Abstract
The exposure of hepatocytes to high concentrations of lipids and carbohydrates and the ensuing hepatocellular injury are termed lipotoxicity and glucotoxicity, respectively. A common denominator is metabolic derangement, especially in regards to intracellular energy homeostasis, which is brought on by glucose intolerance and insulin resistance in tissues. In this review, we highlight the lipids and carbohydrates that provoke hepatocyte injury and the mechanisms involved in lipotoxicity and glucotoxicity, including endoplasmic reticulum stress, oxidative stress and mitochondrial impairment. Through upregulation of proteins involved in various pathways including PKR-like ER kinase (PERK), CCAAT/enhancer-binding homologous protein (CHOP), c-Jun NH2-terminal kinase-1 (JNK), Bcl-2 interacting mediator (BIM), p53 upregulated modulator of apoptosis (PUMA), and eventually caspases, hepatocytes in lipotoxic states ultimately undergo apoptosis. The protective role of certain lipids and possible targets for pharmacological therapy are explored. Finally, we discuss the role of high fructose and glucose diets in contributing to organelle impairment and poor glucose transport mechanisms, which perpetuate hyperglycemia and hyperlipidemia by shunting of excess carbohydrates into lipogenesis.
Keywords: Hepatic lipid, NASH, ER stress, CHOP, JNK, BH3-only proteins, Death receptors, Oxidative stress, Inflammation, Fibrosis
1. INTRODUCTION
Lipotoxicity refers to the harmful effects of high concentrations of lipids and lipid derivatives to cells. Hyper-alimentation with diets rich in lipids and carbohydrates is associated with the development of one of two clinical-histopathological phenotypes of liver fatty accumulation: non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). Clinically, NASH is associated with obesity, metabolic syndrome, insulin resistance and dyslipidemia. Histological features of NASH include hepatic macrovesicular lipid accumulation, chronic inflammation, hepatocyte ballooning, interstitial fibrosis and necro-apoptosis 1. The mechanisms involved in lipotoxicity include endoplasmic reticulum (ER) stress, c-Jun NH2-terminal kinase (JNK)-induced toxicity and BH3-only protein-induced mitochondrial and lysosomal dysfunction 2-6.
Glucotoxicity refers to the toxic effects of hyperglycemia and excess carbohydrate intake on cells and tissues. Glucotoxicity is intrinsically linked to insulin resistance, which facilitates hyperglycemia. Excess carbohydrates can be converted into free fatty acids (FFA) and triglycerides (TG), and subsequently hepatotoxic lipids such as lysophosphatidyl choline (LPC), ceramides, free cholesterol and bile acids (BA) may accumulate. High carbohydrate diets activate several lipogenic enzymes such as acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS) or SCD-1, inducing lipogenesis and steatosis. Recent data indicate that glucotoxicity can be injurious to liver cells by inducing ER stress and hepatocyte cell death. In this review, we explore some of the organelle dysfunction and molecular pathways activated by excessive consumption of carbohydrates and lipids fats, leading to hepatotoxicity.
2. HEPATOTOXIC LIPIDS
Several members of the lipid family have been shown to mediate hepatic lipotoxicity. These include free fatty acids (FFA), triglycerides (TG), lysophosphatidyl choline (LPC) and ceramides, free cholesterol (FC), and bile acids (BA).
2.1 Free fatty acid (FFA) and triglycerides (TG)
2.1.1 Free Fatty acid
FFA with double bonds are referred to as “unsaturated” while those without double bonds are called “saturated”. Palmitate (PA; C16:0), the most common saturated FFA found in animals and plants, is ingested as part of the diet or can be produced by de novo lipid synthesis from excess carbohydrate consumption. Oleate (OA; C18:1), an unsaturated FFA, is commonly present in the Western diet. Several in vitro studies have demonstrated the toxic effects of unsaturated FFA such as PA or stearate on liver cells by inducing apoptosis (vide infra).
Hepatocytes exposed to high circulating FFAs increase uptake in order to clear the FFAs in the blood. FFAs entering the liver are mostly derived from lipolysis of adipose tissue triglyceride in the fasting state (constituting 60% of liver FFAs in NAFLD subjects), de novo lipogenesis (26%) and from hydrolysis of dietary triglycerides (15%) 1. Although the precise mechanisms regulating increased hepatic FFA uptake are unclear, it seems to involve a tetrameric plasma membrane protein complex that comprises plasma membrane fatty acid-binding protein (FABP), caveolin-1, fatty acid translocase (FAT/CD36) and calcium independent membrane phospholipase A2 (iPLA2β) 2. FAT/CD36-mediated incorporation of circulating FFA into hepatocyte vacuoles causes these hepatocytes to resemble adipocytes 3. In the liver of mice and humans with insulin resistance, steatosis and NASH, CD36 is overexpressed via transcriptional regulation by the transcription factor PPARγ. Hepatocytes exposed to PA and OA display increased expression of FAT/CD36 and fatty acid transport protein (FATP)-2 leading to accumulation of diacylglycerol (DAG) or ceramides into the cells 4.
2.1.2 Triglycerides
Triglycerides (TG) are composed of a glycerol molecule with three free fatty acids and represent a form of energy storage. Studies performed in humans have shown that accumulation in excess of hepatic TGs is mainly the result of increased delivery of adipose-derived FFAs to the liver and enhanced de novo lipid synthesis in the liver 1. In contrast hepatic steatosis is only modestly affected by lipid disposal via β-oxidation or very low density lipoproteins (VLDL) export 5or directly by increased dietary lipids 1. Also, consumption of large amounts of carbohydrates can contribute to hepatic steatosis by facilitating lipogenesis and lipid storage as TG. Indeed, rat pups fed a 60% fructose rich diet showed altered lipid profile with increased TG, cholesterol, VLDL and low density lipoproteins (LDL) which was reversed when fed a standard diet 6.
2.1.3 Unlike TG, saturated FFA are toxic to hepatocytes
Treatment of hepatocyte cell lines with OA versus PA showed that while OA generated more hepatocyte steatosis, PA was responsible for higher rates of apoptosis. PA was associated with PPARα activation and impairment of insulin signaling. In addition, PA triggered cell death via JNK-dependent mitochondrial dysfunction and caspase activation 7(Table 1). OA, on the other hand, generated higher formation of TG 7. It appears that TG represent a defense system against the pro-apoptotic effects of large loads of FFA in cells 7,8. Yamaguchi et al. confirmed the protective role of TG by showing that inhibition of dyacylglycerol acetyltransferase 2 (DAGT2), the final catalyst in hepatocyte TG synthesis, generated increased necro-inflammation, increased peroxidation and oxidative stress 9 (Table 1).
Table1.
Lipid types and mechanisms of liver injury.
| Lipid Types | Pathways mediating hepatic injury | References |
|---|---|---|
| Free Fatty Acids (FFA)/ Lysophosphatidyl choline (LPC) |
|
Ricchi, 2009 |
|
Cazanave, 2009; Malhi, 2006; Pagliassotti, 2007 Malhi, 2007 Cazanave, 2011 |
|
|
Lee, 2012; Lin, 2013; Cazanave, 2014 |
|
| Triglycerides (TG) |
|
Ricchi, 2009; Listenberger, 2003; Yamaguchi, 2007 |
| Ceramides |
|
Pagadala, 2012; Schwabe, 2006 |
| Free Cholesterol (FC) |
|
Arguello, 2015; Tomita, 2014 |
|
Musso, 2013 | |
|
Gan, 2014 | |
| Bile Acids |
|
Ferreira, 2014 |
c-Jun NH2 terminal kinase (JNK), p53 upregulated modulator of apoptosis (PUMA), death receptor 5 (DR5), TNFα related apoptosis inducing ligand (TRAIL), endoplasmic reticulum (ER), CAATT enhancer binding homologous protein (CHOP), Bcl-2 interacting mediator (BIM), Kelch like ECH associated protein 1 (Keap1), Reactive oxygen species (ROS), unfolded protein response (UPR), sirtuin 1 (SIRT1).
2.2 Lysophosphatidyl choline (LPC)
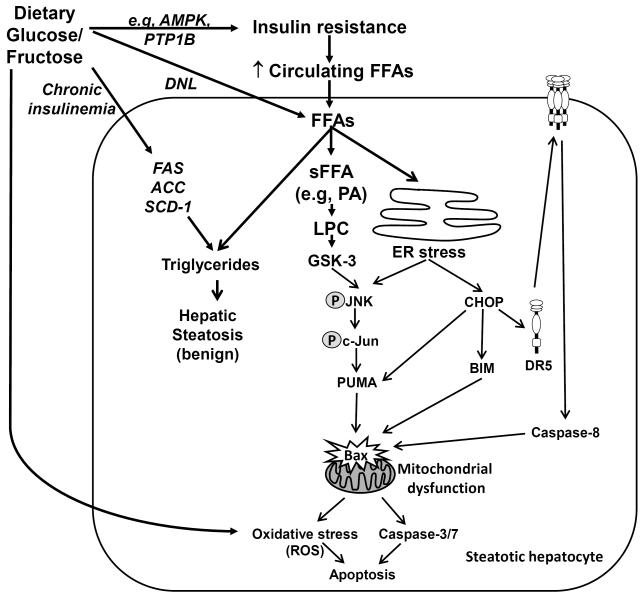
LPC is a class of lipids derived from phosphatidylcholine by partial hydrolysis through the phospholipase A2 (PLA2). LPCs have been implicated in phagocyte chemotaxis and are released secondary to activation of calcium independent PLA2 by caspase 3 when cells undergo apoptosis 10. LPC levels are increased in the liver or plasma of both human NASH patients 11 and animal models of NASH 12,13. Furthermore, treatment of liver cells with PA results in increased intracellular LPC concentration and cell toxicity 14. LPC appears to be a key instigator of lipotoxicity by triggering an ER stress and inducing apoptotic pathways downstream of the activation of JNK or glycogen synthase kinase 3 (GSK3) and the induction of the transcription factor CCAAT/enhancer-binding homologous protein (CHOP), all leading to the upregulation of pro-death proteins, e.g p53 upregulated modulator of apoptosis (PUMA). LPC toxicity was attenuated by inhibition of JNK or GSK3 activity and by knockdown of CHOP 14. In fact, it seems that LPC-induced lipotoxic mechanisms are largely indistinguishable from those of PA, suggesting that FFAs could exert cytotoxicity through generation of LPC 14 (Figure 1).
Figure 1. Molecular mechanisms of hepatocyte lipotoxicity and glutotoxicity.
Insulin resistance, a hallmark of NAFLD, leads to an increase in serum concentration of circulating FFAs. FFAs are transported into the hepatocyte where they can be esterified into neutral triglycerides resulting in hepatic steatosis. Esterification of FFAs represents a buffering mechanism allowing cells to maintain viability in the face of excess non-esterified FFAs exposure. Saturated FFAs (sFFA) in excess are toxic to liver cells and they can accumulate in the endoplasmic reticulum (ER) and induce an ER stress, which in turn induce the transcription factor CHOP and induce JNK activity. Other hepatotoxic lipids such as palmitate (PA)-derived lysophosphatidyl choline (LPC) also mediate JNK-dependent hepatic toxicity downstream of glycogen synthase kinase (GSK)-3 signaling cascades and ER stress induction. Active JNK phosphorylates the transcription factor c-Jun, which cooperates with CHOP to upregulate the transcription of the pro-apoptotic BH3-only protein PUMA. CHOP also mediate the upregulation of another BH3-only protein BIM; and BIM and PUMA cooperate in activating the executioner proapoptotic protein Bax, causing mitochondrial dysfunction, activation of the effector caspases 3/7 and cellular apoptosis. CHOP also upregulates the expression of the death receptor DR5, resulting in increased DR5 cell surface expression which clustering of the receptor leading to recruitment and activation of the executioner caspase-8, which ultimately induces Bax. Saturated FFAs-induced mitochondrial dysfunctions is also mediated by a Bax-dependent permeabilization of lysosomes and release of cathepsin B in the cytosol. Mitochondrial dysfunction also results in generation of reactive oxygen species (ROS) which further induce cellular demise. In addition, excess dietary sugars, resulting in chronic hyperglycemia, can further induce liver toxicity or glucotoxicity by increasing hepatic steatosis via de novo lipogenesis (DNL) and exacerbating insulin resistance and cellular demise, through the activation of oxidative and ER stress responses, and/or downstream the modulation of the activity of AMP-activated protein kinase (AMPK) and protein-tyrosine phosphatase 1B (PTP1B). Also, chronic hyperinsulinemia is associated with hepatic steatosis via the upregulation of hepatic lipogenic gene expression (ACC, FAS, SCD-1).
2.3 Ceramides
Ceramides and their derivative sphingolipids are cell membrane components and biologically active lipids that have been implicated in insulin resistance, oxidative stress and inflammation 15. Increased circulating concentration of ceramides were seen in peripheral blood samples of obese patients with NASH 16. Ceramides can be produced de novo after oxidative stress from palmitoyl co-A and serine through the action of serine palmitoyl co-A transferase (SPT), which is the rate limiting enzyme. Another pathway for generation of ceramides is through the action of neutral sphingomyelinase (N-SMase) hydrolysis of cell membrane sphingomyelin. N-SMase is upregulated by inflammation 17 (Table 1).
Increased pro-inflammatory cytokines such as interleukin (IL)-1 and IL-6 are present in NASH and correlate with augmented ceramide levels 17. Indeed, in the liver, ceramides interacts with TNFα and promotes the release of reactive oxygen species (ROS) by hepatic mitochondria, resulting in apoptosis and worse hepatic inflammation 17,18. Similarly, mice fed a high fat diet with subsequent steatosis displayed an increase in sphyngomyelinase activity 19and in hepatic long chain ceramides (C16 and C18) which were associated with hepatocyte apoptosis 20. Also, increased liver ceramide levels were also associated with the development of hepatic insulin resistance 4,21. The role of ceramides in NAFLD and lipotoxicity is still not fully understood and seems to be cell-type specific. Indeed, saturated FFAs-induced hepatocyte apoptosis was found to be ceramide-independent 22,23, whereas in pancreatic β-cells, ceramides are important mediators of cellular failure and apoptosis and may work as second messenger in cross-talk between the ER and mitochondria 24.
Inhibition of ceramide synthesis, using inhibitors such as myriocin or imipramine, reduced liver injury, steatosis, and improved insulin sensitivity in target hepatic tissue in rodents fed a high fat diet 25,25,26.
2.4 Free Cholesterol (FC) and Bile Acids (BA)
In NAFLD, hepatic free cholesterol (FC) accumulates as a result of enhanced synthesis, increased cholesterol de-esterification and decreased cholesterol export and BA synthesis. Abundant intracellular FC stimulates Kupffer cells and hepatic stellate cells (HSCs) which mediate inflammation and fibrosis as well as mitochondrial dysfunction, generation of ROS, activation of unfolded protein response (UPR) and downstream effects that culminate in hepatocyte apoptosis 27.
In the liver, disruption of cholesterol homeostasis contribute to its accumulation in liver cells and within organelles. Sterol regulatory-element binding protein (SREBP)-2 is the principal regulatory enzyme of hydroxymethylglutaryl-CoA reductase (HMGCoA), which is the key cholesterol synthesis enzyme. SREBP-2 levels are increased in NASH, correlating with increased levels of FC and steroidogenic acute regulatory protein (StAR), a mitochondrial cholesterol transporter 28. Feeding animals with high levels of FC results in accumulation of toxic hepatic oxysterols which contributes to mitochondrial dysfunction and liver injury 29, 30 (Table 1). In another study, mitochondrial FC accumulation led to apoptosis through a toll like receptor 4 (TLR4)-regulated JNK1 pathway that activated the inflammatory mediators high mobility group box 1 protein (HMGB1) 31(Table 1). The key role that cholesterol plays in the development of steatohepatitis has prompted research into the use of utilizing cholesterol lowering medications to protect subjects from developing NASH. Indeed, this was demonstrated recently in a multicenter cohort of 1201 patients where statins were protective against liver damage 32. In vitro and in vivo, fluvastatin attenuated HSC activation and protected against liver fibrosis, inflammation and oxidative stress 33.
BA are end-products of the degradation of cholesterol. Hydrophobic bile acids, including deoxycholic acid (DCA), can trigger hepatic apoptosis and are increased in NASH through a JNK1/p53/sirtuin 1 (SIRT1) pathway 34. Farnesoid X receptors (FXR) are bile acid receptors that inhibits BA synthesis, and FXR agonists can protect from steatohepatitis and improve insulin resistance and hyperlipidemia in obese patients 35,36.
Different lipid families are toxic to hepatocytes and induce lipoapoptosis. Among them, some of the most prominent offenders to the liver are unsaturated FFA, arising from TGs lipolysis in adipose tissue and excess release of FFA into the circulation. Saturated FFA (such as palmitate and stearate) or their end products (LPC) exert their toxicity by triggering several apoptotic processes implicating the activation of ER stress- and JNK-dependent pathways. In addition, the role of ceramides in the liver is still being defined, but it exerts an important effect on insulin resistance. Other lipids, such as FC and BA, contribute to liver inflammation, fibrosis, mitochondrial dysfunctions and apoptosis in the fatty liver disease spectrum.
3. MOLECULAR MECHANISMS OF HEPATOCYTE LIPOTOXICITY
Failure of hepatocytes to dispose of excess FFA results in lipoapoptosis, a cardinal feature of NASH. In hepatocytes, apoptosis can occur via an intrinsic pathway activated by intracellular stress such as oxidative stress or by organelle dysfunctions including ER stress and mitochondrial permeabilization. Hepatocyte apoptosis can also occur via an extrinsic mechanism initiated by binding of death ligands, such as Fas or TRAIL, to their respective receptors. Both intrinsic and extrinsic pathways converge on effector caspases to mediate apoptosis.
3.1 Endoplasmic reticulum (ER) stress
The progression of liver disease from steatosis to NASH is characterized by endoplasmic reticulum (ER) stress37. The ER supports many vital cellular processes, including synthesis, maturation, folding and transport of protein, lipid synthesis and packaging and regulation of calcium homeostasis. Disturbances of any of these processes will initiate an ER stress. The response to an ER stress is initially mediated by the combination of several signaling pathways termed the unfolded protein response (UPR), which serves at first to re-establish ER homeostasis and promote survival by directing unfolded or misfolded proteins towards degradation 38. However, a prolonged activation of the UPR will trigger apoptotic pathways causing cell death. In addition, recent work has shown that lipid saturation of the ER membrane can activate UPR independently of unfolded proteins, supporting a direct role of lipids in ER stress response 39.
The three main UPR-mediated transmembrane proteins activated in ER stress are IRE1/X-box binding protein 1 (XBP1), PRKR-like endoplasmic reticulum kinase (PERK)/eukaryotic translation initiation factor 2α (eIF2α), and activating transcription factor-6 (ATF6) 40. During normal cell function, the transmembrane proteins are bound to the intraluminal chaperone binding immunoglobulin protein (Bip/GRP78). Under stressful conditions induced by accumulation of misfolded or unfolded proteins, depletion of ER-calcium levels or increased free cholesterol in the ER lumen, Bip/GRP78 is released from the UPR transmembrane proteins, resulting in the activation of the IRE1-, PERK- or ATF6-mediated signaling pathways 38. These pathways regulate the expression of genes involved in UPR and ER-assisted degradation (ERAD) with the aim of restoring ER homeostasis. However, these pathways also regulate expression of genes that mediate apoptosis. IRE1 may mediate cell apoptosis by recruiting TNF receptor-associated factor 2 (TRAF2) and activating JNK 41. PERK-mediated apoptosis involves activation of downstream gene GADD153 (also known as CHOP [CCAAT-enhancer-binding protein homologous protein]), while activated ATF6 promotes the expression of XBP1 and CHOP 38,42.
All three ER stress sensing pathways (ATF6α, PERK/eIF2α, IRE1α/XBP1) can regulate the development of microvesicular steatosis in the liver 43. Of note, both XBP1 and eIF2α have been shown to participate in basal and/or diet-induced regulation of lipid metabolism 44,45; and prolonged expression of CHOP can inhibit CCAAT/enhancer-binding protein alpha (C/EBPα) leading to the suppression of genes involved in lipid homeostasis and possibly driving the development of steatosis 43 . It appears that once the liver crosses the threshold from fatty accumulation to NASH, genes involved in apoptosis are upregulated, while ER stress/UPR gene sets are downregulated 46. Liver specific depletion of PERK reduces the transcriptional and translational phases of the UPR with consequent disruption of lipid metabolism and enhanced apoptosis 47.
Upregulation of the transcription factor CHOP, which has low levels of expression under non-stress conditions, plays a critical role in FFA-mediated ER stress-related liver cell death 48,49. Treatment of Huh-7 hepatocytes with the FFA palmitate led to increased interaction between CHOP and the other transcription factor c-Jun which bind to the activator protein-1 (AP-1) binding site within the p53 upregulated modulator of apoptosis (PUMA) promoter. This results in upregulation of the BH3-only protein PUMA, with subsequent Bax activation and cell death 50(Figure 1). Hepatic cells with knockdown of CHOP failed to upregulate PUMA in response to PA insult 50. CHOP has also been implicated in the upregulation of the TRAIL receptor death receptor 5 (DR5) and the other BH3-only protein BIM (Figure 1), both inducing cell death 51, 52.
3.2 JNK-dependent toxicity
Several articles have explored the relationship between free fatty acids and JNK activation, since the JNK pathway is one of the key mediators of insulin resistance and fatty acid-induced hepatotoxicity 50, 53. The JNK pathway is known to be stimulated by both oxidative stress and ER stress. Malhi et al. demonstrated that hepatocyte apoptosis induced by saturated FFAs occurred via a JNK-dependent mechanism involving upregulation of BIM and Bax activation, leading to caspase release and mitochondrial dysfunction and culminating in cell death 54. Hepatocytes contain two JNK genes, JNK1 and JNK2. The isoform JNK1 plays a central role in apoptosis induction by regulating the expression of pro-apoptotic B-cell lymphoma protein 2 (Bcl-2) family BH3-only proteins 50. It is known that JNK1 can phosphorylate c-Jun, which in turn can be integrated into the pro-apoptotic AP-1 complex. It has been demonstrated that saturated FFA, through the activation of JNK1, can upregulate PUMA in both murine and human cellular models 50,55(Table 1). PUMA works cooperatively with BIM to mediate FFA-induced hepatocyte injury (Figure 1). PUMA also activates Bax, which mediates the mitochondrial pathway of apoptosis 55. Non selective inhibition of JNK by SP600125 in vitro led to attenuation of PA-induced PUMA upregulation, resulting in decreased lipoapoptosis 54-56.
3.3 BH3-only proteins
The Bcl-2 family proteins regulate the permeabilization of the mitochondrial membrane, allowing cytochrome c leakage from the mitochondria. The Bcl-2 proteins contain one or more Bcl-2 homology (BH) domains. Anti-apoptotic members of the Bcl-2 family contain all four BH domains, while the proapoptotic members are divided among the multi BH domain members including Bak, Bax and Bok, and the BH3-only proteins including Bad, Bid, BIM, and PUMA 42. Once Bid, a BH3-only protein, is cleaved by caspase-8, it can activate multi-domain members Bak and Bax to form pores in the outer mitochondrial membrane and allow for cytochrome c leakage. Caspase-8 activation is mediated by binding of Fas ligand to its receptor Fas, a death receptor that has been found to be overexpressed in patients with hepatitis C, NASH and other chronic hepatocyte inflammatory conditions 57.
Both BIM and PUMA have been implicated as important contributors of FFA-induced hepatocyte toxicity 50,58; and knockdown of BIM or PUMA in liver cells partially protects against FFA-induced Bax activation, mitochondrial dysfunctions, caspase-3 activation and apoptosis. As discussed in previous above sections, increase in PUMA cellular levels during the lipotoxic insult involves both JNK1 and CHOP-dependent transcriptional regulation of the protein 50. In the context of lipotoxicity, BIM transcriptional upregulation is dependent on the transcription factor Forkhead box O3a (FoxO3a), activated upon dephosphorylation by the protein phosphatase 2A (PP2A) 58. Cellular levels of anti-apoptotic Bcl-2 proteins, such as Bcl-XL and Mcl-1 are also decreased during FFA-induced lipotoxicity 23,59. Decreased expression of Bcl-XL or increased proteasomal degradation of Mcl-1 both contribute to the apoptotic phenotype. 50 Thus, preventing the decrease of Bcl-xL or Mcl-1 by either overexpressing Bcl-XL or a non-degradable form of Mcl-1 partially reduced the lipotoxic insult in liver cells. Whereas BIM and PUMA directly or indirectly activate Bax; the pro-survival proteins Mcl-1 and Bcl-xL inhibit Bax function at the mitochondria by direct interaction with Bax or indirectly by sequestration of activator BH3-only proteins 60. Following Bax activation and permeabilization of mitochondrial membranes, proteins that participate in the execution phase of apoptosis, such as caspases 3, 6 and 7, are activated and trigger DNA damage and cell death. NAFLD severity is reflected by an increase of serum caspase-cleaved cytokeratin–fragments 61; and caspase 3 knockout mice are protected against liver injury induced by a high fat diet 62. Thus, BH3-only proteins can be activated by multiple mediators with the end result of hepatocyte apoptosis 63.
The pan-caspase inhibitor Z-VAD-fmk was found to attenuate saturated FFA-induced apoptosis in mouse hepatocytes 54. Despite a recent phase 2 clinical trial accessing tolerability and efficacy of caspase inhibitor GS9450 in adults with NASH, a longer trial was stopped as a result of drug toxicity 64. The utility of other caspase inhibitors in NASH may need to be investigated.
3.4 Death receptors
The tumor necrosis factor related apoptosis-inducing ligand (TRAIL) receptor 2 or DR5, has been implicated as one of the death receptor signaling pathways in lipotoxicity 52(Figure 1). Exposure to PA induced an increase in DR5 mRNA and protein expression in Huh-7 cells, leading to DR5 clustering into lipid rafts, recruitment of caspase-8, and cell death through activation of Bax 52. In another study, oleic acid (OA) sensitized Huh-7 cells and primary mouse hepatocytes to TRAIL-induced toxicity, via JNK mediated upregulation of DR5 65.
3.5 Mitochondria and oxidative stress
The features of hepatic steatosis include insulin resistance, increased FA beta-oxidation and oxidative stress, while NASH patients show evidence of mitochondrial dysfunctions and structural defects 66. Koliaki et al. showed by using high-resolution respirometry that mitochondrias work harder in obese humans with or without NASH (4.3-5.0 max respiration rate). However, NASH livers had a higher mass of mitochondrias with 30-40% lower respiration rates, with associated insulin resistance, mitochondrial uncoupling, and leaking activity 67. OA exposure of Chang liver cells caused mitochondrial swelling and reduced mitochondrial calcium concentration, leading to generation of reactive oxygen species (ROS), alteration of cell cycle and decreased cell viability. Cells were rescued when pre-treated with the p53 inhibitor pifithrin-alpha (PTA), suggesting a role for p53 in OA-associated oxidative stress 68. The voltage dependent anion channel (VDAC) of mitochondria’s outer membrane can act as an early sensor for lipotoxicity. When exposed to lipid accumulation states, VDAC exhibits less phosphorylation which allows great influx of water and calcium into the organelle. Ultimately, this edema leads to cytochrome c release and cell death 69. Moreover, free cholesterol in livers of diabetic mice with NASH accumulates in the hepatocyte plasma membrane, mitochondria and ER, resulting in mitochondrial permeability transition pore, generation of oxidative stress and apoptosis in a JNK1-dependent manner. At the molecular level, Sab (SH3BP5), a mitochondrial outer membrane JNK docking protein and substrate, undergoes JNK phosphorylation with subsequent impairment of mitochondrial function and apoptosis. These findings were dependent on the PA concentration to which hepatocytes were exposed 70.
A recent study by Gariani et al., demonstrated that high-fat high-sucrose fed mice display lower hepatic NAD+ levels causing reductions in hepatic mitochondrial functions in parallel to increase hepatic weight, steatosis and lipid peroxidation. Enhanced NAD+ salvage may represent a prerequisite for the upregulation of mitochondrial metabolism as an adaptive mechanism in response to chronic hepatic lipid accumulation and glucose intolerance 71. In support of that concept, NAD+ repletion with the NAD+ precursor nicotinamide riboside (NR) prevented or reversed NAFLD in mice fed a high fat-high sucrose diet. NAD+ protective effects were dependent on the induction of the aging-associated histone deacetylases, SIRT1 and SIRT3-mediated mitochondrial unfolded protein response (UPRmt) 72, triggering an adaptive pathway to increase hepatic beta-oxidation and mitochondrial complex content and activity. Thus, NAD+-induced SIRT1 activation regulates various cellular processes including energy metabolism, stress response and mitochondrial functions 68.
Lysosomal permeabilization and release of cathepsin B, a major lysosomal cysteine protease, into the cytosol is observed in human liver tissues from patients with NAFLD 73 and could also contribute to mitochondrial dysfunctions and caspases activation associated with the disease. Treatment of Huh7 cells with PA activates the lysosomal pathway of apoptosis through Bax-dependent permeabilization of lysosomes and release of cathepsin B in the cytosol; and genetic or chemical inhibition of cathepsin B activity partially reduced PA-induced mitochondrial dysfunction and liver cell death 74.
There is crosstalk between the ER and mitochondria. Exposure of primary hepatocytes and H4IIEC3 cells to PA resulted in efflux of calcium from the ER, leading to mitochondrial dysfunction and oxidative stress 75. One of the key mediators of mitochondrial dysfunction is generation of ROS during oxidative stress (Figure 1). CYP2E1 is implicated in hepatocyte injury and progression to NASH by promoting oxidative stress, inflammation, protein modification and insulin resistance 76. A significant amount of CYP2E1 is found in the mitochondria. This enzyme hydrolyzes various small molecules such as FA and ethanol into byproducts (toxic superoxide anion) which alter mitochondrial respiratory chain and damage mitochondrial constituents 77. It has been suggested that the accumulation of ROS occurs downstream of the inner membrane mitochondrial oxidative metabolism, since blocking of cytochrome complex I was associated with no accumulation of ROS in H4IIEC3 rat hepatoma cells exposed to PA 75. Another way by which FFA-mediated oxidative stress induces toxicity is through the Keap1-Nrf2 pathway. Kelch-like ECH-associated protein (Keap1) works as an adaptor for E3 ligase and initiates proteasomal degradation of several proteins. Treatment of liver cells with PA resulted in decreased Keap1 cellular levels as the result of an increased Keap1 degradation through autophagy by a p62 dependent mechanism 78, 79. In addition, short hairpin RNA-mediated knockdown of Keap1 resulted in upregulation of BIM and PUMA, leading to hepatocyte apoptosis 80 (Table 1).
Reduction of oxidative stress is a potential therapeutic strategy for patients with NASH. In the PIVENS trial, vitamin E improved histological and serum biomarkers of liver injury in adults with NASH, with no significant effect on fibrosis over the 2-year study period 81. In the subsequent TONIC trial, NASH resolution was greater in children treated with vitamin E when compared to metformin 82. Also, inhibition of cathepsin B could also represents another potential therapeutic strategy for NAFLD patients. Pharmacological cathepsin B inhibition using cathepsin inhibitors CA07 and E-64 preserved mitochondrial function, reduced oxidative stress, and protected against hepatic steatosis, liver injury, and insulin resistance 74 .
Many molecular mechanisms contribute to lipoapoptosis. This review focused on how organelle dysfunction (i.e. ER stress and mitochondrial impairment) contribute to liver cell injury. Indeed, prolonged ER stress and exhaustion of the UPR mechanisms results in the activation of CHOP and downstream upregulation of the death mediators PUMA, BIM and DR5. Also, ER stress-dependent and -independent activation of JNK is an important pathway activated by FFA, resulting in BIM and PUMA-mediated activation of Bax with subsequent mitochondrial dysfunctions, increased caspase activity and cellular demise. Finally, oxidative stress downstream of mitochondrial impairment also contributes to hepatocyte apoptosis.
4. PROTECTIVE ROLE OF CERTAIN LIPIDS
Despite the hepatotoxic effect of most lipids, there is increasing awareness of the beneficial effects of certain lipids. At present, studies on beneficial lipids are few and far between, calling for more studies in this arena.
4.1 Monounsaturated Fatty acids (MUFAs)
Exposing human and murine hepatocytes to MUFAs generated lipid accumulation, but preserved cell viability. This was thought to be dependent on stearoyl-CoA desaturase-1 (SCD-1), and enzyme that converts the transformation of saturated FA into MUFAs. Thus, MUFAs are a safe form of excess lipid storage 83,84.
Palmitoleate (PO) is a monounsaturated FFA which works as a lipokine in adipose tissue. Recent studies have shown that it has a protective role against apoptosis in Huh-7 cells and primary hepatocyte 85. However, PO did not protect against steatosis, but rather accentuated the lipid accumulation induced by PA in Huh-7 cells. PO inhibited ER stress responses by blocking upregulation of BIM and PUMA, thus protecting hepatocytes against downstream death mediator Bax 85.
4.2 Polyunsaturated Fatty Acids (PUFAs)
PUFAs have recently been heralded as a potential therapy for prevention of NASH. The availability of n-3 long chain PUFAs to the liver is crucial in fat removal from hepatocytes 86. The beneficial effect of n-3 PUFA was suggested by the finding that NASH patients had decreased levels of n-3 PUFA eicosapentaenoic and docosahexaenoic acids 87, which may contribute to the progression of steatosis to steatohepatitis 88. Several clinical trials have demonstrated the beneficial effect of n3-PUFAs eicosapentaenoic acid supplementation in NAFLD patients resulting in improvement in the major histological features of disease activity (steatosis, necro-inflammation, ballooning, fibrosis) 89,90 , suggesting a potential medical application of PUFAs in inhibiting disease progression. Moreover improvement in NASH activity scores correlated with increase in plasma levels of the n3-PUFA alpha-linolenic (ALA) 89. Similar protective effects of PUFAs have been described in vitro using Huh7 cells. Indeed, treatment with the n-6 PUFAs linoleate can prevent PA-induced apoptosis and inflammation in liver cells by inhibiting JNK and nuclear factor Kappa beta (NFκβ) activation and by suppressing the production of the pro-inflammatory cytokine IL-8 91. Another study found that alpha-linolenic acid played a protective role to primary rat hepatocytes exposed to stearic acid through decrease of ER stress markers GRP78, CHOP and GRP94 92. However, the beneficial role of n3-PUFAs in NAFLD remains controversial. Indeed, in a larger double-blind trial (performed in 37 sites in North America) of NAFLD patients receiving ethy-leicosapentanoic acid supplementation versus placebo, there was no significant difference in NASH activity scores, insulin resistance, liver enzymes and other inflammatory markers between groups 93.
As mentioned prior, some lipids work as safe fat storage and even have a protective role against toxic FFAs. Of note, MUFAs are relatively non-toxic to liver cells and can protect hepatocytes against saturated FFA-induced lipotoxicity by redirecting these toxic FFAs into TGs storage. PUFAs, commonly present in fish oil, may also have a protective potential, but further studies are needed to better elucidate this effect.
5) HEPATOTOXIC CARBOHYDRATES AND GLUCOTOXICITY
Epidemiological studies indicate a correlation between high-carbohydrate diets and NAFLD as reviewed by Basaranoglu M. et al. 94. Indeed, high-carbohydrate diets induce lipogenesis and steatosis through the activation of several lipogenic enzymes such as acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS) or SCD-1. The abundant carbohydrate consumption and resultant increased levels of sugars in the blood also have deleterious effects on liver cells, a phenomenon termed glucotoxicity. This concept is intrinsically linked to insulin resistance, which allows these elevated glycemic levels. Insulin resistance is considered the first step in the path toward the development of type II diabetes mellitus (T2DM). Because abundant levels of plasma FFA can also lead to insulin resistance, it is difficult to distinguish between the effects of glucotoxicity and lipotoxicity in vivo. However, in vitro studies have demonstrated that chronic hyperglycemia affects both insulin secretion and action as a consequence of the generation of ROS and the activation of an oxidative stress. Most of the studies in the literature have focused on the deleterious effect of glucotoxicity on pancreatic β-cells, but recent data indicate that glucotoxicity can also be injurious to liver cells and induce an ER stress and hepatocyte cell death.
5.1) Glucose
Glucose is unique as it is utilized by all body tissues to produce energy. After its consumption, approximately 20% of the glucose enters the hepatocyte via the glucose transporter (GLUT2) membrane transporter, where it is either stored as glycogen or undergoes glycolysis. Glucose stimulates insulin release from the pancreas. Indeed, the rest of the glucose (80%) contributes to the increase in glucose levels in the peripheral circulation; thus, in response to this hyperglycemia, insulin is released by the β-cells. Administration of a high-glucose diet to mice for 4 weeks resulted in elevated FA accumulation in the liver, increased insulin resistance and induction of hepatic oxidative stress, all major players in the development of diabetes 95.
5.2) Fructose
Fructose is also a lipogenic sugar, selectively metabolized in the liver where it is directed toward liver glycogen and triglyceride synthesis. Absorbed fructose is delivered to the liver via the portal vein and enters the hepatocyte via GLUT2, but it does not induce insulin release from pancreatic β-cells. The end-products of fructose metabolism in the glycolytic pathway of the liver are glucose, glycogen, lactate, and pyruvate. Many studies have shown that dietary fructose induces lipogenesis in humans, reinforcing the role of fructose in the obesity epidemic 96. Also, administration of high-fructose corn syrup induces deposition of fat in the liver secondary to increase in fatty acyl coenzyme A, diacylglycerol (DAG) and TAG. Indeed, high fructose exposure also increases the lipogenic enzymes FAS, carbohydrate responsive element binding protein (ChREBP) and ACC-1 as well as higher amounts of the fat transporter CD36 and that even prior to any body weight changes in rats 97; and high fructose diet-induced upregulation of FAS and SCD-1 results in higher saturated FA liver content and increased hepatic gluconeogenesis inducing the steatotic phenotype in the liver 98. The lipogenic and pro-inflammatory effects of fructose may be related to its rapid intracellular phosphorylation and release of calcium and uric acid.
Diet high in fructose also reduced insulin sensitivity associated with impaired hepatic insulin action and whole-body glucose disposal. Although high fat diet alone induces insulin resistance, obesity and steatosis with little inflammation and without fibrosis, the combination of a diet with high fat and fructose content exacerbates the liver injury with the presence of hepatic fibrosis, inflammation, endoplasmic reticulum stress and lipoapoptosis. Whether fructose consumption directly contributes to NAFLD pathogenesis is unknown and large prospective studies following subjects with high fructose consumption and the development of NAFLD will be necessary to ascertain fructose contribution to the disease 94.
The toxic effects of high levels of carbohydrates to cells are intrinsically related to insulin resistance and derangement of energy management within cells and tissues. Glucose cannot be properly stored or utilized in the absence of insulin in most tissues. High glucose diets can lead to steatotis in the liver and induction of oxidative stress. Fructose has also been shown to induce lipogenesis and liver steatosis through induction of fatty acyl CoA, DAG and TAG as well as FAS, ChREBP and ACC-1. This happens in addition to upregulation of fat transporter CD36. Prospective studies of fructose’s contribution in NASH particularly, are still needed.
6) HARMFUL EFFECTS OF HEPATOTOXIC CARBOHYDRATES
Several studies indicate that exposure of liver cells to high glucose, fructose or sucrose content induce hepatic insulin resistance in rodent 99,100 which can be the consequence of decreased insulin receptor expression or increased phosphorylation of the insulin receptor substrate (IRS)1 therefore suppressing insulin receptor signaling 101,102. The deleterious effects of chronic hyperglycemia on insulin sensitivity have been linked, among others, to oxidative stress, inflammation and ER stress 103.
Oxidative stress induced by high sugar consumption may contribute to the establishment of an insulin resistant status. Indeed, both fructose and glucose-sweetened liquid consumption upregulates thioredoxin-2 expression in mice, a key protein upregulated in response to mitochondrial oxidative stress 104. Fructose-fed rats harbor liver dysfunctions, high transaminases and increased levels of oxidative markers including higher protein carbonyl and nitrosothiol content in liver 105 as well as diminished levels of total antioxidant status, reduced glutathione, catalase and cellular glutathione peroxidase (GPX1) activity in the liver 106.
Several kinases and phosphatases activated downstream of stress-induced pathways have been identified as important contributor of high carbohydrate diet-induced insulin resistance. First, recent studies suggests that JNK may contribute to fructose-induced antagonism of insulin signaling in the liver. Indeed, hyperactivation of JNK mediates insulin resistance by phosphorylating and inhibiting IRS-1, blocking downstream insulin signaling 107. Thus, hepatic insulin resistance induced by a sucrose-enriched diet in rats was associated with elevated hepatic JNK activity, and treatment of hepatocytes isolated from high sucrose diet-fed rats with a JNK inhibitor improved insulin-stimulated tyrosine phosphorylation of IRS proteins and insulin suppression of glucose release 107. Also, a whole body of data indicate a central role for AMP-activated protein kinase (AMPK) in regulating insulin sensitivity, as sustained decreases in AMPK activity accompany insulin resistance, whereas AMPK activation increases insulin sensitivity. In HepG2 cells exposed to high glucose media, AMPK activity is decreased 108; and decrease in AMPK activity can be mediated by the inhibition of Sirtuin 1 (SIRT1) 109, a redox-regulated NAD-dependent histone/protein deacetylase. Interestingly, heme-oxygenase 1 (HO-1), an endogenous anti-oxidant gene, can attenuate fructose-induced hepatic lipid accumulation and improve insulin sensitivity via the activation of SIRT1 gene expression 109,110. Therefore, the identification of potent and specific AMPK activators may be beneficial for the prevention and treatment of metabolic syndrome–associated disorders. Futhermore, other studies also indicate that sucrose-induced hepatic insulin resistance is associated with increased expression of protein-tyrosine phosphatase 1B (PTP1B) 107. PTP1B inhibits insulin signaling by dephosphorylating the insulin receptor and possibly IRS1; and liver-specific PTP1B-deficiency improves insulin sensitivity in animal model of NASH 111. Thus, PTP1B could also represent an attractive therapeutic target for obesity, diabetes, and metabolic syndrome.
Chronic hyperglycemia can also provoke metabolic perturbations in the liver promoting a low-grade inflammation which contributes to the insulin-resistant state. Thus, high fructose consumption in rats results in hepatic increase of TNFα mRNA expression via NFκβ-dependent transcriptional upregulation 112 . TNFα induces serine phosphorylation of IRS-1 through inhibition of serine phosphatases (such as protein phosphatase 2A or PP2A) or activation of serine kinases (such as JNK or protein kinase C theta) leading to disruption of insulin signaling in the liver 113. Also, suppressors of cytokine signaling (SOCS) are inflammatory mediators that regulate insulin signaling and de novo lipogenesis in the liver. SOCS-1 and 3 are induced by pro-inflammatory cytokines and can inhibit insulin signaling by interfering with IRS-1 tyrosine phosphorylation. SOCS proteins markedly induce de novo fatty acid synthesis in the liver by inhibiting the janus tyrosine kinase (JAK)/signal transduction and activator of transcription proteins (STAT) 3 resulting in the upregulation and activation of SREBP-1c 114. In rats, high-fructose diet increases the amount of SOCS-3 115, and downregulation of SOCS-3 can have beneficial effects on insulin-resistant rat hepatocytes by increasing their insulin sensitivity 116.
More recent data indicate that high-fructose-induced insulin resistance and de novo lipogenesis in the liver of mice were associated with the dual activation of the IRE1/XBP1 and PERK/eIF2α pathways 117,118. Fructose-induced ER stress activation has been linked to the activation of SREBP-1c, associated with the upregulation of lipogenic enzymes genes, with downstream effect on insulin sensitivity 92,119 . High-fructose or sucrose diet can also results in CHOP upregulation and JNK activation 120,121, which further induce cellular apoptosis subsequent to BH3-only proteins BIM and PUMA-mediated mitochondrial dysfunction and caspase 3 activation 122 and glucose can further amplify FA-induced ER stress 123(Table 1).
Thus, chronic hyperglycemia can induce liver toxicity by activating oxidative stress, inflammation and ER stress responses leading to insulin resistance, steatosis and cellular demise (Figure 1). Whether chronic hyperglycemia directly contributes to the insulin resistance, T2DM and obesity characterizing the NAFLD phenotype remains to be clarified.
7. CONCLUSION
NASH represents a growing epidemic worldwide and is predicted to emerge as the main cause of liver cirrhosis, surpassing alcohol and viral hepatitis. Therefore, understanding the molecular mechanisms behind the hepatocellular injury is paramount in efforts to deter this epidemic. Metabolic syndrome, which is intrinsically linked to a diet rich in saturated fats and refined sugars (i.e. fructose and/or glucose), is characterized by glucose intolerance and insulin resistance. This abundance of FFA and carbohydrates generates cytotoxicity to tissues through deregulation of energy storage homeostasis. Several lipotoxic lipids have been described in the literature, including FFA, LPC, ceramides, FC, and BA. The pathways involved in lipotoxicity are related to organelle damage, mainly the mitochondria and endoplasmic reticulum, where, through activation of JNK and BH3-only family proteins, caspases become activated resulting in apoptosis. In contrast, other lipids like MUFAs or PUFAs (e.g., fish oil) can protect the liver from lipotoxicity, although the exact mechanisms by which they exert their hepatoprotective effects are still being delineated.
Several drugs are being studied as potential therapies for NASH. Among these are BA agonists (such as ursodeoxycholic acid), vitamin E, caspase inhibitors, JNK inhibitors, TRAIL antagonists, PUFAs, lipid lowering drugs (statins), drugs that sensitize tissues to glucose (metformin) and AMPK inhibitors. Further research is necessary to continue to explore this intricately woven metabolic web and determine future directions of therapy in the metabolic syndrome spectrum of diseases, including NASH.
ACKNOWLEDGEMENTS
The authors thank John W. Cyrus for his excellent editorial assistance.
This work was supported, in whole or in part, by NIH Grants R01 DK081450 and T32 07150 (AJS).
Abbreviations
- ALA
alpha-linolenic
- ACC
acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- ATF
activating transcription factor
- AP-1
activator protein-1
- BAs
bile acids
- BH
Bcl-2 homology
- BIM
Bcl-2 interacting mediator
- CHOP
CCAAT/enhancer-binding homologous protein
- ChREBP
carbohydrate responsive element binding protein
- CYP2E1
cytochrome P450 2E1
- DAG
diacylglycerides
- DR
death receptor
- ER
endoplasmic reticulum
- ERAD
ER-assisted degradation
- FAS
fatty acid synthase
- FAT
fatty acid translocase
- FATP
fatty acid transport protein
- FC
free cholesterol
- FFAs
free fatty acids
- FXR
farnesoid X receptor
- GLUT2
glucose transporter 2
- HMGB1
high mobility group box 1 protein
- HMGCo-A
hydroxymethylglutaryl-Co-A
- HSCs
hepatic stellate cells
- GSK3
glycogen synthase kinase 3
- IRE1α
inositol-requiring enzyme-1α
- IRS–1
insulin receptor substrate-1
- JNK
c-Jun NH2-terminal kinase
- Keap1
Kelch-like ECH-associated protein
- LPC
lysophosphatidyl Choline
- LDL
low density lipoprotein
- MUFA
monounsaturated fatty acid
- NF-kB
nuclear factor-kappaB
- MCD
methionine choline deficient
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- N-SMase
neutral sphingomyelinasePA, palmitate
- PERK
PKR-like ER kinase
- PLA2
phospholipase A2
- PO
palmitoleate
- PTA
p53 inhibitor pifithrin-alpha
- PTP1B
protein-tyrosine phosphatase 1B
- PUFA
polyunsaturated fatty acid
- PUMA
p53 upregulated modulator of apoptosis
- OA
oleate
- ROS
reactive oxygen species
- SA
stearate
- SCD-1
stearoyl-CoA desaturase-1
- SOCS
suppressor of cytokine signaling
- SREBP-2
sterol regulatory-element binding protein 2
- StAR
steroidogenic acute regulatory protein
- STAT5
signal transducer and activator of transcription 5
- SIRT1
JNK-1/p-53/miR 34a sirtuin 1
- T2DM
type 2 diabetes mellitus
- TAG
triacylglycerides
- TG
triglycerides
- TGF beta
transforming growth factor beta
- TLR4
toll like receptor 4
- TNF
tumor necrosis factor
- TRAIL
TNFα-related apoptosis-inducing ligand
- UFA
unsaturated fatty acid
- UPR
unfolded protein response
- VDAC
voltage dependent anion channel
- VLDL
very low-density lipoprotein particles
Footnotes
Conflict of Interest/Financial disclosure: The authors have no conflicts of interest to report
8. REFERENCES
- 1.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stremmel W, Staffer S, Wannhoff A, Pathil A, Chamulitrat W. Plasma membrane phospholipase A2 controls hepatocellular fatty acid uptake and is responsive to pharmacological modulation: Implications for nonalcoholic steatohepatitis. FASEB J. 2014;28(7):3159–3170. doi: 10.1096/fj.14-249763. [DOI] [PubMed] [Google Scholar]
- 3.Pan X, Wang P, Luo J, et al. Adipogenic changes of hepatocytes in a high-fat diet-induced fatty liver mice model and non-alcoholic fatty liver disease patients. Endocrine. 2015;48(3):834–847. doi: 10.1007/s12020-014-0384-x. [DOI] [PubMed] [Google Scholar]
- 4.Chabowski A, Zendzian-Piotrowska M, Konstantynowicz K, et al. Fatty acid transporters involved in the palmitate and oleate induced insulin resistance in primary rat hepatocytes. Acta Physiol (Oxf) 2013;207(2):346–357. doi: 10.1111/apha.12022. [DOI] [PubMed] [Google Scholar]
- 5.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23(2):201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 6.Cambri LT, Ghezzi AC, Arsa G, Botezelli JD, de Mello MA. Standard short-term diet ameliorates the lipid profile altered by a fructose-rich diet in rats. J Dev Orig Health Dis. 2015;6(4):335–341. doi: 10.1017/S2040174415001026. [DOI] [PubMed] [Google Scholar]
- 7.Ricchi M, Odoardi MR, Carulli L, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24(5):830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 8.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100(6):3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 10.Lauber K, Bohn E, Krober SM, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–730. doi: 10.1016/s0092-8674(03)00422-7. doi: S0092867403004227 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Han MS, Park SY, Shinzawa K, et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49(1):84–97. doi: 10.1194/jlr.M700184-JLR200. doi: M700184-JLR200 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Yaligar J, Gopalan V, Kiat OW, et al. Evaluation of dietary effects on hepatic lipids in high fat and placebo diet fed rats by in vivo MRS and LC-MS techniques. PLoS One. 2014;9(3):e91436. doi: 10.1371/journal.pone.0091436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pathil A, Mueller J, Warth A, Chamulitrat W, Stremmel W. Ursodeoxycholyl lysophosphatidylethanolamide improves steatosis and inflammation in murine models of nonalcoholic fatty liver disease. Hepatology. 2012;55(5):1369–1378. doi: 10.1002/hep.25531. [DOI] [PubMed] [Google Scholar]
- 14.Kakisaka K, Cazanave SC, Fingas CD, et al. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G77–84. doi: 10.1152/ajpgi.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez JA, Siddique MM, Wang ST, Ching J, Shayman JA, Summers SA. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J Biol Chem. 2014;289(2):723–734. doi: 10.1074/jbc.M113.522847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anjani K, Lhomme M, Sokolovska N, et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J Hepatol. 2015;62(4):905–912. doi: 10.1016/j.jhep.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab. 2012;23(8):365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: Role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G583–9. doi: 10.1152/ajpgi.00422.2005. doi: 290/4/G583 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Longato L, Tong M, Wands JR, de la Monte SM. High fat diet induced hepatic steatosis and insulin resistance: Role of dysregulated ceramide metabolism. Hepatol Res. 2012;42(4):412–427. doi: 10.1111/j.1872-034X.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasumov T, Li L, Li M, et al. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS One. 2015;10(5):e0126910. doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konstantynowicz-Nowicka K, Harasim E, Baranowski M, Chabowski A. New evidence for the role of ceramide in the development of hepatic insulin resistance. PLoS One. 2015;10(1):e0116858. doi: 10.1371/journal.pone.0116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291(2):E275–81. doi: 10.1152/ajpendo.00644.2005. doi: 00644.2005 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1339–46. doi: 10.1152/ajpgi.00509.2005. doi: 00509.2005 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janikiewicz J, Hanzelka K, Kozinski K, Kolczynska K, Dobrzyn A. Islet beta-cell failure in type 2 diabetes--within the network of toxic lipids. Biochem Biophys Res Commun. 2015;460(3):491–496. doi: 10.1016/j.bbrc.2015.03.153. [DOI] [PubMed] [Google Scholar]
- 25.Kurek K, Piotrowska DM, Wiesiolek-Kurek P, et al. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 2014;34(7):1074–1083. doi: 10.1111/liv.12331. [DOI] [PubMed] [Google Scholar]
- 26.Babenko NA, Kharchenko VS. Effects of inhibitors of key enzymes of sphingolipid metabolism on insulin-induced glucose uptake and glycogen synthesis in liver cells of old rats. Biochemistry (Mosc) 2015;80(1):104–112. doi: 10.1134/S0006297915010125. [DOI] [PubMed] [Google Scholar]
- 27.Arguello G, Balboa E, Arrese M, Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta. 2015;1852(9):1765–1778. doi: 10.1016/j.bbadis.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Caballero F, Fernandez A, De Lacy AM, Fernandez-Checa JC, Caballeria J, Garcia-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol. 2009;50(4):789–796. doi: 10.1016/j.jhep.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Bellanti F, Mitarotonda D, Tamborra R, et al. Oxysterols induce mitochondrial impairment and hepatocellular toxicity in non-alcoholic fatty liver disease. Free Radic Biol Med. 2014;75(Suppl 1):S16–7. doi: 10.1016/j.freeradbiomed.2014.10.594. [DOI] [PubMed] [Google Scholar]
- 30.Liang T, Alloosh M, Bell LN, et al. Liver injury and fibrosis induced by dietary challenge in the ossabaw miniature swine. PLoS One. 2015;10(5):e0124173. doi: 10.1371/journal.pone.0124173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, Farrell GC. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol. 2014;61(6):1376–1384. doi: 10.1016/j.jhep.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Dongiovanni P, Petta S, Mannisto V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705–712. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Chong LW, Hsu YC, Lee TF, et al. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol. 2015;15 doi: 10.1186/s12876-015-0248-8. 22-015-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira DM, Afonso MB, Rodrigues PM, et al. C-jun N-terminal kinase 1/c-jun activation of the p53/microRNA 34a/sirtuin 1 pathway contributes to apoptosis induced by deoxycholic acid in rat liver. Mol Cell Biol. 2014;34(6):1100–1120. doi: 10.1128/MCB.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3(1) doi: 10.3978/j.issn.2305-5839.2014.12.06. 5-5839.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bechmann LP, Kocabayoglu P, Sowa JP, et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology. 2013;57(4):1394–1406. doi: 10.1002/hep.26225. [DOI] [PubMed] [Google Scholar]
- 37.Puri P, Mirshahi F, Cheung O, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):568–576. doi: 10.1053/j.gastro.2007.10.039. doi: S0016-5085(07)01919-1 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. doi: 7400779 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A. 2013;110(12):4628–4633. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen X, Ellis RE, Sakaki K, Kaufman RJ. Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet. 2005;1(3):e37. doi: 10.1371/journal.pgen.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardo V, Gonzalez-Rodriguez A, Muntane J, Kozma SC, Valverde AM. Role of hepatocyte S6K1 in palmitic acid-induced endoplasmic reticulum stress, lipotoxicity, insulin resistance and in oleic acid-induced protection. Food Chem Toxicol. 2015;80:298–309. doi: 10.1016/j.fct.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 42.Szegezdi E, Macdonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296(5):C941–53. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 43.Rutkowski DT, Wu J, Back SH, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15(6):829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320(5882):1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7(6):520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lake AD, Novak P, Hardwick RN, et al. The adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease. Toxicol Sci. 2014;137(1):26–35. doi: 10.1093/toxsci/kft230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teske BF, Wek SA, Bunpo P, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22(22):4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: Roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298(5):E1027–35. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Singh N, Robinson-Taylor KS, et al. Hepatocyte autophagy is linked to C/EBP-homologous protein, Bcl2-interacting mediator of cell death, and BH3-interacting domain death agonist gene expression. J Surg Res. 2015;195(2):588–595. doi: 10.1016/j.jss.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 50.Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, Gores GJ. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2010;299(1):G236–43. doi: 10.1152/ajpgi.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iurlaro R, Munoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2015 doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 52.Cazanave SC, Mott JL, Bronk SF, et al. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J Biol Chem. 2011;286(45):39336–39348. doi: 10.1074/jbc.M111.280420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52(1):165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. doi: M510660200 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Cazanave SC, Mott JL, Elmi NA, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284(39):26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pagliassotti MJ, Wei Y, Wang D. Insulin protects liver cells from saturated fatty acid-induced apoptosis via inhibition of c-jun NH2 terminal kinase activity. Endocrinology. 2007;148(7):3338–3345. doi: 10.1210/en.2006-1710. doi: en.2006-1710 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Hikita H, Takehara T, Kodama T, et al. Delayed-onset caspase-dependent massive hepatocyte apoptosis upon fas activation in bak/bax-deficient mice. Hepatology. 2011;54(1):240–251. doi: 10.1002/hep.24305. [DOI] [PubMed] [Google Scholar]
- 58.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282(37):27141–27154. doi: 10.1074/jbc.M704391200. doi: M704391200 [pii] [DOI] [PubMed] [Google Scholar]
- 59.Masuoka HC, Mott J, Bronk SF, et al. Mcl-1 degradation during hepatocyte lipoapoptosis. J Biol Chem. 2009;284(44):30039–30048. doi: 10.1074/jbc.M109.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 61.Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol. 2014;60(5):1063–1074. doi: 10.1016/j.jhep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 62.Thapaliya S, Wree A, Povero D, et al. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig Dis Sci. 2014;59(6):1197–1206. doi: 10.1007/s10620-014-3167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heo SH, Kwak J, Jang KL. All-trans retinoic acid induces p53-depenent apoptosis in human hepatocytes by activating p14 expression via promoter hypomethylation. Cancer Lett. 2015;362(1):139–148. doi: 10.1016/j.canlet.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 64.Ratziu V, Sheikh MY, Sanyal AJ, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55(2):419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56(8):1124–1131. doi: 10.1136/gut.2006.118059. doi: gut.2006.118059 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 67.Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21(5):739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Park EJ, Lee AY, Chang SH, Yu KN, Kim JH, Cho MH. Role of p53 in the cellular response following oleic acid accumulation in chang liver cells. Toxicol Lett. 2014;224(1):114–120. doi: 10.1016/j.toxlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Martel C, Allouche M, Esposti DD, et al. Glycogen synthase kinase 3-mediated voltage-dependent anion channel phosphorylation controls outer mitochondrial membrane permeability during lipid accumulation. Hepatology. 2013;57(1):93–102. doi: 10.1002/hep.25967. [DOI] [PubMed] [Google Scholar]
- 70.Win S, Than TA, Le BH, Garcia-Ruiz C, Fernandez-Checa JC, Kaplowitz N. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J Hepatol. 2015;62(6):1367–1374. doi: 10.1016/j.jhep.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Penke M, Larsen PS, Schuster S, et al. Hepatic NAD salvage pathway is enhanced in mice on a high-fat diet. Mol Cell Endocrinol. 2015;412:65–72. doi: 10.1016/j.mce.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 72.Gariani K, Menzies KJ, Ryu D, et al. Eliciting the mitochondrial unfolded protein response via NAD repletion reverses fatty liver disease. Hepatology. 2015 doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldstein AE, Werneburg NW, Canbay A, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40(1):185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 74.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47(5):1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Egnatchik RA, Leamy AK, Noguchi Y, Shiota M, Young JD. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metabolism. 2014;63(2):283–295. doi: 10.1016/j.metabol.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57(4):860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: Mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol. 2011;35(10):630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 78.Lee HS, Daniels BH, Salas E, Bollen AW, Debnath J, Margeta M. Clinical utility of LC3 and p62 immunohistochemistry in diagnosis of drug-induced autophagic vacuolar myopathies: A case-control study. PLoS One. 2012;7(4):e36221. doi: 10.1371/journal.pone.0036221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin X, Li S, Zhao Y, et al. Interaction domains of p62: A bridge between p62 and selective autophagy. DNA Cell Biol. 2013;32(5):220–227. doi: 10.1089/dna.2012.1915. [DOI] [PubMed] [Google Scholar]
- 80.Cazanave SC, Wang X, Zhou H, et al. Degradation of Keap1 activates BH3-only proteins bim and PUMA during hepatocyte lipoapoptosis. Cell Death Differ. 2014;21(8):1303–1312. doi: 10.1038/cdd.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA. 2011;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li L, Lu DZ, Li YM, Zhang XQ, Zhou XX, Jin X. Proteomic analysis of liver mitochondria from rats with nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(16):4778–4786. doi: 10.3748/wjg.v20.i16.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: Role of stearoyl-CoA desaturase. J Biol Chem. 2009;284(9):5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akazawa Y, Cazanave S, Mott JL, et al. Palmitoleate attenuates palmitate-induced bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52(4):586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Badry AM, Graf R, Clavien PA. Omega 3 - omega 6: What is right for the liver? J Hepatol. 2007;47(5):718–725. doi: 10.1016/j.jhep.2007.08.005. doi: S0168-8278(07)00466-7 [pii] [DOI] [PubMed] [Google Scholar]
- 87.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 88.Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med. 2004;37(9):1499–1507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 89.Nogueira MA, Oliveira CP, Ferreira Alves VA, et al. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2015 doi: 10.1016/j.clnu.2015.05.001. doi: S0261-5614(15)00131-4 [pii] [DOI] [PubMed] [Google Scholar]
- 90.Tanaka N, Sano K, Horiuchi A, Tanaka E, Kiyosawa K, Aoyama T. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol. 2008;42(4):413–418. doi: 10.1097/MCG.0b013e31815591aa. [DOI] [PubMed] [Google Scholar]
- 91.Maruyama H, Takahashi M, Sekimoto T, Shimada T, Yokosuka O. Linoleate appears to protect against palmitate-induced inflammation in Huh7 cells. Lipids Health Dis. 2014;13 doi: 10.1186/1476-511X-13-78. 78-511X-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Yang X, Shi H, Dong L, Bai J. Effect of alpha-linolenic acid on endoplasmic reticulum stress-mediated apoptosis of palmitic acid lipotoxicity in primary rat hepatocytes. Lipids Health Dis. 2011;10 doi: 10.1186/1476-511X-10-122. 122-511X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M, EPE-A Study Group No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147(2):377–84.e1. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 94.Basaranoglu M, Basaranoglu G, Bugianesi E. Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surg Nutr. 2015;4(2):109–116. doi: 10.3978/j.issn.2304-3881.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du D, Shi YH, Le GW. Oxidative stress induced by high-glucose diet in liver of C57BL/6J mice and its underlying mechanism. Mol Biol Rep. 2010;37(8):3833–3839. doi: 10.1007/s11033-010-0039-9. [DOI] [PubMed] [Google Scholar]
- 96.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7(5):251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 97.Janevski M, Ratnayake S, Siljanovski S, McGlynn MA, Cameron-Smith D, Lewandowski P. Fructose containing sugars modulate mRNA of lipogenic genes ACC and FAS and protein levels of transcription factors ChREBP and SREBP1c with no effect on body weight or liver fat. Food Funct. 2012;3(2):141–149. doi: 10.1039/c1fo10111k. [DOI] [PubMed] [Google Scholar]
- 98.Maslak E, Buczek E, Szumny A, et al. Individual CLA isomers, c9t11 and t10c12, prevent excess liver glycogen storage and inhibit lipogenic genes expression induced by high-fructose diet in rats. Biomed Res Int. 2015;2015:535982. doi: 10.1155/2015/535982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pagliassotti MJ, Prach PA, Koppenhafer TA, Pan DA. Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am J Physiol. 1996;271(5 Pt 2):R1319–26. doi: 10.1152/ajpregu.1996.271.5.R1319. [DOI] [PubMed] [Google Scholar]
- 100.Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1334–40. doi: 10.1152/ajpregu.2000.279.4.R1334. [DOI] [PubMed] [Google Scholar]
- 101.Bezerra RM, Ueno M, Silva MS, Tavares DQ, Carvalho CR, Saad MJ. A high fructose diet affects the early steps of insulin action in muscle and liver of rats. J Nutr. 2000;130(6):1531–1535. doi: 10.1093/jn/130.6.1531. [DOI] [PubMed] [Google Scholar]
- 102.Rebollo A, Roglans N, Baena M, et al. Liquid fructose down-regulates liver insulin receptor substrate 2 and gluconeogenic enzymes by modifying nutrient sensing factors in rats. J Nutr Biochem. 2014;25(2):250–258. doi: 10.1016/j.jnutbio.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 103.Mozzini C, Garbin U, Stranieri C, et al. Endoplasmic reticulum stress and Nrf2 repression in circulating cells of type 2 diabetic patients without the recommended glycemic goals. Free Radic Res. 2015;49(3):244–252. doi: 10.3109/10715762.2014.997229. [DOI] [PubMed] [Google Scholar]
- 104.Kunde SS, Roede JR, Vos MB, et al. Hepatic oxidative stress in fructose-induced fatty liver is not caused by sulfur amino acid insufficiency. Nutrients. 2011;3(11):987–1002. doi: 10.3390/nu3110987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pooranaperundevi M, Sumiyabanu MS, Viswanathan P, Sundarapandiyan R, Anuradha CV. Insulin resistance induced by high-fructose diet potentiates carbon tetrachloride hepatotoxicity. Toxicol Ind Health. 2010;26(2):89–104. doi: 10.1177/0748233709359273. [DOI] [PubMed] [Google Scholar]
- 106.K S, Senthilkumar GP, Sankar P, Bobby Z. Attenuation of oxidative stress, inflammation and insulin resistance by allium sativum in fructose-fed male rats. J Clin Diagn Res. 2013;7(9):1860–1862. doi: 10.7860/JCDR/2013/6924.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei Y, Pagliassotti MJ. Hepatospecific effects of fructose on c-jun NH2-terminal kinase: Implications for hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2004;287(5):E926–33. doi: 10.1152/ajpendo.00185.2004. [DOI] [PubMed] [Google Scholar]
- 108.Pang J, Gong H, Xi C, Fan W, Dai Y, Zhang TM. Poly(ADP-ribose) polymerase 1 is involved in glucose toxicity through SIRT1 modulation in HepG2 hepatocytes. J Cell Biochem. 2011;112(1):299–306. doi: 10.1002/jcb.22919. [DOI] [PubMed] [Google Scholar]
- 109.Sodhi K, Puri N, Favero G, et al. Fructose mediated non-alcoholic fatty liver is attenuated by HO-1-SIRT1 module in murine hepatocytes and mice fed a high fructose diet. PLoS One. 2015;10(6):e0128648. doi: 10.1371/journal.pone.0128648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Liu X, Gao Y, Li M, et al. Sirt1 mediates the effect of the heme oxygenase inducer, cobalt protoporphyrin, on ameliorating liver metabolic damage caused by a high-fat diet. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.05.018. doi: S0168-8278(15)00345-1 [pii] [DOI] [PubMed] [Google Scholar]
- 111.Agouni A, Mody N, Owen C, et al. Liver-specific deletion of protein tyrosine phosphatase (PTP) 1B improves obesity- and pharmacologically induced endoplasmic reticulum stress. Biochem J. 2011;438(2):369–378. doi: 10.1042/BJ20110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vasiljevic A, Bursac B, Djordjevic A, et al. Hepatic inflammation induced by high-fructose diet is associated with altered 11betaHSD1 expression in the liver of wistar rats. Eur J Nutr. 2014;53(6):1393–1402. doi: 10.1007/s00394-013-0641-4. [DOI] [PubMed] [Google Scholar]
- 113.Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270(40):23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 114.Basaranoglu M, Basaranoglu G, Sabuncu T, Senturk H. Fructose as a key player in the development of fatty liver disease. World J Gastroenterol. 2013;19(8):1166–1172. doi: 10.3748/wjg.v19.i8.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vila L, Roglans N, Alegret M, Sanchez RM, Vazquez-Carrera M, Laguna JC. Suppressor of cytokine signaling-3 (SOCS-3) and a deficit of serine/threonine (ser/thr) phosphoproteins involved in leptin transduction mediate the effect of fructose on rat liver lipid metabolism. Hepatology. 2008;48(5):1506–1516. doi: 10.1002/hep.22523. [DOI] [PubMed] [Google Scholar]
- 116.Ye J, Zheng R, Wang Q, et al. Downregulating SOCS3 with siRNA ameliorates insulin signaling and glucose metabolism in hepatocytes of IUGR rats with catch-up growth. Pediatr Res. 2012;72(6):550–559. doi: 10.1038/pr.2012.123. [DOI] [PubMed] [Google Scholar]
- 117.Ren LP, Chan SM, Zeng XY, et al. Differing endoplasmic reticulum stress response to excess lipogenesis versus lipid oversupply in relation to hepatic steatosis and insulin resistance. PLoS One. 2012;7(2):e30816. doi: 10.1371/journal.pone.0030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chan SM, Sun RQ, Zeng XY, et al. Activation of PPARalpha ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes. 2013;62(6):2095–2105. doi: 10.2337/db12-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang C, Chen X, Zhu RM, et al. Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice. Toxicol Lett. 2012;212(3):229–240. doi: 10.1016/j.toxlet.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 120.Wali JA, Rondas D, McKenzie MD, et al. The proapoptotic BH3-only proteins bim and puma are downstream of endoplasmic reticulum and mitochondrial oxidative stress in pancreatic islets in response to glucotoxicity. Cell Death Dis. 2014;5:e1124. doi: 10.1038/cddis.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhong Y, Wang JJ, Zhang SX. Intermittent but not constant high glucose induces ER stress and inflammation in human retinal pericytes. Adv Exp Med Biol. 2012;723:285–292. doi: 10.1007/978-1-4614-0631-0_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jaiswal N, Maurya CK, Arha D, et al. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis. 2015;20(7):930–947. doi: 10.1007/s10495-015-1128-y. [DOI] [PubMed] [Google Scholar]
- 123.Bachar E, Ariav Y, Ketzinel-Gilad M, Cerasi E, Kaiser N, Leibowitz G. Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic beta-cells via activation of mTORC1. PLoS One. 2009;4(3):e4954. doi: 10.1371/journal.pone.0004954. [DOI] [PMC free article] [PubMed] [Google Scholar]