Abstract
Objectives
Quantitative EEG features have been identified as surrogates and predictors of cognitive decline/dementia, a common feature of progressive Parkinson's disease. The biochemical correlates for altered quantitative EEG features are unknown. Our primary objective was to test the hypothesis that quantitative EEG measures correlate with cortical levels of phosphorylated α-synuclein, a modified form of the synaptic protein α-synuclein, in Parkinson's disease cases, in contrast to other pathology-associated proteins. A secondary objective was to explore the same correlations among cellular fractions of these proteins.
Methods
We used posterior cingulate cortex autopsy tissue from 44 Parkinson's disease subjects with various degrees of cognitive decline, who had undergone EEG. In this brain region, which is a major hub of the default mode network, biochemical measurements for levels of phosphorylated α-synuclein, unmodified α-synuclein, Aβ peptide, phosphorylated tau and key synaptic proteins were analyzed, and data correlated with spectral EEG measures.
Results
Findings revealed significant correlations between background rhythm peak frequency and all bandpower values (highest in delta bandpower) with total phosphorylated α-synuclein, but not any correlation with total α-synuclein, phosphorylated tau protein, Aβ peptide or synaptic proteins. Certain fractions of synaptosomal-associated protein 25 showed correlation with some quantitative EEG measures.
Conclusions
These data show association between increased phosphorylation of α-synuclein and the abnormal EEG signatures of cognitive decline. Results suggest quantitative EEG may provide an in vivo approximation of phosphorylated α-synuclein in Parkinson's disease cortex. This adds to previous evidence that quantitative EEG measures can be considered valid biomarkers of Parkinson's disease cognitive decline.
Search terms: Electroencephalography/EEG, Parkinson's disease, Dementia, α-Synuclein, Biochemistry, Synaptic proteins
Introduction
As new treatments for Parkinson's disease (PD) cognitive decline will hopefully improve PD cortical dysfunction, identifying physiologic biomarkers that reflect clinical change is paramount. Without these biomarkers, progress is stifled for improving therapies for this disabling problem associated with PD progression. Subcortical pathology, diffusely projecting neurotransmitter abnormalities, and intrinsic cortical changes have all been proposed to play important roles.1,2 At autopsy, PD with dementia (PD-D) is associated with higher Lewy type synucleinopathy (LTS) stage that reflects cortical involvement.2-7 LTS develops in diseased brains through the formation of structures primarily composed of aggregated and phosphorylated –α synuclein (p-α-syn). However, the degree to which electroencephalography (EEG), as a non-invasive measure of cortical electrophysiology, changes with cortical LTS biochemistry is unknown.
One method of studying physiological changes in PD patients is quantitative electroencephalography (QEEG). Physiologic changes in cortical activity have been established to correlate with PD cognitive decline.8-13 The resting EEG is simple to acquire, requires minimal patient cooperation, and is not dependent on verbal or motor responses which may be affected in PD. QEEG measures may be ideal biomarkers to complement neuropsychological testing with physiological corroboration for studying cognitive decline among patients with PD.8,11 Because EEG measures the electric dendritic potentials from populations of cortical pyramidal neurons in real time, correlation of QEEG spectral measures with the biochemistry of LTS could provide insight on PD cognitive decline. Recently, the formation of increased amounts of p-α-syn in PD cortex has been studied by our laboratory and others.14,15 Studies reveal that fibrillary or oligomeric α-synuclein can alter cell membrane permeability and hence electrical properties of neurons, so the potential for abnormal deposition of α-synuclein to alter EEG measures in PD exists.16-18
In this study, we tested the hypothesis that QEEG spectral measures correlate with cortical p-α-syn levels in PD. For contrast, we also measured levels of the Alzheimer's disease (AD) pathology-associated proteins (phosphorylated tau and amyloid beta (Aβ) peptide and the synaptic proteins synaptophysin and Synaptosomal-Associated Protein 25 (SNAP-25). We chose posterior cingulate cortex as the brain region for analysis as cingulate cortex is often affected in PD subjects with cognitive decline. The posterior cingulate cortex has been proposed to have a central role in supporting internally-directed cognition and the focus of attention.19 These aspects of cognition can be compromised in PD.1 In PD-D, this area shows atrophy on MRI.20 Moreover, the posterior cingulate cortex is a key hub (i.e. highly interconnected area) for the default mode network, which has been reported using non-invasive methods to show alterations in both PD and AD.21
Subjects and Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The Banner Sun Health Research Institute (BSHRI) and Mayo Clinic Institutional review boards approved all procedures; written informed consent was obtained from each study subject.
Subjects
The PD cohort was studied as part of the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND), a program overseen at BSHRI. AZSAND is part of a Brain and Body Donation Program (BBDP) that performs annual, prospective, standardized, longitudinal assessments until death. These include movement, neuropsychological, and general medical history and physical examination assessments.22 For PD subjects, motor scales, and medications are recorded, and PD medication is converted to levodopa equivalents as per a previous report.8 All PD subjects also undergo pre-mortem biennial digital EEG recording. Upon death, subjects are autopsied in a rapid autopsy program.23 Neuropathological methods employed to diagnose and classify brains used a standardized series of brain regions for diagnosing neurodegenerative disorders including LTS and AD. LTS pathological diagnosis includes staining tissue sections with Hemotoxylin and Eosin dyes for Lewy Bodies, as well as immunohistochemically for p-α-syn.23 After neuropathological findings are available, the designation of PD diagnosis and cognitive state is made in clinical-pathological consensus conference with movement disorder, cognitive neurologists and neuropsychologists.24 This consensus conference, as per previous publications from the AZSAND program, uses pre-mortem data that include a full neuropsychological battery reflecting various cognitive domains: attention, frontal/executive, memory, visuospatial, and language.8,24 Conference participants were blinded to the QEEG results. Likewise, the investigator who analyzed the raw EEG recordings to yield QEEG measures (JNC) was blinded to the conference results. In addition, the mini-mental status exam (MMSE) is used as a measure of global cognitive performance. Only cases with neuropathological diagnosis of PD were used in this study. Clinical PD diagnostic criteria were as per previous reports and Movement Disorder Society guidelines were used for PD-MCI and PD-D.8,25,26 The cohort data were obtained from our database by searching the most recent EEG recorded before death, along with the other data and consensus determinations associated in that assessment epoch. The use of the most recent EEG before death was consistent with the objective to investigate the QEEG as surrogate for biochemical abnormalities at autopsy.
We excluded a total of 10 cases that had one or more criterion of: a) EEG examinations that were greater than 2.5 years before death, b) excessive muscle artifact in the EEG, c) deep brain stimulation, and d) barbiturate, benzodiazepine, neuroleptic, acetylcholinesterase inhibitors, or anti-seizure medication on day of EEG that might influence the EEG. Forty-four cases met all criteria, consisting of 14 PD and 30 PD-D.
EEG recording
Recordings during relaxed wakefulness were obtained from PD patients in the “on” state, seated in a recliner with eyes closed as previously described.8 Briefly, Ag/AgCl EEG electrodes were placed on the scalp using twenty standard 10-20 EEG electrode positions. Recordings were referenced to the Fz electrode, with a right mastoid electrode serving as ground. Electrode impedances were closely monitored and kept below 5 kohms. Data were acquired using the Neuroscan Synamps2 system (Compumedics, Charlotte, NC, USA) at a sampling rate of 1000 Hz and a bandpass of 1–200 Hz. An EEG technician and neurophysiologist (JNC) were present during the entire recording session to observe the behavioral state of the patient and to monitor on-line for signal quality. Patients were asked every minute if they were awake, and drowsiness was further excluded by monitoring for background changes and slow eye movements of drowsiness during the course of the recording. When muscle, eye movement/blink, or other artifacts were identified, the patient was coached until an artifact-free signal was obtained. The final recorded sample consisted of an awake and artifact-free period of approximately 150-180 seconds.
EEG data processing
The EEG data were processed off-line using Neuroscan EDIT software (Compumedics, Charlotte, NC, USA). Consecutive, non-overlapping, 4096-point epochs were created from the continuous data, allowing for a frequency resolution of 0.244 Hz. Each epoch was visually inspected for artifacts; rejection due to artifacts was uncommon due to the monitoring of the online acquisition. The number of epochs accepted for further processing ranged from 30-40. The epochs were passed through a 10% cosine window, processed with a fast Fourier transform (FFT), and averaged to produce an averaged FFT power spectrum for each electrode. EEG was analyzed for frequency domain QEEG measures of background rhythm frequency (BRF); and global relative power in delta, theta, alpha, and beta bands. The BRF was defined as dominant frequency peak in the power spectra of the posterior parietal and occipital electrodes (e.g.s. P3, P4, Oz) as determined by visual inspection of the FFT average. Frequency bands were designated as follows: delta =2.5–3.9 Hz; theta =4–7.9 Hz; alpha=8–12.9 Hz; beta=13–30 Hz. The global (over multiple electrodes) relative (%) EEG bandpower for each of the four frequency bands was calculated (using all electrodes except Fp1, Fp2, A1, and A2) as a percentage of total EEG power between 2.5 and 30 Hz from the FFT average.
Brain tissue homogenization and protein extraction
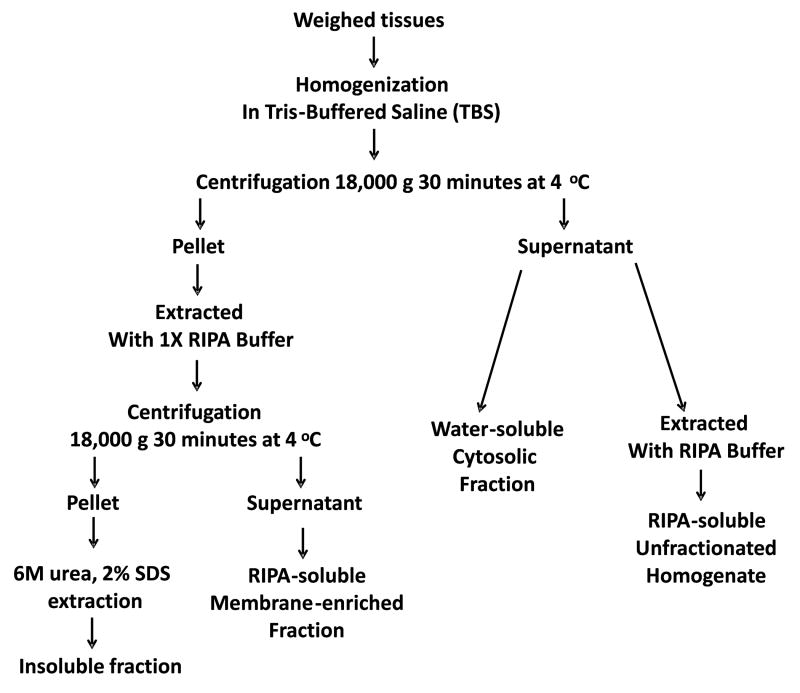
Frozen cortical brain tissue samples from posterior cingulate cortex were provided by the AZSAND BBDP. The extraction and fractionation of the grey matter of posterior cingulate cortices followed our previously published procedures, including fractionation methods for total (unfractionated), cytosol, membrane and insoluble fractions (Figure 1).14,15 The reason to explore measuring α-synuclein and p-α-syn in different fractions is that normal α-synuclein is in cytosol fractions, but as it becomes more aggregated, it becomes enriched first in membrane and then insoluble fractions.14,15
Figure 1. Fractionation methods for total (unfractionated), membrane, cytosol, and insoluble fractions.
Western blotting procedure
The western blot procedures were as previously employed.14,15 Details of antibodies used in this study are available upon request. A previously characterized rabbit polyclonal antibody raised against p-α-syn (phosphorylated at serine 129) was used for immunodetection of p-α-syn.3,14,15,27 Total α-synuclein was measured by western blot procedures using a non-phosphorylation-dependent antibody that detects an epitope between amino acid 121-125 of human α-synuclein (Syn 211, Life Technologies). The intensity of p-α-syn and total α-synuclein bands at 16 kD (monomeric) were quantified. Other proteins analyzed include Aβ peptide, phosphorylated tau, and synaptic proteins (SNAP-25 and synaptophysin). The level of β-actin in each sample (detected with Clone AC-15, Sigma, 1:10,000) was used for normalization purposes. The intensity of the immunoreactive bands for all antibodies were produced by reaction of blots with chemiluminescent substrate were measured using a direct imaging CCD camera system (Fluorochem Q, Protein Simple, San Jose, CA) and AlphaView Software. This system has a dynamic range of 4 logs to ensure linearity of dose-response. This was confirmed by using multiple exposures of each analysis.
Analysis and Statistics
The associations between total levels of p-α-syn protein, other proteins of interest and QEEG values, MMSE, Hoehn and Yahr (H&Y) stage, and the UPDRS Part I Thought Disorder score were assessed by using Pearson correlation coefficient. We used H&Y stage to investigate the relationship of these protein measurements to motor function in our PD subjects. To study thought disorder, including hallucinations, we also correlated the protein measurements with the UPDRS Part I Thought Disorder score: 0=None; 1=Vivid Dreaming; 2=“Benign” hallucinations with insight retained; 3=Occasional to frequent hallucinations or delusions, without insight, could interfere with daily activities; 4=Persistent hallucinations, delusions, or florid psychosis, not able to care for self. The Bonferroni correction was used to account for the 48 comparisons in the primary analysis, defining significance at P<.001.
The same Pearson correlation method was used to secondarily explore the relationships between the protein levels of interest and cellular compartments.
Results
Study Subjects
Demographic and clinical data for the 44 PD cases are shown (Table 1). 30 subjects had PD-D, 6 had PD-MCI, and 8 were cognitively normal. Thus, a spectrum of cognitive performance was represented. The percentage of demented subjects and relatively low mean MMSE scores are both consistent with the known accumulative PD-D prevalence of 60-80%.2 Likewise, the UPDRS “off” motor score and H&Y stage reflect the advanced PD state of autopsied cases.
Table 1. Demographics of PD Subjects used in Study.
| Variable | Mean ± SD |
|---|---|
| Cases (n) | 44 |
| PMI (h) | 3.8 (2.6) |
| Age at death (y) | 81.1 (6.8) |
| % Female | 32% |
| EEG to death interval (y) | 1.15 (0.58) |
| Levodopa equivalents (mg) | 732 (390) |
| Hoehn and Yahr stage | 3.4 (1.1) |
| UPDRS part III (off state) | 39 (16) |
| MMSE | 23.6 (4.8) |
| % demented | 68% |
| apoE (% E4 alleles) | 13.9% |
Biochemical Analyses
The findings of this study are demonstrated in the relationship between QEEG measures (BRF and bandpower for delta, theta, alpha, and beta), MMSE scores, H&Y stage, Thought disorder score, and the levels of proteins quantified in unfractionated (total) brain homogenates (Table 2). The primary analysis shows that total levels of p-α-syn has significant correlation with BRF, delta and alpha bandpower, but the highest correlation is with delta bandpower at 0.64. Total p-α-syn measures reflect the sum of p-α-syn in the total brain homogenate. There are negative correlations of p-α-syn fractions with BRF and faster frequency band alpha, whereas positive correlations were associated with slow frequency band delta. P-α-syn total levels showed a significant correlation with MMSE of -.51, but no significant correlation with the H&Y motor staging of or the Thought disorder score (Table 2). In contrast, the total levels of unmodified α-syn showed no correlation with QEEG measures. We also measured Aβ peptide and p-tau in the same samples; these proteins are associated with AD-pathology. Total Aβ peptide and p-Tau levels did not correlate with QEEG measures. The synaptic proteins synaptophysin and SNAP25 (total) did not correlate with QEEG measures.
Table 2. r correlation measures between unfractionated biochemical, QEEG measures, MMSE score, H&Y stage, and UPDRS part I Thought Disorder.
| Variable (Total) | BRF | Delta | Theta | Alpha | Beta | MMSE | H & Y | Thought |
|---|---|---|---|---|---|---|---|---|
| α-Syn | -.16 | .09 | .11 | -.10 | -.11 | .04 | -.16 | .22 |
| P-α-Syn | -.52* | .64* | .33 | -.51* | -.34 | -.51* | .34 | -.19 |
| Abeta Peptide | -.13 | .27 | -.07 | -.10 | .02 | -.17 | -.18 | -.12 |
| P-Tau | -.23 | .23 | .13 | -.17 | -.15 | .09 | .07 | -.10 |
| Synaptophysin | .09 | -.21 | .04 | .13 | -.09 | .14 | -.20 | .04 |
| SNAP-25 | .04 | -.13 | -.02 | -.05 | .22 | -.04 | .01 | .10 |
Legend: Total: unfractionated brain extract. BRF: background rhythm frequency. Bandpower labels Delta: 2.5-4.0 Hz, Theta: 4-8 Hz, Alpha: 8-13 Hz, Beta: 13-30 Hz, MMSE: Mini mental status exam cognitive score. H&Y: Hoehn and Yahr stage. Thought: UPDRS part I Thought Disorder. Significance level:
P<.001 with Bonferroni correction for multiple comparisons.
In the exploratory analysis for the secondary objective, there are significant correlations of QEEG measures with p-α-syn fractions but not with unmodified α-syn fractions (Table 3). The biochemical levels of p-α-syn measured in different types of cellular fractions correlate significantly with all five QEEG measures except cytosolic p-α-syn, which only correlated with BRF, alpha, and delta measures. Membrane-associated and insoluble forms of p-α-syn measure α-synuclein in a more aggregated and abnormal conformation than the normal protein. Among different types of protein extracts, membrane-associated p-α-syn levels showed highest correlation with all QEEG measures (0.67). The overall pattern of positive and negative correlations for p-α-syn fractions is identical to those for unfractionated p-α-syn (Tables 2 and 3). MMSE scores correlated negatively with membrane-associated and cytosolic p-α-syn levels, but not with p-α-syn in the insoluble fraction, which contains the most highly aggregated (detergent-insoluble) (i.e. Lewy body and neurite) types of α-synuclein. Aβ peptide and p-tau fraction levels did not show any correlation with QEEG measures (Table 3). Among synaptic proteins measured, pre-synaptic protein SNAP-25 in membrane fraction had significant negative correlations with theta bandpower (r=-0.35), whereas membrane-associated (r=0.40) and cytosolic SNAP-25 (r=0.31) both correlated with beta bandpower (Table 3). Membrane-associated SNAP-25 also positively correlated with MMSE scores (r=0.32).
Table 3. r correlation measures between fractionsated biochemical and QEEG measures.
| Variable | BRF | Delta | Theta | Alpha | Beta |
|---|---|---|---|---|---|
| α-SYNUCLEIN | |||||
| Membrane | .12 | -.12 | .12 | .03 | -.15 |
| Cytoplasmic | -.00 | -.09 | .12 | .03 | -.17 |
| PHOSPHORYLATED α-SYNUCLEIN | |||||
| Membrane | -.56** | .67** | .51** | -.64** | -.50** |
| Cytoplasmic | -.50** | .48** | .30 | -.44** | -.28 |
| Insoluble | -.58** | .48** | .56** | -.64** | -.37* |
| ABETA PEPTIDE | |||||
| Membrane | -.19 | .25 | .03 | -.23 | .08 |
| Cytoplasmic | -.19 | .21 | -.05 | -.12 | .08 |
| Insoluble | -.16 | .23 | .08 | -.18 | -.06 |
| PHOSPHORYLATED TAU | |||||
| Cytoplasmic | -.00 | .10 | .02 | .03 | -.18 |
| SYNAPTOPHYSIN | |||||
| Membrane | .06 | -.23 | .16 | .06 | -.15 |
| Cytoplasmic | .09 | -.22 | -.11 | .12 | .20 |
| SNAP-25 | |||||
| Membrane | .22 | -.24 | -.35* | .26 | .40** |
| Cytoplasmic | .11 | -.04 | -.29 | .14 | .31* |
Legend: Membrane: Detergent-soluble fraction. Cytoplasmic: Tris buffer soluble-fraction. Insoluble: Detergent insoluble fraction. BRF: background rhythm frequency. Bandpower labels Delta: 2.5-4.0 Hz, Theta: 4-8 Hz, Alpha: 8-13 Hz, Beta: 13-30 Hz. Significance level:
P<.05;
P<.01.
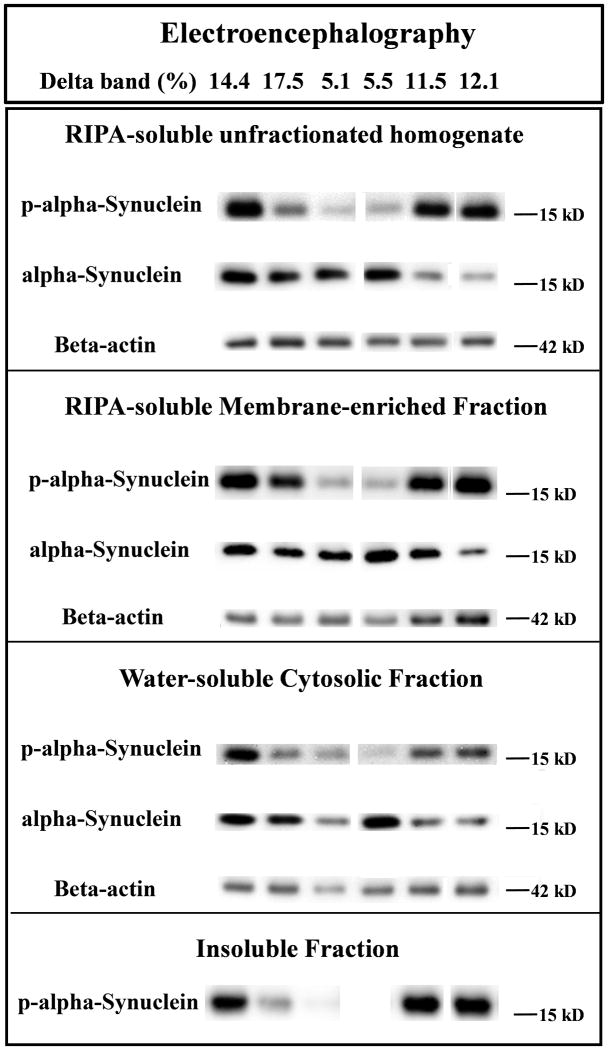
A representative image of western blot immunoreactive bands from total and different fractions of human brain extracts from six subjects is shown along with the QEEG measure delta bandpower (Figure 2). This demonstrates visually how well QEEG measure correlates with the intensity of the p-α-syn gel band (Figure 2).
Figure 2. Representative immunoreactive western blot band images compiled from different fractions of human brain extracts.
Six subject examples of gel bands corresponding to p-α-syn, unmodified α-syn, and β-actin. The QEEG measure delta bandpower is given at the top for each subject, showing visual correlation with the intensity of the p-α-syn gel band.
Discussion
This study examined which biochemical protein species known to be important in PD-D are correlated with the QEEG measures in PD cingulate cortex, which we and other have shown to be surrogate and predictive biomarkers of PD cognitive decline.8,11,28 We discovered that EEG spectral physiology measures from PD subjects correlated strongly with total levels of p-α-syn in posterior cingulate cortex, but not with measures of total unmodified α-synuclein. Specifically, p-α-syn levels positively correlated with increases in delta bandpower, and negatively correlated with decreases in BRF and alpha bandpower, which together are the QEEG signature changes in PD cognitive decline.11 Interestingly, the strongest correlation was with delta bandpower, which was recently found to be highly associated with PD-D incidence.8 Specificity of our results is suggested by the observation that p-α-syn and the other proteins of interest did not show significant correlation with motor staging or with thought disorder/hallucination scores. In our exploratory analysis of how p-α-syn portioned with cellular compartments, all p-α-syn cortical tissue fractions showed strong correlation with QEEG measures, so it is not yet clear which p-α-syn compartment(s) are the primary driver of the association. These results support a significant connection between cortical electrophysiology (EEG), biochemistry (p-α-syn level), and neuropsychological performance (MMSE) more than with the motor H&Y scores. Indeed, results suggest QEEG may provide an in vivo approximation of phosphorylated α-synuclein in Parkinson's disease cortex. This adds to previous evidence that QEEG measures can be considered valid biomarkers of PD cognitive decline.
Since the initial identification of p-α-syn as the major component of Lewy bodies and associated pathology, mechanisms as to how α-synuclein, both unmodified and modified, could affect neurons in PD have been suggested.29-31 Recent studies have implicated aggregated α-synuclein as an inducer of endoplasmic reticulum stress and mitochondrial dysfunction.32-34 Model systems have shown that formation of p-α-syn enhances neurotoxicity in nigral neurons.35 Enhanced toxicity mediated by p-α-syn was also observed in a Drosophila PD model.36,37 Moreover, p-α-syn accumulation start in cortical areas before histologic evidence of Lewy bodies and Lewy neurites.14 Increasing attention has been given to α-synuclein's ability to disrupt neuronal membrane structure and alter neuronal electrophysiology in PD.17 It is somewhat controversial as to whether or not α-synuclein produces increased permeability by forming membrane pores or by distortion of the lipid bilayer.16,18,38 In either case, if phosphorylation of α-synuclein facilitates an abnormal interaction with neuronal, synaptic, and/or dendritic membranes, then the resulting altered electrophysiology may explain its correlation with QEEG abnormalities. Thus, the early presence of p-α-syn in synucleinopathy, along with its strong correlation with EEG brainwave abnormalities that track with PD cognitive decline, suggests that p-α-syn may be involved in the mechanism of PD-D. However, this hypothesis requires further investigation.
In our study, neither Aβ peptide nor phosphorylated tau had significant correlation with any QEEG spectral measure. These were surprising results, since 68% of the PD cases had dementia, and AD pathology is thought to contribute to PD-D in at least some cases.2 It is possible that, at least for the posterior cingulate cortex, the biochemistry of AD pathology is dissociated from the production of EEG rhythm abnormalities when compared to p-α-syn. This is consistent with another study from our laboratory that found higher levels of α-synuclein with the presence of PD motor cortex hyperexcitability, while AD pathology biochemistry markers were equal between the groups.35 Studies show that PD cortical dysfunction and dementia correlate with greater cortical LTS histopathology.3,4,39 The current results from this study go further to show that the building blocks of LTS (i.e. p-α-syn) correlates more strongly with physiologic cortical dysfunction, as shown by QEEG, than do AD pathology biochemistry markers. Our cases had a relatively small proportion of apoE4 allele cases so this may have affected the influence of AD pathology biochemistry markers. It has been suggested that AD and LTS biochemical abnormal proteins can interact in significant manners to accelerate pathology in biochemistry and pathology in samples from PD and dementia with Lewy bodies 6. Thus, it is still possible that AD pathology biochemistry influences PD cortical dysfunction through such interaction with LTS, but further study is needed to clarify the mechanism.
We found correlation between the abundant synaptic protein SNAP-25 and beta (positive) and theta (negative) bandpower, whereas synaptophysin had no significant correlation. These correlations were present mostly for the membrane fraction and not for the total value. The significant correlations are consistent with the notion that decreases in SNAP-25 correlates with dysfunctional changes in EEG activity. α-synuclein is a chaperone protein of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex at the synapse.40 A certain degree of α-synuclein association with membranes is needed to form functional SNARE complexes.41 SNAP-25 is a primary component of the presynaptic SNARE complex. An abnormal distribution of SNARE proteins have been shown in presynaptic terminals in human PD cases.42 The hypothesis that increased association of phosphorylated α-synuclein with synaptic SNARE might cause synaptic dysfunction, which in turn leads to abnormal EEG activity remains to be proven. The interaction found here of p-α-syn with SNAP-25 is only based on the correlative analysis. Co-localization of these proteins in tissue sections needs to be investigated biochemically and will be part of a future study. Given that the synapse is the substrate by which neurons communicate, and that EEG records electrical activity from connected neuron populations, it will be useful to further study the role that synaptic associated synucleinopathy plays in PD cortical dysfunction.
It is of interest that the cortical area examined here, the posterior cingulate cortex is a key hub location of the default node network.43 Other major locations of the default mode network are the medial frontal, lateral parietal, and medial temporal cortical areas. This network is so named because these hubs are active during relative resting state and de-activate during cognitive tasks. The default mode network has been shown to have alterations in both AD and PD in functional imaging studies.21 Default mode network abnormalities appear to be associated with cognitive performance in non-demented PD.44 However, activity within the default mode network via functional MRI was recently demonstrated to be higher in PD with hallucinations than without hallucinations between PD groups of equal cognitive performance.45,46 In the Franciotti et al. study, which was highly controlled, the authors provide evidence that the presence of hallucinations need to be considered when studying the default mode network in PD cognitive dysfunction.46 Our study used different methodology, which prevents a valid comparison of results. In our present investigation, the severity of thought disorder/hallucinations did not correlate with the biochemical measurements. Global network disruption has been correlated with PD cognitive decline using magnetoencephalography.13 More research is needed to determine whether certain cortical areas are more critical than others for causing widespread cortical EEG abnormalities in PD. Our study only examined tissue from the posterior cingulate cortex, so we do not know whether other locations would have the same or different results. However, if specific cortical areas such as the posterior cingulate cortex drive widespread cortical dysfunction in PD, then this raises the possibility that targeting key network locations in PD-D may become a focus for experimental therapeutics.47
Acknowledgments
John N. Caviness, M.D.-conception, organization, execution, writing of the first draft, review and critique; Lih-Fen Lue, Ph.D.- conception, organization, execution, writing of the first draft, review and critique; Joseph G. Hentz, M.S.-statistical analysis design, execution, review and critique; Christopher T. Schmitz, B.A.-execution, review and critique; Charles H. Adler, M.D., Ph.D.-review and critique; Holly A. Shill, M.D.-review and critique; Marwan N. Sabbagh, M.D.-review and critique; Thomas G. Beach, M.D., Ph.D.-execution, review and critique; Douglas G. Walker, Ph.D.- conception, organization, execution, writing of first draft, review and critique.
Financial Disclosures: John N. Caviness reports grants from Michael J. Fox Foundation for Parkinson's disease research; Joseph G Hentz reports grants from Michael J. Fox Foundation for Parkinson's disease research. Charles H. Adler reports personal fees from Abbvie, Acadia, Impax, Ipsen, Lundbeck, Merz, Novartis, Teva, and Xenoport. LihFen Lue, Thomas Beach and Douglas Walker reports grants from National Institute for Neurological Disorders and Stroke and National Institute on Aging; personal fees from Avid Radiopharmaceuticals, personal fees from GE Healthcare. Christopher T. Schmitz, Holly A. Shill, Marwan N. Sabbagh, Thomas G. Beach, and Douglas G. Walker have nothing to disclose.
Study funding: Michael J. Fox Foundation for Parkinson's Disease Research. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson's Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer's Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson's Research.
Footnotes
Study Financial Disclosure/Conflict of Interest concerning the research related to the manuscript: The authors have nothing to disclose.
References
- 1.Caviness JN, Lue L, Adler CH, Walker DG. Parkinson's disease dementia and potential therapeutic strategies. CNS Neurosci Ther. 2011;17:32–44. doi: 10.1111/j.1755-5949.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman JG, Williams-Gray C, Barker RA, Duda JE, Galvin JE. The spectrum of cognitive impairment in Lewy body diseases. Mov Disord. 2014;29:608–621. doi: 10.1002/mds.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson DW, Fujishiro H, Orr C, et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S1–5. doi: 10.1016/S1353-8020:-2. [DOI] [PubMed] [Google Scholar]
- 5.Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov Disord. 2014;29:634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swirski M, Miners JS, de SR, et al. Evaluating the relationship between amyloid-beta and alpha-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson's disease. Alzheimer's Res Ther. 2014;6:77–0077. doi: 10.1186/s13195-014-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbagh MN, Adler CH, Lahti TJ, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23:295–297. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caviness JN, Hentz JG, Belden CM, et al. Longitudinal EEG changes correlate with cognitive measure deterioration in Parkinson's disease. J Parkinsons Dis. 2015;5:117–124. doi: 10.3233/JPD-140480. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca LC, Tedrus GM, Letro GH, Bossoni AS. Dementia, mild cognitive impairment and quantitative EEG in patients with Parkinson's disease. Clin EEG Neurosci. 2009;40:168–172. doi: 10.1177/155005940904000309. [DOI] [PubMed] [Google Scholar]
- 10.Serizawa K, Kamei S, Morita A, et al. Comparison of quantitative EEGs between Parkinson disease and age-adjusted normal controls. J Clin Neurophysiol. 2008;25:361–366. doi: 10.1097/WNP.0b013e31818f50de. [DOI] [PubMed] [Google Scholar]
- 11.Bousleiman H, Zimmermann R, Ahmed S, et al. Power spectra for screening parkinsonian patients for mild cognitive impairment. Ann Clin Transl Neurol. 2014;1:884–890. doi: 10.1002/acn3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olde Dubbelink KT, Stoffers D, Deijen JB, et al. Resting-state functional connectivity as a marker of disease progression in Parkinson's disease: A longitudinal MEG study. Neuroimage Clin. 2013;2:612–9. doi: 10.1016/j.nicl.2013.04.003. eCollection@2013.:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olde Dubbelink KTE, Hillebrand A, Stoffers D, Deijen JB, et al. Disrupted brain network topology in Parkinson's disease: a longitudinal magnetoencephalography study. Brain. 2014;137:197–207. doi: 10.1093/brain/awt316. [DOI] [PubMed] [Google Scholar]
- 14.Lue LF, Walker DG, Adler CH, et al. Biochemical increase in phosphorylated alpha-synuclein precedes histopathology of Lewy-type synucleinopathies. Brain Pathol. 2012;22:745–756. doi: 10.1111/j.1750-3639.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker DG, Lue LF, Adler CH, et al. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol. 2013;240:190–204. doi: 10.1016/j.expneurol.2012.11.020. Epub@2012 Nov 28.:190-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferkorn CM, Jiang Z, Lee JC. Biophysics of α-Synuclein Membrane Interactions. Biochimica et Biophysica Acta. 2012;1818(2):162–71. doi: 10.1016/j.bbamem.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantini J, Yahi N. The Driving Force of Alpha-Synuclein Insertion and Amyloid Channel Formation in the Plasma Membrane of Neural Cells: Key Role of Ganglioside-and Cholesterol-Binding Domains. Advances in Experimental Medicine and Biology. 2013;991:15–26. doi: 10.1007/978-94-007-6331-9_2. [DOI] [PubMed] [Google Scholar]
- 18.Stockl MT, Zijlstra N, Subramaniam V. α-Synuclein Oligomers: an Amyloid Pore? Mol Neurobiol. 2013;47:613–621. doi: 10.1007/s12035-012-8331-4. [DOI] [PubMed] [Google Scholar]
- 19.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple-Alford JC, Anderson TJ. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;83:188–194. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- 21.Rektorova I. Resting-state networks in Alzheimer's disease and Parkinson's disease. Neurodegenerative diseases. 2014;13:186–188. doi: 10.1159/000354237. [DOI] [PubMed] [Google Scholar]
- 22.Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–412. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beach TG, Adler CH, Sue LI, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015 Jan 26; doi: 10.1111/neup.12189. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, Evidente VG, Shill HA, Adler CH. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22(9):1272–7. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 25.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task force guidelines. Movement Disorders. 2012;27(3):349–56. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disorders. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 27.Obi K, Akiyama H, Kondo H, et al. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Klassen B, Hentz J, Shill H, et al. Quantitative Electroencephalography as a Predictor for Parkinson's Disease Dementia. Neurology. 2011;77:118–124. doi: 10.1212/WNL.0b013e318224af8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson JP, Walker DE, Goldstein JM, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara H, Hasegawa M, Dohmae N, et al. Alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 31.Okochi M, Walter J, Koyama A, et al. Constitutive phosphorylation of the Parkinson's disease associated alpha-synuclein. J Biol Chem. 2000;275:390–397. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- 32.Colla E, Coune P, Liu Y, et al. Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J Neurosci. 2012;32:3306–3320. doi: 10.1523/JNEUROSCI.5367-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, Lee MK. Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J Neurosci. 2012;32:3301–3305. doi: 10.1523/JNEUROSCI.5368-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaltieri M, Longhena F, Pizzi M, Missale C, Spano P, Bellucci A. Mitochondrial Dysfunction and alpha-Synuclein Synaptic Pathology in Parkinson's Disease: Who's on First? Parkinsons Dis. 2015:108029. doi: 10.1155/2015/108029. Epub@2015 Mar 31.:108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bentea E, Van der Perren A, Van LJ, et al. Nigral proteasome inhibition in mice leads to motor and non-motor deficits and increased expression of Ser129 phosphorylated alpha-synuclein. Front Behav Neurosci. 2015;9:68. doi: 10.3389/fnbeh.2015.00068. eCollection@2015.:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Periquet M, Wang X, et al. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Invest. 2009;119:3257–3265. doi: 10.1172/JCI39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellucci A, Navarria L, Zaltieri M, Missale C, Spano P. Alpha-Synuclein synaptic pathology and its implications in the development of novel therapeutic approaches to cure Parkinson's disease. Brain Res. 2012;1432:95–113. doi: 10.1016/j.brainres.2011.11.031. Epub@2011 Nov@19.:95-113. [DOI] [PubMed] [Google Scholar]
- 39.Caviness JN, Lue LF, Beach TG, et al. Parkinson's disease, cortical dysfunction, and alpha-synuclein. Mov Disord. 2011;26:1436–1442. doi: 10.1002/mds.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burre J, Sharma M, Sudhof TC. Alpha-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA. 2014;111:E4274–E4283. doi: 10.1073/pnas.1416598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Reitbock P, Anichtchik O, Bellucci A, et al. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson's disease. Brain. 2010;133:2032–2044. doi: 10.1093/brain/awq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;22:1689–1707. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Disbrow EA, Carmichael O, He J, Lanni KE, Dressler EM, Zhang L, Malhado-Chang N, Sigvardt KA. Resting State Functional Connectivity is Associated with Cognitive Dysfunction in Non-Demented People with Parkinson's Disease. Journal of Parkinson's Disease. 2014;4:453–465. doi: 10.3233/JPD-130341. [DOI] [PubMed] [Google Scholar]
- 45.Yao N, Chang RS, Cheung C, Pang S, Cheung C, Lau KK, Suckling J, Rowe JB, Yu K, Mak HK, Chua SE, Ho SL, McAlonan GM. The Default Mode Network is Disrupted in Parkinson's Disease with Visual Hallucinations. Human Brain Mapping. 2014;35:5658–5666. doi: 10.1002/hbm.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franciotti R, Pizzi SD, Perfetti B, Tartaro A, Bonanni L, Thomas A, Weis L, Biundo R, Antonini A, Onofrj M. Default Mode Network Links to Visual Hallucinations: A Comparison Between Parkinson's Disease and Multiple System Atrophy. Movement Disorders. 2015;30:1237–1247. doi: 10.1002/mds.26285. [DOI] [PubMed] [Google Scholar]
- 47.Caviness JN. Pathophysiology of Parkinson's disease behavior-a view from the network. Parkinsonism and related disorders. 2014;20S1:S39–S43. doi: 10.1016/S1353-8020(13)70012-9. [DOI] [PubMed] [Google Scholar]