Abstract
Age-related deficits in motor and cognitive functioning may be driven by perturbations in calcium (Ca2+) homeostasis in nerve terminals, mechanisms that are also thought to mediate the neurotoxicity of methylmercury (MeHg). Calcium-channel blockers (CCBs) protect against MeHg toxicity in adult mice, but little is known about their efficacy in other age groups. Two age groups of BALB/c mice were exposed to 0 or 1.2 mg/kg/day MeHg and 0 or 20 mg/kg/day of the CCB nimodipine for approximately 8.5 months. Adults began exposure on postnatal day (PND) 72 and the retired breeders on PND 296. High-rate operant behavior was maintained under a percentile schedule, which helped to decouple response rate from reinforcer rate. Responding was analyzed using a log-survivor bout analysis approach that partitioned behavior into high-rate bouts separated by pauses. MeHg-induced mortality did not depend on age but nimodipine neuroprotection was age-dependent, with poorer protection occurring in older mice. Within-bout response rate (a marker of sensorimotor function) was more sensitive to MeHg toxicity than bout-initiation rate (a marker of motivation). Within-bout rate declined almost 2 months prior to overt signs of toxicity for the MeHg-only retired breeders but not adults, suggesting greater delay to toxicity in younger animals. Motor-based decrements also appeared in relatively healthy adult MeHg + NIM animals. Aging appeared to alter the processes underlying Ca2+ homeostasis thereby diminishing protection by nimodipine, even in mice that have not reached senescence. The study of MeHg exposure presents an experimental model by which to study potential mechanisms of aging.
Keywords: aging, methylmercury, nimodipine, calcium homeostasis, bout analysis, motor function
1. Introduction
Methylmercury (MeHg) is a global pollutant and the primary concerns about its health effects are due to its neurotoxicity [1]. Prenatal exposures produce diffuse central nervous system (CNS) damage and cognitive dysfunction [2,3,4] whereas adult-onset exposures produce relatively focal damage that appears in the primary motor cortex, sensory regions of the cerebral cortex, cerebellar granule cells, and dorsal root ganglion and results in motor dysfunction [5,6,7]. Methylmercury-induced disruptions of intracellular signaling and cell death have been linked, at least in part, to dysregulation of Ca2+ homeostasis inside nerve terminals [8,9]. Similarly, neuronal degeneration during aging is thought to be mediated by changes in the level of intracellular Ca2+ [10,11,12]. Chronically elevated levels of intracellular Ca2+ in neurons and reduced ability to buffer Ca2+ levels during normal aging provoke subtle age-associated declines and mild impairment [12,13,14,15,16]. Chronic MeHg exposure, acting to disrupt Ca2+ homeostasis, may exacerbate age-related declines in motor or cognitive functioning and accelerate normal or neurodegenerative aging, as has been noted with MeHg [17].
The excess intracellular Ca2+ produced by MeHg and aging suggests that preventing increased Ca2+ influx into intracellular cytosol could be neuroprotective. Calcium channel blockers reduce intracellular Ca2+ by blocking Ca2+ channels located in neuronal cell membranes, cerebral and peripheral vasculature, and cardiac smooth muscle [18,19,20,21]. Nimodipine, a 1,4-dihyrdopyridine CCB, is an L-type Ca2+ blocker with excellent selectivity for the CNS [22], and is an ideal candidate to protect against Ca2+-mediated CNS insults like MeHg exposure. In vitro [23,24] and in vivo [24,25,26] studies support this notion. For example, Bailey et al. [25] and Hoffman & Newland [26] found that chronic nimodipine (2-20 mg/kg/day) afforded dose-dependent neuroprotection in adult BALB/c mice chronically exposed to 2.6 mg/kg/day MeHg. Nimodipine attenuated or blocked deficits in an incremental repeated acquisition (IRA) procedure [25], wheel-running and rotarod performance, and mortality [26]. CCBs, including nimodipine, also attenuate or block selective signs of normal aging [27,28,29,30,31] and other CNS insults [32,33,34,35,36,37; c.f. 38).
It is difficult to separate motor from motivational components of behavior in models of neurotoxicant-induced motor deficits because the behavior is closely coupled to the motivation to engage in it [39]. Procedures that produce high-rate responding, such as fixed-ratio, variable-ratio, and differential reinforcement of high rate schedules (DRH) inherently link reinforcement rate to response rate. Thus, impairment may produce a positive feedback loop wherein response rate decrements drive reductions in reinforcer rate which could, in turn, further reduce response rate, confounding motor deficits with the consequences of reinforcer loss. In the current study we separated motor and motivational influences first by manipulating the contingency linking responding to the delivery of reinforcers and second by using an analytical approach capable of differentially estimating the contribution of motoric and motivational components of behavior may be advantageous.
We used both percentile (PCNT) and differential reinforcement of high rate (DRH) schedules to maintain high-rate nose-poking. The PCNT schedule is particularly appealing because it titrates the response criterion in real-time according to the subject’s recent performance [40,41]. As response rate declines, a PCNT schedule relaxes the response criterion, making it easier to obtain reinforcers. This allows behavior to contact reinforcement even in the face of impairment, disentangling the effect of reinforcer loss on response rate with MeHg- or aging-induced decreases in responding. In contrast, response rate decrements under the DRH schedule generally lead to a direct decrease in reinforcer delivery. To separate further reinforcer rate from motor deficits, criterion responses were reinforced under a random interval 30s (RI 30s) schedule of reinforcement, which randomly reinforced criterion response patterns at an average rate of two reinforcers per min.
The analytical approach to separating influences over behavior was based on the observation that high-rate behavior typically occurs as bouts of response bursts separated by intervals during which the animal is disengaged from the target behavior, is to use a dynamic analysis that breaks a response epoch second-by-second into bouts. [42-47]. The key response unit on which this analysis is based is the interresponse time (IRT). A bout comprises a run of short IRTs while the initiation of a new bout typically terminates a long IRT. We used a log-survivor analysis, described in detail by Shull and colleagues [42-44], to partition these IRTs into two distinct distributions. The short IRTs produced by response bursts, or within-bout responses, serve as an index of motor function. In contrast, the long IRTs that represent inter-bout intervals, the inverse of which is bout-initiation rate, serve as an index of the motivation to engage in the target behavior. These interpretations are supported empirically by studies that show that changes in motivating operations like food deprivation selectively affect bout-initiation rate [41,47] whereas manipulations that makes responding more difficult [45] or compounds like MeHg [26] and pentobarbital [46] with known motoric effects preferentially affect within-bout rate. This analysis assumes that responding can be described as three orthogonal components, within-bout rate, bout-initiation rate, and bout length [42-44], which is supported by Hoffman & Newland’s [26] reconstruction of overall response rate in control and MeHg-exposed mice by a linear combination of these three terms derived from a change-point analysis.
The present study used a log-survivor bout analysis approach to disentangle motoric from motivational deficits in high-rate nose-poking induced by chronic MeHg exposure and neuroprotection by nimodipine in two age cohorts of male BALB/c mice.
2. Material and methods
2.1 Subjects
Adult and retired breeder male BALB/c mice (N=112) were purchased from Harlan Laboratories (Indianapolis, IN) and housed in an Optimice® rack system in an AAALAC-accredited temperature- and humidity-controlled vivarium that was maintained on a 12-hour light-dark cycle (lights on at 6:00am). Two age cohorts, two MeHg water concentrations, and two nimodipine diets produced a 2 (age) × 2 (MeHg) × 2 (nimodipine) full factorial design with 12-16 mice per exposure group by age.
2.1.1 Adults
The adult cohort (n=51) arrived at 49 days of age. Upon arrival, mice were housed in pairs in clear polycarbonate cages, separated by a clear Plexiglas© divider that prevented physical contact, but allowed visual, olfactory, and auditory interaction. Due to the aggressiveness of adult male BALB/c mice [48], animals remained separated for the duration of the study. Their weight was maintained at approximately 24 g by feeding approximately 2.5 g standard rodent chow per animal per day, adjusted according to their body mass, with free access to water except during experimental sessions. After 4 months, adults transitioned to a final target weight of approximately 26 g by feeding approximately 3.0 g rodent chow per day.
2.1.2 Retired breeders
The retired breeder age cohort (n=63) arrived at 273 days of age and were housed in the same manner as the adults. Upon arrival, they weighed 26-30g, which was reduced and maintained at a final target weight of approximately 26 g by feeding approximately 3.0g standard rodent chow per animal per day with free access to water except during experimental sessions.
2.2 Methylmercury and nimodipine exposure
Methyl mercuric chloride (CH3HgCl) was procured from Alfa-Aesar (Ward Hill, MA, USA) and dissolved into water to produce the water solutions. Nimodipine was procured from Sigma-Aldrich (St. Louis, MO) and mixed into standard rodent chow manufactured by Purina TestDiets and based on a 5LL2 laboratory chow diet. Based on measurements of water and food consumption and weight (data not shown), MeHg exposure corresponded to approximately 0 and 1.2 mg/kg/day of Hg and nimodipine exposure corresponded to approximately 0 and 20.0 mg/kg/day. Exposures began at 72 and 296 days of age for adults and retired breeders, respectively, and continued for 253 days until animals were 325 and 549 days of age. Experimental procedures for both age groups began on the first day of exposure.
2.3 Apparatus
Experiments were conducted in standard Med Associates Inc. modular operant conditioning chambers (St. Albans, VT, product #ENV-007). Each chamber measured 30.5 cm L × 24.1 cm W × 29.2 cm H and contained two stainless steel front and back walls and two Plexiglas© side walls. Mounted on the front wall were two nose-poke holes (Left and Right), separated by a pellet dispenser. Above each nose-poke hole was a yellow LED. Interrupting an infrared beam in the nose-poke hole registered a response. The pellet dispenser delivered 20mg sucrose pellets. Chambers had two Sonalert™ tones (2900 and 4500 Hz, nominally) calibrated to an amplitude of 70 dB for presentation of auditory stimuli. Located near the ceiling of the chamber on the back wall was a single 2.8-W house light. Sound-attenuating cabinets enclosed operant chambers with a fan to circulate air for ventilation.
2.4 Procedure
Experimental sessions were conducted in the operant chambers described above. With the exception of autoshaping, all sessions lasted 32.5 min and occurred 4 days per week. In addition to nose-poking, wheel-running and rotarod tests were conducted Fri – Sun; these data are described in a separate manuscript (see Shen et al., under review [48]).
2.4.1 Autoshaping
Nose-poking at two spatially distinct locations (left and right) was autoshaped for both age cohorts. The autoshaping procedure has been described in detail previously [see 49 and 50] and was implemented without modification for both age groups. Autoshaping sessions ended when animals met a specific response criterion or after 4hr whichever occurred first.
2.4.2 Percentile (PCNT)
The PCNT schedule of reinforcement used in this study was designed to generate high-rate operant behavior while adjusting the response criterion according to an animal’s ability to respond. Each left nose-poke terminated an IRT that then was compared with the 10 previous IRTs (i.e., a look-back window of 10 IRTs or 11 responses). Responses that terminated IRTs shorter than 50% of the previous 10 met the high-rate criterion (10:0.5) and were paired with a 0.2 s tone. These criterion responses were reinforced under a random interval (RI) 30 s schedule; a schedule that produces reinforcers unpredictably but at relatively constant overall rate of approximately 2/min. Criterion nose-pokes, including those followed by reinforcement, were paired with the same tone.
2.4.3 Differential reinforcement of high rate (DRH)
Under the DRH schedule, bursts of responses to the right nose-poke hole were reinforced. Criterion bursts eligible for reinforcement consisted of a 9-response burst that started and ended within 4 s (9:4). Criterion bursts of responses were paired with a 0.2 s tone and reinforced under an RI 30 s schedule of reinforcement as described in section 2.4.2.
2.4.4 Training
Initially, only the PCNT schedule was available for animals to respond under, which was counterbalanced across left and right nose-pokes. Sessions began with the illumination of the nose-poke hole and the corresponding LED light. Reinforcement initially followed criterion IRTs using a dense schedule that was slowly thinned as response rates increased (see Table 1). Each session consisted of six 5 min components during which the PCNT schedule was active. Between components, subjects experienced a blackout lasting 30 s during which nose-poke holes and LED lights were not illuminated and there were no programmed consequences for nose-pokes.
Table 1. Multiple schedule training.
Training progression for the PCNT and DRH components of the high-rate multiple schedule.
| Percentile (PCNT) | Differential reinforcement (DRH) | |||
|---|---|---|---|---|
|
| ||||
| Look-back window | RI value | Response-burst requirement | RI value | Sessions |
| 10 (50%) | 5” | N/A | N/A | 12 |
| 10 (50%) | 10” | N/A | N/A | 1 |
| 10 (50%) | 20” | N/A | N/A | 4 |
| 10 (50%) | 20” | 2 (1”) | 5” | 2 |
| 10 (50%) | 30” | 4 (2”) | 5” | 2 |
| 10 (50%) | 30” | 6 (3”) | 5” | 4 |
| 10 (50%) | 30” | 9 (4”) | 5” | 2 |
| 10 (50%) | 30” | 9 (4”) | 10” | 2 |
| 10 (50%) | 30” | 9 (4”) | 20” | 2 |
| 10 (50%) | 30” | 9 (4”) | 30” | Final values |
The zigzag line represents when both schedules reached their final values.
The DRH schedule was added after response rates increased under the PCNT schedule to form a multiple schedule of reinforcement. Components within a single session alternated between PCNT and DRH schedules to produce six 5 min components (3x each schedule), each separated by a 30 s blackout. The first component of each session alternated between PCNT and DRH schedules across days. Similar to the PCNT, the initial RI schedule used during DRH components was shorter, producing a dense schedule of reinforcement. The DRH criteria for reinforcement were more rigid relative to the PCNT schedule, and thus the response criterion slowly incremented to its full value. Table 1 shows the progression of the two schedules from training until final values.
2.5 Humane endpoints
Mice were inspected daily and their body weight measured before every experimental session. Any mice that appeared ill or that displayed overt signs of MeHg toxicity (weight loss, limb clasping, severe motor dysfunction) were placed under 24hr observation in a heated cage with access to food and the attending veterinarian was consulted. Every effort was undertaken to keep mice alive, provided it did not prolong distress. Animals that met predefined criteria were euthanized according to procedures approved by the Auburn University Institutional Animal Care and Use Committee (IACUC).
2.6 Brain mercury (Hg) concentration
Brains were taken when an animal was euthanized either due to MeHg neurotoxicity or at the end of the study. Cold-vapor atomic absorption was performed by the Michigan State University Diagnostic Center for Population and Animal Health (DCPAH) to determine whole-brain Hg concentration (total Hg). Brains from following exposure groups were analyzed: adult MeHg-only (n=12), retired breeder MeHg-only (n=12), adult MeHg + NIM (n=12), retired breeder MeHg + NIM (n=12), adult control (n=4), retired breeder control (n=4), and finally adult NIM-only (n=4) and retired breeder NIM-only (n=4).
2.7 Data analysis
2.7.1 Survival Analysis
Mantel-Cox survival analysis was used to determine differences in mortality between age and exposure groups. Multiple comparison tests were made by applying the Holm-Sidak correction (shown in parentheses).
2.7.2 Bout Analysis
The microstructure of responding during PCNT and DRH schedules was assessed using log-survivor bout analysis. IRTs were collected separately for the PCNT and DRH schedules, aggregated within-session from three components for each schedule, and sorted from shortest to longest. Physical constraints prevented IRTs shorter than 0.02” but an unbiased log-survivor analysis requires a Y intercept at the shortest IRT so the entire distribution was shifted to the left by 0.02” by subtracting each IRT by that amount. The resulting IRT distributions were fitted to the bi-exponential model described in Eq. 1.
| (1) |
Based on the findings of Johnson et al. [46], Eq. 1 was log-transformed to provide a better fit of the data and is described by Eq. 2.
| (2) |
Here, Y(t) represents the proportion of IRTs > t sec; p is the proportion of all IRTs that occur between bouts; (1 − p) is the proportion of all IRTs that occur within bouts. Using nonlinear least squares, estimates for the parameters bout-initiation rate (b), within-bout response rate (w), and bout length (1/p) were obtained for each individual subject after each session. The model requires at least 50 responses to produce reliable bout parameters estimates, and this occurred for all subjects after 18 sessions under the PCNT schedule and 25 sessions under the DRH schedule. Following training, differences in parameter estimates between schedules (PCNT and DRH) were minor and thus, for brevity, only data from the PCNT schedule are reported. Also, bout length was either as sensitive or less sensitive to MeHg exposure as bout-initiation rate, and always less sensitive than within-bout rate so, for brevity, it is not discussed.
2.7.3 Event analysis
Response patterns changed with experience under the schedules but age-related differences between unexposed control mice remained for a majority of the study. To accommodate these differences, analyses of exposure-related effects were performed on a session-by-session basis. The performance of individual mice was compared with the performance of their age-matched unexposed control group. After each session, raw parameter estimates from individual subjects were standardized using the mean and SD of the age-matched control group from that session to produce Z-score units. The threshold for impairment was designated as a Z-score at or below -1.0 (i.e., at least one SD unit below the control mean for that day). We chose impairment as 1 SD below the mean because performance of healthy-aging adults that is 1.0-1.5 SD below the mean of adults on memory and learning tasks generally meets criteria for age-associated mild cognitive impairment [51,52,53,54] (for a review see Jekel et al., 2015 [55]). Impairment was defined as a Z-score of less than -1.0 and the latency to impairment was determined as the time at which a Z-score dropped below -1.0 for at least 75% of the remaining sessions.
Latency to impairment was obtained for each animal on each dependent measure and submitted to Mantel-Cox analysis (Holm-Sidak correction). Animals that survived until the end of the study were censored. This is the same approach used for survival analyses but termed “event” analysis to avoid confusion. For individual animals, the latency to impairment for each dependent measure was also compared with latency to mortality, using a difference score, to identify which measures, if any, served as early and reliable predictors of MeHg toxicity and nimodipine neuroprotection. These data were analyzed using an analysis of variance (ANOVA).
Statistical analyses were conducted using RS/1 software (Brooks Automation, Chelmsford, MA), Systat v.13, and SigmaPlot for Windows v.12.5. Graphs were created using SigmaPlot for Windows v.12.5 and tables were created using Microsoft Excel v.14.
3. Results
3.1 Mortality
Animals were tracked for 262 days (nose-poking was tracked for 253 days). Figure 1 shows mortality for all exposure groups. The Mantel-Cox test found a statistically significant difference among the eight curves [χ2 (7) = 89.45, p<0.01]; p-values from multiple comparison tests are shown in parentheses.
Figure 1.
Survival analysis plots (e.g., mortality) are shown for adult (solid lines) and retired breeder (dashed lines) age cohorts. Each colored line represents a different exposure group; black = control, green = NIM-only, red = MeHg-only, and blue = MeHg + NIM. Asterisks denote groups that were significantly different from age-matched control and NIM-only groups (p<0.01).
For adults, the MeHg-only group (p<0.01) but not MeHg + NIM (p=0.52) or NIM-only (p=0.85) groups differed from control. The adult MeHg-only group also differed from the MeHg + NIM group (p<0.01). For retired breeders, both the MeHg-only and the MeHg + NIM groups differed from control (p<0.01 and p=0.04, respectively), but they did not differ from each other (p=0.87). Comparing between ages, adult and retired breeder MeHg-only groups were not different (p=0.99), but MeHg + NIM groups were significantly different (p=0.03), with adults surviving longer than retired breeders. Median mortality in exposure days (25th and 75th percentiles in parentheses) could be determined for three groups: adult MeHg-only, 111 days (102-138); retired breeder MeHg-only, 107 days (90-143); retired breeder MeHg + NIM, 138 days (108-232)
3.2 Age differences in behavior
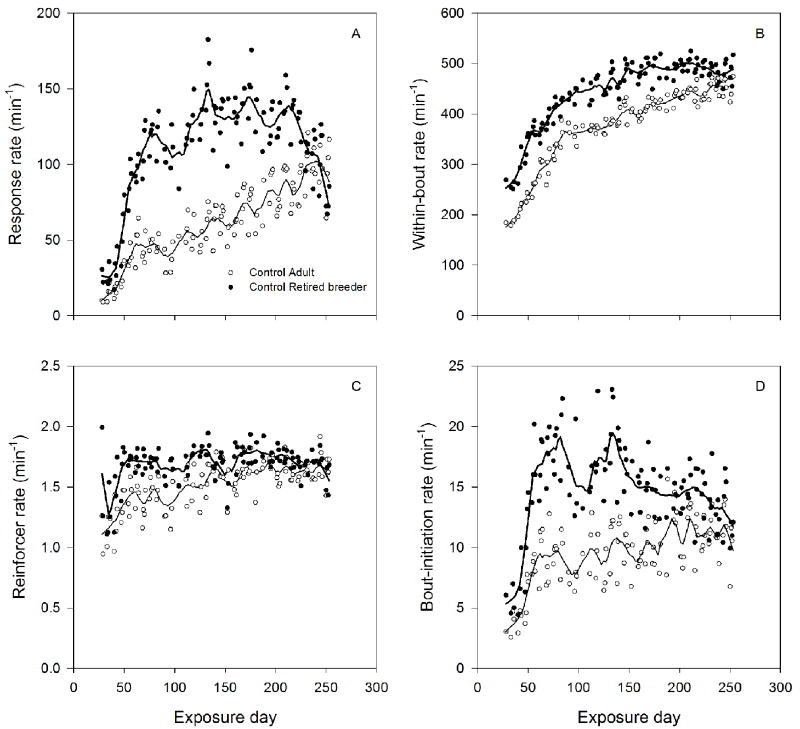
Figure 2A-D shows session averages of within-bout rate (A), bout-initiation rate (B), response rate (C), and reinforcer rate (D) for unexposed control animals. Lines represent the best fit of a LOESS smoothing algorithm. There were large, systematic differences between control adult and retired-breeders from the outset that persisted throughout the study. Retired breeders had higher overall response rates than adults (C), a result of higher within-bout (A) and bout-initiation rates (B). Despite the wide range of response-rate differences across sessions, the RI 30” schedule of reinforcement in both the PCNT and DRH (not shown, as explained in section 2.7.2) components produced a relatively constant reinforcer rate across sessions (D), although it was slightly lower for adults early in training. A decline in overall response rates (C) in the retired breeders was associated with a decline in the rate at which bouts were initiated (B), not within-bout rate (A). To reveal specific influences of age on responding, Figure 3A-D shows these same dependent measures plotted as a function of chronological age, starting at 100 days of age for the adults and 324 days of age for the retired breeders. Interestingly, following acquisition, the curve for the retired breeders seems to be a nearly seamless extension of the curve for the adults for overall response rate (C) and within-bout rate (A) and nearly so for the bout-initiation rate (B).
Figure 2.
Mean within-bout rate (A), bout-initiation rate (B), response rate (C), and reinforcer rate (D) as a function of exposure day under the PCNT schedule. Open circles and filled triangles represent adult and retired breeder age cohorts, respectively. Lines represent the best fit of LOESS smoothing algorithm.
Figure 3.
Mean within-bout rate (A), bout-initiation rate (B), response rate (C), and reinforcer rate (D) as a function of chronological age under the PCNT schedule. Open circles and filled triangles represent adult and retired breeder age cohorts, respectively. Lines represent the best fit of LOESS smoothing algorithm.
3.3 Methylmercury toxicity and nimodipine neuroprotection
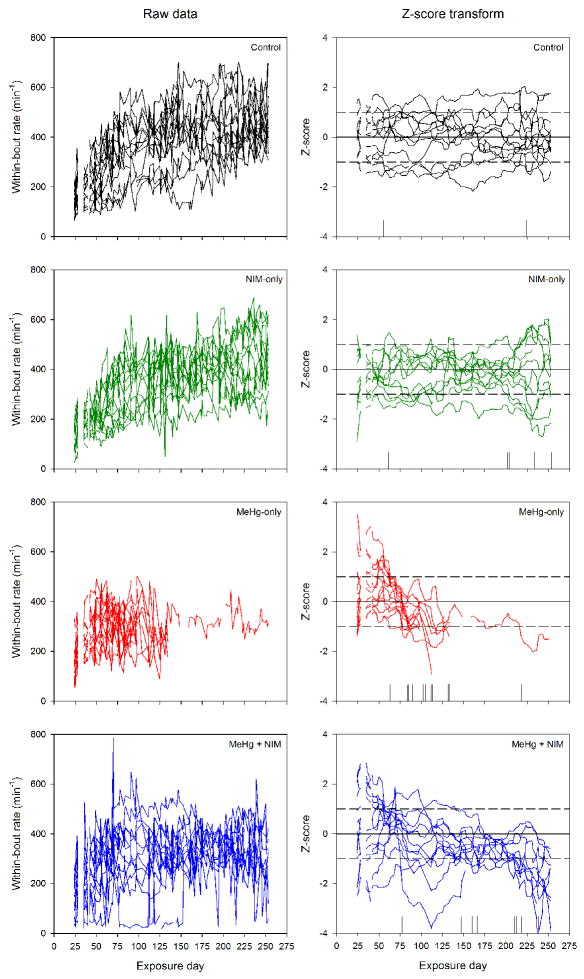
Figure 4 shows within-bout rates for adults and exemplifies the event analysis. Each line within a plot corresponds to an individual subject. The left column presents raw parameter estimates of within-bout rate for control, NIM-only, MeHg-only, and MeHg + NIM exposure groups (from top to bottom). The right column shows these rates normalized as Z-score units calculated from the mean and standard deviation of the control group. This approach allows each exposure group to be compared with age- and experience-matched control mice. Vertical lines along the abscissa represent the time at which an animal met criteria for impairment. These latencies to impairment were analyzed using a Mantel-Cox analysis, which are shown in Fig. 5 and 6.
Figure 4.
An example of the statistical methods used in the event analysis. The left column shows raw parameter estimates of within-bout rate as a function of exposure day for individual subjects (each line represents one subject) from the adult cohort (top to bottom: control, NIM-only, MeHg-only, and MeHg + NIM). On a session-by-session basis, individual animal performance was standardized using the mean and SD of the control group to produce Z-scores. These Z-scores are shown in the right column for the same exposure groups with dashed lines demarcating ±1 SD. For standardized plots (right side), vertical lines on the abscissa represent the latency to impairment for an individual animal.
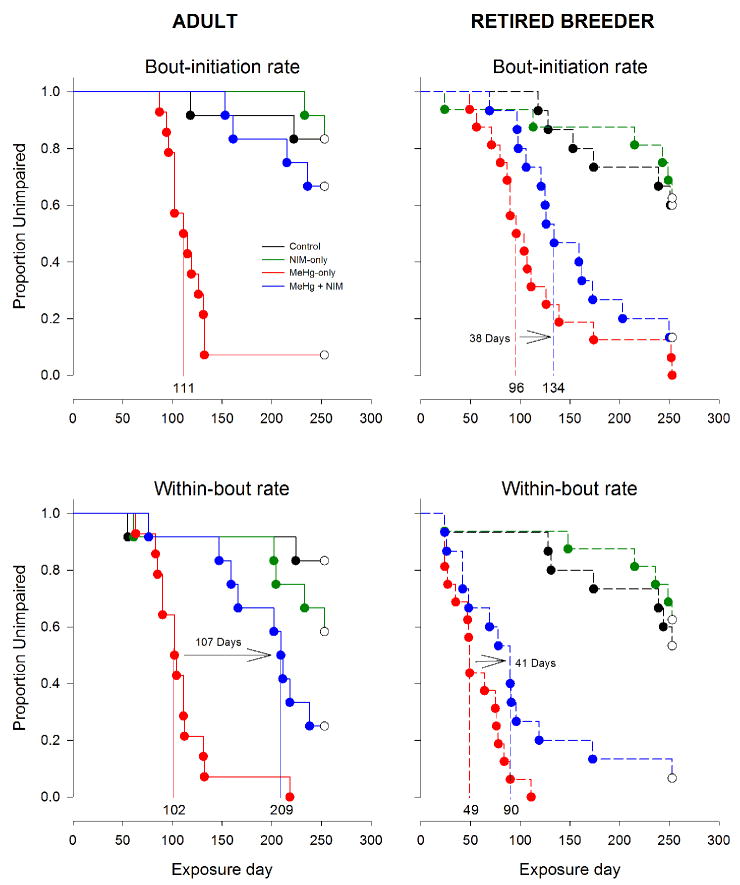
Figure 5.
Event analyses (survival analysis) for bout-initiation rate (top panel) and within-bout rate (bottom panel), separated by age (left and right). Shown near the abscissa of each Kaplan-Meier plot are the median latencies to impairment for the MeHg-only and MeHg + NIM exposure groups, as well as the difference between the two groups. Exposure groups: black = control, green = NIM-only, red = MeHg-only, and blue = MeHg + NIM.
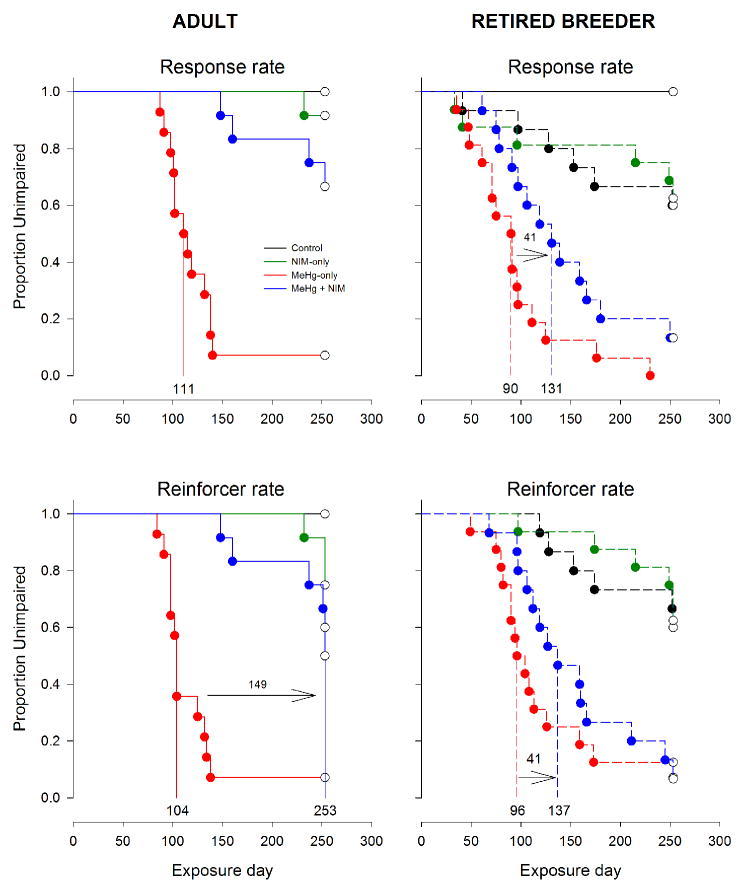
Figure 6.
Event analyses (survival analysis) for response rate (top panel) and reinforcer rate (bottom panel), separated by age (left and right). The format is the same as Fig. 5. Note that for response rate, too few adult MeHg + NIM animals reached impairment to determine median latency to impairment. Exposure groups: black = control, green = NIM-only, red = MeHg-only, and blue = MeHg + NIM.
Figure 5 show the results of the event analyses for bout-initiation and within-bout rates and Fig. 6 shows the results for overall response- and reinforcer rates. The median latency to impairment is marked on the abscissa for key exposure groups and the difference between latencies between MeHg and MeHg + NIM groups, in days, is also shown on the graphs. Note that median latencies could not be calculated for adult bout-initiation rate (Fig. 5) and response rate (Fig. 6) for adult MeHg-only and MeHg + NIM groups since so few subjects showed deficits on these measures. Multiple comparisons tests revealed a number of significant differences among exposure groups, catalogued in detail in Table 2. While every dependent measure was eventually affected by MeHg, the time-course of disruption was not the same for each measure. For brevity and clarity the specific statistics are omitted in the narrative below but all effects described are associated with an omnibus p-value of less than 0.01.
Table 2. Event analysis: Multiple pairwise comparisons.
Multiple pairwise comparison tests from the event analysis (Mantel-Cox).
| Age | Group 1 | Group 2 | Response rate | Reinforcer rate | Bout-initiation rate | Within-bout rate | Bout length |
|---|---|---|---|---|---|---|---|
| Adult | Control | NIM | 0.78 | 0.51 | 0.93 | 0.77 | 0.85 |
| Control | MeHg | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Control | MeHg + NIM | 0.28 | 0.07 | 0.94 | 0.06 | 0.48 | |
| NIM | MeHg | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| NIM | MeHg + NIM | 0.60 | 0.78 | 0.91 | 0.52 | 0.85 | |
| MeHg | MeHg + NIM | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
|
| |||||||
| Retired breeder | Control | NIM | 0.88 | 0.95 | 0.97 | 0.97 | 0.99 |
| Control | MeHg | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Control | MeHg + NIM | 0.07 | <0.01 | 0.03 | 0.01 | 0.02 | |
| NIM | MeHg | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| NIM | MeHg + NIM | 0.04 | <0.01 | 0.02 | <0.01 | <0.01 | |
| MeHg | MeHg + NIM | 0.18 | 0.92 | 0.69 | 0.18 | 0.71 | |
|
| |||||||
| Adult vs. Retired breeder | MeHg | MeHg | 0.41 | 0.97 | 0.98 | <0.01 | 0.85 |
| MeHg + NIM | MeHg + NIM | 0.01 | 0.01 | 0.02 | 0.01 | <0.01 | |
Bold and italicized p-values represent a significant difference between Group 1 and Group 2.
First, control and NIM-only groups did not differ in either age group. Second, for adults, MeHg-only mice differed from both control and NIM-only groups on all dependent measures but MeHg + NIM did not differ from control or NIM-only groups on any measure and MeHg-only and MeHg + NIM groups differed on all dependent measures. That is, for the adults the MeHg + NIM mice resembled controls, not MeHg-exposed mice.
Third, for retired breeders, MeHg-only and MeHg + NIM mice differed from both control and NIM-only groups on all dependent measures with the exception that control and MeHg + NIM mice did not differ on response rate. Fourth, MeHg-only and MeHg + NIM did not differ on any measure. That is, for the retired breeders, MeHg + NIM mice largely resembled MeHg-exposed mice and differed from controls. Finally, between ages, MeHg-only groups did not differ on any measure except within-bout rate and the MeHg + NIM groups differed on all dependent measures.
3.4 Predicting impairment
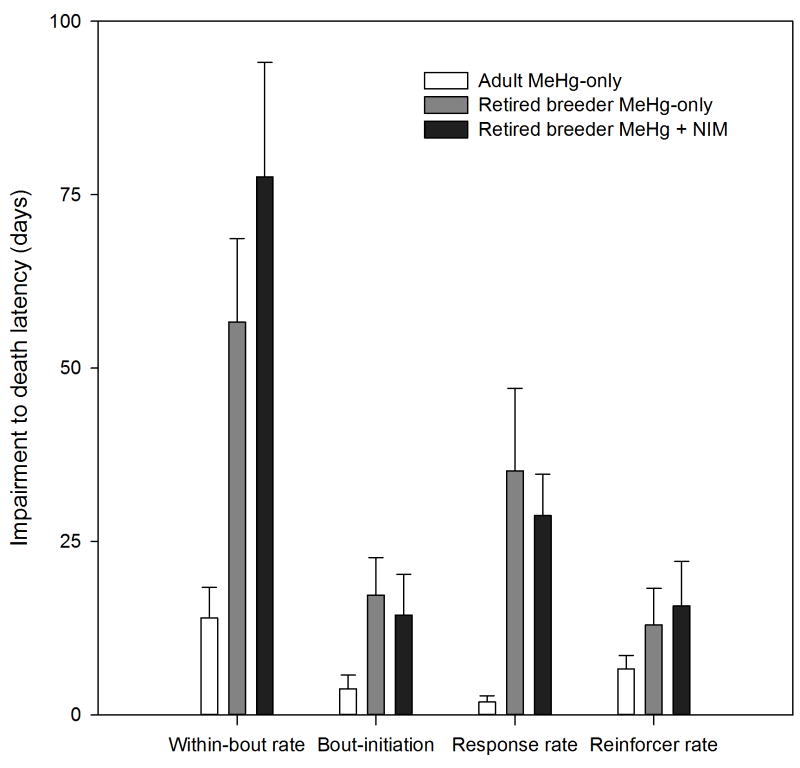
The measure with the shortest latency to impairment, i.e., the most sensitive to MeHg, was within-bout rate for all exposure groups (Fig. 5, 6). For adult and retired breeder MeHg-only groups, the median latency to impairment was 102 and 49 days, respectively, and for adult and retired breeder MeHg + NIM groups, the median latency to impairment was 209 and 90 days, respectively. To test the hypothesis that within-bout rate is the earliest reliable predictor of MeHg-induced behavior impairment, we compared latency to impairment and latency to mortality for all animals that died; a long latency is associated with an early marker of impairment. This generated a quantitative measure of prediction for each dependent measure. Figure 7 shows the latency from impairment to death for adult and retired breeder MeHg-only groups and the retired breeder MeHg + NIM for bout-initiation rate, reinforcer rate, response rate, and within-bout rate. Adult MeHg + NIM animals were omitted because so few died (see Fig. 1). A mixed ANOVA revealed a Dependent Measure X Group interaction [F(6,108) = 6.35, p<0.01]. Post-hoc tests revealed that for within-bout rate and response rate, the latency from impairment to death was shorter for the adult MeHg-only group than the retired breeder MeHg-only (p<0.01) and MeHg + NIM groups (p<0.01).
Figure 7.
The bar chart show the average latency (±SEM) from impairment to death for four measures. Note that the adult MeHg + NIM group is excluded because so few animals died from MeHg toxicity (see text for details). The latency from impairment to death was longest for within-bout rate (*, all p’s<0.001). For within-bout rate and response rate, the average latency for adult MeHg-only animals was significantly shorter compared to retired breeder MeHg-only and MeHg + NIM animals (#, all p’s<0.001)
3.5 Brain Hg concentrations
Control and NIM-only samples had undetectable brain Hg concentrations (< 0.1 ppm). Adult MeHg-only animals had average brain concentrations of 24.5 ± 1.39 ppm (± SEM) when they were euthanized due to MeHg toxicity. Adult MeHg + NIM animals had average brain Hg concentrations of 17.3 ± 1.27 ppm; most of these were taken at the end of the study and the mice showed few or no overt signs of toxicity. Retired breeder MeHg and MeHg + NIM animals had average brain Hg concentrations of 22.2 ± 0.67 and 20.0 ± 1.7 ppm, respectively, when they were euthanized due to MeHg toxicity. Because brain Hg concentrations for the MeHg + NIM adults were obtained at the end of the study, these Hg levels were not compared to those taken earlier in the study when animals were euthanized due to MeHg toxicity. Two-tailed t-tests revealed no significant difference in brain Hg levels between MeHg-only adult and retired breeders [t(22)=1.432, p=0.17] or between MeHg-only and MeHg + NIM retired breeders [t(22)=1.205, p<0.24].
4. Discussion
The present study was designed to characterize the role of age in determining sensitivity to the neurotoxic effects of chronic MeHg exposure and protection by nimodipine. The effects of low- and high exposure levels are qualitatively similar but quantitatively different [56]. Higher exposure levels produce short latencies to the onset of neurotoxicity and greater spread between relatively sensitive and insensitive endpoints. The exposure regimen used here, which was relatively high, could model lower exposure levels but more rapidly. With low exposure levels the delay to toxicity is longer, but the signs of exposure are the same [56]. The current study found that: 1) chronic adult-onset exposure to 1.2 mg/kg/day MeHg produced age-independent mortality in male BALB/c mice, 2) the latency to MeHg-induced motor deficits was age-dependent with earlier impairment occurring in older animals 3) 20 mg/kg of nimodipine afforded age-dependent neuroprotection from MeHg insult with greater protection in younger animals, but the drug had no effect when administered alone, 4) a log-survivor bout analysis divorced motor and motivational aspects of high-rate nose-poking, and 5) decrements in within-bout rate, a putative measure of motor function, were most sensitive to MeHg and served as an early predictor of neurotoxicity.
4.1 Age differences
Throughout the study the retired breeders responded more than adults, although this discrepancy dissipated as the study progressed. The adults may have been less engaged in nose-poking for sucrose, reflecting differences in motivation or reinforcer efficacy or engagement in behavior incompatible with nose-poking. In support of this notion, the higher response rate seen in the retired breeders (see Fig. 2 and 3) was driven entirely by a higher bout-initiation rate, which is especially sensitive to motivational manipulations such as satiation/deprivation and response cost [40,41]. Within-bout rate between the two age groups was indistinguishable Adults eventually reached the same response and bout-initiation rates as the retired breeders when they reached comparable ages, as revealed by Fig. 3, providing support for the idea that the motivational variables affect behavior in an age-dependent manner. Response rate declined as retired breeders aged and this was due to a decrease in bout-initiation rate while reinforcer rate and within-bout rate remained constant. This suggests that aged retired breeders paused longer between bouts and were less likely to initiate a high-rate response bout that was reinforced by sucrose (Figs. 2-3).
4.2 Chronic nimodipine
There were no detectable effects of nimodipine on mortality or high-rate nose-poking within adult and retired breeder groups, consistent with previous studies from our laboratory [25, 26]. Nimodipine and similar L-type CCBs produce measureable vascular [28], electrophysiological [57], and neurochemical changes [58,59]. Evidence suggests nimodipine may facilitate select forms of learning [59,60,61,62,63] in healthy adult animals, although other studies with nimodipine contradict these results [64,65]. Nimodipine does reliably attenuate learning deficits associated with normal aging [27,29,30,36,66,67] and protects against CNS insult in experimental models of Parkinson’s disease [68,69,70,71]. Here, over the course of 8.5 months nimodipine did not affect any dependent measure in animals that aged from 2.5-10 months (adults) and 9.5-18 months (retired breeders).
4.3 Motor dysfunction
Within-bout rate, a measure of motor function derived from the log-survivor bout analysis, was the most sensitive measure to MeHg exposure (Fig. 5-7; Table 2). Between age groups, deficits appeared 53 days earlier in retired breeders than adults in the MeHg-only group and 119 days earlier in retired breeders than adults in the MeHg + NIM group. Within age, nimodipine significantly delayed MeHg’s effect on within-bout rate by 107 days for the adult MeHg + NIM mice compared to only 53 days for the retired breeders.
In general, these findings agree with previous studies [26,53,72,73,74,75] and support the notion that adult-onset MeHg produces primary and early degradation of motor function followed by secondary cognitive deficits. Importantly, the motivation to respond was not affected even when the ability to respond was impaired. In past studies, e.g., Bailey et al. [25], declines in performance-based measures were coupled with commensurate motor deficits, suggesting that impaired motor function influences the motivation to engage in active behavior. Recently, Hoffman & Newland [26], using a bout analysis approach to parse motor and motivational contributions to wheel-running, noted that MeHg diminished the speed of wheel-running, indicative of a diminished motor function, but it did not diminish the motivation to engage in wheel-running even as animals met criteria for euthanasia.
Our report (Fig. 5, 6, 7) supports the conclusions of Hoffman & Newland [26], as log-survivor estimates of within-bout rate and bout-initiation rate of nose-poking were differentially sensitive to the effects of MeHg. Further support is derived from the observation that median latency to reduced reinforcer rate, a motivation-based measure that is not included in the log-survivor model, was at or near the median latency for bout-initiation rate. Also, nimodipine delayed MeHg effects on nose-poking in a similar manner to Hoffman & Newland’s [26] finding that nimodipine delayed MeHg effects on wheel-running and rotarod performance. Running is a natural act for which its reinforcement is inextricably embedded in the act itself, referred to as automatic reinforcement [76]. In contrast, nose-poking is a relatively contrived behavior and the reinforcement of nose-poking is easily divorced from the act. Taken together, motor deficits do not appear to affect the influence of a reinforcer (intrinsic or extrinsic), or motivation. They also suggest that nimodipine’s protection does not depend on the function or topography of the motor act, which stands in contrast to some drug effects (see Johnson et al. [46]).
This longitudinal study of chronic MeHg exposure provided direct evidence that MeHg-induced behavior deficits are age-dependent. To illustrate this point, Figure 7 shows that, on average, latency from within-bout rate and response rate impairment to death was 43 and 33 days longer, respectively, for retired breeders than adults. Together, these findings suggest an exaggerated delay to neurotoxicity in younger mice and supports the hypothesis that the delayed neurotoxicity of MeHg [77,78,79] is linked to the cumulative impact of cellular dysfunction that results from disruptions in Ca2+ homeostasis. Both Bailey et al. [25] and Hoffman & Newland [26] reported a complete block of MeHg’s effects by 20 mg/kg/day nimodipine after 160 days of exposure. The present study ran longer and showed that MeHg’s neurotoxicity eventually appeared. Thus, nimodipine delayed but did not prevent MeHg neurotoxicity even in the adult mice.
4.4 Methylmercury-induced mortality and nimodipine neuroprotection
Methylmercury-induced mortality was age-independent whereas nimodipine neuroprotection was age-dependent, with greater protection afforded to adults than retired breeders (Fig. 1). The fact that MeHg-induced mortality was age-independent suggests that the mechanisms underlying MeHg’s disruption of behavior may differ from those related to its mortality. Nimodipine’s protection from MeHg-induced mortality supports the hypothesis that chronic MeHg toxicity is due, at least in part, to disruption of Ca2+ homeostasis. The observation that it was age-dependent provides support for the notion that age-induced perturbation of Ca2+ signaling mitigated nimodipine’s effects. Together with Hoffman & Newland [26], in which adult-onset chronic MeHg (2.6 mg/kg/day) induced mortality after a median of approximately 97 days, these results show that survival is dose-related.
There was no difference in brain Hg concentration among animals euthanized due to severe MeHg toxicity: adult MeHg-only and retired breeder MeHg-only and MeHg + NIM mice. For these three groups, these brain Hg levels represent a lethal concentration. In contrast, adult MeHg + NIM animals had, on average, brain Hg concentrations that were 7.15 ppm lower than adult MeHg-only animals, although no statistical test was performed because the conditions under which the brains were taken were different between the two groups. A similar result was reported by Bailey et al. [25], using adult male BALB/c mice chronically exposed to 2.6 mg/kg/day MeHg and 20 mg/kg/day nimodipine. In both studies, brain samples from adult MeHg + NIM animals were collected at the end of the study from relatively healthy mice and do not represent a lethal dose. In most cases, this meant that adult MeHg + NIM animals were exposed to MeHg longer than MeHg-only animals but acquired less Hg in their brains. Nonetheless, in both studies the MeHg + NIM mice had lower brain Hg concentrations.
Together, these findings suggest that nimodipine acts to reduce the bioavailability of MeHg, an effect that may be mediated by age. For example, CCBs could affect the uptake of MeHg in the gut, its elimination, its passage across the blood-brain barrier, or its retention in the brain. The mechanism(s) of action by which nimodipine lowers brain Hg is not clear at this time but it does indicate that nimodipine acts, at least in part, by altering the toxicokinetics of MeHg.
4.5 Mechanisms of aging and methylmercury neurotoxicity
MeHg neurotoxicity is thought elevate Ca2+ concentration in nerve terminals in two distinct temporal phases, the first phase a result of Ca2+ release from intracellular stores (i.e., mitochondria and smooth endoplasmic reticulum) and the second a result of an influx of extracellular Ca2+ via voltage-gated Ca2+ channels [80,81]. These distinct phases may, in part, contribute to the delayed neurotoxicity of MeHg. The more subtle Ca2+-related changes that occur during normal aging, which do not directly lead to cell death, likely include increased Ca2+ release from intracellular stores via inositol(1,4,5) triphosphate (IP3) receptors and ryanodine (Ry) receptors, increased Ca2+ influx through L-type Ca2+ channels, increased amplitude and duration of the Ca2+-dependent, K+-mediated afterhyperpolarization (i.e., a shift from shorter to longer afterhyperpolarizations during which actions potentials cannot be produced), reduced NMDA receptor-mediated Ca2+ influx, and reduced Ca2+ buffering capacity [16,82,83]. Thus, reduced protection by nimodipine in older animals may be due chronic MeHg exposure taxing an already perturbed Ca2+ system, which could accelerate signs of aging or neurodegeneration. This supposition is supported by our finding of motor impairment in relatively healthy MeHg + NIM adults after a lengthy exposure regimen (253 days). Theoretically, a higher dose of nimodipine would be needed to offset chronic MeHg exposure in aging animals and completely block deficits in younger animals.
5. Conclusion
The findings reported here showed that MeHg produced significant behavior deficits and mortality in two age groups of mice and nimodipine attenuated these deficits in an age-dependent manner. Log-survivor bout analysis of high-rate operant behavior, which parses responding into motoric and motivational components, identified components of the molecular structure of behavior differentially sensitive to chronic MeHg exposure and nimodipine neuroprotection. Chronic MeHg exposure produced relatively similar mortality between the two age groups. Relative to mortality, motor deficits were the earliest signs to appear and, even in the face of significant decreases in response speed, mice continued to initiate bouts of nose-poking, which suggests that MeHg did not diminish the motivation to respond. The younger adults experienced a longer delay to toxicity than older retired breeders. Nimodipine attenuated MeHg-induced mortality and behavior deficits, but protection was substantially diminished in older animals. One methodological contribution is the use of an event analysis to quantify behavior changes in a situation in which attrition diminishes sample size over the course of a study. The finding of age-dependent nimodipine neuroprotection provides evidence that MeHg exposure in aging animals may tax and already-perturbed Ca2+ signaling system.
Highlights.
Young- and older adult mice were exposed chronically to MeHg and/or nimodipine
MeHg-induced mortality was age-independent
Nimodipine afforded greater protection against MeHg-induced mortality in younger mice
MeHg affected the maximum rate of nose-poking but not the motivation to do so
Older mice had a shorter latency to impairment
Nimodipine delayed motor deficits by 100+ days in younger compared with older mice
Acknowledgments
The authors would like to thank Megan Arnold and Steven Boomhower for their assistance with this research project. In addition, we are grateful for the assistance provided by numerous undergraduate students in the laboratory.
Support for this research was provided by the National Institutes of Health [Grant ES R01 003299].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew Nathanael Shen, Department of Psychology, Behavioral Toxicology Lab, Auburn University.
Craig Cummings, Department of Psychology, University of Alabama.
Derek Pope, Department of Psychology, Behavioral Toxicology Lab, Auburn University.
Daniel Hoffman, Department of Psychology, Indiana University Southeast.
M. Christopher Newland, Department of Psychology, Behavioral Toxicology Lab, Auburn University.
References
- 1.Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, et al. Methylmercury exposure and health effects in humans: a worldwide concern. AMBIO: A Journal of the Human Environment. 2007;36(1):3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Castoldi AF, Onishchenko N, Johansson C, Coccini T, Roda E, Vahter M, et al. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Regulatory Toxicology and Pharmacology. 2008;51(2):215–229. doi: 10.1016/j.yrtph.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Rice DC, Gilbert SG. Effects of developmental exposure to methyl mercury on spatial and temporal visual function in monkeys. Toxicology and applied pharmacology. 1990;102(1):151–163. doi: 10.1016/0041-008x(90)90092-9. [DOI] [PubMed] [Google Scholar]
- 4.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental health perspectives. 2000;108(Suppl 3):511. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eto K. Review article: Pathology of Minamata disease. Toxicologic Pathology. 1997;25(6):614. doi: 10.1177/019262339702500612. [DOI] [PubMed] [Google Scholar]
- 6.Möller-Madsen B. Localization of mercury in CNS of the rat. Iii. Oral administration of methylmercuric chloride (ch3hgcl) Fundamental and Applied Toxicology. 1991;16(1):172–187. [PubMed] [Google Scholar]
- 7.Itoh K, Korogi Y, Tomiguchi S, Takahashi M, Okajima T, Sato H. Cerebellar blood flow in methylmercury poisoning (Minamata disease) Neuroradiology. 2001;43(4):279–284. doi: 10.1007/s002340000462. [DOI] [PubMed] [Google Scholar]
- 8.Atchison WD. Is chemical neurotransmission altered specifically during methylmercury-induced cerebellar dysfunction? Trends in pharmacological sciences. 2005;26(11):549–557. doi: 10.1016/j.tips.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. The FASEB Journal. 1994;8(9):622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- 10.Khachaturian Z. Towards theories of brain aging Handbook of studies on psychiatry and old age. Elsevier Amsterdam; 1984. pp. 7–30. [Google Scholar]
- 11.Khachaturian ZS. The role of calcium regulation in brain aging: reexamination of a hypothesis. Aging Clinical and Experimental Research. 1989;1(1):17–34. doi: 10.1007/BF03323872. [DOI] [PubMed] [Google Scholar]
- 12.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiological reviews. 1998;78(1):99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 13.Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiology of aging. 1987;8(4):329–343. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- 14.Iacopino AM, Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proceedings of the National Academy of Sciences. 1990;87(11):4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murchison D, Griffith WH. Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging cell. 2007;6(3):297–305. doi: 10.1111/j.1474-9726.2007.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging cell. 2007;6(3):307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environmental health perspectives. 2002;110(Suppl 5):851. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellemann P, Schade A, Towart R. Dihydropyridine receptor in rat brain labeled with [3H] nimodipine. Proceedings of the National Academy of Sciences. 1983;80(8):2356–2360. doi: 10.1073/pnas.80.8.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen C, McCarthy R. Nimodipine block of calcium channels in rat anterior pituitary cells. The Journal of physiology. 1987;387:195. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haws CW, Gourley JK, Heistad DD. Effects of nimodipine on cerebral blood flow. Journal of Pharmacology and Experimental Therapeutics. 1983;225(1):24–28. [PubMed] [Google Scholar]
- 21.Kazda S, Towart R. Nimodipine: a new calcium antagonistic drug with a preferential cerebrovascular action. Acta neurochirurgica. 1982;63(1-4):259–265. doi: 10.1007/BF01728880. [DOI] [PubMed] [Google Scholar]
- 22.Scriabine A, Schuurman T, Traber J. Pharmacological basis for the use of nimodipine in central nervous system disorders. The FASEB Journal. 1989;3(7):1799–1806. [PubMed] [Google Scholar]
- 23.Limke TL, Bearss JJ, Atchison WD. Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor-linked pathway. Toxicological Sciences. 2004;80(1):60–68. doi: 10.1093/toxsci/kfh131. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto M, Ikegami N, Nakano A. Protective effects of Ca2+ channel blockers against methyl mercury toxicity. Pharmacology & toxicology. 1996;78(3):193–199. doi: 10.1111/j.1600-0773.1996.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 25.Bailey JM, Hutsell BA, Newland MC. Dietary nimodipine delays the onset of methylmercury neurotoxicity in mice. Neurotoxicology. 2013;37:108–117. doi: 10.1016/j.neuro.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman DJ, Newland MC. Chronic methylmercury exposure decreases the ability, but not the motivation to run: A microstructural analysis and protection by nimodipine. Neurotoxicology. 2016;54:127–129. doi: 10.1016/j.neuro.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Batuecas A, Pereira R, Centeno C, Pulido JA, Hernández M, Bollati A, et al. Effects of chronic nimodipine on working memory of old rats in relation to defects in synaptosomal calcium homeostasis. European journal of pharmacology. 1998;350(2):141–150. doi: 10.1016/s0014-2999(98)00250-7. [DOI] [PubMed] [Google Scholar]
- 28.De Jong G, De Weerd H, Schuurman T, Traber J, Luiten P. Microvascular changes in aged rat forebrain. Effects of chronic nimodipine treatment. Neurobiology of aging. 1990;11(4):381–389. doi: 10.1016/0197-4580(90)90003-i. [DOI] [PubMed] [Google Scholar]
- 29.Levere T, Walker A. Old age and cognition: enhancement of recent memory in aged rats by the calcium channel blocker nimodipine. Neurobiology of aging. 1992;13(1):63–66. doi: 10.1016/0197-4580(92)90010-u. [DOI] [PubMed] [Google Scholar]
- 30.Solomon PR, Wood MS, Groccia-Ellison ME, Yang B-Y, Fanelli RJ, Mervis RF. Nimodipine facilitates retention of the classically conditioned nictitating membrane response in aged rabbits over long retention intervals. Neurobiology of aging. 1995;16(5):791–796. doi: 10.1016/0197-4580(95)00093-t. [DOI] [PubMed] [Google Scholar]
- 31.Thompson L, Deyo R, Disterhoft J. Nimodipine enhances spontaneous activity of hippocampal pyramidal neurons in aging rabbits at a dose that facilitates associative learning. Brain research. 1990;535(1):119–130. doi: 10.1016/0006-8993(90)91830-a. [DOI] [PubMed] [Google Scholar]
- 32.Bork K, Wurm F, Haller H, Strauss C, Scheller C, Gnanapragassam VS, et al. Neuroprotective and Neuroregenerative Effects of Nimodipine in a Model System of Neuronal Differentiation and Neurite Outgrowth. Molecules. 2015;20(1):1003–1013. doi: 10.3390/molecules20011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haile M, Galoyan S, Li Y-S, Cohen BH, Quartermain D, Blanck T, et al. Nimodipine-Induced Hypotension but Not Nitroglycerin-Induced Hypotension Preserves Long-and Short-Term Memory in Adult Mice. Anesthesia & Analgesia. 2012;114(5):1034–1041. doi: 10.1213/ANE.0b013e31824b2b05. [DOI] [PubMed] [Google Scholar]
- 34.Iimuro Y, Ikejima K, Rose ML, Bradford BU, Thurman RG. Nimodipine, a dihydropyridine-type calcium channel blocker, prevents alcoholic hepatitis caused by chronic intragastric ethanol exposure in the rat. Hepatology. 1996;24(2):391–397. doi: 10.1002/hep.510240217. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Hu X, Liu Y, Bao Y, An L. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2009;56(3):580–589. doi: 10.1016/j.neuropharm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Veng LM, Mesches MH, Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein α 1D (Ca v 1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Molecular Brain Research. 2003;110(2):193–202. doi: 10.1016/s0169-328x(02)00643-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Zheng S, Dong F, Wang Z. Nimodipine improves regional cerebral blood flow and suppresses inflammatory factors in the hippocampus of rats with vascular dementia. Journal of International Medical Research. 2012;40(3):1036–1045. doi: 10.1177/147323001204000322. [DOI] [PubMed] [Google Scholar]
- 38.Riekkinen M, Schmidt B, Kuitunen J, Riekkinen P. Effects of combined chronic nimodipine and acute metrifonate treatment on spatial and avoidance behavior. European journal of pharmacology. 1997;322(1):1–9. doi: 10.1016/s0014-2999(96)00976-4. [DOI] [PubMed] [Google Scholar]
- 39.Newland MC. Motor function and the physical properties of the operant: applications to screening and advanced techniques. Neurotoxicology: Approaches and methods. 1995:265–299. [Google Scholar]
- 40.Alleman HD, Platt JR. Differential reinforcement of interresponse times with controlled probability of reinforcement per response. Learning and Motivation. 1973;4(1):40–73. [Google Scholar]
- 41.Galbicka G, Platt JR. Parametric manipulation of interresponse-time contingency independent of reinforcement rate. Journal of Experimental Psychology: Animal Behavior Processes (Washington, DC) 1986;12(4):371–380. [PubMed] [Google Scholar]
- 42.Shull RL, Gaynor ST, Grimes JA. Response rate viewed as engagement bouts: Effects of relative reinforcement and schedule type. Journal of the Experimental Analysis of Behavior. 2001;75(3):247–274. doi: 10.1901/jeab.2001.75-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shull RL, Gaynor ST, Grimes JA. Response rate viewed as engagement bouts: Resistance to extinction. Journal of the Experimental Analysis of Behavior. 2002;77(3):211–231. doi: 10.1901/jeab.2002.77-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shull RL, Grimes JA. Bouts of responding from variable-interval reinforcement of lever pressing by rats. Journal of the Experimental Analysis of Behavior. 2003;80(2):159–171. doi: 10.1901/jeab.2003.80-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brackney RJ, Cheung THC, Neisewander JL, Sanabria F. The Isolation of Motivational, Motoric, and Schedule Effects on Operant Performance: A Modeling Approach. Journal of the Experimental Analysis of Behavior. 2011;96(1):17–38. doi: 10.1901/jeab.2011.96-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson JE, Bailey JM, Newland MC. Using pentobarbital to assess the sensitivity and independence of response-bout parameters in two mouse strains. Pharmacology Biochemistry and Behavior. 2011;97(3):470–478. doi: 10.1016/j.pbb.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Smith TT, McLean AP, Shull RL, Hughes CE, Pitts RC. Concurrent performance as bouts of behavior. Journal of the Experimental Analysis of Behavior. 2014;102(1):102–125. doi: 10.1002/jeab.90. [DOI] [PubMed] [Google Scholar]
- 48.Shen AN, Cummings C, Hoffman D, Pope D, Arnold M, Newland MC. Chronic methylmercury exposure: age-dependent neurotoxicity and nimodipine neuroprotection of wheel-running and rotarod performance in BALB/c mice. (under review) [Google Scholar]
- 49.Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: effects on a spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27(5):721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paletz EM, Day JJ, Craig-Schmidt MC, Newland MC. Spatial and visual discrimination reversals in adult and geriatric rats exposed during gestation to methylmercury and n– 3 polyunsaturated fatty acids. Neurotoxicology. 2007;28(4):707–719. doi: 10.1016/j.neuro.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crook T, Bartus RT, Ferris SH, Whitehouse P, Cohen GD, Gershon S. Age-associated memory impairment: Proposed diagnostic criteria and measures of clinical change—report of a national institute of mental health work group 1986 [Google Scholar]
- 52.Levy R. Aging-associated cognitive decline. International Psychogeriatrics. 1994;6(01):63–68. [PubMed] [Google Scholar]
- 53.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 54.Schönknecht P, Pantel J, Kruse A, Schröder J. Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects. American Journal of Psychiatry. 2005 doi: 10.1176/appi.ajp.162.11.2071. [DOI] [PubMed] [Google Scholar]
- 55.Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, et al. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review, Alzheimer’s Research & Therapy. 2015;7(17) doi: 10.1186/s13195-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heath JC, Banna KM, Reed MN, Pesek EF, Cole N, Li J, et al. Dietary selenium protects against selected signs of aging and methylmercury exposure. Neurotoxicology. 2010;31(2):169–179. doi: 10.1016/j.neuro.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lashgari R, Motamedi F, Asl SZ, Shahidi S, Komaki A. Behavioral and electrophysiological studies of chronic oral administration of L-type calcium channel blocker verapamil on learning and memory in rats. Behavioural brain research. 2006;171(2):324–328. doi: 10.1016/j.bbr.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 58.Kabuto H, Yokoi I, Mori A, Murakami M, Sawada S. Neurochemical changes related to ageing in the senescence-accelerated mouse brain and the effect of chronic administration of nimodipine. Mechanisms of ageing and development. 1995;80(1):1–9. doi: 10.1016/0047-6374(94)01542-t. [DOI] [PubMed] [Google Scholar]
- 59.Levy A, Kong RM, Stillman MJ, Shukitt-Hale B, Kadar T, Rauch TM, Lieberman HR. Nimodipine improves spatial working memory and elevates hippocampal acetylcholine in young rats. Pharmacology Biochemistry and Behavior. 1991;39(3):781–786. doi: 10.1016/0091-3057(91)90164-w. [DOI] [PubMed] [Google Scholar]
- 60.Hoffmeister F, Benz U, Heise A, Krause HP, Neuser V. Behavioral effects of nimodipine in animals. Arzneimittel-Forschung. 1981;32(4):347–360. [PubMed] [Google Scholar]
- 61.Martin LJ, Fournier NM, Galic MA, Emond MH. Chronic administration of the L-type calcium channel blocker nimodipine can facilitate the acquisition of sequence learning in a radial-arm maze. Behavioural pharmacology. 2004;15(2):133–139. doi: 10.1097/00008877-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 62.McMonagle-Strucko K, Fanelli RJ. Enhanced acquisition of reversal training in a spatial learning task in rats treated with chronic nimodipine. Pharmacology Biochemistry and Behavior. 1993;44(4):827–835. doi: 10.1016/0091-3057(93)90013-j. [DOI] [PubMed] [Google Scholar]
- 63.Quartermain D, Garcia deSoria V, Kwan A. Calcium channel antagonists enhance retention of passive avoidance and maze learning in mice. Neurobiology of learning and memory. 2001;75(1):77–90. doi: 10.1006/nlme.1999.3958. [DOI] [PubMed] [Google Scholar]
- 64.Clements MP, Rose SP, Tiunova A. ω-Conotoxin GVIA disrupts memory formation in the day-old chick. Neurobiology of learning and memory. 1995;64(3):276–284. doi: 10.1006/nlme.1995.0010. [DOI] [PubMed] [Google Scholar]
- 65.Maurice T, Bayle J, Privat A. Learning impairment following acute administration of the calcium channel antagonist nimodipine in mice. Behavioural pharmacology. 1995 [PubMed] [Google Scholar]
- 66.Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243(4892):809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- 67.Fundaro’ A. Behavioural effects of chronic administration of nimodipine in grouped or individually housed rats. Progress in Neuropsychopharmacology & Biological Psychiatry. 1995;19(2):299–312. [Google Scholar]
- 68.Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiology of disease. 2011;43(2):364–371. doi: 10.1016/j.nbd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kupsch A, Gerlach M, Pupeter SC, Sautter J, Dirr A, Arnold G, et al. Pretreatment with nimodipine prevents MPTP-induced neurotoxicity at the nigral, but not at the striatal level in mice. NeuroReport. 1995;6(4):621–625. doi: 10.1097/00001756-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Kupsch A, Sautter J, Schwarz J, Riederer P, Gerlach M, Oertel WH. 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain research. 1996;741(1):185–196. doi: 10.1016/s0006-8993(96)00917-1. [DOI] [PubMed] [Google Scholar]
- 71.Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Annals of neurology. 2010;67(5):600–606. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bellum S, Thuett KA, Bawa B, Abbott LC. The effect of methylmercury exposure on behavior and cerebellar granule cell physiology in aged mice. Journal of Applied Toxicology. 2013;33(9):959–969. doi: 10.1002/jat.2786. [DOI] [PubMed] [Google Scholar]
- 73.Carvalho MC, Franco JL, Ghizoni H, Kobus K, Nazari EM, Rocha JB, Farina M, et al. Effects of 2, 3-dimercapto-1-propanesulfonic acid (DMPS) on methylmercury-induced locomotor deficits and cerebellar toxicity in mice. Toxicology. 2007;239(3):195–203. doi: 10.1016/j.tox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 74.Dietrich MO, Mantese CE, dos Anjos G, Souza DO, Farina M. Motor impairment induced by oral exposure to methylmercury in adult mice. Environmental toxicology and pharmacology. 2005;19(1):169–175. doi: 10.1016/j.etap.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe C, Satoh H. Evolution of our understanding of methylmercury as a health threat. Environmental Health Perspectives. 1996;104(Suppl 2):367. doi: 10.1289/ehp.96104s2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaughan ME, Michael JL. Automatic reinforcement: An important but ignored concept. Behaviorism. 1982;10(2):217–227. [Google Scholar]
- 77.Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environmental health perspectives. 2005:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiss B, Reuhl K. Delayed neurotoxicity: a silent toxicity. Neurological disease and therapy. 1994;26:765–765. [Google Scholar]
- 79.Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environmental Health Perspectives. 2002;110(Suppl 5):851. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marty MS, Atchison WD. Pathways Mediating Ca 2+ Entry in Rat Cerebellar Granule Cells Followingin VitroExposure to Methyl Mercury. Toxicology and applied pharmacology. 1997;147(2):319–330. doi: 10.1006/taap.1997.8262. [DOI] [PubMed] [Google Scholar]
- 81.Edwards JR, Marty MS, Atchison WD. Comparative sensitivity of rat cerebellar neurons to dysregulation of divalent cation homeostasis and cytotoxicity caused by methylmercury. Toxicol Appl Pharmacol. 2005;208(2005):222–232. doi: 10.1016/j.taap.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 82.Landfield PW. ‘Increased calcium-current’ hypothesis of brain aging. Neurobiology of aging. 1987;8(4):346–347. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- 83.Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging cell. 2007;6(3):267–273. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]