To the Editor
Eczema Vaccinatum (EV) is a life threatening complication of exposure to smallpox vaccination in patients with atopic dermatitis (AD) characterized by dissemination of vaccinia virus (VV) in skin and internal organs1. We have shown that BALB/c mice inoculated with VV at sites of allergic skin inflammation elicited by epicutaneous (EC) sensitization with ovalbumin (OVA) exhibit features of EV2. They include satellite lesions and VV dissemination to internal organs. EV features were absent in mice inoculated with VV in control skin EC sensitized with saline, suggesting that allergic skin inflammation predisposes to VV dissemination2, and consistent with the known role of the Th2 cytokine IL-4 in promoting VV dissemination3. The levels of the Th2-promoting epithelial cell-derived cytokines thymic stromal lymphopoietin (TSLP) and interleukin (IL)-33 are increased in the skin lesions and serum of patients with AD4. We have used our mouse model of AD to examine the potential role of these cytokines in EV.
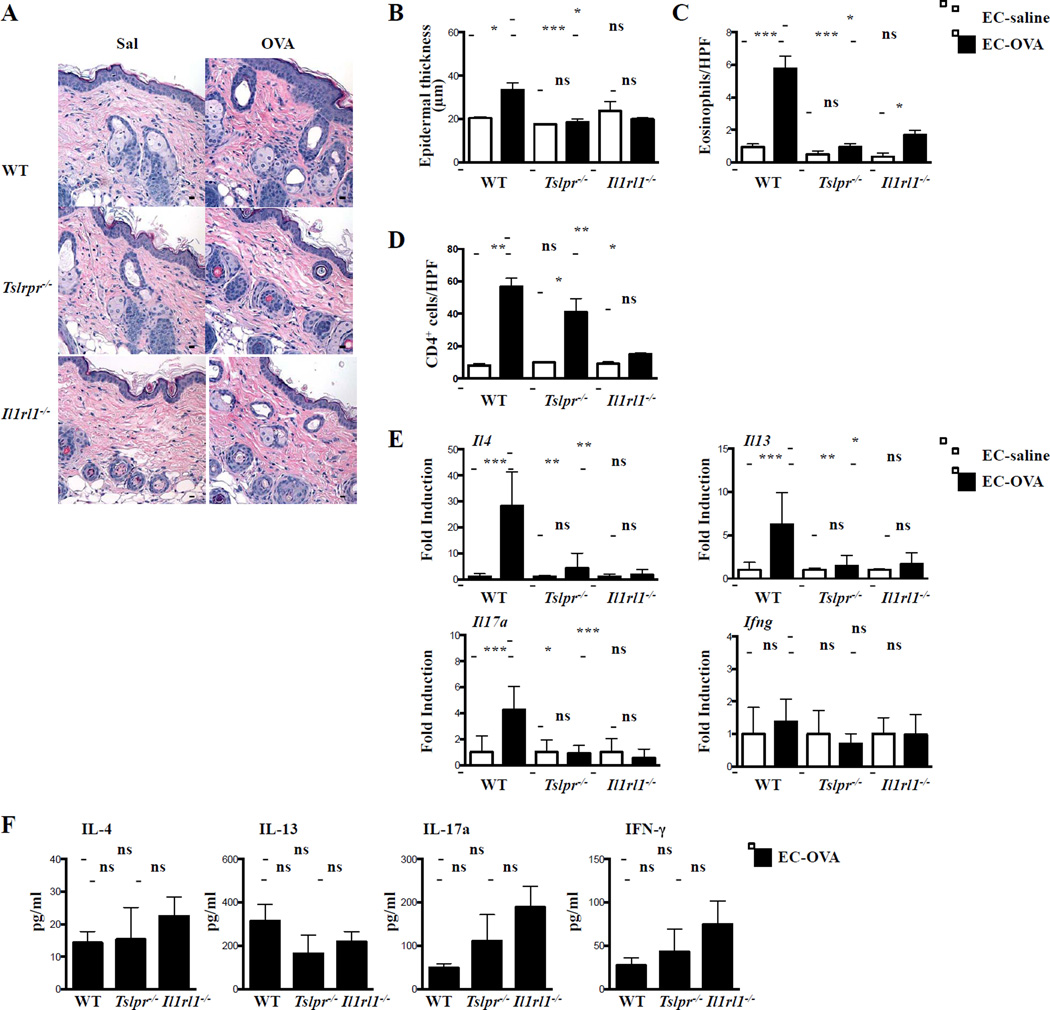
Wild-type (WT), Tslpr−/−, and IL-33 receptor-deficient Il1rl1−/− mice on BALB/c background were EC sensitized with OVA or saline over 7 weeks5. As previously reported5, WT mice EC sensitized with OVA exhibited an increase in epidermal thickening, numbers of dermal eosinophils and CD4+ cells, and levels of mRNA expression for Il4, Il13, Il17a, but not Ifng, compared to saline sensitized controls (Fig. 1A–D). In contrast, Tslpr−/− and Il1rl1−/− mice EC sensitized with OVA failed to develop skin inflammation, except for comparable numbers of dermal CD4+ cells in Tslpr−/− mice, as previously reported5, and a small increase in the number of dermal eosinophils in Il1rl1−/− mice (Fig. 1A–D). This was not due to an impaired systemic cytokine response to OVA. In response to in vitro re-stimulation with OVA, splenocytes from Tslpr−/− and Il1rl1−/− mice secreted comparable amounts of IL-4, IL-13, and IL-17A, as well as IFNγ as splenocytes from WT controls (Fig. 1E), consistent with previous observations in Tslpr−/− and Il33−/− mice5,6. Thus, in contrast to other modes of immunization7, the systemic Th2 response to EC sensitization is not dependent on TSLP or IL-33. Importantly, our findings indicate that IL-33 and TSLP play non-redundant roles in promoting the expression of Th2 and IL-17A cytokines in EC sensitized skin. Intradermal injection of anti-TSLP neutralizing antibody inhibits the development of allergic skin inflammation following cutaneous antigen challenge of previously sensitized WT mice5, suggesting that local expression of TSLP and possibly IL-33 may be important for the upregulation of Th2 and IL-17A cytokine expression by immune cells in EC sensitized skin.
Figure 1. Impaired allergic skin inflammation in Tslpr−/− and Il1rl1−/− mice.
A. Representative H&E sections. B. Epidermal thickness. C, D. Numbers of eosinophils/HPF (C) and CD4+ cells/HPF (D). E. Cytokine mRNA expression relative to saline-exposed skin of the same genotype. F. OVA-specific splenocyte cytokine production. Scale bars: 100 μm. n = 5 per group. *P < .05, **P < .01 and ***P < .001. ns, not significant. Results were analyzed by nonparametric one-way ANOVA.
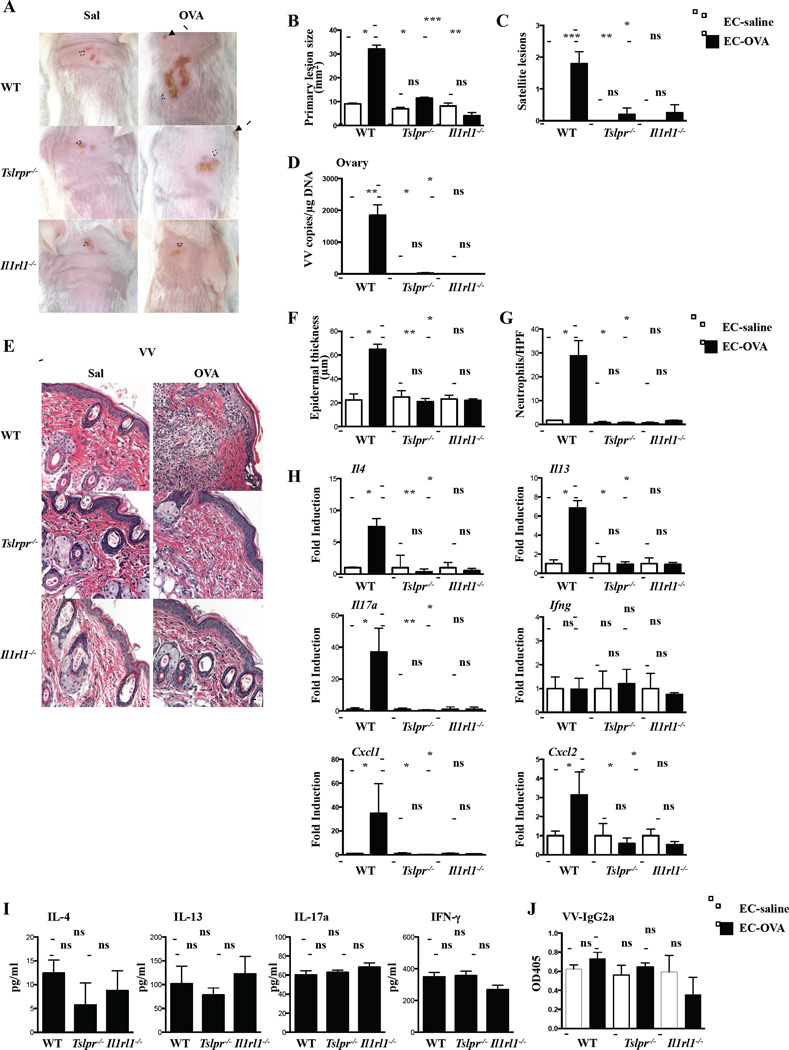
To examine the role of TSLP and IL-33 in the response to cutaneous VV inoculation, Tslpr−/− and Il1rl1−/− mice were inoculated in EC sensitized skin sites with VV Western Reserve strain (ATCC, VR-1454) by skin scarification, using 107 PFU/mouse, and euthanized seven days later. As previously reported2, VV inoculation in OVA-sensitized skin of WT mice resulted in significantly larger and erosive primary lesions, significantly more satellite lesions, and significantly greater recovery of VV genomes from the ovaries compared to VV inoculation in saline-sensitized skin (Figure 2A–D). In contrast, VV inoculation in OVA-sensitized skin of Tslpr−/− and Il1rl1−/− mice resulted in no significant increase in the size of the primary lesion, number of satellite lesions, or recovery of VV genomes from the ovaries compared to VV inoculation in saline-sensitized skin (Figure 2A–D). All three parameters were comparable in saline sensitized skin of Tslpr−/−, Il1rl1−/− and WT mice. No VV genomes could be detected in the inoculated-skin EC sensitized with saline or OVA 7 days after VV inoculation. In previous studies, no or small numbers of VV genomes were detected in VV inoculated sensitized skin EC sensitized with saline or OVA2, 8.
Figure 2. EV features are ameliorated in Tslpr−/− and Il1rl1−/− mice inoculated with VV in OVA sensitized skin.
A. Gross appearance. Dashed circles indicate primary lesions, and arrows indicate satellite lesions. B. Area of primary lesions. C. Number of satellite lesions. D. Viral load. E. Representative H&E sections. Scale bars: 100 μm. F. Epidermal thickness. G. Number of neutrophils/HPF. H. Cytokine mRNA expression relative to infected saline-exposed skin. I. VV-specific splenocyte cytokine production2. J. VV-specific IgG2a serum levels. n = 5 per group. *P < .05, **P < .01 and ***P < .001. ns, not significant. Results were analyzed by nonparametric one-way ANOVA.
As previously reported2, 8, VV inoculation in OVA-sensitized skin of WT mice resulted in significantly increased epidermal thickness caused significantly more dermal infiltration with neutrophils and significantly higher expression of Il4, Il13, Il17a, Cxcl1 and Cxcl2, but not Infg, compared to VV inoculation in saline-sensitized skin (Fig. 2E–H). In contrast, VV inoculation in OVA-sensitized skin of Tslpr−/− and Il1rl1−/− mice resulted in no significant increase in epidermal thickness, dermal infiltration with neutrophils, or cytokine mRNA expression compared to VV-inoculation in saline-sensitized skin (Fig. 2E–H). The response of Tslpr−/− and Il1rl1−/− mice to VV inoculation in saline-sensitized skin was comparable to that of WT controls. Th2 and Th17 cytokines promote, while IFNγ and IgG2a antibody limit VV replication2, 3, 9. Perturbations in the systemic response to VV are unlikely to explain the failure of OVA-sensitized VV-inoculated Tslpr−/− and Il1rl1−/− mice to develop skin inflammation and their resistance to VV dissemination. VV stimulated cytokine production by splenocytes and VV-specific IgG2a antibody levels were comparable in Tslpr−/−, Il1rl1−/− mice, and WT controls (Fig. 2I–J).
IL-4 and IL-17A promote VV replication2, 3. The failure of Tslpr−/− and Il1rl1−/− mice to upregulate the expression of these cytokines in OVA-sensitized skin may have constrained local viral replication early after VV inoculation, resulting in a minimal-size primary lesion, virtual lack of satellite lesions and undetectable viral dissemination to the ovaries, as observed following VV inoculation in saline-sensitized skin. IL-17A is highly upregulated following VV inoculation in OVA sensitized skin sites, and is critical for neutrophil infiltration and the development of erosive skin lesions in our EV model2. The failure to upregulate Il17a, Cxcl1, and Cxcl2, mRNA expression in VV-inoculated OVA-sensitized skin of Tslpr−/− and Il1rl1−/− mice likely underlies the failure of neutrophils to be recruited to the VV inoculation site and thereby the absence of erosive skin lesions in these mice.
TSLP and IL-33 expression is upregulated in AD skin lesions4. Genetic variants in TSLP are associated with AD and eczema herpeticum10, supporting the involvement of TSLP in the control of viral skin infection in AD. It has been suggested that TSLP and IL-33 drive AD via group 2 innate lymphoid cells (ILC2s)4. Comparable numbers of dermal CD4+ cells in Tslpr−/− mice, but not in Il1rl1−/− mice, suggest that TSLP and IL-33 may drive the development of allergic skin inflammation by different mechanisms. Identification of the targeted cells by TSLP and IL-33 in our model, which may include ILC2s, dendritic cells, basophils, mast cells, and Th2 cells requires future investigations. Our results in a mouse model of AD suggest that TSLP and IL-33 may play a key role in the development of AD and EV. The clinical relevance of our findings needs to be established by examining whether blockade of these two epithelial cytokines is effective in in AD patients and in preventing EV.
Summary.
TSLP and IL-33 play an important role in the development of AD and EV. Blocking TSLP or IL-33 may attenuate the severity of AD and EV.
Acknowledgments
Funding: This work was supported by NIH grant HHSN272201000020C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engler RJ, Kenner J, Leung DY. Smallpox vaccination: Risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357–365. doi: 10.1067/mai.2002.128052. [DOI] [PubMed] [Google Scholar]
- 2.Oyoshi MK, Elkhal A, Kumar L, Scott JE, Koduru S, He R, et al. Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proc Natl Acad Sci U S A. 2009;106:14954–14959. doi: 10.1073/pnas.0904021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 5.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita H, Arae K, Unno H, Toyama S, Motomura K, Matsuda A, et al. IL-25 and IL-33 Contribute to Development of Eosinophilic Airway Inflammation in Epicutaneously Antigen-Sensitized Mice. PLoS One. 2015;10:e0134226. doi: 10.1371/journal.pone.0134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oyoshi MK, Wang JY, Geha RS. Immunization with modified vaccinia virus Ankara prevents eczema vaccinatum in a murine model of atopic dermatitis. J Allergy Clin Immunol. 2011;128:890–892. e3. doi: 10.1016/j.jaci.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 10.Gao PS, Rafaels NM, Mu D, Hand T, Murray T, Boguniewicz M, et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol. 2010;125:1403–1407. e4. doi: 10.1016/j.jaci.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]