Abstract
Background
The association between baseline renal impairment (RI) and the prognosis of diffuse large B-cell lymphoma (DLBCL) was previously not defined. The aim of this study was to evaluate the prognostic value of RI in patients with DLBCL treated with three-weekly rituximab plus cyclophosphamide, Adriamycin, vincristine, and prednisolone immunochemotherapy (R-CHOP21).
Methods
Patients with newly diagnosed de novo DLBCLs treated with ≥1 cycle of R-CHOP21 were analyzed retrospectively. Pretreatment blood samples were collected and the glomerular filtration rate (GFR) was calculated. RI was defined by a GFR of <60 mL/min/1.73 m2 according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Results
Of the 185 patients enrolled in the present study, 19 patients (10.3%) had RI. The reasons for baseline RI were pre-existing CKD (N=5), acute kidney injury due to either obstruction (N=2) or electrolyte imbalance (N=2) related to DLBCL, and undefined causes (N=10). Patients with baseline RI showed inferior overall survival (OS) compared to those without RI (P<0.001). In multivariate analysis, RI was identified as an International Prognostic Index (IPI)-independent prognostic indicator. A baseline hemoglobin level of <10 g/dL and the presence of RI effectively discriminated a portion of the patients with far inferior event-free survival and OS among the patients having high or high-intermediate risk cancers according to either the standard- or the National Comprehensive Cancer Network-IPI.
Conclusion
Pretreatment RI was an independent prognostic marker for inferior OS in patients with DLBCL treated with R-CHOP21 immunochemotherapy.
Keywords: Diffuse large B-cell lymphoma, Renal insufficiency, Prognosis, Rituximab
INTRODUCTION
Patients who are diagnosed with malignancies often have some degree of renal impairment (RI) at the time of diagnosis [1,2]. The evaluation of 4,684 patients with various types of cancers from 15 centers in France by the Renal Insufficiency and Cancer Medication (IRMA) study group [1] showed that 7.2% of the patients had serum creatinine levels >110 µmol/L (1.24 mg/dL). However, the proportion of patients with any degree of abnormal renal function or RI had increased to 57.4% and 52.9%, when assessed by the Cockcroft-Gault and abbreviated Modification of Diet in Renal Disease (MDRD) study formula, respectively [3]. Considering this common incidence, the baseline renal function should be accurately assessed in every patient with a newly diagnosed cancer, including those having normal serum creatinine levels, in order to avoid renal toxicity during treatment through the adequate dosage adjustment of chemotherapeutic or other agents [2,3,4].
In the field of oncology, the role of renal function as a prognostic indicator was previously suggested in patients with advanced cancers receiving palliative or end-of-life care [5,6]. However, recently, its prognostic value has been assessed in patients with newly diagnosed cancers. The baseline RI, assessed by the blood urea nitrogen (BUN) level, was significantly associated with the overall survival (OS) in patients with advanced stage non-small cell lung cancer [7], or with early mortality in patients with resectable pancreatic adenocarcinoma [8].
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma among adults [9,10]. Although DLBCL can be further classified according to the cell-of-origin [11], this molecular classification currently cannot be implemented in routine clinical practice. The International Prognostic Index (IPI) is a useful clinical parameter and has been widely used for the prediction of overall treatment outcome in patients with DLBCL, even in the rituximab era [12]. However, there is still a need for novel and easy-to-use, as well as reliable biomarkers for better prognostication.
In the present study, we evaluated the prognostic value of baseline RI in patients with DLBCL who were uniformly treated with a three-weekly dose of rituximab plus cyclophosphamide, Adriamycin, vincristine, and prednisolone (R-CHOP21) immunochemotherapy.
MATERIALS AND METHODS
Patients and treatment
The inclusion criteria for the current study were as follows: 1) patients newly diagnosed with DLBCL according to the 2008 World Health Organization classification criteria; 2) patients aged ≥20 years; 3) patients treated with ≥1 cycle of R-CHOP21 as previously described at the Gachon University Gil Medical Center, Incheon, Korea [13]; and 4) patients with a complete set of clinical, laboratory, and radiologic data for use in the retrospective analysis. Patients were excluded if they had DLBCLs: 1) of primary or concomitant central nervous system (CNS); 2) with human immunodeficiency virus infection; or 3) transformed from preexisting or previously diagnosed indolent lymphoma. Dose reduction was determined at the physicians' discretion; however, patients having a dose reduction of >25% or those having cyclophosphamide and/or Adriamycin omitted from their treatment regimens were excluded.
Ann Arbor stage I or II patients received 3–4 cycles of R-CHOP21 followed by involved-site radiotherapy or 6–8 cycles of R-CHOP21. Patients having advanced stage cancers received 6–8 cycles of R-CHOP21. Following the completion of 6–8 cycles of R-CHOP21, patients underwent a final response evaluation with both computed tomography (CT) and 18F-fluorodeoxyglucose-positron emission tomography/CT. Tumor response was evaluated according to the revised response criteria for malignant lymphomas [14]. After the completion of the planned R-CHOP21 treatment, involved-site radiotherapy or upfront autologous stem cell transplantation for high-risk patients could be performed at the physician's discretion. For patients who achieved complete response (CR), an outpatient department-based surveillance was performed every 2–3 months for the first 2 years, and 4–6 months thereafter. A pathologic diagnosis was mandatory to confirm the relapse of DLBCL.
The present study was approved by the institutional review board (IRB) of the Gachon University Gil Medical Center (approval number: GAIRB2016-023). All procedures performed in the current study were in accordance with the ethical standards of the IRB and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Considering the retrospective nature of this study with minimal risk, the IRB waived the need for informed consent.
The assessment of renal function and anemia
Pretreatment blood samples were collected and laboratory analysis was performed to determine the concentrations of BUN and serum creatinine. If a patient had serial BUN or creatinine measurements before the initiation of treatment, the lowest values were selected according to the assumption that the reversible component of RI caused by dehydration could be mitigated with fluid use at the time of lymphoma diagnosis or staging workup. Because the use of corticosteroids elevates the level of blood BUN, pretreatment with any type of corticosteroid before the administration of R-CHOP21 was thoroughly investigated for each patient. For patients who were pretreated with corticosteroids, only the serum BUN and creatinine measurements obtained before the administration of corticosteroids were selected.
The glomerular filtration rate (GFR) was calculated according to the MDRD study formula [15] and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [16]. The cut-offs for the classification of renal function were adapted from the 2002 Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease [17], which were as follows: ≥90 mL/min/1.73 m2 for normal GFR (corresponding to CKD stage 1), 60–89 mL/min/1.73 m2 for mildly reduced GFR (CKD stage 2), 30–59 mL/min/1.73 m2 for moderately reduced GFR (CKD stage 3), 15–29 mL/min/1.73 m2 for severely reduced GFR (CKD stage 4), and <15 mL/min/1.73 m2 for a GFR that necessitates dialysis for end-stage kidney failure (CKD stage 5). In the present study, RI was defined by a GFR of <60 mL/min/1.73 m2 according to the CKD-EPI. Baseline anemia was estimated as previously described [13].
Statistical analysis
Event-free survival denoted the time from treatment initiation to disease progression or to the discontinuation of treatment for any reason (e.g., toxicity, patient preference, or death from any cause) [18]. OS denoted the time from treatment initiation to death from any cause. Survival analyses were performed using the Kaplan–Meier method and the survival rates were compared using the log-rank test. Variables dichotomized from continuous variables were compared using the Fisher exact test or the Pearson chi-square test, as appropriate. Multivariate analysis was performed with a backward Cox regression model, only including the variables that returned P-values <0.1 in univariate analysis. Each P-value was two-sided and all P-values <0.05 were considered statistically significant.
RESULTS
Patient characteristics and overall treatment outcomes
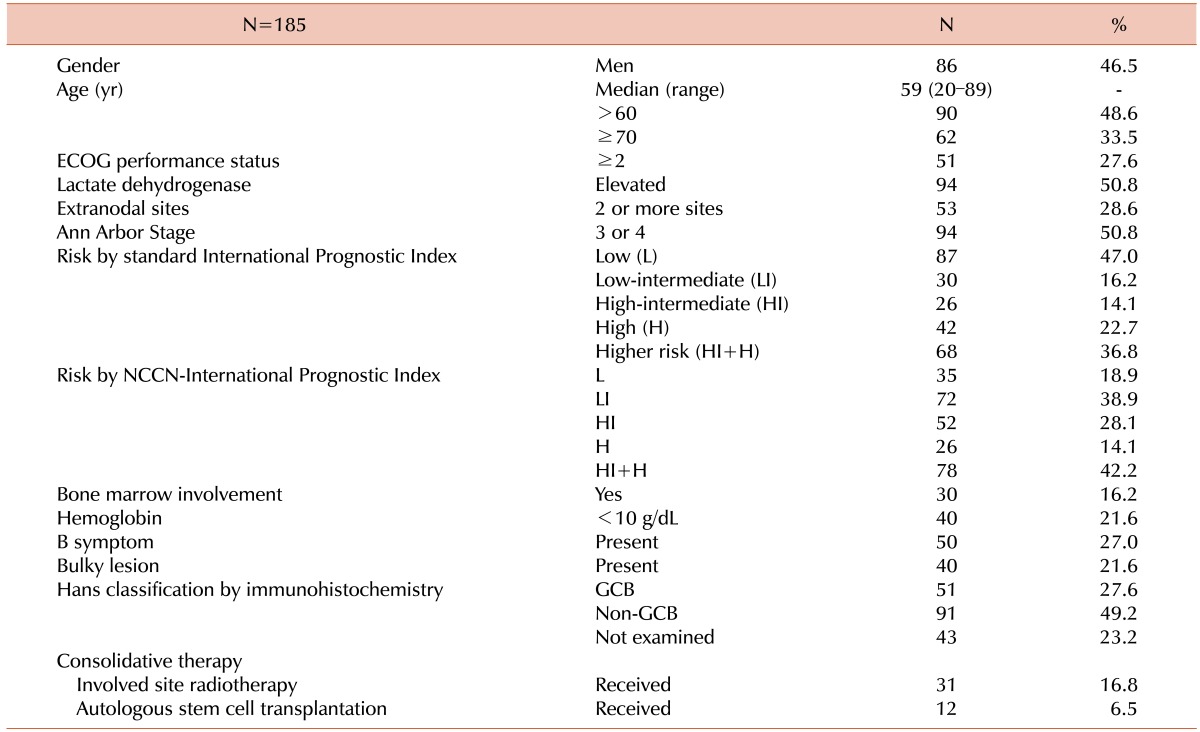
In total, 185 patients who were treated between March 2006 and January 2015 were included in the current retrospective analysis. Detailed characteristics of the included patients are summarized in Table 1.
Table 1. Patient characteristics.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NCCN, National Comprehensive Cancer Network; GCB, germinal center B-cell-like.
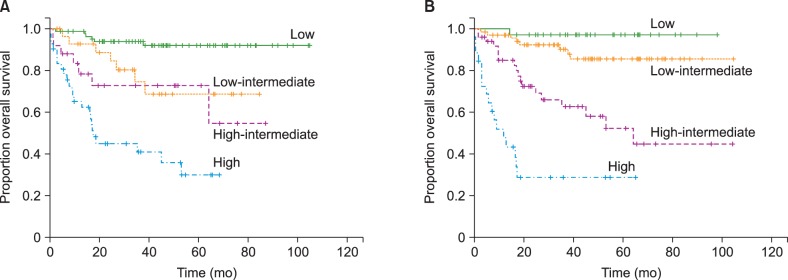
Of the 166 eligible patients, 144 patients (86.7%) achieved CR, 15 patients (9.0%) showed partial response, and 7 patients (4.2%) were refractory to R-CHOP21 (progressive disease) in the final response evaluation. During the 44.1-month median follow-up period (95% confidence interval [CI], 37.5–50.7), 53 patients (28.6%) developed any event, which included 7 patients who developed disease progression with R-CHOP21 treatment; 10 patients who developed treatment-related mortality (TRM) with R-CHOP21; 32 patients who developed DLBCL relapse after the achievement of CR; and 4 patients who died from causes other than DLBCL or DLBCL treatment after CR (1 patient died of acute myocardial infarction, 1 of aortic aneurysmal rupture, 1 of severe pulmonary embolism, and 1 of hepatocellular carcinoma progression). Among the 144 patients who achieved CR after R-CHOP21 immunochemotherapy, the 2-year and 4-year relapse-free survival rates were 81.9% and 79.5%, respectively. Among the patients who were analyzed, 44 patients (23.8%) died, which included 10 patients who died due to TRM during R-CHOP21 immunochemotherapy, 1 patient due to TRM of upfront autologous stem cell transplantation, and 4 patients due to deaths from other causes (as described above). The remaining 29 patients died of disease progression. The 3-year event-free survival and OS were 71.1% and 76.5%, respectively. Both the standard IPI and the National Comprehensive Cancer Network (NCCN)-IPI showed good prognostication. However, the NCCN-IPI was more efficient in discriminating between patients of low-intermediate and high-intermediate risk. In addition, the patients classified as the high-risk group according to the NCCN-IPI showed poorer OS compared to those classified as high-risk according to the standard IPI (Fig. 1).
Fig. 1. Kaplan–Meier graphs for overall survival according to (A) the standard International Prognostic Index and (B) the National Comprehensive Cancer Network International Prognostic Index.
Renal impairment
Twenty-three patients (12.4%) were pretreated with corticosteroids and the lowest blood BUN and creatinine levels measured before the initiation of corticosteroids were included. Seventeen patients were identified to have RI according to the MDRD study formula. Because 2 additional patients were determined to have RI according to the CKD-EPI formula (from a GFR of 61.8 and 61.9 mL/min/1.73 m2, respectively, according to the MDRD study formula; to a GFR of 56.4 and 56.8 mL/min/1.73 m2, respectively, according to the CKD-EPI formula), 19 patients had RI according to CKD-EPI formula. Of the 19 patients, 5 patients were identified to have had pre-existing CKD through the review of past medical records. Two patients had post-renal azotemia, one via the invasion of the ureter, and the other via the renal parenchymal involvement of the lymphoma with obstruction of the renal pelvis. Two patients had hypercalcemia and/or hyperuricemia, suggestive of acute kidney injury (AKI) due to an electrolyte imbalance caused by lymphoma. We could not identify the cause of RI among the other 10 patients who were aged ≥70 (range, 71–89) years, except for 2 patients aged 49 and 69 years, respectively.
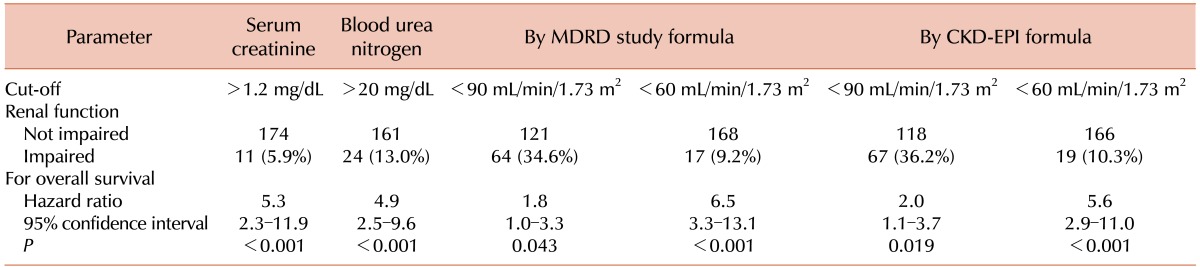
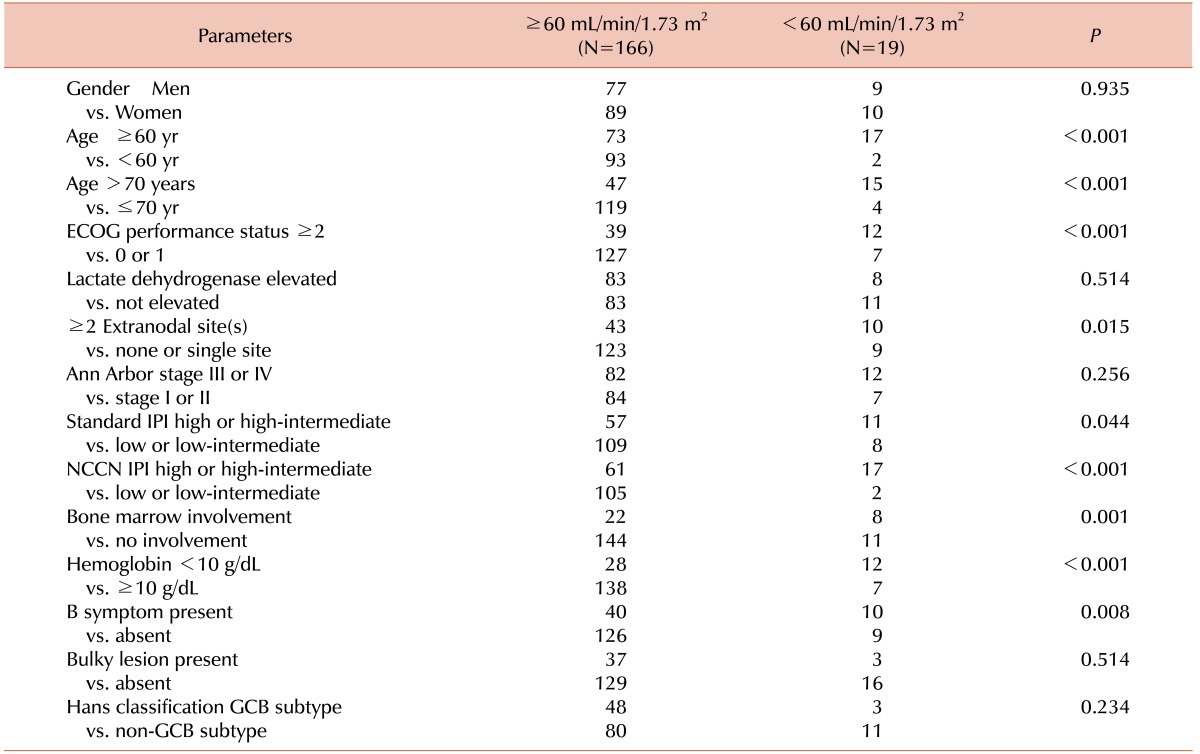
The OS was analyzed according to the GFR using both the MDRD study formula and the CKD-EPI formula (Table 2). The MDRD study formula and the CKD-EPI formula showed similar results. RI was selected according to the CKD-EPI formula for further analysis. The relationship between RI and the baseline characteristics is shown in Table 3.
Table 2. Baseline renal function estimated using several parameters in the analyzed patients.
Abbreviations: MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Table 3. The relationship of renal impairment defined by the CKD-EPI formula to the baseline characteristics of the patients analyzed.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; NCCN, National Comprehensive Cancer Network; GCB, germinal center B-cell-like.
9 out of 19 patients (47.4%) with RI underwent an initial dose reduction (only a reduction of ≤25%) for cyclophosphamide and/or Adriamycin, whereas only 28 out of 138 patients (16.9%) without RI did (P =0.002). The proportion of patients who underwent a subsequent dose reduction of the two drugs did not differ between patients with and without RI (23.5% of patients with RI vs. 38.1% patients without RI, P =0.235).
Renal impairment and survival
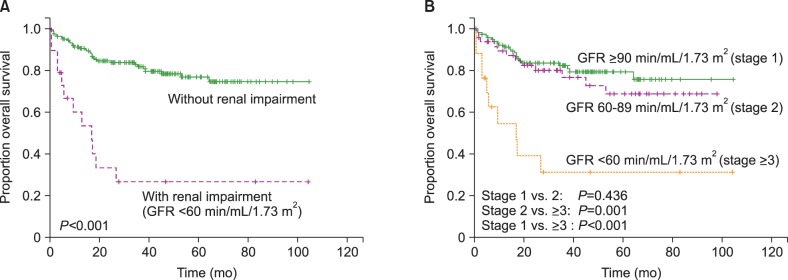
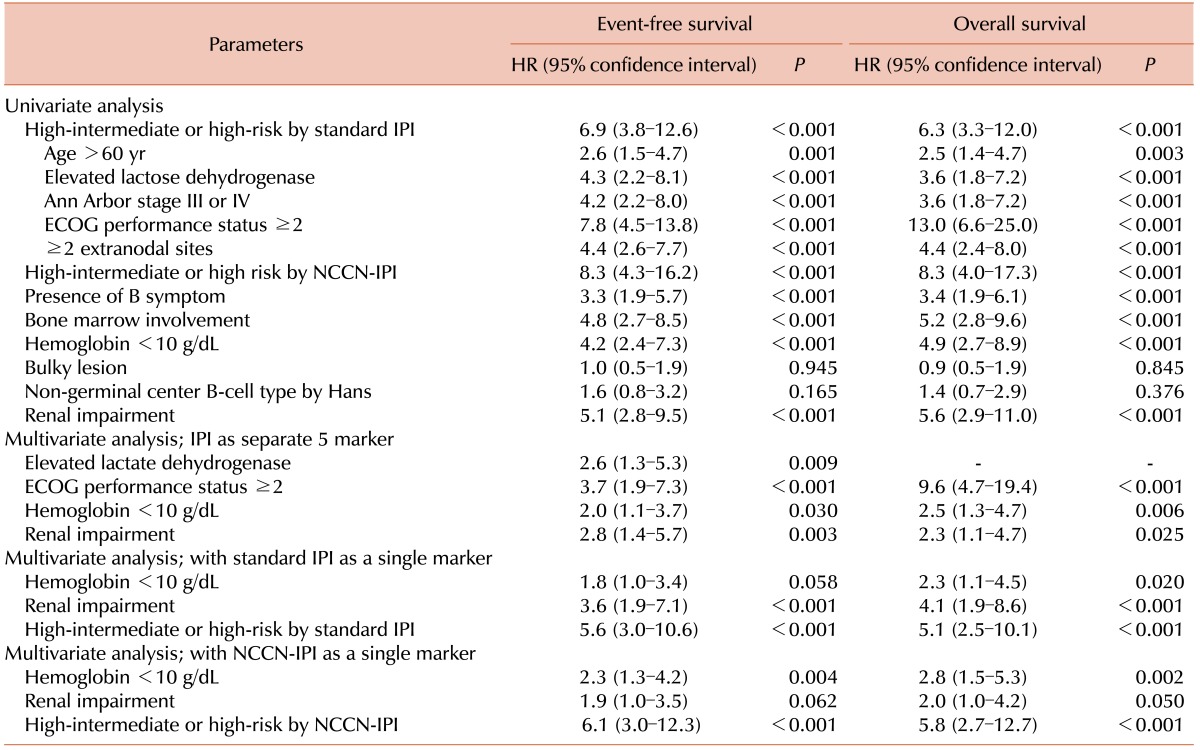
Patients with RI showed inferior OS compared to those without RI (3-yr OS, 26.7% vs. 81.8%, P <0.001; Fig. 2A). The OS did not differ between patients with GFR ≥90 mL/min/1.73 m2 and those with GFR ×60–89 mL/min/1.73 m2 (3-yr OS 82.4% vs. 76.7%, P =0.436; Fig. 2B), suggesting that only RI defined by a GFR of <60 mL/min/1.73 m2 is a meaningful prognostic indicator of OS. In multivariate analysis, RI was an IPI-independent prognostic biomarker (Table 4).
Fig. 2. Kaplan–Meier graph of the patients analyzed according to renal function.
Table 4. Univariate and multivariate analysis for event-free and overall survival.
Abbreviations: HR, Hazard Ratio; IPI, International Prognostic Index; ECOG, Eastern Cooperative Oncology Group; NCCN, National Comprehensive Cancer Network.
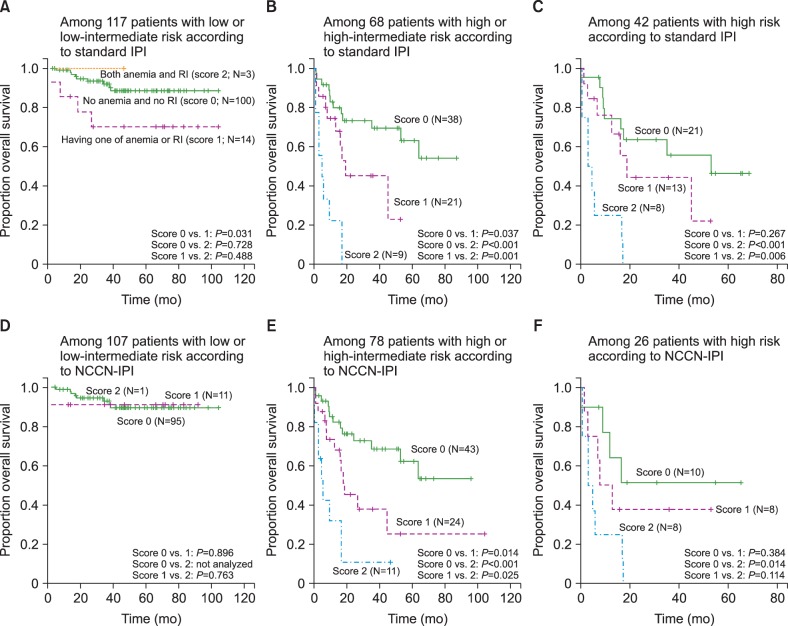
Among patients who were classified as high or high-intermediate risk according to the standard IPI, patients who had neither anemia of hemoglobin <10 g/dL nor RI, had superior OS compared to those with one or both. However, this was not the case for patients who were classified as low or low-intermediate risk according to the standard IPI (Fig. 3A, Fig. 3B). These findings were maintained when the analysis was restricted to high-risk patients according to standard IPI (Fig. 3C). The use of NCCN-IPI instead of standard IPI showed similar results (Fig. 3D-F).
Fig. 3. Kaplan–Meier curves according to subgroups defined by either the standard International Prognostic Index (IPI) (A–C) or the National Comprehensive Cancer Network-IPI (D–F).
DISCUSSION
In the present study, the baseline RI was independently associated with inferior survival in patients with DLBCL treated with R-CHOP21. In combination with baseline hemoglobin <10 g/dL, which we previously suggested as a potential prognostic marker [13], RI enhanced prognostication among patients who were classified as high-risk according to either the standard or the NCCN-IPI.
Several large studies have already reported an association between RI in either patients with pre-defined CKD or impaired renal function at baseline, and higher risk of cancer-related death [19,20,21]. Na and colleagues, who retrospectively reviewed 8,223 cancer patients with at least one baseline serum creatinine measurement, reported that CKD was associated with an increased risk of cancer-related mortality [adjusted hazard ratio, 1.12 for patients with 30 ≤ GFR <60 mL/min/1.73 m2 (P =0.04) and 1.75 for those with GFR <30 mL/min/1.73 m2 (P <0.001)] [20]. Iff and colleagues conducted a prospective population-based cohort study in 4,077 patients. In the analysis of 307 subjects with cancer-related death in the cohort, it was reported that each 10 mL/min/1.73 m2 decrease in the GFR was associated with a 18% increase in cancer-related death, irrespective of the age, gender, smoking status, blood pressure, and fasting blood glucose level. They also reported that among the subjects who were diagnosed with cancer after the initiation of the study, the overall risk of mortality increased by at least 14% for each 10 mL/min/1.73 m2 decrease in the GFR [21]. However, because cancer-related death involves other contributing factors, including smoking status, alcohol drinking, diet, and environmental factors, such large-scale studies also have limitations in that several confounding factors might not be adequately reflected. In addition, each cancer has its own specific risk factors and in some cases, a few specific risk factors might be more predominant among the others. For this reason, the actual impact of RI on cancer-related mortality needs to be evaluated for every cancer type through the integration of the already known prognostic or confounding factors.
Considering the fact that the reported prevalence rate for CKD stage ≥3 was 5.0% in Korea in 2006 [22], the 10.3% incidence rate of RI in this study is higher. The incidence of RI is higher among cancer patients compared to that of the normal population [3]. In the present study, only 5 patients had pre-existing CKDs, comprising 2.7% of the 185 analyzed patients, and 26.3% of the 19 patients with RI. Excluding the 9 patients with pre-existing CKDs or AKI, the causes of RI in the remaining 10 patients were not defined. They might be previously undiagnosed CKD patients or patients with AKI related to lymphoma. Because 8 of them were elderly patients, we assumed that age-related GFR decline could be a major cause of their RI. By all accounts, RI in patients with DLBCL can be considered in three aspects: 1) CKD, 2) AKI, and 3) age-related impairment of renal function.
CKD is defined by a GFR of <60 mL/min/1.73 m2 and/or kidney damage for at least 3 months [17]. CKD has many adverse effects on the human body. As GFR declines, oxidation products such as advanced glycation end products increase via oxidative stress. They induce cytokine production and monocyte activation, resulting in a pro-inflammatory condition [23]. Inflammation causes sarcopenia and malnutrition, and alters the structure of the vascular endothelium to favor atherogenesis [24]. Therefore, patients with CKD are vulnerable to cardiovascular disease, muscle wasting, hormonal imbalance, anemia, and infection caused by impaired immunity. In addition, there appears to be a relationship between CKD and frailty, which cannot be fully explained by inflammation, sarcopenia, anemia, and overt cardiovascular disease, possibly caused by subclinical vascular disease [25].
Some of the analyzed patients might have AKI caused by lymphoma. For patients with lymphoma who did not have CKD, the development of AKI might occur in several ways [26]. First, anatomic factors such as obstruction caused by compression, and the invasion or infiltration of the kidney or the other components of the urinary system can cause RI. Second, some patients with lymphoma have metabolic abnormalities such as hypercalcemia caused by osteolytic metastasis or the release of parathyroid hormone-related peptide; or hyperuricemia with high tumor burden might cause AKI. Because these conditions imply higher tumor burden, AKI as well as CKD might have contributed towards inferior survival.
In the present study, RI was strongly associated with age >60 years (P =0.001) as well as age ≥70 years (P =0.001). We expected this result because GFR steadily decreases with age [27]. However, a notable finding is that in multivariate analysis including five individual IPI factors, RI was an independent prognostic factor for OS whereas age >60 years was not (Table 4). A similar trend was observed when we included age ≥70 years or even age ≥75 years instead of age >60 years (Data now shown). Based on these results, we could cautiously assume that RI might have a role in the indication of the biological age of patients with DLBCL. It should be further evaluated in future studies.
In the present study, patients with RI underwent more frequent initial dose reduction of cyclophosphamide and/or Adriamycin compared to those without RI. Although we did not calculate the dose intensity of the individual patients for each drug, such frequent dose reductions could potentially cause lower dose intensity and might contribute to the inferior treatment outcomes. One might argue that RI did not actually have a prognostic significance and appeared to do so due to insufficient dose intensity. However, we believe that the impact of lower dose intensity would be limited, because the degree of initial dose reduction was limited to ≤25%, and patients without RI underwent more frequent dose reduction beyond the first cycle compared to those with RI (38.1% vs. 23.5%), although statistically insignificant. Dose reduction, which is very common in clinical practice, is not solely decided according to renal function. A poor performance status, frailty caused by old age or comorbidity, and several other factors might also affect the decision.
The recently introduced NCCN-IPI refined categorization of age and lactate dehydrogenase, and the identification of disease involvement at specific extranodal lesions, resulted in the better discrimination of low and high-risk DLBCL patients treated with rituximab-containing immunochemotherapy compared to standard IPI [28]. The results of the present study were consistent with that of the above-mentioned study. Instead of dichotomizing the age at 60 years, the NCCN-IPI classified patients into 3 groups by 15–20-year increments of age, and indicated higher risk in the group having patients with higher age (score 1 for patients with age >40 years to ≤60 years, score 2 for those with an age >60 years to ≤75 years, and score 3 for those with age >75 years), because the effect of continuous age on survival was linear [28]. Therefore, the gradual decline of GFR is associated with aging.
The present study had several limitations. First, it is a retrospective analysis including a modest number of patients. We used a single measured value of serum creatinine and estimated GFR accordingly. Therefore, it might not represent the genuine kidney function for some patients. The cell-of-origin is an IPI-independent prognostic biomarker in patients with DLBCL. However, the difference of survival between the germinal-center B-cell (GCB) and the non-GCB subtypes could not be reproduced in the present study. It might be explained by two reasons. One possibility is that the small patient number made it impossible to show statistically significant difference of survival between the two groups. In the present study, 23.2% of the patients lacked immunohistochemical staining results for the Hans classification. Second, as previously suggested, the Hans classification is a useful, but not an ideal surrogate of the genuine cell-of-origin, which can be acquired by gene expression profiling [29].
Despite its limitations, the present study adequately demonstrated that RI could be a useful indicator for predicting worse OS. To the best of our knowledge, only a few previous studies have evaluated RI as a prognostic indicator in patients with DLBCL. In addition, along with anemia, a subgroup of very poor outcome patients was identified among the high-intermediate or high-risk patients according to either the standard IPI or the NCCN-IPI. Hemoglobin and serum creatinine are markers that are easy to test at almost no additional cost.
In conclusion, pre-treatment RI was an independent prognostic marker for inferior OS in patients with DLBCL treated with the R-CHOP21 therapy. In combination with a hemoglobin of <10 g/dL, RI enhanced prognostication in patients classified as higher-risk according to either the standard IPI or the NCCN-IPI. This result should be validated in future large-scale studies.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Launay-Vacher V. Epidemiology of chronic kidney disease in cancer patients: lessons from the IRMA study group. Semin Nephrol. 2010;30:548–556. doi: 10.1016/j.semnephrol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Launay-Vacher V. Cancer and the kidney: individualizing dosage according to renal function. Ann Oncol. 2013;24:2713–2714. doi: 10.1093/annonc/mdt431. [DOI] [PubMed] [Google Scholar]
- 3.Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of Renal Insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110:1376–1384. doi: 10.1002/cncr.22904. [DOI] [PubMed] [Google Scholar]
- 4.Kurita GP, Lundström S, Sjøgren P, et al. Renal function and symptoms/adverse effects in opioid-treated patients with cancer. Acta Anaesthesiol Scand. 2015;59:1049–1059. doi: 10.1111/aas.12521. [DOI] [PubMed] [Google Scholar]
- 5.Durand JP, Mir O, Coriat R, Cessot A, Pourchet S, Goldwasser F. Validation of the Cochin Risk Index Score (CRIS) for life expectancy prediction in terminally ill cancer patients. Support Care Cancer. 2012;20:857–864. doi: 10.1007/s00520-011-1163-3. [DOI] [PubMed] [Google Scholar]
- 6.Gwilliam B, Keeley V, Todd C, et al. Development of prognosis in palliative care study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ. 2011;343:d4920. doi: 10.1136/bmj.d4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang K, Lai Y, Axelrod R, et al. Modeling the overall survival of patients with advanced-stage non-small cell lung cancer using data of routine laboratory tests. Int J Cancer. 2015;136:382–391. doi: 10.1002/ijc.28995. [DOI] [PubMed] [Google Scholar]
- 8.Sohal DP, Shrotriya S, Glass KT, et al. Predicting early mortality in resectable pancreatic adenocarcinoma: A cohort study. Cancer. 2015;121:1779–1784. doi: 10.1002/cncr.29298. [DOI] [PubMed] [Google Scholar]
- 9.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–1692. doi: 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi HG, Kim JS, Suh C, et al. Clinical features and survival outcomes of patients with diffuse large B-cell lymphoma: analysis of web-based data from the Korean Lymphoma Working Party Registry. Blood Res. 2013;48:115–120. doi: 10.5045/br.2013.48.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 12.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 13.Hong J, Woo HS, Kim H, et al. Anemia as a useful biomarker in patients with diffuse large B-cell lymphoma treated with R-CHOP immunochemotherapy. Cancer Sci. 2014;105:1569–1575. doi: 10.1111/cas.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–266. [PubMed] [Google Scholar]
- 18.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 19.Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol. 2011;6:1121–1128. doi: 10.2215/CJN.09011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Na SY, Sung JY, Chang JH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol. 2011;33:121–130. doi: 10.1159/000323740. [DOI] [PubMed] [Google Scholar]
- 21.Iff S, Craig JC, Turner R, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis. 2014;63:23–30. doi: 10.1053/j.ajkd.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Lim CS, Han DC, et al. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci. 2009;24(Suppl):S11–S21. doi: 10.3346/jkms.2009.24.S1.S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–2532. [PubMed] [Google Scholar]
- 24.Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12:1549–1557. doi: 10.1681/ASN.V1271549. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671.e2. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coggins CH. Renal failure in lymphoma. Kidney Int. 1980;17:847–855. doi: 10.1038/ki.1980.97. [DOI] [PubMed] [Google Scholar]
- 27.Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419–428. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutiérrez-García G, Cardesa-Salzmann T, Climent F, et al. Geneexpression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117:4836–4843. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]