To the Editor
Low vitamin D levels have been associated with increased asthma exacerbations and decreased lung function (reviewed by Paul et. al1) Studies have suggested that vitamin D may play a role in modulating the response to steroids in patients with steroid resistant asthma2, 3. T cells, monocytes and dendritic cells (DC) express the vitamin D receptor (VDR) as well as 25-hydroxyvitamin-D3-1α-hydroxylase required to convert 25(OH)-vitamin D to its active form, 1,25(OH)2-vitamin D. Addition of exogenous vitamin D to activated T cells and myeloid DC in vitro inhibits the expression inflammatory cytokines and induces a tolerogenic phenotype4.
We performed an ancillary study as part of the Vitamin D Add-on Therapy Enhances Corticosteroid Responsiveness in Asthma (VIDA) trial (NCT01248065)5. In this trial, vitamin D insufficient asthmatics with persistent symptoms despite low dose inhaled corticosteroids (ICS) received vitamin D or placebo with inhaled ciclesonide for 12 weeks, at which time the ICS was tapered and the number of exacerbations and treatment failures determined. Peripheral blood was collected at the 3rd visit (V3) prior to randomization and 12 weeks later at the 6th visit (V6), prior to the ICS taper.
Intracellular cytokine staining with fluorescently conjugated antibodies against IL-4, IFNγ, IL-10 or IL-17A was performed on whole blood after stimulation with phorbol-12-myristate-13-acetate and ionomycin. Isolated PBMCs were also stimulated with α-CD3/CD28. Culture supernatants and plasma were assayed for IL-2, IL-4, IL-6, IL-10, IL-17, TNFα, IFNγ and TGFβ. If there was sufficient sample, CD14+ monocytes were isolated and stimulated with lipopolysaccharide and IL-6, IL-10 and IL-12p40 levels measured. PBMC were also stained with monoclonal antibodies (mAb) to allow for phenotypic characterization of monocyte and DC populations.
Initial samples were collected at V3 from 100 participants, but samples were not available at the 2nd time point for several participants (Supplemental Figure E1). Only paired V3 and V6 samples were analyzed. The baseline characteristics of participants (Table E1) and baseline vitamin D levels were comparable (placebo= 19.4 ± 6.5, vitamin D = 19.3 ± 6.3 ng/ml). At V6, there was no change in vitamin D levels in the placebo group (18.9 ± 8.6 ng/ml), whereas the mean vitamin D level increased to 42.3 ± 10.9 ng/ml in those randomized to vitamin D (p< 0.001 as compared to placebo). Ninety percent of the vitamin D-treated participants in the sub-study achieved a 25(OH)-vitamin D level ≥ 30 ng/ml.
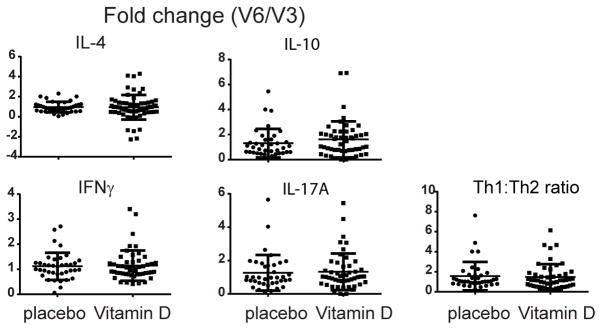
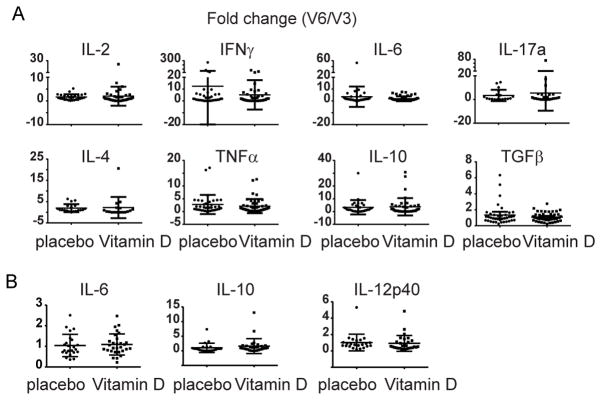
The fold change in the percentage of CD4+ T cells expressing IFNγ, IL-10 or IL-17 from V3 to V6 was not different between vitamin D- or placebo-treated participants (Figure 1). Although there was a small decrease in the percentage of IL-4 secreting cells in the placebo group from V3 to V6 (3.2 ± 1.6 to 2.6 ± 1.2, p=.03), there was no significant change in the ratio of Th1 to Th2 cells (p = 0.55) (Figure 1 and Supplemental Figure E2). Serum cytokine levels were also similar between groups (Supplemental Table E2) as was cytokine secretion of PBMC following in vitro stimulation with α-CD3/CD28 (Figure 2A and Supplemental Figure E3). No significant differences were observed between groups in the production or fold change of IL-6, IL-10 or IL-12p40 in the culture supernatants of CD14+ monocytes stimulated with LPS (Figure 2B and Supplemental Figure E4). Supplementation with vitamin D did not alter the percentage of monocytes or their subsets, expression of the activation marker CD40 or the chemokine receptor CX3CR1, which has been shown to correlate with an inflammatory phenotype (data not shown)6, 7. In addition, no differences were detected in the percentage or ratio of peripheral blood plasmacytoid or myeloid DC, cell surface expression of HLA-DR or fold change in HLA-DR expression in either treatment group (data not shown). All unadjusted comparisons between the placebo and vitamin D-treated participants were confirmed by adjusted ANCOVA results. In addition, analysis comparing only those participants that achieved vitamin D sufficiency to placebo yielded the same conclusions (data not shown).
Figure 1. Intracellular cytokine analysis of CD4+ T cells.
Peripheral blood samples were obtained prior to (V3) and after (V6) 12 weeks of treatment with placebo or vitamin D. Intracellular cytokine staining of CD4+ T cells was performed and the percentage of CD4+ T cells expressing IL-4, IFNγ, IL-10 or IL-17A determined. Shown is the fold change from V3 to V6, calculated as the ratio of the V6 value divided by the V3 value for each sample pair. There was no difference in this ratio between vitamin D- and placebo-treated participants. The Th1:Th2 ratio, calculated by dividing the percentage of CD4/IFNγ positive by the percentage of CD4/IL-4 positive cells, was also not different. Shown are individual data points and lines representing the mean ± standard deviation.
Figure 2. Cytokine secretion by stimulated PBMCs and monocytes.
A) PBMCs were isolated from samples collected either before (V3) or after (V6) vitamin D supplementation or placebo, and stimulated with α-CD3 and α-CD28 for 48 hours. Secreted cytokines were analyzed in the culture supernatant by cytokine bead array or ELISA (TGFβ only). The fold change from V3 to V6 was calculated by dividing the value at V6 by the value at V3 for each sample pair. B) CD14+ monocytes were isolated from PBMC from vitamin D-deficient asthmatics before (V3) and after (V6) vitamin D supplementation or placebo and stimulated with LPS for 48 hours. IL-6, IL-10 and IL-12p40 production was determined by ELISA. The fold change from V6 to V3 (V6/V3) for each sample pair was calculated and shown are individual data points and lines representing the mean ± standard deviation.
In vitro data has suggested that CD4+ T cells from steroid resistant asthmatics may not augment IL-10 production in response to steroids, and that exogenous vitamin D can reverse this defect2. Although we did not directly measure this, we found that in vivo treatment of vitamin D insufficient asthmatics with vitamin D had no detectable effect on CD4+ T cell cytokine profiles or the phenotype of monocytes or DC.
The samples for this study were obtained as part of a clinical trial that examined whether treatment with vitamin D in symptomatic asthmatics that were insufficient would provide a steroid sparing effect5. In that trial, vitamin D supplementation did not decrease the rate of first treatment failure or exacerbation although when only those that achieved sufficiency in vitamin D levels were analyzed, vitamin D supplementation was associated with a decrease in treatment failures and exacerbations. However, analysis of this subgroup in our assays did not alter our findings (data not shown).
In contrast to in vitro studies with exogenous vitamin D exposure, we found no detectable effect when vitamin D insufficient subjects were supplemented in vivo. There are several important limitations to these data. Vitamin D3 was used in this trial, which must be converted to its active form, whereas most in vitro studies utilize the active form, 1,25(OH)2-vitamin D3. Vitamin D levels are affected by the serum concentration of VDR, which differs in African American and Caucasian populations, though vitamin D bioavailable levels do not appear to differ8. Cellular expression of VDR and 1α-hydroxylase may differ between subjects and influence the cellular level of the active compound. It is possible that other aspects of immune or structural lung cell function such as the generation Tregs or of the antibacterial cathelicidin may have been altered by vitamin D supplementation4, 9. We cannot exclude the possibility that treatment for longer periods of time, or with calcitriol, or to higher serum vitamin D levels, might have yielded different results.
Our data suggests that in vivo supplementation with vitamin D in insufficient asthmatics has no effect on T cell, monocyte or DC function. The role for vitamin D supplementation as an adjunctive immunomodulatory therapy remains to be established.
Methods
Participants
Participants that consented to the parent VIDA trial and that met eligibility criteria were invited to participate in this substudy. Informed consent was obtained and peripheral blood collected at the 3rd parent study visit (V3) prior to randomization and again 12 weeks later at the 6th parent study visit (V6), prior to beginning the ICS taper. Samples were shipped overnight in insulated packaging to the research laboratories at Brigham and Women’s Hospital (M.C., Boston, MA) or Washington University in St Louis (JMG, St Louis, MO). Institutional Review Board approval was obtained from each participating institution. This trial was registered with clinicaltrials.gov as part of the parent trial (NCT01248065).
Analysis CD4 T cell function
Intracellular cytokine staining was performed using the FastImmune protocol for staining of samples of whole blood (BD Biosciences, San Jose, CA). Briefly, 500 μl aliquots of whole blood were stimulated with phorbol 12-myristate 13-acetate (PMA, 50 ng/ml) and ionomycin (1 μg/ml) in the presence of brefeldin A (10 μg/ml) for 5 hours at 37 °C. Samples were subsequently stained with allophycocyanin (APC) conjugated α-CD4 followed by hypotonic RBC lysis, fixed and permeabilized and then stained with fluorescently conjugated antibodies against either IL-4, IFNγ, IL-10 or IL-17A and analyzed on a FacsCalibur flow cytometer (Becton Dickinson, Mountainview, CA). Data was analyzed using WinList version 7 software (Verity Software Corporation, Topsham ME). Gating strategy for CD4+ T cell analysis: Live cells were gated on by forward vs side scatter, which were then gated on the CD4+ subset. The percentage of live, CD4+ cells were then analyzed to determine the percentage of cells expressing each intracellular cytokine. Isotype controls were used to determine the background level and gates were set to exclude negative cells.
Plasma was collected by centrifugation of heparinized blood and stored at −80 °C for later analysis and for use in in vitro culture. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation (Ficoll-Paque PLUS, GE Healthcare, Pittsburgh, PA), and an aliquot reserved for flow cytometry. PBMCs were cultured at 5×105/ml in complete media containing 10% autologous serum and replicate cultures were either unstimulated or stimulated with α-CD3 (1 μg/ml) and α-CD28 (1 μg/ml) for 48 hours. Plasma and culture supernatants were assayed for IL-2, IL-4, IL-6. IL-10, IL-17, TNFα and IFNγ by Cytokine Bead Array according to the manufacturer’s instructions (BD Biosciences, San Jose, CA). TGFβ was assayed by ELISA (eBioscience, San Diego, CA) following acidification of the sample.
Monocyte isolation and in vitro stimulation
PBMC and autologous serum for culture were isolated as described above and an aliquot of cells reserved for flow cytometry. If there was sufficient sample, monocytes were isolated from PBMC using CD14 magnetic MicroBeads (Miltenyi Biotec, San Diego, CA) as per the manufacturer’s instructions. Monocytes were cultured at 5 × 105/ml in RPMI 1640 containing 20% autologous serum, L-glutamine and penicillin-streptomycin and stimulated with 10 ng/ml lipopolysaccharide (LPS, E. coli 011:B4, Sigma, St Louis, MO) for 48 hours. Supernatants were stored at −80°C until analysis. IL-6, IL-10 and IL-12p40 levels were determined by ELISA (eBioscience, San Diego, CA).
Monocyte and dendritic cell flow cytometry
PBMC were stained with the following directly conjugated fluorescent monoclonal antibodies (mAb) to allow for phenotypic characterization of monocyte and dendritic cell populations: Anti-CD14-fluorescein (FITC), anti-CD1a-phycoerythrin (R-PE), anti-CX3CR1-PerCp-eFluor710, anti-CD40-allophycocyanin (APC), anti-CD16- (PE-Cy7), anti-CD11c-PerCP-Cy5.5, anti-CD1c-APC and anti-CD123-PE-Cy7; anti-HLA-DR-APC-Cy7, Lin1 (CD3, CD14, CD16, CD19, CD20, CD56)- FITC and appropriate fluorescently conjugated isotype controls (eBioscience, San Diego, CA). Data was acquired on a FACS Canto II (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR). Myeloid (CD11c+HLA-DR+) and plasmacytoid (CD123+HLA-DR+) DC were identified in the lineage (Lin1)− HLA-DR+ PBMC population after live cell gating.
Statistical analysis
Within-subject comparison of all outcomes from V3 to V6 was performed using 2-tailed paired t-tests. Between-subject comparison of the placebo versus vitamin D treated participants was performed using Mann-Whitney U-test for non-parametric data (GraphPad Prism v6, GraphPad Software Inc, San Diego, CA). In addition, the change in each study outcome from V3 to V6 was also compared between placebo and vitamin D treated participants via an analysis of covariance (ANCOVA) model with adjustment for center, BMI, and race (SAS v9.3, SAS Institute, Research Triangle Park, NC). The unadjusted and adjusted analyses were then repeated to compare between those vitamin D treated participants who reached vitamin D sufficiency (25(OH) vitamin D ≥ 30 ng/ml) after 12 weeks of treatment versus the placebo group.
Supplementary Material
Acknowledgments
We thank Richard Martin, MD, (National Jewish, Denver CO), Sally Wenzel, MD (University of Pittsburgh), Lewis Smith, MD (Northwestern University), Jerry Krishnan, MD, PhD (University of Illinois at Chicago), Julian Solway, MD (University of Chicago), Christine Sorkness, PharmD (University of Wisconsin, Madison), Homer Boushey, MD (University of California, San Francisco), Monica Kraft, MD (Duke University), Stephen Peters, MD PhD (Wake Forest University) and Craig LaForce MD (North Carolina Clinical Research) and the staff and coordinators at each of the clinical sites for their efforts in recruiting participants and diligence in collecting and shipping samples to the research laboratories.
Sources of funding: This work was funded as a subaward of the NIH AsthmaNet grant (U10HL098115) from the National Heart, Lung and Blood Institute.
Abbreviations
- ICS
inhaled corticosteroids
- LPS
lipopolysaccharide
- mAb
monoclonal antibodies
- Tregs
regulatory T cells
- VIDA
Vitamin D Add-on Therapy Enhances Corticosteroid Responsiveness in Asthma
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. Vitamin D and asthma. Am J Respir Crit Care Med. 2012;185:124–32. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Dimeloe S, Richards DF, Chambers ES, Black C, Urry Z, et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax. 2014;69:508–15. doi: 10.1136/thoraxjnl-2013-203421. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083–91. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 7.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–72. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 8.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D- binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.