and to non-alcoholic steatohepatitis (NASH) [7, 8]. The major therapeutic approaches in patients with lipodystrophy are those used in the related metabolic syndrome, and include, diet, insulin, and oral anti-diabetic and lipid lowering agents that are approved by the Food and Drug Administration (FDA). The major problem with this approach is that the metabolic disturbance is severe and does not respond well to these conventional therapies.
Table 1.
Metabolic complications of lipodystrophy
| Glucose metabolism |
| Insulin resistance |
| Diabetes |
| Acanthosisnigricans |
| Lipid metabolism |
| High triglycerides |
| Eruptive xanthomata |
| Pancreatitis |
| Nonalcoholic fatty liver disease (NAFLD) |
| Steatosis→ Nonalcoholic steatohepatitis (NASH)→ Cirrhosis |
| Reproductive |
| Polycystic ovarian syndrome (PCOS) |
| Abnormal gonadotropin secretion |
Conventional treatment of lipodystrophy
Standard therapies can be utilized in treating metabolic complications in patients with generalized lipodystrophy with modest effects. The fundamental treatment for patients with generalized lipodystrophy centers on diet. An ideal diet for children over the age of two is comprised of 55% carbohydrates, 20% protein, and 25% fat (1/3 monounsaturated, 1/3 polyunsaturated, 1/3 saturated fats). Children need appropriate caloric intake to support growth. This must be done with caution because excess saturated fat and carbohydrates may promote hyperlipidemia. In addition, excess caloric intake will lead to increased triglyceride synthesis, and potentially increased hepatic steatosis in these patients. The weight goals for growing children need to be tempered against the fact that they do not have adipose tissue, and thus their weight comparatively will be lower than the age-matched US cohort. Typically, the caloric need in patients with generalized lipodystrophy is 1800–2200 calories/day after peak growth has been achieved.
Regarding diabetes therapy, metformin has been used effectively in children as early as 6 months of age, although its use is off label in children under 10 years of age. Doses we employ are 40–60 milligram/kilogram/day (mg/kg/day) for children less than 30 kilogram (kg). For children and adults greater than 30 kg, doses of 2 grams (g) daily are well tolerated. Thiazolidinediones have been used with good results in patients with partial lipodystrophy, but have not been studied in generalized lipodystrophy [9]. Other hypoglycemic agents such as sulfonylureas, acarbose, or GLP-1 analogs have not been used extensively in lipodystrophy.
In cases where glycemic targets are unable to be met with oral agents, exogenous insulin is indicated. Typically, given the severity of insulin resistance, patients with lipoatrophic diabetes require >3 units/kg/day of insulin. Thus, concentrated U-500 insulin is the most efficient and effective form of insulin in patients with lipodystrophy. U-500 insulin is especially useful in patients who lack subcutaneous tissue and require large volumes of insulin. Insulin glargine should be avoided in these patients since it requires formation of a crystal structure in adipose tissue to provide its long acting property.
To treat the severe hypertriglyceridemia in patients with lipodystrophy, the standard therapies recommended are fibrates, statins, and omega-3 fatty acids.
For proteinurea, given the background of diabetes, medications such as angiotensin-converting enzyme (ACE) inhibitors are recommended to protect the kidneys.
In women with oligomenorrhea or amenorrhea, caution is recommended in the use of exogenous estrogen, as this may lead to a significant rise in blood triglycerides. In women needing contraception, barrier methods are best tolerated, but progesterone delivered through intrauterine devices (IUD) has also been useful with minimal adverse effects on blood triglyceride levels.
Discovery of leptin and its development as a pharmaceutical
Leptin was discovered by Dr. Jeffrey Friedman in 1994 [10]. Leptin is produced and secreted by fat cells and its concentration in blood is a function of the adipose tissue mass. While the existence of a circulating factor that regulates food intake had long been postulated by the work of Coleman in the ob/ob mouse [11], the identity of the protein now allowed for its production, and most importantly, its preparation as a pharmaceutical.
General characteristics of leptin
Leptin is a 16 kilodaltons (kDa) protein that consists of 167 amino-acids and has a 4-helix bundle motif, a structure seen in cytokines [12]. Leptin is primarily produced in adipose tissue to signal the status of energy reserves [13]. Circulating leptin levels are proportional to the amount of body fat. Leptin levels also change with nutritional state with a notable decrease during fasting [14]. Leptin is secreted in a pulsatile fashion and follows a circadian rhythm, with the highest levels between midnight and early morning and lowest levels at midafternoon [15]. Leptin levels are generally higher in women who have a higher percent body fat. Multiple other factors affect leptin expression and secretion, including glucose, insulin, glucocorticoids, and proinflammatory cytokines that all increase leptin production [16].
Leptin exerts its effect via binding to leptin receptors (ObR) that belong to the class I cytokine receptor family with a single membrane-spanning domain [12]. Leptin receptors are located in the central nervous system, especially in the hypothalamus, and several peripheral organs, including pancreatic islets, liver, skeletal muscle, and adipose tissue [12]. Leptin suppresses appetite, inhibits insulin secretion and lipogenesis, and stimulates lipolysis in the liver, just to mention a few of its actions [16].
Metreleptin is a recombinant human leptin analogue produced in E.coli that differs from native human leptin by the addition of a methionine residue at its amino terminus (http://www.myalept.com/pdfs/pi_myalept.pdf). Metreleptin is a 147 amino-acid chain composed of a non-glycosylated polypeptide with one disulfide bond between Cys-97 and Cys-147 and a molecular weight of approximately 16.15 kDa. There is limited data on pharmacokinetics of metreleptin in generalized lipodystrophy patients. Based on the metreleptin the Tmax of metreleptin was 4 hours (range: 2 to 8 hours) following single-dose administration of metreleptin in 5 lipodystrophy patients. Following a single subcutaneous dose of 0.01 to 0.3 mg/kg metreleptin in healthy subjects, the peak serum leptin concentration (Cmax) occurred at 4.0 to 4.3 hours, and the half-life was 3.8 to 4.7 hours. Preclinical data indicates that renal clearance is the major route of metreleptin elimination.
The mechanisms by which metreleptin corrects the metabolic derangements seen in lipodystrophy have largely been studied in rodent models [17]. In rodent studies, these actions appear to be mediated by the regulation of a number of transcription factors and other metabolic intermediates [16]. Thus, there are two general ways that leptin exerts its clinical effect: the first is to decrease energy intake. The second, shown thus far only in pair-fed rodents, is a direct action to decrease insulin resistance.
Leptin replacement in lipodystrophy
Following the failure of metreleptin treatment in clinical trials of patients with conventional obesity, in which circulating leptin levels are high due to the expanded fat mass [18], the focus shifted to use of metreleptin in conditions in which circulating leptin levels are low. In extremely rare patients with congenital leptin deficiency, metreleptin treatment normalized hyperphagia, body weight, and hypothalamic dysfunction [19, 20]. Lipodystrophy is another condition associated with low leptin levels, and because of our center for the study of syndromic forms of insulin resistance, we were well-positioned to examine the efficacy of metreleptin in this patient population. The lipodystrophies represent a human model of the common, obesity-associated metabolic syndrome, and present an opportunity for a personalized or precision therapy based on the defined target of hypoleptinemia [17].
From 2000 to 2014, 55 patients with generalized forms of lipodystrophy were treated with metreleptin at the Clinical Center of the NIH (Table 2). This study led to the FDA approval of metreleptin for the treatment of generalized forms of lipodystrophy (Table 3) in February, 2014. In the first seven patients treated at the NIH, and two additional patients contributed by Dr. A. Garg, it immediately became apparent that metreleptin was remarkably effective in controlling metabolic parameters that had not responded to conventional therapy [21]. Further studies over the ensuing years continued to validate this initial response [22–24]. A recent meta-analysis showed that metreleptin decreased fasting glucose (standardized mean difference [SMD] 0.75, range 0.36–1.13, P = 0.0001), HbA1c (SMD 0.49, range 0.17–0.81, P = 0.003), triglycerides (SMD 1.00, range 0.69–1.31, P < 0.00001), and liver volume (SMD 1.06, range 0.51–1.61, P = 0.0002) [25].
Table 2.
Types of lipodystrophy treated with metreleptin at the National Institutes of Health
| Genetic Forms of Lipodystrophy | ||
| Inheritance (genes) | Endogenous leptin level mean ± SD (range) (ng/mL) |
|
| Congenital generalized lipodystrophy (CGL) |
Autosomal recessive (AGPAT2, BSCL2) |
1.17 ± 0.8 (0.25–3.3) |
| Familial partial lipodystrophy (FPL) |
Usually autosomal dominant (LMNA, PPARγ, unknown) |
5.9 ± 3.0 (0.95–12.3) |
| Atypical progeria | Autosomal recessive (LMNA, ZMPSTE24, unknown) |
1.08 ± 0.5 (0.33–1.8) |
| Acquired Forms of Lipodystrophy | ||
| Acquired generalized lipodystrophy (AGL) |
- | 1.05 ± 0.7 (0.25–3.3) |
| Acquired partial lipodystrophy (APL) |
- | 7.5 ± 6.8 (0.61–16.9) |
Table 3.
Food and Drug Administration Review of Metreleptin
|
| Indications and Usage |
|
| Limitations of Use |
|
The overall responses to metreleptin are shown in Table 3. The initial response to metreleptin therapy reported by patients is a sense of satiety or satiation [26]. This may result in some degree of weight loss over the first 4–6 months of therapy, but there is usually a stabilization of weight thereafter [27]. Since patients with generalized lipodystrophy do not become obese, it is not always appreciated that they are extremely hyperphagic. The next major effects of metreleptin treatment are improvement in the marked dyslipidemia, including extreme elevation of triglycerides in serum and ectopic tissues [28], as well as insulin resistance and diabetes. As shown in Table 4 and Figure 1, metreleptin has major effects in improving these metabolic derangements. Further, in young women, metreleptin decreases androgen production and normalizes gonadotropin secretion [29]. Interestingly, metreleptin does not result in an elevation in the HDL levels which are very low in patients with generalized lipodystrophy. This is in contrast to treatments for the obesity related metabolic syndromes, which increase HDL levels reciprocally with reductions in triglycerides [30]. NASH is common in patients with generalized forms of lipodystrophy, and metreleptin has been shown to improve the NASH score in these patients and produce some degree of stabilization in patients with more advanced fibrosis. In addition, these patients may have proteinuric renal disease and the proteinuria significantly decreases with metreleptin therapy [31, 32].
Table 4.
Summary of Metreleptin Effects in Generalized Lipodystrophy
| Clinical Parameter | Major Effects |
|---|---|
| Appetite and body weight | Decreased |
| Insulin resistance | Decreased |
| Diabetes | Decreased A1C Decreased insulin doses |
| Hypertriglyceridemia | Decreased |
| Steatohepatitis | Improved |
| Reproduction | Normalized menstrual cycles Increased fertility |
| Kidney disease | Decreased hyperfiltration Decreased protein excretion |
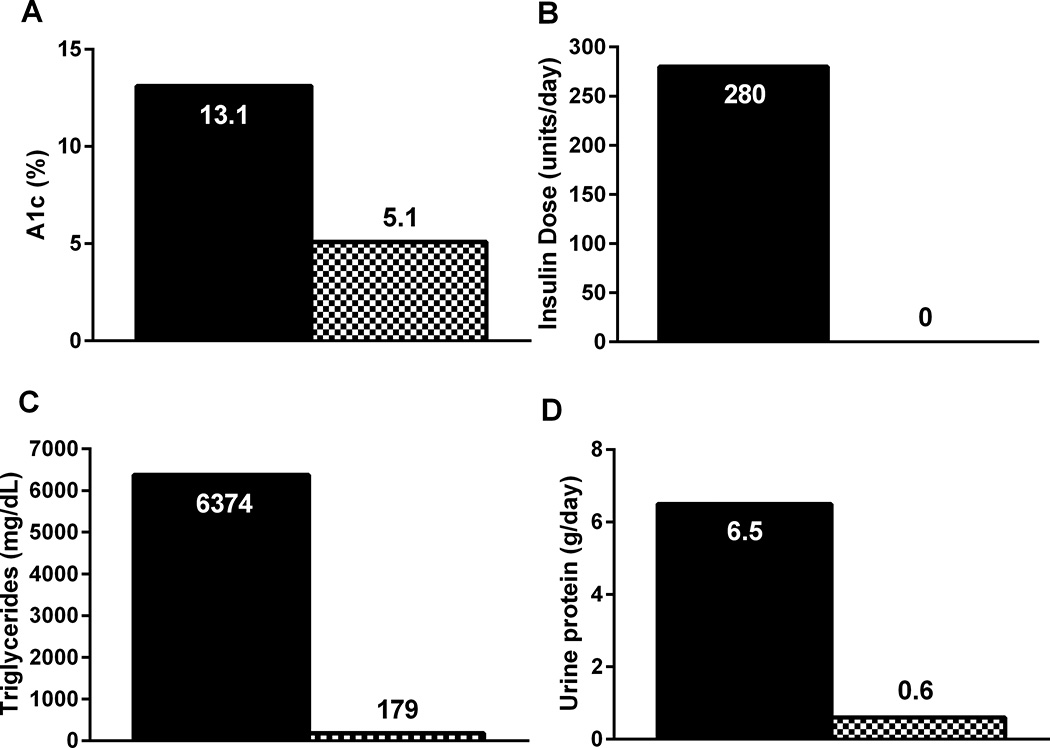
Figure 1.
Dramatic effects of one year of metreleptin treatment in a 21 year old woman with congenital generalized lipodystrophy and a history of poorly controlled diabetes, severe hypertriglyceridemia, and nephrotic range proteinuria. Black bars show clinical parameters prior to metreleptin, and black and white checkered bars after 1 year of metreleptin treatment. Metreleptin dramatically improved A1c (A) despite discontinuation of insulin (B). Metreleptin also greatly decreased fasting triglycerides (C) and 24 hour urine protein excretion (D).
Using the NIH protocol, Japanese investigators demonstrated the same favorable response of metreleptin in their lipodystrophy patients, and their studies led to official approval of metreleptin therapy for all types of lipodystrophy in Japan [33, 34].
Clinical use of metreleptin
The following comments reflect only the interpretation of data, views, and opinions of the authors of this manuscript and in no way should be viewed as policy of Aegerion Pharmaceuticals or the NIH:
Metreleptin Administration
When it is determined that a patient has generalized lipodystrophy and has metabolic complications associated with the hypoleptinemic state (insulin resistance, hypertriglyceridemia, hyperphagia), leptin replacement therapy is warranted. Importantly, conventional therapies should be optimized prior to initiation of metreleptin therapy. Providers located in the United States need to enroll in the Myalept Injection Risk Evaluation and Mitigation Strategy program (REMS). REMS is a strategy to manage known or potential serious risks associated with drug product and is required by the FDA to ensure that the benefits of a drug outweigh its risks. Providers can learn more about the program and enroll at www.myalept.com. Once enrolled, the prescriber fills out the prescription form on the same website.
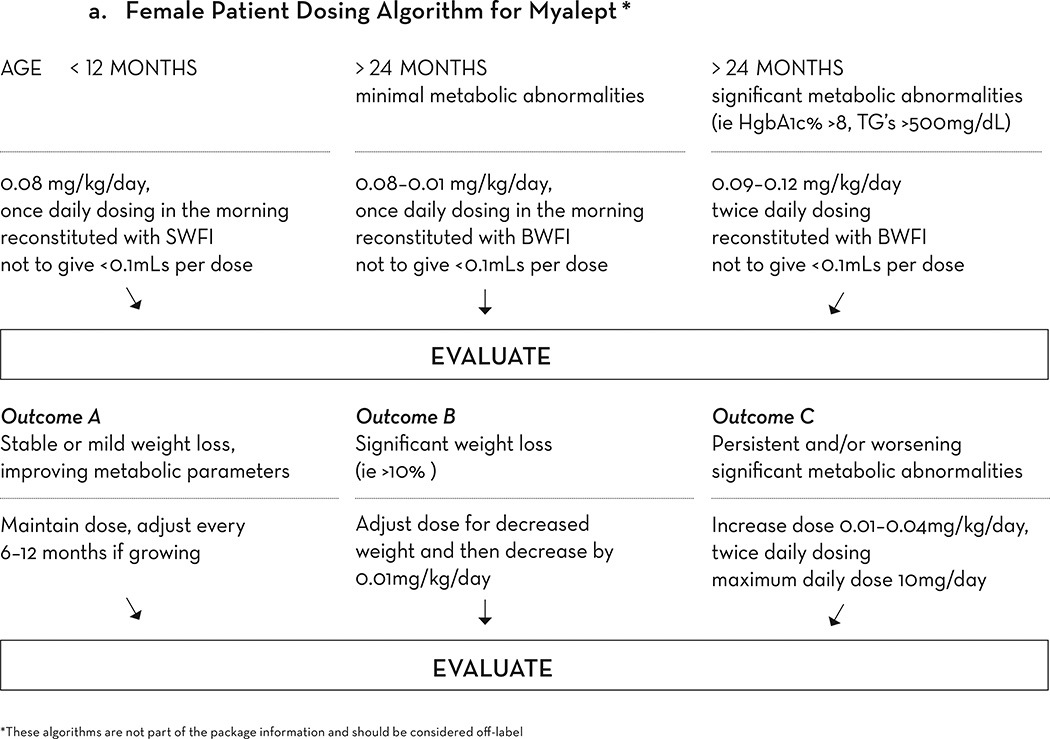
The prescribing information recommends a starting dose of 5 mg every 24 hours in women, and 2.5 mg every 24 hours in men, with weight based dosing for children less than 40 kg. At the NIH, we follow a weight-based dosing guideline which is not part of the dosing schema in the US label. Based on our 15 years of experience, for female patients we suggest that the typical dose required is between 0.09 mg/kg/day and 0.15 mg/kg/day. For male patients, the typical dose requirement is between 0.06 and 0.09 mg/kg/day. Occasionally, doses as large as 0.24 mg/kg/day are required to achieve maximal effect (Figure 2a).
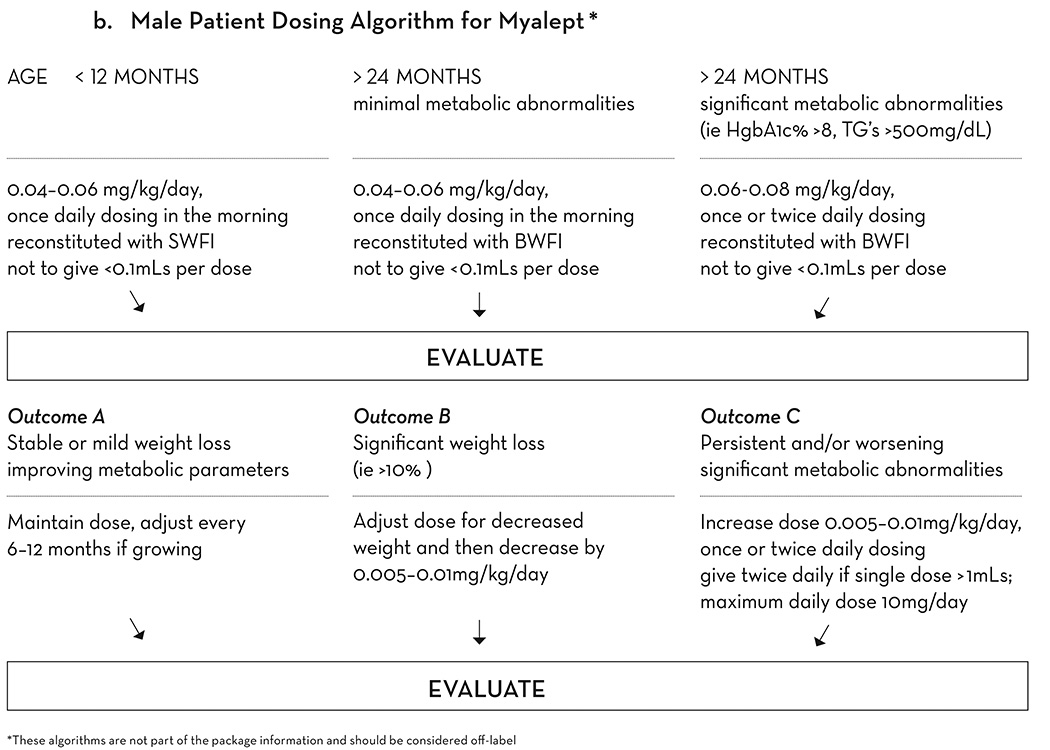
Figure 2.
a. Weight-based dosing algorithm for Myalept for female patients. This algorithm is not part of the package information and should be considered off-label. SWFI, sterile water for injection. BWFI, bacteriostatic water for injection.
b. Weight-based dosing algorithm for Myalept for male patients. This algorithm is not part of the package information and should be considered off-label. SWFI, sterile water for injection. BWFI, bacteriostatic water for injection.
If the total daily dose of metreleptin for a patient is calculated to be less than 0.8 ml, the starting dose recommended is as follows: begin with a once daily dosing of 0.09 mg/kg/day for female patients and 0.06 mg/kg/day for male patients. If the total daily dose is greater than 0.8 ml, it is more effective to divide the dose every 12 hours (Q12H), although again this use is outside of the US label. The patient’s history of compliance should be taken into account when designing the dosing regimen, as there is greater risk of decreased compliance with Q12H dosing (Figure 2b).
The prescription form for metreleptin also includes a section for supplies needed for the reconstitution and administration of the drug. Since patients with generalized lipodystrophy do not have adipose tissue, the shortest needles are recommended not only with regards to appropriate placement of the medication in the space below the dermis and above the muscle, but also to improve patient compliance. A needle length of 8 mm or less is recommended for metreleptin injections but only insulin syringes are available in this length. Thus, the dose is expressed as follows: “metreleptin 4 mg, inject 0.8 ml (80 unit markings on the syringe) under the skin once daily.” Write the dose as mg, volume to be injected, and then the corresponding dose markings on the insulin syringe. Providers should prescribe insulin syringes when possible, as volumetric syringes are 27 g and one-half inch length, and there is great risk for intramuscular (IM) injection with this length needle in this patient population.
The prescription is faxed into the Myalept REMS program, (fax number on prescription form) and then forwarded to the Accredo pharmacy. The pharmacy will review prescription through the patient’s insurance, and work directly with the patient on shipment parameters.
Response to metreleptin administration and dose adjustment
After metreleptin initiation, metabolic changes typically occur within 4–6 weeks. One of the expected early changes the patient or family will note is decreased appetite. This is accompanied with a decrease in weight. Some weight loss should be tolerated even in growing children. Based on the NIH experience, no dose change is needed if weight loss is less than 10% of the patient’s body weight. If more than 10% loss of body weight occurs in a patient, the dose should be adjusted for the new weight without changing the overall mg/kg/day dosing. If the weight loss continues 1 month after the adjustment for weight, then the overall mg/kg/day dosing should be decreased by 0.05–0.1 mg/kg/day.
After 6 months of therapy, if weight loss continues, continue to initially adjust the dose for weight, and then decrease by 0.01–0.02 mg/kg/day. Dose changes should be made no more than every 4 weeks, and metabolic parameters should be monitored.
After 4–6 months on therapy, if no metabolic improvements are observed, the metreleptin dose should be increased by 0.02–0.04 mg/kg/day. Additionally, the dose may be given Q12H, although this is outside of the US label. The patient may also benefit from having a diabetes educator monitor how the patient/family reconstitute and administer the metreleptin to ensure proper metreleptin administration.
If a patient was extremely hyperglycemic prior to metreleptin initiation, this can also lead to delayed response to metreleptin therapy. Hyperglycemia can stimulate hyperphagia, and this will be difficult to overcome with metreleptin therapy alone. Thus, if the hemoglobin A1c (HbA1c) is >9%, U-500 insulin should be initiated prior to, or concomitantly with, metreleptin to help expedite the metabolic improvements with metreleptin. If the patient is already on exogenous insulin at the time of metreleptin initiation, a change in the insulin doses typically will not occur until 4–6 weeks after metreleptin initiation, and then the provider must communicate with the patient/family regarding insulin dose reduction to minimize the risk of hypoglycemia.
Over the 15 years of the NIH metreleptin trial, it cannot be overstated that the greatest barrier to metreleptin efficacy in patients with generalized lipodystrophy was patient/family non-adherence to the prescribed regimen. This non-adherence could occur at any age, but most notably during the teenage years. To minimize non-adherence, it is critical to use the shortest injection needles possible, and to work closely with the patient/family on the dosing and regimen. Although once daily regimens are helpful when compliance is an issue, twice daily regimens are more effective in producing the desired physiological effect of leptin replacement. Exogenous insulin therapy may be necessary when metreleptin therapy is initiated. Again, U-500 insulin is the best form of insulin to administer to this group of patients as a 2–3 injection/day regimen. Some patients may be able to discontinue exogenous insulin therapy completely after metreleptin therapy is established.
While metreleptin has not been approved in for use in pregnant women, several pregnancies have occurred in patients receiving metreleptin, and metreleptin has been continued at pre-pregnancy doses during pregnancy and lactation without obvious teratogenicity [35]. There are no data on metreleptin use in the elderly.
Safety and tolerability
Safety data submitted as part of the FDA approval of metreleptin were based on 100 patients with generalized or partial lipodystrophy treated with metreleptin at NIH and other sites, as well as safety data from clinical trials in over 500 obese patients[18].
The most frequent treatment-related adverse events were weight loss, abdominal pain, hypoglycemia, fatigue, headache, decreased appetite, and injection site reactions (bruising and urticaria). In the instances of hypoglycemia, patients were on concomitant exogenous insulin therapy or insulin secretagogues. Dose adjustments or cessation of exogenous insulin therapy may be necessary. Such patients need to closely monitor their blood glucose with initiation of metreleptin therapy and/or any increases in metreleptin therapy.
Hypersensitivity reactions (urticaria, rash, anaphylaxis), have been observed and need to be reported promptly to the provider. In our experience with cutaneous reactions at the NIH, antihistamine medications were effective. Occasionally, temporary dose reductions in metreleptin may be needed. The provider needs to note, that due to lack of subcutaneous fat, metreleptin injection will normally cause a raised bleb under the skin, which slowly diminishes as the medication is absorbed. Worrisome injection site reactions have redness that persists for at least 6 hours (often more than 24 hours), itch, and recur with each injection.
The metreleptin US label has two boxed warnings. The first is the risk of neutralizing antibodies. Anti-metreleptin antibodies with in vitro neutralizing activity have been identified in a small number of patients treated with metreleptin. These antibodies may lead to loss of efficacy of metreleptin, and carry a theoretical risk of inhibiting the activity of endogenous leptin. The latter effect could induce a phenotype similar to congenital leptin deficiency; however, it likely has little clinical relevance in patients with generalized lipodystrophy who have very low endogenous leptin. Severe infections (sepsis) were observed in two patients in the NIH study who developed in vitro neutralizing antibodies to leptin. However, both patients had risk factors for sepsis (advanced cirrhosis and very poor dental hygiene) and thus it remains unclear if antibodies to leptin increase the risk of sepsis in patients without underlying risk factors. Risks of neutralizing antibodies to leptin should be weighed by the provider along with the benefits of therapy.
T-cell lymphoma has been reported in patients with acquired generalized lipodystrophy. Three cases of T-cell lymphoma occurred in patients receiving metreleptin therapy. One patient with a pre-therapy cutaneous lesion progressed to T-cell lymphoma while on treatment. Lymphoma has also been reported in lipodystrophy patients who have not received metreleptin. Since this has been seen only in patients with other autoimmune features, it is most likely that lymphoma is a manifestation of autoimmunity and unrelated to treatment [36]. However, given the uncertainty about metreleptin and lymphoma, providers should consider the risks and benefits of treatment with acquired forms of lipodystrophy, particularly in patients with hematologic abnormalities prior to therapy. Leptin has also been proposed to be involved in the pathogenesis of obesity associated cancers, such as breast and colon cancer; however, convincing human data to support this are lacking [37].
Progression or development of autoimmune disease, including both membranoproliferative glomerulonephritis [31] and autoimmune hepatitis [8], has been reported during metreleptin treatment, but it is not clear that the drug played a role versus the natural history of underlying autoimmunity in these patients.
The relevance of rodent model systems in humans
Metreleptin was discovered in a rodent model and therefore rodent models have been essential both in discovery and understanding of a number of actions of leptin [10]. Further the initial beneficial effect of leptin therapy was shown in mouse models [38]. However, rodent models may not always have relevance for the human condition with respect to either the action of leptin or potential adverse effects. For instance, it has been suggested that leptin may be the mediator of hypertension in common forms of obesity. This is based on a rodent model of sympathetic stimulation by leptin with resulting changes in blood pressure [39]. Human studies, on the other hand, show no evidence that metreleptin exacerbates hypertension and therefore this appears to be a clear issue in which the rodent model does not predict human behavior [40].
A second example is the suggestion that leptin administration to rodents may lead to liver fibrosis [41, 42], but again this is unlikely to apply to humans. Non-alcoholic steatohepatitis, which is a fibrosing condition of the liver, may actually be improved by metreleptin therapy [7, 8]. A third example is inflammatory conditions in the central nervous system of rodents that occur in the presence of leptin but not in its absence. Though leptin may have some immunologic effect in rodents or humans with congenital leptin deficiency, the effects of metreleptin on immunologic parameters in patients with lipodystrophy is minimal [43]. A fourth example is leptin’s effect in bone. While there are several different effects of leptin administration on bone in rodents, our studies suggest that this has no relevance in the human condition [44].
Expert Commentary and 5 year view
Metreleptin is approved by the FDA only for generalized forms of lipodystrophy. Metreleptin has also been approved in Japan for treatment of the lipodystrophies. Otherwise, metreleptin is used only under two conditions: experimental studies and compassionate use protocols.
Based on preclinical and clinical research, there are two conditions that standout as an unmet need for metreleptin in that the drug provides unique benefits. The first is congenital leptin deficiency where the benefit of metreleptin therapy has been clearly demonstrated [45, 46]. The second is the various forms of partial lipodystrophy. We have shown that a subset of patients with partial lipodystrophy may have the same baseline abnormalities as patients with generalized forms of lipodystrophy and that under certain conditions they respond to metreleptin [24]. These conditions include serum leptin levels of ≤4 ng/ml and serum triglycerides levels of >300–500 mg/dl, usually with associated diabetes. Thus, using appropriate metabolic stratification, a subgroup of patients with partial lipodystrophy clearly benefit from metreleptin.
Metreleptin has also shown partial benefit in ameliorating diabetes in patients with insulin receptor defects and extreme insulin resistance [47]. In these cases there are very few other treatment options. There have been other preliminary studies that have suggested a potential role for metreleptin. These include HIV (highly active anti-retroviral therapy-induced) lipodystrophy which generally has a less severe metabolic profile than other forms of partial lipodystrophy [48]. Other conditions include non-alcoholic steatohepatitis with low serum leptin levels, hypogonadotropic hypogonadism induced by extreme exercise, and issues related to bone health [49]. Type-1 diabetes responds to leptin therapy in rodents [50], but the translation of this to humans is unclear.
While metreleptin is unique in these lipodystrophic patients with extreme metabolic dysfunction in that it affects lipids, insulin resistance and diabetes, other classes of drugs are under development that may affect triglycerides. These include antisense compounds that lower apolipoprotein C-III [51]. Thus, at this point in time there are conditions where the benefit and need for metreleptin are clear, and other areas that need further development.
Key Issues.
Lipodystrophy
Conventional treatment of lipodystrophy
Discovery of leptin and its development as a pharmaceutical
General characteristics of leptin
Leptin replacement in lipodystrophy
Clinical use of metreleptin
Safety and tolerability
The relevance of rodent model systems in humans
Future directions for metreleptin therapy
Acknowledgments
The authors were supported by NIH intermural support.
We are grateful to the nurses and clinical fellows who have cared for our patients. We are also grateful to the following pharmaceutical companies who have made metreleptin available to us: Amgen, Bristol Myers Squib, Astra Zeneca and Aegerion.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1. Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–3325. doi: 10.1210/jc.2011-1159. Clincal review of the heterogenous group of diseases termed the lipodystrophies. This review explores both genetic and acquired disorders characterized by selective loss of body fat and predisposition to insulin resistance.
- 2. Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–1234. doi: 10.1056/NEJMra025261. Clinical trial demonstrating the lack of effectiveness of metreleptin therapy in obese individuals with high endogenous circulating leptin.
- 3. Wachslicht-Rodbard H, et al. Heterogeneity of the insulin-receptor interaction in lipoatropic diabetes. J Clin Endocrinol Metab. 1981;52(3):416–425. doi: 10.1210/jcem-52-3-416. Clinical trial of nine female patients with lipodystrophy demonstrating the efficacy of metreleptin therapy to amerliorate metabolic complications such as insulin resistance, hypertriglyceridemia, and hyperglycemia.
- 4. Agarwal AK, et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88(10):4840–4847. doi: 10.1210/jc.2003-030855. Study demonstrates the baseline characteristics of patients with generalized forms of lipodystrophy versus partial forms of lipodystrophy and their respective responses to metreleptin therapy.
- 5. Speckman RA, et al. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet. 2000;66(4):1192–1198. doi: 10.1086/302836. Study that shows that chronic leptin treatment improves insulin-stimulated hepatic and peripheral glucose metabolism in severely insulin-resistant lipodystrophic patients. Improved insulin action was associated with marked reduction in hepatic and muscle triglyceride content.
- 6. Payne F, et al. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci U S A. 2014;111(24):8901–8906. doi: 10.1073/pnas.1408523111. Clinical study showing the efficacy of metreleptin therapy of seven Japanese patients with generalized lipodystrophy, two acquired and five congential, treated with metreleptin.
- 7. Javor ED, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41(4):753–760. doi: 10.1002/hep.20672. This was the first demonstration of the efficacy of meterelptin therapy to improve meteabolic features in a lipodystrophic mouse model.
- 8. Safar Zadeh E, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131–137. doi: 10.1016/j.jhep.2013.02.007. First demonstration that a hypo-leptinemic state characterized by extreme obesity responds by significant weight reduction to metreleptin therapy.
- 9.Arioglu E, et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133(4):263–274. doi: 10.7326/0003-4819-133-4-200008150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 11.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16(10):1097–1099. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 12.Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89(3):991S–997S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frederich RC, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 14.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 15.Minocci A, et al. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord. 2000;24(9):1139–1144. doi: 10.1038/sj.ijo.0801385. [DOI] [PubMed] [Google Scholar]
- 16.Moon HS, et al. Leptin's role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34(3):377–412. doi: 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorden P, et al. Is there a human model for the 'metabolic syndrome' with a defined aetiology? Diabetologia. 2010;53(7):1534–1536. doi: 10.1007/s00125-010-1719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heymsfield SB, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282(16):1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 19.Farooqi IS, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paz-Filho G, et al. Congenital leptin deficiency and thyroid function. Thyroid Res. 2009;2(1):11. doi: 10.1186/1756-6614-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 22.Javor ED, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(7):1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 23.Chong AY, et al. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53(1):27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 24.Diker-Cohen T, et al. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–1810. doi: 10.1210/jc.2014-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez AJ, et al. Leptin replacement therapy for the treatment of non-HAART associated lipodystrophy syndromes: a meta-analysis into the effects of leptin on metabolic and hepatic endpoints. Arq Bras Endocrinol Metabol. 2014;58(8):783–797. doi: 10.1590/0004-2730000003174. [DOI] [PubMed] [Google Scholar]
- 26.McDuffie JR, et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab. 2004;89(9):4258–4263. doi: 10.1210/jc.2003-031868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran SA, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism. 2004;53(4):513–519. doi: 10.1016/j.metabol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Petersen KF, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lungu AO, et al. Insulin resistance is a sufficient basis for hyperandrogenism in lipodystrophic women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2012;97(2):563–567. doi: 10.1210/jc.2011-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph J, et al. Lipid regulation in lipodystrophy versus the obesity-associated metabolic syndrome: the dissociation of HDL-C and triglycerides. J Clin Endocrinol Metab. 2014;99(9):E1676–E1680. doi: 10.1210/jc.2014-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javor ED, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89(7):3199–3207. doi: 10.1210/jc.2003-032140. [DOI] [PubMed] [Google Scholar]
- 32.Musso C, et al. Spectrum of renal diseases associated with extreme forms of insulin resistance. Clin J Am Soc Nephrol. 2006;1(4):616–622. doi: 10.2215/CJN.01271005. [DOI] [PubMed] [Google Scholar]
- 33.Ebihara K, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92(2):532–541. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 34.Ebihara K, Masuzaki H, Nakao K. Long-term leptin-replacement therapy for lipoatrophic diabetes. N Engl J Med. 2004;351(6):615–616. doi: 10.1056/NEJM200408053510623. [DOI] [PubMed] [Google Scholar]
- 35.Maguire M, et al. Pregnancy in a woman with congenital generalized lipodystrophy: leptin's vital role in reproduction. Obstet Gynecol. 2012;119(2 Pt 2):452–455. doi: 10.1097/AOG.0b013e31822cecf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown RJ, et al. Lymphoma in acquired generalized lipodystrophy. Leuk Lymphoma. 2015:1–6. doi: 10.3109/10428194.2015.1040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strong AL, et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimomura I, et al. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401(6748):73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 39.Simonds SE, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159(6):1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown RMC, Gorden P. Leptin Does Not Mediate Hypertension Associated With Human Obesity. Cell. 2015 doi: 10.1016/j.cell.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reitman ML. Leptin in the liver: a toxic or beneficial mix? Cell Metab. 2012;16(1):1–2. doi: 10.1016/j.cmet.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imajo K, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16(1):44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Oral EA, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab. 2006;91(2):621–628. doi: 10.1210/jc.2005-1220. [DOI] [PubMed] [Google Scholar]
- 44.Christensen JD, et al. Bone mineral content in patients with congenital generalized lipodystrophy is unaffected by metreleptin replacement therapy. J Clin Endocrinol Metab. 2014;99(8):E1493–E1500. doi: 10.1210/jc.2014-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farooqi IS, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 46.Paz-Filho G, Mastronardi CA, Licinio J. Leptin treatment: facts and expectations. Metabolism. 2015;64(1):146–156. doi: 10.1016/j.metabol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Brown RJ, Cochran E, Gorden P. Metreleptin improves blood glucose in patients with insulin receptor mutations. J Clin Endocrinol Metab. 2013;98(11):E1749–E1756. doi: 10.1210/jc.2013-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsoukas MA, Farr OM, Mantzoros CS. Leptin in congenital and HIV-associated lipodystrophy. Metabolism. 2015;64(1):47–59. doi: 10.1016/j.metabol.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366(9479):74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 50.Wang MY, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2010;107(11):4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaudet D, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371(23):2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]