Abstract
Background
Recent genome-wide association studies have identified single nucleotide polymorphisms in the protein αT-catenin (αT-cat; CTNNA3) that correlate with both steroid-resistant atopic asthma and asthmatic exacerbations. α-catenins are important mediators of cell-cell adhesion and αT-cat is predominantly expressed in cardiomyocytes. In the lung, αT-cat appears exclusively expressed in the cardiomyocytes surrounding pulmonary veins, but its contribution to atopic asthma remains unknown.
Objective
To understand the role of αT-cat in the pathogenesis of asthma.
Methods
We utilized αT-cat knockout mice and a house dust-mite extract (HDM) model of atopic asthma, with assessment by forced oscillation, bronchoalveolar lavage, and histologic analysis.
Results
We found that the genetic loss of αT-cat in mice largely attenuated HDM-induced airway inflammation and airway hyperresponsiveness to methacholine. Mice lacking αT-cat exposed to HDM extract showed reduced pulmonary vein inflammation, specifically near the large veins surrounded by cardiac cells. The proximity of airways to pulmonary veins correlated with the severity of airway goblet cell metaplasia, suggesting that pulmonary veins may influence the inflammatory milieu of adjacent airways. Loss of αT-cat led to the compensatory upregulation of αE-cat, which itself has a defined anti-inflammatory function.
Conclusion
These data mechanistically support previous clinical and genetic associations between αT-cat and the development of atopic asthma, and suggest that pulmonary veins may have an under-appreciated role in allergic airway inflammation.
Keywords: αT-catenin, CTNNA3, pulmonary vein, cardiomyocyte, cell-cell adhesion
INTRODUCTION
About 9% of children in the US suffers from asthma(1), making it the most common chronic illness of childhood. Additionally, 5-10% of these asthmatics have a more severe form of the disease that is resistant to steroid treatment, and although comprising the minority of asthma patients, they account for about 50% of all asthma-related healthcare costs(2). How this disease differs from steroid-responsive asthma, and how new treatments may be tailored for steroid-resistant asthma, are largely unknown. Recently, a genome-wide association (GWA) study found that polymorphisms in alpha-T-catenin (αT-cat; CTNNA3) were associated with steroid-resistance in asthma, where children with asthma carrying the minor haplotype failed to benefit from inhaled corticosteroids(3). Additionally, an independent GWA study found that αT-cat polymorphisms were also associated with the rate of asthmatic exacerbations(4).
α-catenins are essential F-actin-binding proteins of the cadherin/catenin adhesion complex, the major cell-cell adhesion system in tissues throughout the body(5). Epithelial (E), neural (N), and testis/heart (T) forms of this family have been described(6). αT-cat appears to be the most dispensable member of the family, as αT-cat knockout (KO) mice are viable and fertile(7). However, with age, these mice develop cardiomyopathy with decreased ejection fraction(7), consistent with the abundant expression of αT-cat in heart, and its recent link to familial arrythmogenic right ventricular cardiomyopathy(8). Our group has previously shown that αT-cat is present in the lungs, but in contrast to the more ubiquitously expressed αE-cat (found in lung epithelial, smooth muscle and endothelial cell types), αT-cat is restricted to the cardiomyocyte cell layer that surrounds pulmonary veins(9). Pulmonary veins have long been observed to become inflamed in murine models of asthma(10), but their role in regulating airway inflammation is largely not known, perhaps because the pulmonary veins develop separately from the sheath containing the arteries and airways of the lung(11). Despite their developmental independence, we have shown that subsets of airways can be found adjacent to the pulmonary veins in the mouse lung(9).
In addition to the recent links to atopic asthma outcomes, αT-cat polymorphisms have also been recently associated in humans with variability in forced vital capacity (a measure of lung function)(12) and with the incidence and severity of diisocyanate-induced occupational asthma in two independent and ethnically distinct population cohorts(13, 14). Indeed, our group previously found that αT-cat KO mice show altered lung mechanics, specifically in the generation of an enlarged pressure volume curve, and were more susceptible to diisocyanate asthma(9). The study that follows continues our experimental interrogation of the numerous genetic associations between αT-cat and asthma(3, 4) by determining the contribution of this cardiac junction protein to the pathogenesis of atopic asthma, which may be particularly relevant to forms of asthma resistant to steroid therapy.
METHODS
Histology
Mice were euthanized using 60mg/kg sodium pentobarbital and tracheostomized with an angiocatheter. Mouse heart and lungs were removed together, perfused with saline, inflated through the catheter with 10% neutral buffered formalin, fixed in 10% neutral buffered formalin, and embedded in paraffin blocks(15). For immunofluorescence, tissue sections were deparaffinized and antigens were retrieved by boiling in citrate buffer for 30 minutes. Endogenous mouse IgG was blocked with goat-anti mouse Fab fragment (Jackson ImmunoResearch, 115-007-003). Fluorescent images were captured using a Zeiss Axioplan epifluorescence microscope. Primary antibodies used were: BD Biosciences mouse anti-αE-cat (#610193), Sigma mouse anti-desmin (D1033), and rabbit anti-αT-cat (polyclonal #952, kindly provided by Frans van Roy, Ghent University, Belgium). For quantification, the intensity of each cardiomyocyte junction was measured using ImageJ from exposure-matched images. H&E staining was performed by the Northwestern University Mouse Pathology Core Facility.
Mice
All experiments using animals were approved by the Northwestern University IACUC. αT-cat KO C57BL/6 mice (obtained from Dr. Glenn Radice, Thomas Jefferson University, Philadelphia, PA) were bred with C57BL/6 WT to create heterozygote, C57BL/6 breeders. WT and αT-cat KO mice used for experimentation were littermates or descendants from littermates, and were genotyped similarly as shown previously(16). αT-cat-KO specific primers, which generate separate bands in KO and WT mice, were used to genotype using PCR: F: 5’-TCTATTTTTGAGGCTGTCG-3’; R: 5’-CAAACTTATGCGTGGTG-3’. αT-cat KO was confirmed by PCR, distinguishing from heterozygotes, showing absence of band generated by WT-specific primers: F: 5’-CCACCCCTGATATGACCTGTAG-3’; R: 5’-TCCCCAGGAATCAAGTCGTT-3’
HDM-model of asthma
House Dust Mite Extract, whole body Dermatophagoides Pteronyssinus (XPB70D3A25, Lot Number 254527; 2588.75 mcg allergen, 66.7mg protein, and 7775 EU LPS), was provided by Greer Labs (Lenoir, NC). Female C57BL/6 mice at least 6-weeks-old underwent intranasal injections of 20μL of 50μg of HDM extract in phosphate buffered saline while under isoflurane anesthesia(17). Intranasal injections occurred about every other day for three weeks (Fig. 1A). The day after the final challenge, mice underwent forced oscillation, bronchoalveolar lavage, and/or tissue fixation.
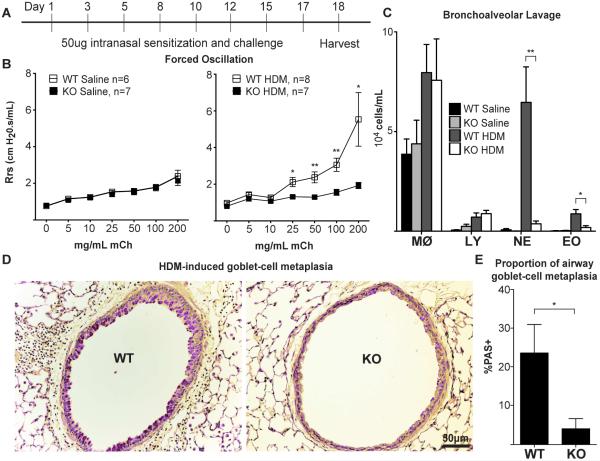
Figure 1. αT-cat is necessary for the development of HDM-induced murine model of atopic asthma.
(A) HDM-asthma model, with female mice at least 6 weeks old, with (B) Airway resistance measurements by forced oscillation, (C) airway inflammation measured by BAL, and (D) goblet cell metaplasia measured by PAS-staining (purple). Airways with the greatest goblet cell metaplasia from each mouse were quantified in (E) to avoid bias in choosing a “representative airway” (WT, n = 5; KO, n = 6). MØ=macrophage, LY=lymphocyte, NE=neutrophil, EO=eosinophil. *p<0.05, **p<0.01, Student’s t-test. Error bars=S.E.M.
Forced Oscillation
To measure airway hyperresonsiveness, mice were sedated using 60mg/kg sodium pentobarbital and tracheostomized with a catheter. After successful anesthesia, mechanical ventilation was started using the Flexivent system, per manufacturers instructions (Emka Technologies, Falls Church, VA). Next, the mice were paralyzed with an I.P. injection of 50μL of 10mg/mL rocuronium. After no independent breaths were recorded, the forced oscillation protocol was begun, with nebulized exposure to doses of methacholine from 0-200mg/mL in phosphate buffered saline. Airway resistance values shown are the maximum resistance of the repeated measurements during each exposed dose.
Bronchoalveolar Lavage
Mice were lavaged through the tracheal catheter with 0.8mL of saline fluid and aspirated. Cell counts were measured using Trypan-Blue exclusion and the automated Countess system (ThermoFisher Scientific, Grand Island, NY). To measure the cell differential, 200μL of BAL fluid was placed in Cytospin funnels (Block Scientific, Inc., Bellport, NY), centrifuged at 2000rpm for 5 minutes onto a glass slide. Cells were stained by Wright-Giemsa and manually counted and identified.
Periodic Acid-Schiff staining and quantification
PAS staining kit was obtained from Sigma (395B-1KT) (St. Louis, MO). Per manufacturer’s instructions, tissue sections were deparaffinized and stained with both periodic acid solution and Schiff’s reagent. For quantification, the area of PAS-positive staining was measured and compared to the total airway epithelium area using ImageJ. For Figure 1, the highest PAS-positive airways from WT and KO mice were used for quantification. For Figure 5, all available PAS-positive airways were quantified.
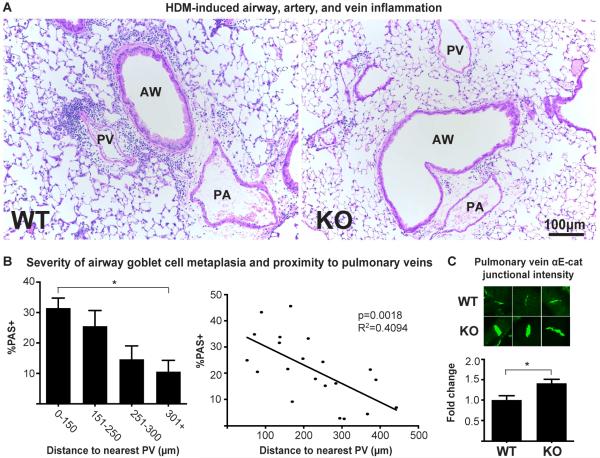
Figure 5. The pulmonary veins are an important mediator of airway inflammation.
(A) H&E-stained airways of both WT and αT-cat KO mice exposed to HDM, showing inflammation from the PV in WT mice. (B) Percent PAS-positive staining as a function of distance to the nearest PV. Each of the 21 data points in the regression analysis represents a single PAS-positive airway from HDM-treated WT mice, one tissue section each, from Figure 1 (n=5 mice). (C) Measurement of raw intensity of αE-cat in PV junctions of WT (n=50) and KO (n=98) mice exposed to HDM, normalized to WT. AW=airway, PA=Pulmonary artery, PV=Pulmonary vein. *p<0.05, Student’s t-test. Error bars=S.E.M.
Airway and Pulmonary Vein Inflammation Quantification
For the pulmonary vein inflammation quantification, the inflammatory cuff area, pulmonary vein muscle layer (for large veins), and lumen size (for small veins) was quantified using ImageJ. Only veins with intact inflammatory cuffs, not interrupted by proximity to airways, such as the vein/airway seen in Figure 5A, were measured. For Figure 4, the largest inflammatory cuff of all measured was utilized for quantification. For the airway goblet cell metaplasia comparison to vein proximity measured in Figure 5, all available PAS-positive airways from all WT mice shown in Figure 1 were quantified as described above. The distance to the nearest pulmonary vein was quantified using ImageJ.
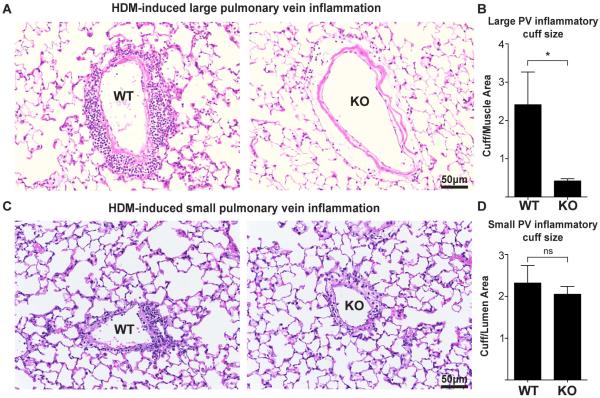
Figure 4. αT-cat loss decreases large, but not small pulmonary vein inflammation.
H&E-stained large (A) and small (C) PVs of both WT and αT-cat KO mice exposed to HDM. (B & D) Inflammatory cuff size was measured by dividing the area by the thickness of the muscle (large vein) or lumen size (small vein). AW=airway, PA=Pulmonary artery, PV=Pulmonary vein. *p<0.05, Student’s t-test, n=5. Error bars=S.E.M.
Statistics
Statistical analysis was performed using Student’s t-test, two-tailed and unpaired, and standard linear regression, with a p-value of less than 0.05 considered significant. Calculations were performed using GraphPad Prism software (Graph Pad Software Inc., La Jolla, CA).
RESULTS
αT-cat is necessary for HDM-induced atopic asthma
Since a majority of atopic asthmatics show hypersensitivity to house dust mite (HDM) (18), we modeled atopic asthma using a murine model driven by exposure to HDM modified from Clarke et al (19). Briefly, wild-type (WT) and αT-cat knockout (KO) mice were exposed to 50μg HDM extract intranasally over 3 weeks (Fig. 1A). To determine changes in airway hyperresponsiveness (AHR), mouse airway resistance was measured in response to nebulized methacholine using the Flexivent mechanical ventilation system. While there was no significant difference in the baseline AHR between saline-treated WT and αT-cat KO mice, WT mice exposed to HDM showed a significantly greater AHR than αT-cat KO mice (Fig. 1B). Consistent with this observation, WT mice exposed to HDM displayed significantly greater neutrophil and eosinophil infiltration than αT-cat KO mice (Fig. 1C), along with characteristic asthmatic airway changes in mucous cell metaplasia assessed using Periodic Acid-Schiff (PAS) staining of lung tissue (Fig. 1D&E). Thus, the loss of αT-cat appears to be protective in the HDM model of atopic asthma.
αT-cat is found in the cardiomyocytes of large, but not small, pulmonary veins
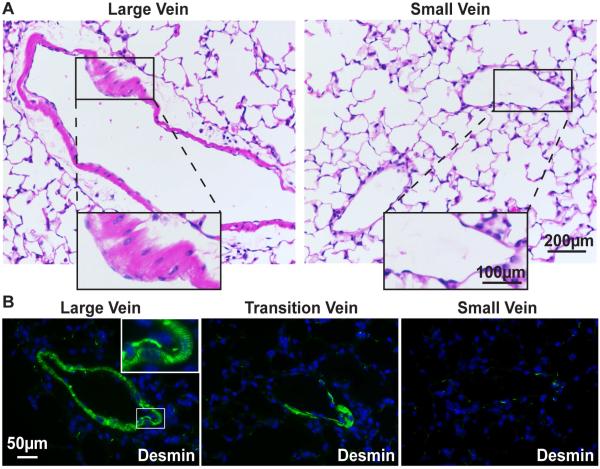
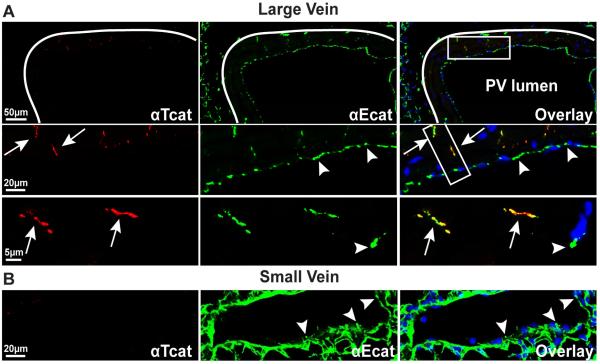
H&E staining reveals that regions of the large pulmonary veins are ensheathed by morphologically distinct cardiomyocytes, whereas small veins only contain endothelial cells (Fig. 2A). Evidence that these muscle cells are truly cardiomyocytes, rather than smooth muscle, is supported by immunostaining for the muscle-specific intermediate filament protein, desmin, which shows characteristic striated organization stereotypic to cardiac cells (Fig. 2B, left panel).. As these veins transition to smaller vessels, the cardiomyocytes are lost (Fig. 2B, right panels). In large veins, αT-cat can be detected in cardiomyocyte cell-cell junctions, but is absent from small veins (Fig. 3A, arrows). In contrast, αE-cat is expressed in both endothelial cells and cardiac cells distal to the large vessel lumen, and is the only α-cat present in small pulmonary veins (Fig. 3B, arrowheads). Consistent with the cardiomyocyte-restricted expression of αT-cat in the lung, there are no obvious perturbations in epithelial cell-cell junctions in the lungs of αT-cat KO mice, which show similar abundance and localization of both β-cat (Supplemental Fig. 1) and αE-cat (Supplemental Fig. 2) compared to WT mice.
Figure 2. Only the large pulmonary veins contain cardiomyocytes.
(A) H&E-stained large and small pulmonary veins. Large vein inset shows region containing morphologically characteristic cardiac cells. Small vein inset shows absence of this layer. (B) Immunofluorescence image of large and small pulmonary veins stained with the intermediate filament protein desmin (green), marking cardiomyocyte striations (inset, double-arrow) and demonstrating the loss of cardiac cells in small veins. Nuclei are stained blue with Hoechst dye.
Figure 3. αT-cat is expressed only in the large, cardiomyocyte-containing pulmonary veins.
Immunofluorescence of large (A) and small (B) pulmonary veins double-labeled for both αT-cat (red) and the more ubiquitously expressed αE-cat (green). Note αE-cat was detected in endothelial junctions (arrowheads) of both large and small veins, as well as the cardiomyocyte junctions (arrows) of large veins. αT-cat was found only in cardiomyocyte junctions of the large veins (arrows). White line defines outer cardiomyocyte boundary of the large pulmonary vein. Boxed insets (right) are vertically arranged as progressive increases in magnification to show cardiac junction staining. Nuclei are stained blue with Hoechst dye.
αT-cat is necessary for pulmonary vein inflammation
Since αT-cat expression in the lung is only detected in cardiomyocytes lining the pulmonary veins (PV)(9), we examined changes to the pulmonary venous system in response to HDM exposure. In WT mice, robust inflammatory cuffs formed around PV sheathed in cardiomyocytes after exposure to HDM, whereas αT-cat KO mice showed only sparse inflammation around these veins (Fig. 4A&B). To assess whether this inflammatory difference was specific to the presence of these cardiac cells, we examined the small PV where these cells are absent. Interestingly, we found that inflammatory cuffs around these small PV did not differ between the WT and αT-cat KO mice exposed to HDM (Fig. 4C&D). Thus together, these data indicate that the loss of αT-cat may lead to a cardiac cell-specific inhibition of inflammation around the PV. These findings also imply that these vessels may significantly contribute to airway inflammation (Fig. 1C).
Pulmonary veins may be important mediators of airway inflammation
If αT-cat is necessary for PV inflammation, how PV inflammation could potentially drive airway inflammation and AHR (Fig 1) is less clear. We previously reported that some PV run adjacent to airways(9), which could, in principle, connect PV inflammation and asthmatic airway disease. Indeed, among the WT mice, airway inflammation was noticeably more severe when directly adjacent to a PV (Fig. 5A). We quantified goblet cell metaplasia, a correlate of airway inflammation, as a function of its distance to the nearest PV. From this analysis, airways closest to PV showed significantly more severe airway goblet cell metaplasia than airways furthest from PV (Fig. 5B). When these data were analyzed by scatter-plot, regression analysis showed a significant relationship between the severity of airway inflammation and its proximity to a PV. These associative data suggest the possibility that αT-cat expression in cardiomyocytes along PV may contribute in an important way to allergic airway inflammation and disease.
The mechanism by which αT-cat loss protects veins from inflammation may relate to its compensation by the related molecule, αE-cat, as a previous study showed that αT-cat KO induced a compensatory increase in the expression αE-cat in the heart(7). Similar to this study, we observed a compensatory increase in the expression of αE-cat in PV cardiomyocytes of αT-cat KO mice when compared to WT (Fig. 5C). Since αE-cat is known to play an anti-inflammatory role in other tissues through an ability to attenuate NF-kB signaling(20, 21), we speculate that the reduction in inflammation around αT-cat KO PV may be due the compensatory up-regulation and anti-inflammatory activity of αE-cat.
DISCUSSION
A role for cardiomyocyte-containing pulmonary veins in asthma
We show that the cardiac junction protein αT-cat is necessary for the development of several classic manifestations of asthma, including airway inflammation, goblet cell metaplasia and increased resistance to methacholine challenge, in a commonly used murine model of allergic asthma. Our findings mechanistically support several human GWA studies linking αT-cat (CTNNA3) to steroid-resistant asthma in children(3) asthmatic exacerbations in children(4), and occupational asthma in adults (13, 14). Importantly, we found that loss of αT-cat is associated with a dramatic reduction in PV inflammation in the HDM murine model of atopic asthma, suggesting that cardiomyocytes within PV may contribute to the inflammatory milieu of adjacent airways. Although αT-cat is also expressed in cardiomyocytes of the heart(7), it is difficult to rationalize how its loss in the heart could to lead to reduced inflammation along brochovascular bundles in HDM-challenged lungs. Indeed, while αT-cat KO mice demonstrate an age-dependent cardiac enlargement and reduction in ejection fraction(7), these effects would be expected to enhance rather than reduce airway hyperresponsiveness. Thus, we reason that the contribution of αT-cat to asthma relates to its expression within PV that co-mingle with airways in the bronchovascular bundles. Ultimately distinguishing a PV-specific role for αT-cat will require that we uncover molecular differences between cardiomyocytes of the PV and heart to enable its selective deletion from each compartment. We and others have been unable to demonstrate expression of αT-cat in the smooth muscle or inflammatory cells in the lung(6, 9). Additionally, the low dose of HDM injected into the mice likely preferentially affected the large airways(22), but inflammatory cells tend to penetrate the lung through small peripheral capillaries(23). Therefore, the origin of the inflammatory cells surrounding the PV, particularly the large PV, and how αT-cat may affect their trafficking, remains unknown.
The protective effect on airway hyperresponsiveness seen in αT-cat KO mice contrasts with that observed previously in a mouse model of occupational asthma(9). When αT-cat KO mice were exposed to the chemical toluene diisocyanate, they developed increased AHR as quantified by plethysmography(9). However, this chemical model was limited in only inducing minimal inflammation(9), as opposed to the robust airway inflammation demonstrated by HDM models of atopic asthma. Therefore, differences between these murine models may account for the differential contribution of αT-cat to asthma phenotypes.
Remarkably, the most recent human genomic data identifying polymorphisms associated with asthma exacerbations in children(4) further support the notion of a cardiac cell/PV contribution to asthma, with αT-cat (CTNNA3) as the top-hit and two other associated genes with direct and established cardiac cell/PV functions(4). These included the sarcomere structural protein, titin, which is important for the contraction of cardiac tissues(24) and the secreted vascular guidance protein, semaphorin 3d, whose primary role is in patterning and development of the PV(25). Thus, our evidence that the cardiomyocyte cell adhesion protein, αT-cat, is necessary for the pathogenesis of asthma in the HDM murine model, together with the unexpected observation that titin and Semaphorin 3d are also associated with asthmatic exacerbations(4), suggest that PV structures or their cardiomyocyte constituents may be an important contributor to allergic airway disease.
Supplementary Material
KEY MESSAGES.
Mutations in αT-catenin have been shown to confer an increased risk of steroid resistant asthma in children.
In the lung, the expression of αT-catenin is restricted to cardiac cells adjacent to the pulmonary vein.
Genetic loss of αT-catenin reduces pulmonary vein and airway inflammation and attenuates the increase in airway hyperresponsiveness in an established murine model of allergic asthma.
αT-catenin-expressing cardiac cells adjacent to the pulmonary veins may play an unappreciated role in the pathogenesis of allergic asthma.
CAPSULE SUMMARY.
Polymorphisms in the cardiac protein αT-catenin (CTNNA3) have been associated with steroid-resistance and exacerbations in asthmatics. Using a murine model, we demonstrate that αT-catenin is permissive for the development of asthma, potentially through its regulation of pulmonary vein inflammation.
ACKOWLEDGEMENTS
We thank Robert Schleimer and Jing Liu (Northwestern University) for discussions and Annette S. Flozak for reading the manuscript. S.S.F. was supported by the National Institutes of Health (T32CA09560, F30 ES024622) and an American Heart Association pre-doctoral fellowship (15PRE21850010). C.J.G. is supported by the National Institutes of Health (GM076561).
ABBREVIATIONS
- AHR
airway hyperresponsiveness
- αT-cat
Alpha-T-catenin
- BAL
bronchoalveolar lavage
- GWA study
Genome-wide association study
- HDM
house dust mite
- KO
knockout
- PAS
Periodic-Acid Schiff
- PV
pulmonary vein
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: the authors have declared that no conflict of interest exists.
REFERENCES
- 1.Akinbami LJ, Moorman JE, Liu X. Asthma Prevalence, Health Care Use, and Mortality: United States, 2005–2009. National Health Statistics Reports. 2011 Jan 12;2011(32) [PubMed] [Google Scholar]
- 2.Adcock IM, Ito K. Steroid resistance in asthma: a major problem requiring novel solutions or a non-issue? Curr Opin Pharmacol. 2004 Jun;4(3):257–62. doi: 10.1016/j.coph.2004.02.001. PubMed PMID: 15140417. [DOI] [PubMed] [Google Scholar]
- 3.Perin P, Potocnik U. Polymorphisms in recent GWA identified asthma genes CA10, SGK493, and CTNNA3 are associated with disease severity and treatment response in childhood asthma. Immunogenetics. 2014 Mar;66(3):143–51. doi: 10.1007/s00251-013-0755-0. PubMed PMID: 24407380. [DOI] [PubMed] [Google Scholar]
- 4.McGeachie MJ, Wu AC, Tse SM, Clemmer GL, Sordillo J, Himes BE, et al. CTNNA3 and SEMA3D: Promising loci for asthma exacerbation identified through multiple genome-wide association studies. The Journal of allergy and clinical immunology. 2015 Jun 12; doi: 10.1016/j.jaci.2015.04.039. PubMed PMID: 26073756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991 May 31;65(5):849–57. doi: 10.1016/0092-8674(91)90392-c. PubMed PMID: 1904011. [DOI] [PubMed] [Google Scholar]
- 6.Janssens B, Goossens S, Staes K, Gilbert B, van Hengel J, Colpaert C, et al. alphaT-catenin: a novel tissue-specific beta-catenin-binding protein mediating strong cell-cell adhesion. J Cell Sci. 2001 Sep;114:3177–88. doi: 10.1242/jcs.114.17.3177. Pt 17. PubMed PMID: 11590244. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Goossens S, van Hengel J, Gao E, Cheng L, Tyberghein K, et al. Loss of alphaT-catenin alters the hybrid adhering junctions in the heart and leads to dilated cardiomyopathy and ventricular arrhythmia following acute ischemia. J Cell Sci. 2012 Feb 15;125:1058–67. doi: 10.1242/jcs.098640. Pt 4. PubMed PMID: 22421363. Pubmed Central PMCID: 3311935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hengel J, Calore M, Bauce B, Dazzo E, Mazzotti E, De Bortoli M, et al. Mutations in the area composita protein alphaT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. European heart journal. 2013 Jan;34(3):201–10. doi: 10.1093/eurheartj/ehs373. PubMed PMID: 23136403. [DOI] [PubMed] [Google Scholar]
- 9.Folmsbee SS, Morales-Nebreda L, Van Hengel J, Tyberghein K, Van Roy F, Budinger GR, et al. The cardiac protein alphaT-catenin contributes to chemical-induced asthma. American journal of physiology Lung cellular and molecular physiology. 2015 Feb 1;308(3):L253–8. doi: 10.1152/ajplung.00331.2014. PubMed PMID: 25480337. Pubmed Central PMCID: 4340121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wachtel MS, Shome G, Sutherland M, McGlone JJ. Derivation and validation of murine histologic alterations resembling asthma, with two proposed histologic grade parameters. BMC immunology. 2009;10:58. doi: 10.1186/1471-2172-10-58. PubMed PMID: 19878549. Pubmed Central PMCID: 2777149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013 Aug 29;500(7464):589–92. doi: 10.1038/nature12358. PubMed PMID: 23873040. Pubmed Central PMCID: 3758448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong BA, Li J, McDonough JM, Wei Z, Kim C, Chiavacci R, et al. Gene network analysis in a pediatric cohort identifies novel lung function genes. PloS one. 2013;8(9):e72899. doi: 10.1371/journal.pone.0072899. PubMed PMID: 24023788. Pubmed Central PMCID: 3759429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SH, Cho BY, Park CS, Shin ES, Cho EY, Yang EM, et al. Alpha-T-catenin (CTNNA3) gene was identified as a risk variant for toluene diisocyanate-induced asthma by genome-wide association analysis. Clin Exp Allergy. 2009 Feb;39(2):203–12. doi: 10.1111/j.1365-2222.2008.03117.x. PubMed PMID: 19187332. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein DI, Kashon M, Lummus ZL, Johnson VJ, Fluharty K, Gautrin D, et al. CTNNA3 (alpha-catenin) gene variants are associated with diisocyanate asthma: a replication study in a caucasian worker population. Toxicol Sci. 2013 Jan;131(1):242–6. doi: 10.1093/toxsci/kfs272. PubMed PMID: 22977168. Pubmed Central PMCID: 3537126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam AP, Herazo-Maya JD, Sennello JA, Flozak AS, Russell S, Mutlu GM, et al. Wnt coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014 Jul 15;190(2):185–95. doi: 10.1164/rccm.201401-0079OC. PubMed PMID: 24921217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S, et al. Alpha-Catenins Control Cardiomyocyte Proliferation by Regulating Yap Activity. Circ Res. 2014 Oct 10; doi: 10.1161/CIRCRESAHA.116.304472. PubMed PMID: 25305307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. Journal of immunology. 2004 Nov 15;173(10):6384–92. doi: 10.4049/jimmunol.173.10.6384. PubMed PMID: 15528378. [DOI] [PubMed] [Google Scholar]
- 18.Augusto A, Litonjua STW. Risk factors for asthma. UpToDate. 2015 [Google Scholar]
- 19.Clarke DL, Davis NH, Campion CL, Foster ML, Heasman SC, Lewis AR, et al. Dectin-2 sensing of house dust mite is critical for the initiation of airway inflammation. Mucosal immunology. 2014 May;7(3):558–67. doi: 10.1038/mi.2013.74. PubMed PMID: 24129160. Pubmed Central PMCID: 3998635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piao HL, Yuan Y, Wang M, Sun Y, Liang H, Ma L. alpha-catenin acts as a tumour suppressor in E-cadherin-negative basal-like breast cancer by inhibiting NF-kappaB signalling. Nature cell biology. 2014 Mar;16(3):245–54. doi: 10.1038/ncb2909. PubMed PMID: 24509793. Pubmed Central PMCID: 3943677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobielak A, Fuchs E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proceedings of the National Academy of Sciences of the United States of America. 2006 Feb 14;103(7):2322–7. doi: 10.1073/pnas.0510422103. PubMed PMID: 16452166. Pubmed Central PMCID: 1413714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MA, Stabenow JM, Parvathareddy J, Wodowski AJ, Fabrizio TP, Bina XR, et al. Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PloS one. 2012;7(2):e31359. doi: 10.1371/journal.pone.0031359. PubMed PMID: 22384012. Pubmed Central PMCID: 3286442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011 Sep 20;108(38):15990–5. doi: 10.1073/pnas.1110144108. PubMed PMID: 21880956. Pubmed Central PMCID: 3179042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010 May 18;121(19):2137–45. doi: 10.1161/CIRCULATIONAHA.109.860171. PubMed PMID: 20479164. Pubmed Central PMCID: 2905226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degenhardt K, Singh MK, Aghajanian H, Massera D, Wang Q, Li J, et al. Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Nature medicine. 2013 Jun;19(6):760–5. doi: 10.1038/nm.3185. PubMed PMID: 23685842. Pubmed Central PMCID: 3746328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.