Abstract
Prenatal alcohol exposure (PAE) can induce physical malformations and behavioral abnormalities that depend in part on the developmental timing of alcohol exposure. The current studies employed a mouse FASD model to characterize the long-term behavioral and brain structural consequences of a binge-like alcohol exposure during neurulation; a first-trimester stage when women are typically unaware that they are pregnant. Time-mated C57BL/6J female mice were administered two alcohol doses (2.8 g/kg, four hours apart) or vehicle starting at gestational day 8.0. Male and female adolescent offspring (postnatal day28–45) were then examined for motor activity (open field and elevated plus maze), coordination (rotarod), spatial learning and memory (Morris water maze), sensory motor gating (acoustic startle and prepulse inhibition), sociability (three-chambered social test), and nociceptive responses (hot plate). Regional brain volumes and shapes were determined using magnetic resonance imaging. In males, PAE increased activity on the elevated plus maze and reduced social novelty preference, while in females PAE increased exploratory behavior in the open field and transiently impaired rotarod performance. In both males and females, PAE modestly impaired Morris water maze performance and decreased the latency to respond on the hot plate. There were no brain volume differences; however, significant shape differences were found in the cerebellum, hypothalamus, striatum, and corpus callosum. These results demonstrate that alcohol exposure during neurulation can have functional consequences into adolescence, even in the absence of significant brain regional volumetric changes. However, PAE-induced regional shape changes provide evidence for persistent brain alterations and suggest alternative clinical diagnostic markers.
Keywords: Fetal Alcohol Spectrum Disorder, Behavior, Mouse, Brain, Ethanol
1. Introduction
It is well known that alcohol exposure during pregnancy can harm the developing fetus. Yet, many pregnant women continue to drink alcohol and alcohol exposure during pregnancy remains the single most preventable cause of birth defects. The constellation of physical and behavioral abnormalities that are caused by prenatal alcohol exposure (PAE) are classified under the term fetal alcohol spectrum disorders (FASDs), which ranges in severity from fetal alcohol syndrome (FAS) to alcohol-related neurodevelopmental disorder (ARND) or neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE). While distinctive facial features are critical to the diagnosis of FAS, the vast majority of individuals with PAE are not facially dysmorphic but can nonetheless suffer from persistent and pervasive behavioral impairments, including attention, memory, and executive function deficits (Mattson et al., 2011) as well as hyperactivity and motor incoordination (Jones and Smith, 1973, Hanson et al., 1976, Glass et al., 2014). Additionally, prenatal alcohol-exposed individuals are more susceptible to developing externalizing behavioral and mood disorders related to anxiety, depression, and substance abuse (Mattson and Riley, 2000, O’Connor and Paley, 2006, Alati et al., 2006). Unfortunately, the behavioral outcomes following PAE are extremely variable, frustrating attempts at consistent and comprehensive diagnosis.
The wide variety of physical and behavioral manifestations of FASDs is likely due to the interaction between factors such as genetics, maternal nutrition, and the amount, pattern and timing of alcohol exposure (Bailey et al., 2004, Riley et al., 2011). Since many of these variables are difficult to delineate in human studies, animal models are invaluable to understanding specific contributions to the overall FASD phenotype. The C57BL/6J (C57) mouse has proven especially useful for understanding the importance of the developmental timing of early acute binge-like alcohol exposure and studies in this strain have demonstrated that gastrulation and neurulation are two early developmental periods when the embryo is critically sensitive to alcohol (Sulik, 2005). In human development, these periods occur in the middle of the third week of gestation and end of the third week to early fourth week of gestation, respectively, times before most women realize that they are pregnant. Thus, even if a woman abstains from drinking alcohol once she realizes she is pregnant, inadvertent alcohol exposures during gastrulation and neurulation are likely.
During gastrulation, acute binge-like alcohol exposure can cause the loss of midline facial and brain structures, defects that fall within the holoprosencephaly spectrum (Godin et al., 2010a, Godin et al., 2011) and are recognized as the classic FAS face (Sulik et al., 1981). In contrast, alcohol exposure during neurulation induces subtle defects of the fetal face and brain, most notably a smaller cerebellum and right hippocampus, and a relatively expanded hypothalamic/diencephalon area, septal region, pituitary and cerebral ventricles, as well as shape alterations in the cortex, hippocampus, and striatum (Parnell et al., 2009, O’Leary-Moore et al., 2010, Parnell et al., 2013). The variety of effects of early gestational PAE on the fetal brain suggests that there may also be complementary behavioral changes. Unfortunately, the severity of brain defects following gastrulation PAE precludes a thorough examination because many of these mice do not survive after birth.
A few studies have examined the consequences of acute neurulation-stage alcohol exposure on a limited number of postnatal behavioral outcomes. Some studies have shown evidence for delayed sensorimotor development (Endres et al., 2005, Schambra et al., 2015), altered exploratory behavior (Endres et al., 2005) impaired spatial learning or memory during adolescence or adulthood (Summers et al., 2006, Incerti et al., 2010, Minetti et al., 1996, Summers et al., 2008), suggesting altered hippocampal function, but others have not (Sadrian et al., 2014). The research described herein was designed to further understand the consequences of neurulation alcohol exposure by examining a comprehensive battery of behavioral tests in adolescent male and female C57 mice that were exposed to acute alcohol injections on GD8, the beginning of neurulation. Based on the structural changes observed in the fetal brain, particularly the expansion of the septum and reduction of the cerebellum and hippocampus, it was hypothesized that GD8 alcohol exposure would affect measures of motor coordination, exploratory behavior, learning, and social behavior. In addition, to determine if the structural changes in the fetal brain would persist into adolescence, brain regional volumes and shapes were analyzed by magnetic resonance imaging.
2. Materials and methods
2.1. Mice
All experiments were conducted following the guidelines of the National Institutes of Health using methods approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. Female C57BL/6J mice (n=41treated dams) were obtained from The Jackson Laboratory (Bar Harbor, ME), weighed approximately 20g upon arrival, and were housed in groups of five or fewer in standard polycarbonate cages with cob bedding and cotton nesting material. Rodent chow (Isopro RMH 3000; Purina, St. Louis, MO) and tap water were freely available through the cage lid. Timed matings with male C57BL/6J mice began three hours into the light cycle and lasted one to two hours; gestational day 0, 0 hr (GD0) was defined as the beginning of the breeding period in which a copulation plug was detected. At GD8, separate groups of pregnant dams were administered two intraperitoneal (i.p.) injections, 4 hrs apart, of 23.7% (v/v) ethyl alcohol (Pharmaco-Aaper, Brookfield, CT) in a lactated Ringer’s solution at a dose of 2.8 g/kg, or the equivalent volume of the vehicle alone. Peak blood alcohol levels following this procedure have been shown to be approximately 380 mg/dl, 30 min after the second alcohol injection (Parnell et al., 2009). Alcohol- and control-treated litters were housed with their dams, culled to a maximum of 8 pups/litter at postnatal day 3 (PD3), and left undisturbed until weaning at PD28 when they were housed in same sex groups with their littermates. To control for litter effects, one to two male and female mice were randomly selected from each litter for subsequent behavioral experiments conducted between PD28–45 or for ex vivo imaging conducted at PD45.
2.2. Procedures
Behavioral experiments were conducted every weekday in the UNC Behavioral Phenotyping Core during the light portion of the 12:12hr light:dark schedule by experimenters who were blinded to the treatment conditions. The behavioral testing battery was performed in 14 male (8 litters) and 14 female (8 litters) vehicle-exposed mice and 12 male (8 litters) and 12 female (8 litters) prenatal alcohol-exposed mice. All mice were tested in the following testing order: rotarod trials 1–3; elevated plus maze; open-field; rotarod trials 4 and 5; acoustic startle/pre-pulse inhibition; Morris water maze acquisition; Morris water maze reversal; hot plate. Social behaviors were available from separate mice (Veh males n=11; PAE males n=12; Veh females n=11; PAE females n=11) tested on PD 28.
2.2.1. Rotarod
To measure motor coordination and balance, mice were placed on a rotating barrel of a rotarod apparatus (Ugo-Basile, Stoelting Co., Wood Dale, Il) which progressively accelerated from 3 rpm to 30 rpm during the maximum of a 5-min test. An observer recorded the latency to fall off or rotate around the top of the barrel during three repeated trials on the first day of testing and two repeated trials on the second day of testing. Each trial was separated by about 45 s.
2.2.2. Elevated Plus Maze
To measure exploration of an environment containing typically preferred and avoided spaces, mice were placed in the center of a metal maze elevated 50 cm above the floor that contained two open arms (30 cm length) and two closed arms (20 cm high walls). During the 5-min test, an observer recorded the number of entries and time spent in each of the arms. These data were used to calculate the percent of open arm time [(open arm time/total arm time) × 100], the total arm entries, and the percent of open arm entries [(open arm entries/total arm entries) × 100].
2.2.3. Open Field
To measure spontaneous motor activity and exploration of a novel environment, the mice were placed in the corner of an illuminated open field (41×41×30 cm) housed within a sound-attenuated chamber. The open field was equipped with upper and lower grids of photobeams for the detection of both horizontal and vertical activity as well as the position of the mouse in the chamber (Versamax System: Accuscan Instruments, Columbus, OH) which were calculated at 5-min intervals over the 60-min test. The primary measures reported were the time spent, and distance traveled, in the center of the chamber.
2.2.4. Acoustic Startle/Prepulse Inhibition
To measure the whole body startle response to a sudden loud noise and the effects of a preceding, less intense noise on the startle response, mice were tested with the San Diego Instruments SR-Lab system consisting of a Plexiglass cylinder atop of a piezoelectric transducer housed in a sound attenuating chamber containing a houselight, fan, and loudspeaker for the presentation of acoustic stimuli (background 70 dB). The transducer quantified vibrations caused by the mouse’s movements in the cylinder. Following a 5-min habituation period, the test session had 42 trials (six repetitions of seven different types): no-stimulus baseline trials; acoustic startle alone (40ms; 120dB); and prepulse trials (20ms; 74, 78, 82, 86, or 90dB) beginning 100ms before the startle stimulus. Trial types were presented randomly with an average inter-trial interval of 15 s (range: 10–20 s). The startle amplitude for each trial was the peak response during a 65-ms sampling window from the onset of the startle stimulus.
2.2.5. Morris Water Maze
To measure the ability to learn and remember the location of a hidden platform to escape from an aversive environment, mice were placed in one of four quadrants of a large, opaque water-filled, circular pool (122 cm diameter) in a room with distinct visual cues. The pool contained an escape platform (12 cm diameter) in a fixed location submerged just beneath the surface of the water. Each mouse had 60 s to find the platform and received 4 trials per day for 5 days to learn the location of the platform. If the platform was not found in 60 s, the mouse was placed on the platform for 10 s and then given the next trial. Each trial began in a different quadrant in a randomized order. Following acquisition of the platform location, a 60-s probe trial was conducted with the platform removed. During the reversal phase, the platform location switched to the opposite quadrant and the mice learned the new location over five days and a second probe trial was conducted. Movements of the mice were measured using video tracking (Ethovision, Noldus Information Technology, Wageningen, the Netherlands), calculating the latency to find the platform, swimming speed, and the durations of time spent near the wall or in the center of the pool. Latency to find the platform on each day and the percent of time spent in each quadrant of the probe trials were calculated as the primary dependent variables.
2.2.6. Hot Plate
To measure the sensitivity to a noxious, thermal stimulus, mice were placed on a 55 °C hot plate (IITC Life Science, Woodland Hills, CA) for a maximum of 30 s, while an observer recorded the latency for the mouse to lift or lick one of its hind legs from the surface.
2.2.7. Social Preference Testing
To measure the preference for a social stimulus over a non-social stimulus and the preference for a novel social stimulus over a familiar social stimulus, mice were placed in the center of a three-chamber Plexiglass box (60 × 42 × 20 cm) fitted with retractable doorways in the dividing walls. During an initial 10-min habituation phase, the doors were removed and the mice were allowed to freely explore the chambers. In the 10-min sociability phase, an unfamiliar same sex adult C57 mouse was placed in a wire screened protective cage in one of the side chambers while an empty protective cage was placed in the other side. In the final 10-min social novelty phase, a second unfamiliar conspecific was placed in the empty protective cage. Time spent in each chamber and the numbers of chamber entries were recorded using customized data acquisition software.
2.2.8. Image Processing
C57 male and female mice (Veh males=6;PAE males=5; Veh females= 5; PAE females= 6; from a total of 13 litters) were weaned at PD 28 and left undisturbed until GD45. They were deeply anesthetized with tribromo-ethanol (250 mg/kg, i.p.) and perfused with heparinized physiological saline followed by 10% formalin containing the MR contrast agent ProHance (10:1 formalin:ProHance). The brains were post-fixed in formalin overnight at 4°C and rehydrated in 0.1M phosphate buffered saline with ProHance (100:1 PBS:ProHance) at 4°C until they were scanned on a 9.4T magnet at the Duke University Center for In Vivo Microscopy. The field of view was 22mm × 11mm × 11mm, the TR was 100ms, and the TE was 11.82ms.
The data were processed using an in-house pipeline consisting of unbiased, atlas based, regional segmentation (Budin et al., 2013). The images were rigidly aligned (translation and rotation) (Johnson et al., 2007) to an external template for the C57 mouse (The C57 Brookhaven Atlas, Ma et al., 2005) and then skull-stripped using an atlas-based tissue classification method (Oguz et al., 2011). After skull-stripping, the images were deformably co-registered in a group-wise manner to compute an unbiased population average with diffeomorphic non-rigid registration using Advanced Normalization Tools (ANTS) (Avants et al., 2009). The C57 Brookhaven atlas (Ma et al., 2005) segmentation was then registered to the population average and then propagated to the individual subjects for region based analysis of volume. Each subject’s final segmentation was carefully checked by an anatomical expert for quality control.
2.3. Statistical Analysis
Behavioral data from male and female mice were analyzed separately because prenatal alcohol exposure was the primary independent variable of interest. Unpaired t-tests compared prenatal alcohol- and vehicle-exposed mice for the percentage of open arm time, percentage of open arm entries, and total arm entries on the elevated plus maze as well as the response latency on the hot plate. For all other measures two-way, mixed analyses of variance (ANOVAs) were performed with prenatal exposure as the between- and time or trial as the within- subjects factors. The data from the rotarod trials 1–3 were analyzed separately from trials 4–5. Significant F-tests were further analyzed with post-hoc Bonferroni comparisons with alpha levels set at p<0.05.
Region-based statistics were calculated for each subject and included volumes, means, and standard deviations of the intensity in the segmented regions or over the whole mask. Regions of interest (ROIs) used for this study include: cerebellum; corpus callosum; hippocampus; hypothalamus; septum; striatum; thalamus; and 3rd ventricle. Spherical HARMonic representation Point Distributed Models (SPHARM-PDM)(Styner et al., 2006) were used to generate 3D meshes of the ROIs which were extracted from each subject’s atlas segmentation. ROIs were used as inputs to generate first a spherical harmonic representation of 3D shape and then a correspondent triangulated surface. After ensuring data integrity and correspondence establishment, individual PDMs of regions were grouped by treatment condition (data from male and female brains were combined). 3D models were separated into different treatment groups and compared using a permutation testing-based multivariate analysis of covariance (Paniagua et al., 2009). The outputs of these analyses include heat maps indicating areas of statistically significant shape differences and color-coded projections of those differences between the mean 3D shape of control and ethanol-exposed subjects groups. P-value maps were corrected for false positives using a false-discovery rate (FDR) correction (α=0.05) applied for multiple comparisons. The magnitude and direction of these shape changes were visualized in Shape Population Viewer and in-house open source software for the visualization of 3D shapes (https://www.nitrc.org/projects/shapepopviewer/).
3. Results
3.1. Gross morphology
PAE did not affect post-natal body weights in either sex. The average body weights across test days for male mice were: 18.9±0.3 and 18.9±0.5 for vehicle- and alcohol-exposed mice, respectively; and 16.0±0.3 and 16.0±0.2 for vehicle- and alcohol-exposed females, respectively. No gross morphological or neurobehavioral anomalies were observed in any of the mice.
3.2. Rotarod
The latency to fall off the rotarod apparatus increased over successive trials for all groups of mice (Table 1). PAE did not affect rotarod performance in the male mice. However, in the female mice, there was a significant interaction between prenatal exposure and trial on the first day of testing (F2,48 = 4.5; p=0.02; Table 1) such that PAE reduced the latency to fall off the rotarod during the first trial only (p=0.02).
Table 1.
Effects of GD8 Alcohol Exposure on the Latency (s) to Fall off the Rotarod in Adolescent Male and Female Mice.
| Rotarod Trial | 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|---|
| Males | ||||||
| Veh | 151±23 | 160±25 | 191±19 | 200±21 | 241±17 | |
| ALC | 132±21 | 161±24 | 187±29 | 211±25 | 270±17 | |
| Females | ||||||
| Veh | 172±26 | 153±28 | 242±22 | 225±24 | 243±25 | |
| ALC | 96±11* | 191±20 | 257±15 | 230±22 | 243±24 |
All data are expressed as mean (± SEM). Trials 1–3 were conducted on Day 1, and trials 4–5, on Day 2 of testing. Asterisk denotes significance vs. Veh, p<0.05.
3.3. Elevated plus maze and open field
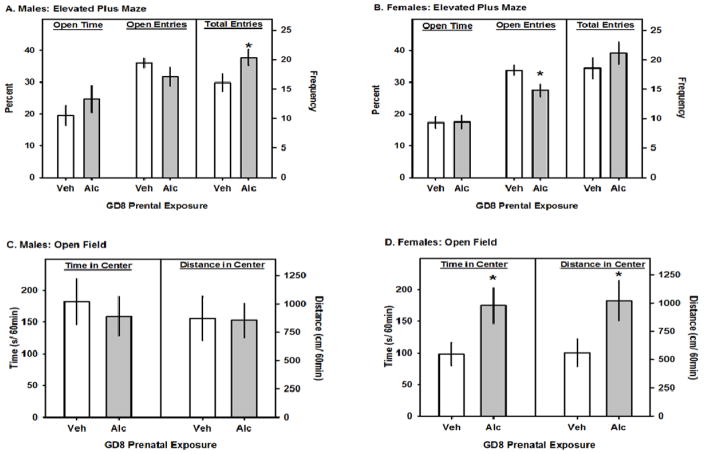
PAE increased the total number of entries made by male mice into the open and closed arms (t24= 2.1; p=0.048; Fig 1A), but did not affect the total entries made by the female mice (Fig 1B). However, in the female mice PAE decreased the percent of open arm entries (t24= 2.5; p=0.019; Fig 1A) but did not affect the duration of time spent on the open arms (Fig 1A and Fig 1B). On the open field test, PAE did not affect any activity measure in the male mice (Fig 1C). However, as indicated by significant main effects of PAE in the female mice, PAE increased the amount of time spent (F1,264 = 5.6; p=0.03; Fig 1D), and the distance traveled (F1,264 = 4.7; p=0.04; Fig 1D), in the center of the open field. PAE did not significantly affect any other open field activity measure.
Fig. 1.
The effects of GD8 alcohol exposure on elevated plus maze and open field behavior. A and B. In each panel the left pairs of bars represent the percentage of time on the open arms and correspond to the left y-axis. The center pairs of bars represent the percentage of total arm entries that were made onto the open arms and correspond to the left y-axis. The total arm entries are represented by the right pair of bars and correspond to the right y-axis. C and D. In each panel the left pairs of bars represent the total time that was spent in the center of open field and correspond to the left y-axis. The right pairs of bars represent the total time distance (cm) that was travelled in the center of open field and correspond to the right y-axis. All data are means ± 1 SEM and asterisks denote significance vs. Veh, p<0.05.
3.4. Social preference
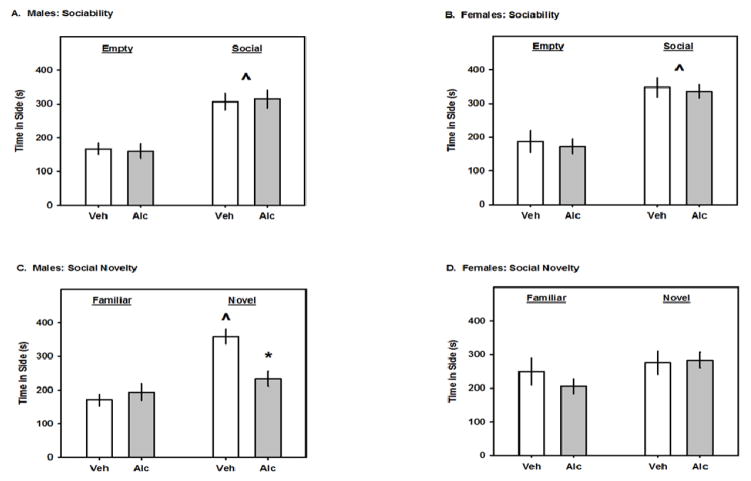
In the sociability test, all groups of mice spent more time on the chamber side with the social partner than they did with the empty cage; there were no significant effects of PAE on sociability (Fig 2A and 2B). However, in the social novelty preference test, there was a significant interaction between prenatal exposure and the time spent in each side for male (F1,20 = 7.22; p=0.01; Fig 2C), but not female mice. Subsequent post-hoc analysis revealed that vehicle-treated adolescent male spent significantly more time on the side with the novel partner than with the familiar partner (p<0.001), while prenatal alcohol exposed mice did not. As compared to vehicle exposure, PAE significantly decreased the time spent on the side with the novel partner (p<0.001). There were no significant effects of PAE in adolescent female mice (which did not prefer the novel partner).
Fig. 2.
The effects of GD8 alcohol exposure on social behaviors in the three-chambered test. A and B. In each panel, the left and right pairs of bars represent the time spent on the side that contained the non-social, or the social stimulus, respectively. C and D. In each panel, the left and right pairs of bars represent the time spent on the side that contained the familiar, or novel, social stimulus, respectively. All data are means ± 1 SEM. Carets denote significance vs. Empty or Familiar and asterisks denote significance vs. Veh, p<0.05.
3.5. Acoustic startle/Prepulse inhibition
All mice reacted to the acoustic startle stimulus and demonstrated inhibition of startle responses with presentation of aprepulse, dependent on the decibel level (Table 2). PAE did not significantly affect any measure of startle reactivity or pre-pulse inhibition.
Table 2.
Effects of GD8 Alcohol Exposure on Startle Response and Pre-Pulse Inhibition (PPI) in Adolescent Male and Female Mice
| Trial | Baseline | Startle | PP 74 dB | PP78 dB | PP82 dB | PP 86 dB | PP 90 dB | |
|---|---|---|---|---|---|---|---|---|
| Males | ||||||||
| Veh | 9.8±0.9 | 576±31 | 504±37 | 441±35 | 419±37 | 376±39 | 315±37 | |
| ALC | 11.6±1.3 | 531±45 | 446±49 | 422±41 | 412±56 | 343±44 | 292±48 | |
| Females | ||||||||
| Veh | 10.8±1.1 | 545±42 | 485±39 | 446±27 | 445±30 | 374±27 | 316±22 | |
| ALC | 8.9±0.9 | 462±35 | 410±31 | 415±38 | 382±33 | 335±30 | 297±33 |
Data are expressed as mean amplitude (± SEM).
3.6. Morris water maze
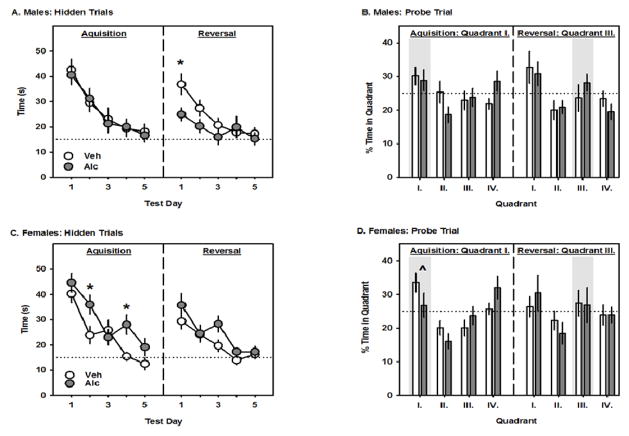
During the acquisition phase, all groups of mice found the location of the hidden platform (quadrant I) faster on the 5th day of testing than they did on the 1st day. While PAE did not affect the latency to find the hidden platform in the male mice (Fig 3A), there was a significant interaction between PAE and test day in the female mice (F4,96 = 3.4; p=0.01; Fig 3C). Post-hoc analysis revealed that PAE increased the latency to find the hidden platform on the second and fourth test days. When the location of the hidden platform was switched to the opposite quadrant, there was a significant interaction between PAE and test day in the male (F4,96 = 4.2; p=0.004; Fig 3A) but not the female mice (Fig 3C). Post-hoc analysis revealed that PAE significantly decreased the latency to find the hidden platform on the first test day. During the acquisition probe test, there was a trend for an interaction between PAE and quadrant (p=0.066) and a significant main effect of quadrant in the female mice (F3,72 = 12.6: p<0.001; Fig 3D) but not the male mice (Fig 3B). Post-hoc analysis revealed that when collapsed across prenatal treatment, female mice spent more time in the quadrant that previously contained the hidden platform (quadrant I) as compared to quadrants II and III. During the reversal probe, neither the male nor female mice spent significantly more time in the quadrant that had been the new location of the hidden platform (Figure 3B and 3D).
Fig. 3.
The effects of GD8 alcohol exposure on Morris water maze performance. A and C. The left and right panels represent the latency (s) to find the hidden platform during the acquisition and reversal phases, respectively. B and D. The left and right panels represent the percentage of time spent in each quadrant during the probe trials for the acquisition and reversal phases, respectively. All data are means ± 1 SEM and asterisks denote significance vs. Veh, while the caret denotes a significance vs. other quadrants, p<0.05. The horizontal dotted lines represent a 15 s criterion during the hidden trials and an equal quadrant preference during the probe trials. Shaded bars highlight the correct quadrant for each phase.
3.7. Hot plate
PAE significantly reduced the latency to lift or lick the hindpaw on the hot plate test in both the male (t24=3.2; p=0.003; Fig 4) and female (t24=2.8; p=0.01; Fig 4) mice.
Fig. 4.
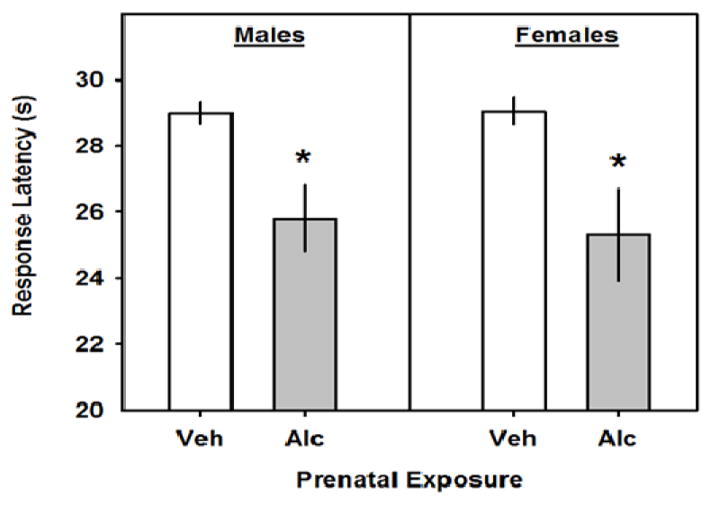
The effects of GD8 alcohol exposure on the latency to respond on the hot plate. The left and right pairs of bars represent the latency to lift or lick the hindpaw for male and female mice, respectively. All data are means ± 1 SEM and asterisks denote significance vs. Veh.
3.8. Brain volumes and shapes
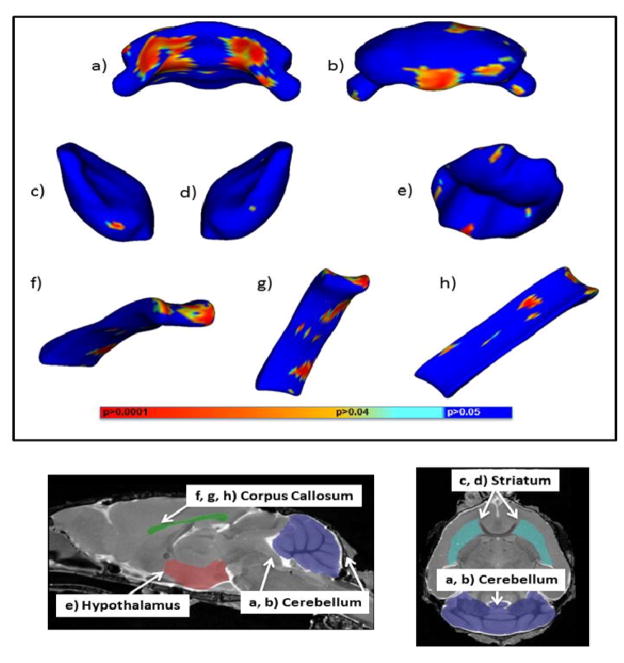
PAE did not significantly affect the volume of any of the brain regions analyzed (Table 3) in either the male or female mice. However, when the data from both sexes were combined, PAE was found to significantly affect the shape of the striatum, hypothalamus, cerebellum, and corpus callosum. In the cerebellum, PAE, as compared to vehicle treatment, significantly reduced the anterior medial area, on both left and right sides of the aqueduct and central gray, while it significantly increased the posterior lower medial area of the cerebellum (Figure 5A). PAE significantly also increased the size of the ventral edge of the left striatum and an area near the medial preoptic area on the anterior face of the hypothalamus (Figure 5 B&C). In the corpus callosum, PAE significantly increased the size of the splenium and the ventral surface of the midbody of the corpus callosum (Fig 6A) while it significantly decreased the size of the dorsal surface of the midbody (Fig 6B). PAE also significantly increased the dorsal surface of the genu, while it significantly decreased the ventral surface of the genu (Fig 6C).
Table 3.
Effects of GD8 Alcohol Exposure on Regional Brain Volumes in Adolescent Male and Female Mice
| Treatment Group | ||||
|---|---|---|---|---|
| Brain Region | Veh Males | ALC Males | Veh Females | ALC Females |
| Cerebellum | 53.8 ± 0.9 | 52.2 ± 1.2 | 52.6 ± 2.2 | 50.1 ± 3.0 |
| Corpus callosum | 0.92 ± 0.03 | 0.96 ± 0.08 | 0.98 ± 0.06 | 1.0 ± 0.07 |
| Hippocampus | 22.8 ± 0.8 | 22.3 ± 0.6 | 22.6 ± 1.0 | 22.9 ± 0.7 |
| Hypothalamus | 8.4 ± 0.4 | 8.8 ± 0.3 | 7.9 ± 0.4 | 7.7 ± 0.2 |
| Septum | 2.1 ± 0.08 | 2.1 ± 0.07 | 2.3 ± 0.09 | 2.1 ± 0.08 |
| Striatum | 21.5 ± 0.6 | 21.9 ± 0.8 | 22.1 ± 0.6 | 22.2 ± 0.5 |
| Thalamus | 24.0 ± 0.7 | 23.6 ± 0.2 | 23.3 ± 0.8 | 23.8 ± 0.4 |
| Third ventricle | 0.47 ± 0.06 | 0.41 ± 0.1 | 0.46 ± 0.06 | 0.53 ± 0.06 |
All data are reported in mm3 and expressed as the mean ± SEM.
Fig. 5.
The effects of GD8 alcohol exposure on regional brain shapes. Top panel. Areas portrayed in blue are statistically unchanged from vehicle, while areas in other colors are significantly different between alcohol and vehicle treated mice. a and b) portray the anterior and posterior views of the cerebellum. c and d) portray the left and right striatum. e) portrays the anterior view of the hypothalamus. f, g, and h) portray the anterior, ventral, and dorsal views of the corpus callosum The colored bars indicate levels of significance. Bottom panel. Parasagittal and horizontal MRI images of a PD 45 mouse brain highlighting regions in which significant shape changes had occurred.
4. Discussion
The current results demonstrate that acute, binge-like alcohol exposure during the early gestational phase of neurulation can have consequences on several types of adolescent male and female mouse behavior. Exploratory and social behaviors were most affected by GD8 PAE, while the significant changes in motor coordination, learning and memory, and reactivity to a noxious stimulus were relatively minor. Interestingly, while GD8 PAE did not induce significant changes in regional brain volumes during adolescence, the detection of shape changes in major structures suggests that brains were indeed altered and illustrates the fact that an apparent absence of structural abnormalities on clinical neuroimaging assessments does not preclude the possibility of brain damage. Taken together with the results of prior fetal brain imaging studies, the current results suggest that GD8 PAE-induced malformations of the fetal brain initiate a course of heterogeneous behavioral alterations that can be measured in adolescent male and female mice.
4.1 GD8 PAE Effects on Exploration
Exploratory behavior is an essential component of an animal’s behavioral repertoire and alterations in activity are frequently observed developmental consequences of prenatal teratogen exposures. The two exploratory behavior assays included in the current test battery were the elevated plus maze and open field test, both of which have regions that mice tend to prefer (i.e. closed arms and the edges of the open field) or avoid (i.e. open arms and the center of the open field). In the female mice, PAE disinhibited the avoidance of the center of the open field, as indicated by a greater time spent, and distance travelled (ca. 80% for each measure), in the center of the open field. There was no change detected in the open field exploratory behavior of male mice, perhaps because of the already high level of baseline exploration. However, PAE increased total activity on the elevated plus maze by about 25%. A trend for increased elevated plus maze activity was also observed in the prenatal alcohol exposed female mice and the lower percent of open arm entries reflects an increased number of closed arm entries. Although hyperactivity is often a symptom of FASD, the fact that the activity increase in the male and female mice was specific to different tests, and therefore a different set of environmental stimuli, suggests that GD8 alcohol exposure alone does not induce general hyperactivity. Behavior on the elevated plus maze and in the center region of the open field can be interpreted as related to fear and anxiety and it is possible that PAE may impair the evaluation of novel stimuli as safe or threatening. Similar phenotypes of disinhibited activity have been observed in mouse models for novelty seeking and impulsivity (e.g. Matzel et al., 2008, Loos et al., 2009) and may be related to the tendency of prenatal alcohol-exposed people to engage in high risk behaviors such as drug abuse.
4.2 GD8 PAE Effects on Social Behaviors
Social deficits are increasingly recognized as being common in individuals who have been prenatally exposed to alcohol (Kully-Martens et al., 2012). In animal models, a variety of PAEs affect mother-pup interactions, play behavior, aggressive behavior, sexual behavior and social preferences (Kelly et al., 2000). In the current study, the three-chambered social test was selected because of its demonstrated sensitivity to the influences of genetic mutations, especially those related to autism spectrum disorders (Kas et al., 2014). All groups of mice spent more time with the social partner than they did the non-social object indicating that PAE did not affect “sociability”. However, in the social novelty phase, which requires more complex sensory processing, PAE inhibited the preference for social novelty partner in male mice by about 35%. Surprisingly, neither group of female mice preferred the novel mouse, precluding the ability to detect a suppressive effect of PAE. The selective effect of PAE on social novelty in the male mice is similar to a number of mouse models for neurodevelopmental disorders (e.g. Brunssen et al., 2013, Ey et al., 2011) in which sensory processing and social motivations have been impaired. The neural circuitry associated with altered social behavior in prenatal alcohol-exposed rodents is thought to include the somatosensory cortex, frontal cortex, amygdala, and nucleus accumbens (Lawrence et al., 2008, Hamilton et al., 2010a, Hamilton et al., 2010b). Although specific volume defects were not observed following GD8 alcohol exposure, shape defects of the corpus callosum suggest some abnormal wiring may have occurred that would impact function. Hemispheric connectivity has been associated with altered social behavior both in humans and other mouse studies (Minshew and Williams, 2007, Brunssen et al., 2013). Additionally, the expansion of the third ventricle in the fetal brain studies as well as the shape changes currently observed in the adolescent anterior hypothalamus indicate that hypothalamic function may be compromised which would also likely alter social motivations. Further in depth analyses of adolescent brains will help uncover cytoarchitectural changes associated with changes in social preferences.
4.3 GD8 PAE Effects on Sensory Reactivity
Sensory processing defects have been noted in FAS and FASD individuals (Jirikowic et al., 2008) and the current experiments tested whether GD8 alcohol exposure affected acoustic startle, pre-pulse inhibition, and hindpaw response to a thermal stimulus. Although PAE did not affect acoustic startle or pre-pulse inhibition, PAE significantly reduced the latency to react to the thermal stimulus of the hot plate in male and female mice by about 14%. This apparent increased nociceptive sensitivity is similar to findings in adolescent rats that had been exposed to alcohol during the postnatal period (Rogers et al., 2004), suggesting that prenatal alcohol exposure can alter the perception of and/or reactivity to noxious stimuli. However, adult C57 mice that had been exposed to alcohol during early gestation were not more sensitive to thermal stimulation (Sanchez Vega et al., 2013, Fish et al., 2015) indicating that recovery of nociceptive thresholds following PAE, like other teratogens exposure, may be possible with age and/or environmental experience (Schneider et al., 2006). Hyper-responsiveness to stressors has been shown in adults following fetal alcohol exposure and it is possible that the altered nociceptive thresholds observed in the current study are related to exaggerated stress reactivity (e.g. Weinberg et al., 2008).
4.4 GD8 PAE Effects on Motor Coordination
Motor coordination impairments are frequently observed symptoms of FAS and FASD and these can be observed in other animal models of prenatal alcohol exposure (Schneider et al., 2011). Based on cerebellar volumes in the fetal brain, GD8 alcohol was expected to induce deficits on the rotarod test. In the current study, prenatal alcohol exposed female mice fell off the rotarod about 44% faster than did the vehicle exposed female mice, on the first rotarod trial only. Since the rotorod was the first test of the battery, it is possible that the reactivity to the novelty of being handled by the experimenter could have influenced motor performance selectively in the prenatal alcohol exposed female mice. Performance improved equally in all groups of mice over successive trials, indicating that the initial deficit could be overcome with practice. This also suggests that more severe motor defects may have been present earlier in postnatal life and that these could have ameliorated with repeated motoric experiences. Surprisingly, unlike the fetal brains, there were no differences in the cerebellar volumes of adolescent mice, suggesting a compensatory growth occurred during the late pre-natal, and/or post-natal periods. Post-natal structural recovery of several brain regions has been shown in a mouse model of alcohol exposure throughout pregnancy and, in that study, motor deficits were detected in the absence of concurrent structural defects (Abbott et al., 2016). As was suggested by those authors, structural defects that are apparent very early in development may be most predictive of later behavioral impairments. The changes in cerebellar shape imply possible alterations in migration patterns and while these may have influenced motor coordination, further detailed histological studies as well as tests of finer motor function (El Shawa et al., 2013, Abbott et al., 2016) will be necessary to precisely test the significance of the fetal cerebellar deficits and adolescent cerebellar shape changes.
4.5 GD8 PAE Effects on Learning and Memory
Learning deficits are a hallmark of FAS and FASD and in the current study there was evidence that GD8 alcohol exposure produced aminor impairment of Morris water maze performance. Mice can use both spatial and non-spatial strategies to find the hidden platform location and the relatively poor performance of both the male and female mice during the probe trials suggests that these adolescent mice relied heavily on non-spatial strategies (Schenk, 1985). Nonetheless, during the acquisition phase of the procedure prenatal alcohol-exposed females took significantly longer (ca. 35%) to find the hidden platform on two of the test days, indicating a performance deficit. Following acquisition, when the location of the platform was switched to the opposite quadrant, prenatal alcohol-exposed male mice took about 32% less time to find the hidden platform than did the vehicle-exposed mice. This suggests that PAE could have impaired memory retention for the previously correct quadrant and/or shifted the male mice to a more aggressive search strategy which would increase the probability of finding the platform in its new location. Continued evaluation of altered learning and memory in prenatal alcohol exposed mice will require experimentally naïve mice and tests such as fear-conditioning (Brady et al., 2012, Caldwell et al., 2014) which may be less affected by potential differences in motor ability. It is also noteworthy that while GD8 alcohol exposure reduced the size of the hippocampus in fetal mice, neither volume nor significant shape changes were detected in the adolescent hippocampus indicating this region’s capacity for post-natal recovery.
4.6 Sex Differences
Male and female offspring often have different responses to teratogens (for review see, DiPietro and Voegtline, 2015), and in animal models, there are several reports that show sex differences in the behavioral and neuroendocrinological measures after PAE (e.g. McGivern et al., 1984, Zimmerberg et al., 1991, Weinberg et al., 2008, Varlinskaya and Mooney, 2014, Wieczorek et al., 2015). Although not statistically significant, vehicle-treated female mice in the current study tended to be more active on the elevated plus maze, less active in the center of the open field, and have less social novelty preference, as compared to vehicle-treated male mice. PAE essentially diminished these baseline sex differences in exploratory and social behaviors; PAE increased the activity of male mice on the elevated plus to the level of female mice, increased the activity of female mice in the center of the open field to the level of the male mice, and reduced the social novelty preference of male mice to the level of female mice. A possible explanation for why one sex showed an effect of PAE and why the other did not, is that ceiling and floor effects limited the behavioral variability of each test. It is possible that adjusting the testing parameters to minimize the baseline sex differences could enable PAE effects to be detected equally in both male and female mice. However, this would not explain the sex difference in performance on the rotarod or in the water maze. Moreover, sex differences following acute GD7 alcohol exposure were observed in adult mice even in the absence of baseline behavioral differences between males and females on the elevated plus maze, forced swim test, and light:dark box activity (Wieczorek et al., 2015).
The sex differences in adolescent behavior following early gestational alcohol are likely due to the interaction between the initial sensitivity of female and male embryos to alcohol and the altered sensitivity to future developmental events (e.g. development of the genital ridge, Sry expression, and prenatal androgen exposure). Structural abnormalities in the hypothalamus and pituitary are common in young fetuses after early gestational alcohol (Godin et al., 2010b, O’Leary-Moore et al., 2010, Parnell et al., 2013) and these may become functional differences as sexual differentiation takes place. Since the sex differences detected in the current study were observed before the typical onset of puberty, they are more likely to reflect interaction between PAE and in utero hormone exposure, rather than the levels of gonadal steroids circulating at the time of testing. There is evidence that alcohol exposure throughout gestation can alter the sensitivity to in utero androgen exposure (McGivern et al., 1988), which can “masculinize” and “feminize” brain regions and behavior measured as early as PD3 in rats (Zimmerberg and Reuter, 1989). Whether PAE during neurulation, a time well before the peak of fetal androgen exposure (Ward and Weisz, 1980), alters subsequent prenatal sensitivity to steroids to initiate a sex-specific behavioral trajectory is not known and is an important area for future study. Finally, there is evidence during neurulation that male mouse embryos are older, as determined by somite number, than are female mouse embryos (Seller and Perkins-Cole, 1987, Brook et al., 1994), suggesting that acute alcohol exposure could alter different developmental events in males and females solely on the basis of age. Studies aimed at understanding the immediate consequences of acute alcohol exposure during neurulation will help further interpret the sex differences in adolescent behavioral outcomes.
5. Conclusions
The results of these experiments are clinically significant in two important ways. First, it is necessary to recognize and disseminate the fact that a single binge-like alcohol exposure during the early embryonic period, a time before women typically recognize that they are pregnant, can have lasting behavioral consequences in a mouse model. Second, the finding of PAE-induced neurofunctional abnormalities in the absence of gross brain volumetric changes (albeit with shape alterations) further substantiates the need for more research on the full spectrum of PAE’s effects on behavior and cognition. That functional deficits can occur without major structural changes suggests that children with potential PAE cannot be examined by neuroimaging techniques alone. Additionally, this study aids in discovering the pathological mechanisms that underlie the behavioral deficits. For example, the cerebellar abnormalities in alcohol-exposed mice are likely the result of cell death along the rostal rhombic lip (Dunty et al., 2001). However, cell death is unlikely to be the causative mechanism for the alcohol-rostral midline enlargements observed in both the fetal and adolescent brain. Continued analysis of the pathological events following binge-like exposures will provide possible mechanisms for these structural and functional defects.
Highlights.
An acute neurulation stage alcohol exposure affected motor activity and social behavior, and had minor effects on Morris water maze performance and hot plate reactivity in adolescent male offspring
An acute neurulation stage alcohol exposure affected motor activity, and had minor effects on Morris water maze performance and hot plate reactivity in adolescent female offspring
The behavioral effects occurred in the absence of differences in regional brain volumes as measured by MRI
The shapes of the cerebellum, hypothalamus, striatum and corpus callosum were significantly altered in neurulation stage alcohol-exposed adolescents
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABBOTT CW, KOZANIAN OO, KANAAN J, WENDEL KM, HUFFMAN KJ. The Impact of Prenatal Ethanol Exposure on Neuroanatomical and Behavioral Development in Mice. Alcoholism, clinical and experimental research. 2016;40:122–33. doi: 10.1111/acer.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALATI R, AL MAMUN A, WILLIAMS GM, O’CALLAGHAN M, NAJMAN JM, BOR W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Archives of general psychiatry. 2006;63:1009–16. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- AVANTS B, TUSTISON N, SONG G, GEE J. ANTS: Advanced open-source normalization tools for neuroanatomy. 2009. [Google Scholar]
- BAILEY BN, DELANEY-BLACK V, COVINGTON CY, AGER J, JANISSE J, HANNIGAN JH, SOKOL RJ. Prenatal exposure to binge drinking and cognitive and behavioral outcomes at age 7 years. American journal of obstetrics and gynecology. 2004;191:1037–43. doi: 10.1016/j.ajog.2004.05.048. [DOI] [PubMed] [Google Scholar]
- BRADY ML, ALLAN AM, CALDWELL KK. A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcoholism, clinical and experimental research. 2012;36:457–66. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOK FA, ESTIBEIRO JP, COPP AJ. Female predisposition to cranial neural tube defects is not because of a difference between the sexes in the rate of embryonic growth or development during neurulation. Journal of medical genetics. 1994;31:383–7. doi: 10.1136/jmg.31.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNSSEN SH, MOY SS, TOEWS AD, MCPHERSON CA, HARRY GJ. Interleukin-6 (IL-6) receptor/IL-6 fusion protein (Hyper IL-6) effects on the neonatal mouse brain: possible role for IL-6 trans-signaling in brain development and functional neurobehavioral outcomes. Brain, behavior, and immunity. 2013;27:42–53. doi: 10.1016/j.bbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUDIN F, HOOGSTOEL M, REYNOLDS P, GRAUER M, O’LEARY-MOORE SK, OGUZ I. Fully automated rodent brain MR image processing pipeline on a Midas server: from acquired images to region-based statistics. Frontiers in neuroinformatics. 2013;7:15. doi: 10.3389/fninf.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL KK, GOGGIN SL, TYLER CR, ALLAN AM. Prenatal alcohol exposure is associated with altered subcellular distribution of glucocorticoid and mineralocorticoid receptors in the adolescent mouse hippocampal formation. Alcoholism, clinical and experimental research. 2014;38:392–400. doi: 10.1111/acer.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIPIETRO JA, VOEGTLINE KM. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNTY WC, JR, CHEN SY, ZUCKER RM, DEHART DB, SULIK KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcoholism, clinical and experimental research. 2001;25:1523–35. [PubMed] [Google Scholar]
- EL SHAWA H, ABBOTT CW, 3RD, HUFFMAN KJ. Prenatal ethanol exposure disrupts intraneocortical circuitry, cortical gene expression, and behavior in a mouse model of FASD. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:18893–905. doi: 10.1523/JNEUROSCI.3721-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDRES M, TOSO L, ROBERSON R, PARK J, ABEBE D, POGGI S, SPONG CY. Prevention of alcohol-induced developmental delays and learning abnormalities in a model of fetal alcohol syndrome. American journal of obstetrics and gynecology. 2005;193:1028–34. doi: 10.1016/j.ajog.2005.05.052. [DOI] [PubMed] [Google Scholar]
- EY E, LEBLOND CS, BOURGERON T. Behavioral profiles of mouse models for autism spectrum disorders. Autism research: official journal of the International Society for Autism Research. 2011;4:5–16. doi: 10.1002/aur.175. [DOI] [PubMed] [Google Scholar]
- FISH EW, WIECZOREK LA, PARNELL SE. Neurobehavioral Consequences of Early Gestational Binge-Like Alcohol Exposure: Age-Related Alterations in Male and Female C57bl/6j Mice. Alcoholism-Clinical and Experimental Research. 2015;39:165a–165a. [Google Scholar]
- GLASS L, GRAHAM DM, DEWEESE BN, JONES KL, RILEY EP, MATTSON SN. Correspondence of parent report and laboratory measures of inattention and hyperactivity in children with heavy prenatal alcohol exposure. Neurotoxicology and teratology. 2014;42:43–50. doi: 10.1016/j.ntt.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODIN EA, DEHART DB, PARNELL SE, O’LEARY-MOORE SK, SULIK KK. Ventromedian forebrain dysgenesis follows early prenatal ethanol exposure in mice. Neurotoxicology and teratology. 2011;33:231–9. doi: 10.1016/j.ntt.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODIN EA, O’LEARY-MOORE SK, KHAN AA, PARNELL SE, AMENT JJ, DEHART DB, JOHNSON BW, ALLAN JOHNSON G, STYNER MA, SULIK KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcohol Clin Exp Res. 2010a;34:98–111. doi: 10.1111/j.1530-0277.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODIN EA, O’LEARY-MOORE SK, KHAN AA, PARNELL SE, AMENT JJ, DEHART DB, JOHNSON BW, ALLAN JOHNSON G, STYNER MA, SULIK KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcoholism, clinical and experimental research. 2010b;34:98–111. doi: 10.1111/j.1530-0277.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON DA, AKERS KG, RICE JP, JOHNSON TE, CANDELARIA-COOK FT, MAES LI, ROSENBERG M, VALENZUELA CF, SAVAGE DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behavioural brain research. 2010a;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON DA, CANDELARIA-COOK FT, AKERS KG, RICE JP, MAES LI, ROSENBERG M, VALENZUELA CF, SAVAGE DD. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behavioural brain research. 2010b;214:66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON JW, JONES KL, SMITH DW. Fetal alcohol syndrome. Experience with 41 patients. JAMA. 1976;235:1458–60. [PubMed] [Google Scholar]
- INCERTI M, VINK J, ROBERSON R, WOOD L, ABEBE D, SPONG CY. Reversal of alcohol-induced learning deficits in the young adult in a model of fetal alcohol syndrome. Obstetrics and gynecology. 2010;115:350–6. doi: 10.1097/AOG.0b013e3181cb59da. [DOI] [PubMed] [Google Scholar]
- JIRIKOWIC T, KARTIN D, OLSON HC. Children with fetal alcohol spectrum disorders: a descriptive profile of adaptive function. Canadian journal of occupational therapy. Revue canadienne d’ergotherapie. 2008;75:238–48. doi: 10.1177/000841740807500411. [DOI] [PubMed] [Google Scholar]
- JOHNSON HJ, HARRIS G, WILLIAMS K. BRAINSFit: Mutual information registrations of whole-brain 3D images, using the Insight Toolkit. The Insight Journal 2007 [Google Scholar]
- JONES KL, SMITH DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- KAS MJ, GLENNON JC, BUITELAAR J, EY E, BIEMANS B, CRAWLEY J, RING RH, LAJONCHERE C, ESCLASSAN F, TALPOS J, NOLDUS LP, BURBACH JP, STECKLER T. Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: current status and future perspectives. Psychopharmacology. 2014;231:1125–46. doi: 10.1007/s00213-013-3268-5. [DOI] [PubMed] [Google Scholar]
- KELLY SJ, DAY N, STREISSGUTH AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicology and teratology. 2000;22:143–9. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULLY-MARTENS K, DENYS K, TREIT S, TAMANA S, RASMUSSEN C. A review of social skills deficits in individuals with fetal alcohol spectrum disorders and prenatal alcohol exposure: profiles, mechanisms, and interventions. Alcoholism, clinical and experimental research. 2012;36:568–76. doi: 10.1111/j.1530-0277.2011.01661.x. [DOI] [PubMed] [Google Scholar]
- LAWRENCE RC, BONNER HC, NEWSOM RJ, KELLY SJ. Effects of alcohol exposure during development on play behavior and c-Fos expression in response to play behavior. Behavioural brain research. 2008;188:209–18. doi: 10.1016/j.bbr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOOS M, VAN DER SLUIS S, BOCHDANOVITS Z, VAN ZUTPHEN IJ, PATTIJ T, STIEDL O, SMIT AB, SPIJKER S. Activity and impulsive action are controlled by different genetic and environmental factors. Genes, brain, and behavior. 2009;8:817–28. doi: 10.1111/j.1601-183X.2009.00528.x. [DOI] [PubMed] [Google Scholar]
- MA Y, HOF PR, GRANT SC, BLACKBAND SJ, BENNETT R, SLATEST L, MCGUIGAN MD, BENVENISTE H. A three-dimensional digital atlas database of the adult C57BL/6J mouse brain by magnetic resonance microscopy. Neuroscience. 2005;135:1203–15. doi: 10.1016/j.neuroscience.2005.07.014. [DOI] [PubMed] [Google Scholar]
- MATTSON SN, CROCKER N, NGUYEN TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTSON SN, RILEY EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcoholism, clinical and experimental research. 2000;24:226–31. [PubMed] [Google Scholar]
- MATZEL LD, BABIARZ J, TOWNSEND DA, GROSSMAN HC, GRUMET M. Neuronal cell adhesion molecule deletion induces a cognitive and behavioral phenotype reflective of impulsivity. Genes, brain, and behavior. 2008;7:470–80. doi: 10.1111/j.1601-183X.2007.00382.x. [DOI] [PubMed] [Google Scholar]
- MCGIVERN RF, CLANCY AN, HILL MA, NOBLE EP. Prenatal alcohol exposure alters adult expression of sexually dimorphic behavior in the rat. Science. 1984;224:896–8. doi: 10.1126/science.6719121. [DOI] [PubMed] [Google Scholar]
- MCGIVERN RF, RAUM WJ, SALIDO E, REDEI E. Lack of prenatal testosterone surge in fetal rats exposed to alcohol: alterations in testicular morphology and physiology. Alcoholism, clinical and experimental research. 1988;12:243–7. doi: 10.1111/j.1530-0277.1988.tb00188.x. [DOI] [PubMed] [Google Scholar]
- MINETTI A, AROLFO MP, VIRGOLINI MB, BRIONI JD, FULGINITI S. Spatial learning in rats exposed to acute ethanol intoxication on gestational day 8. Pharmacology, biochemistry, and behavior. 1996;53:361–7. doi: 10.1016/0091-3057(95)02035-7. [DOI] [PubMed] [Google Scholar]
- MINSHEW NJ, WILLIAMS DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Archives of neurology. 2007;64:945–50. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’CONNOR MJ, PALEY B. The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. Journal of pediatric psychology. 2006;31:50–64. doi: 10.1093/jpepsy/jsj021. [DOI] [PubMed] [Google Scholar]
- O’LEARY-MOORE SK, PARNELL SE, GODIN EA, DEHART DB, AMENT JJ, KHAN AA, JOHNSON GA, STYNER MA, SULIK KK. Magnetic resonance microscopy-based analyses of the brains of normal and ethanol-exposed fetal mice. Birth defects research. Part A, Clinical and molecular teratology. 2010;88:953–64. doi: 10.1002/bdra.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGUZ I, LEE J, BUDIN F, RUMPLE A, MCMURRAY M, EHLERS C, CREWS F, JOHNS J, STYNER M. Automatic Skull-stripping of Rat MRI/DTI Scans and Atlas Building. Proceedings of SPIE--the International Society for Optical Engineering. 2011;7962:7962251–7962257. doi: 10.1117/12.878405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANIAGUA B, STYNER M, MACENKO M, PANTAZIS D, NIEHAMMER M. Local shape analysis using MANCOVA. The Insight Journal. 2009:1–21. [Google Scholar]
- PARNELL SE, HOLLOWAY HT, O’LEARY-MOORE SK, DEHART DB, PANIAQUA B, OGUZ I, BUDIN F, STYNER MA, JOHNSON GA, SULIK KK. Magnetic resonance microscopy-based analyses of the neuroanatomical effects of gestational day 9 ethanol exposure in mice. Neurotoxicology and teratology. 2013;39:77–83. doi: 10.1016/j.ntt.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARNELL SE, O’LEARY-MOORE SK, GODIN EA, DEHART DB, JOHNSON BW, ALLAN JOHNSON G, STYNER MA, SULIK KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 8. Alcoholism, clinical and experimental research. 2009;33:1001–11. doi: 10.1111/j.1530-0277.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RILEY EP, INFANTE MA, WARREN KR. Fetal alcohol spectrum disorders: an overview. Neuropsychology review. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS DT, BARRON S, LITTLETON JM. Neonatal ethanol exposure produces a hyperalgesia that extends into adolescence, and is associated with increased analgesic and rewarding properties of nicotine in rats. Psychopharmacology. 2004;171:204–11. doi: 10.1007/s00213-003-1574-z. [DOI] [PubMed] [Google Scholar]
- SADRIAN B, LOPEZ-GUZMAN M, WILSON DA, SAITO M. Distinct neurobehavioral dysfunction based on the timing of developmental binge-like alcohol exposure. Neuroscience. 2014;280:204–19. doi: 10.1016/j.neuroscience.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ VEGA MC, CHONG S, BURNE TH. Early gestational exposure to moderate concentrations of ethanol alters adult behaviour in C57BL/6J mice. Behavioural brain research. 2013;252:326–33. doi: 10.1016/j.bbr.2013.06.003. [DOI] [PubMed] [Google Scholar]
- SCHAMBRA UB, GOLDSMITH J, NUNLEY K, LIU Y, HARIRFOROOSH S, SCHAMBRA HM. Low and moderate prenatal ethanol exposures of mice during gastrulation or neurulation delays neurobehavioral development. Neurotoxicology and teratology. 2015;51:1–11. doi: 10.1016/j.ntt.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHENK F. Development of place navigation in rats from weaning to puberty. Behavioral and neural biology. 1985;43:69–85. doi: 10.1016/s0163-1047(85)91510-9. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER ML, MOORE CF, ADKINS MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychology review. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER T, TURCZAK J, PRZEWLOCKI R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: issues for a therapeutic approach in autism. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:36–46. doi: 10.1038/sj.npp.1300767. [DOI] [PubMed] [Google Scholar]
- SELLER MJ, PERKINS-COLE KJ. Sex difference in mouse embryonic development at neurulation. Journal of reproduction and fertility. 1987;79:159–61. doi: 10.1530/jrf.0.0790159. [DOI] [PubMed] [Google Scholar]
- STYNER M, OGUZ I, XU S, BRECHBUHLER C, PANTAZIS D, LEVITT JJ, SHENTON ME, GERIG G. Framework for the statistical shape analysis of brain structures using SPHARM-PDM. The Insight Journal. 2006;1071:242–250. [PMC free article] [PubMed] [Google Scholar]
- SULIK KK. Genesis of alcohol-induced craniofacial dysmorphism. Experimental biology and medicine. 2005;230:366–75. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- SULIK KK, JOHNSTON MC, WEBB MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–8. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- SUMMERS BL, HENRY CM, ROFE AM, COYLE P. Dietary zinc supplementation during pregnancy prevents spatial and object recognition memory impairments caused by early prenatal ethanol exposure. Behavioural brain research. 2008;186:230–8. doi: 10.1016/j.bbr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- SUMMERS BL, ROFE AM, COYLE P. Prenatal zinc treatment at the time of acute ethanol exposure limits spatial memory impairments in mouse offspring. Pediatric research. 2006;59:66–71. doi: 10.1203/01.pdr.0000190573.23893.13. [DOI] [PubMed] [Google Scholar]
- VARLINSKAYA EI, MOONEY SM. Acute exposure to ethanol on gestational day 15 affects social motivation of female offspring. Behavioural brain research. 2014;261:106–9. doi: 10.1016/j.bbr.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD IL, WEISZ J. Maternal stress alters plasma testosterone in fetal males. Science. 1980;207:328–9. doi: 10.1126/science.7188648. [DOI] [PubMed] [Google Scholar]
- WEINBERG J, SLIWOWSKA JH, LAN N, HELLEMANS KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. Journal of neuroendocrinology. 2008;20:470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIECZOREK L, FISH EW, O’LEARY-MOORE SK, PARNELL SE, SULIK KK. Hypothalamic-pituitary-adrenal axis and behavioral dysfunction following early binge-like prenatal alcohol exposure in mice. Alcohol. 2015;49:207–17. doi: 10.1016/j.alcohol.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERBERG B, REUTER JM. Sexually dimorphic behavioral and brain asymmetries in neonatal rats: effects of prenatal alcohol exposure. Brain research. Developmental brain research. 1989;46:281–90. doi: 10.1016/0165-3806(89)90291-5. [DOI] [PubMed] [Google Scholar]
- ZIMMERBERG B, SUKEL HL, STEKLER JD. Spatial learning of adult rats with fetal alcohol exposure: deficits are sex-dependent. Behavioural brain research. 1991;42:49–56. doi: 10.1016/s0166-4328(05)80039-7. [DOI] [PubMed] [Google Scholar]