Abstract
Nonalcoholic fatty liver disease (NAFLD) has rapidly become the most common form of chronic liver disease in the United States affecting approximately 80–100 million Americans. NAFLD includes a spectrum of diseases ranging from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) to fibrosis and eventually cirrhosis. Patients with NASH and significant fibrosis on liver biopsy have an increased risk for liver-related morbidity and mortality compared to those with NAFL. Due to the high prevalence of NAFLD and its progressive nature, there has been an urgent need to develop reliable noninvasive tests that can accurately predict the presence of advanced disease without the need for liver biopsy. These tests can be divided into those that predict the presence of NASH and those that predict the presence of fibrosis. In this review, we provide a concise overview of different noninvasive methods for staging the severity of NAFLD.
Keywords: Nonalcoholic steatohepatitis, fibrosis, oxidative stress, apoptosis, noninvasive markers, liver biopsy
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in Western countries and is estimated to affect one in three adults and one in ten children in the United States [1–3]. Moreover, the prevalence of this condition in certain high risk groups is extremely high reaching 90% in severely obese individuals undergoing bariatric surgery and 70% in patients with type 2 diabetes [4, 5]. The term NAFLD includes a spectrum of diseases ranging from nonalcoholic fatty liver (NAFL), which is considered a benign form of the disease with low risk of progression, to the aggressive form of nonalcoholic steatohepatitis (NASH) which can progress to fibrosis and eventually cirrhosis [6]. Indeed, recent data have shown that NASH is the third most common indication for liver transplantation in adults in the United States and is expected to become the leading indication over the next 1–2 decades [7].
Despite its high prevalence and potential for progression to end-stage liver disease, current practice guidelines do not support screening for NAFLD in adults attending primary care clinics or high-risk groups attending diabetes or obesity clinics due to uncertainties surrounding diagnostic tests and treatment options [8]. However, with the development of new reliable methods to quantify liver steatosis [9, 10] and the rapid pace for drug discovery of new therapeutic agents to treat NASH and fibrosis [11], it is anticipated that screening for NAFLD in high risk populations will become the standard of care in the near future. This will lead to the identification of a larger number of subjects with NAFLD requiring further identification of those with NASH and liver fibrosis to be targeted by intensive lifestyle modifications and new drugs.
In this review we will discuss biomarkers, predictive models, and imaging studies that can help with identifying those high-risk patients with NASH and fibrosis within the spectrum of NAFLD.
2. Noninvasive Assessment of Nonalcoholic Steatohepatitis (NASH)
NASH is a serious condition that can progress to cirrhosis and its complications including portal hypertension and hepatocellular carcinoma [6, 12, 13]. Cirrhosis develops in 21% to 28 % of NASH patients compared to only 3% of patients with NAFL [6]. Indeed, a recent meta-analysis demonstrated that compared to NAFL, the hazard ratio for liver-related mortality was 5.7 times higher for patients with NASH [14] indicating that the natural history of NAFLD-related liver morbidity and mortality depends on the histological severity as determined by the presence of NASH. It is important for the clinician to realize that neither liver enzymes nor currently used imaging studies can accurately predict the presence of NASH.
2.1. Biomarkers of NASH
2.1.1. Biomarkers of Hepatocyte Apoptosis
Increased hepatocyte apoptosis in the liver is a central mechanism that contributes to disease progression to NASH and the development of fibrosis [15]. Apoptosis occurs via two pathways: extrinsic mediated by death receptors such as Fas and intrinsic mediated by mitochondrial damage. Both pathways can eventually lead to the activation of caspase 3 which cleaves different intracellular substrates including the intermediate filament protein cytokeratin 18 (CK18). CK18 fragments that are generated by caspase 3 can be measured in the serum using the M30 antigen-monoclonal antibody ELISA and are significantly higher in patients with NASH compared to those with NAFL [16, 17]. Serum concentration of CK18 fragments as a noninvasive marker of the presence of NASH has been extensively validated in multiple studies with a pooled area under the receiver operating characteristic curve (AUROC) of 0.82 [95% confidence interval (CI) 0.76–0.88] [14] and has been recognized as the most promising single noninvasive test for this purpose by the AASLD guidelines for the diagnosis and management of NAFLD [8]. However, it should be noted that this assay is not commercially available yet and that there is no well-established CK18 fragment cutoff value for identifying NASH because each study utilized a study-specific cutoff value. More recently, our group has shown that circulating CK18 fragment levels correlated with the presence of NASH and its individual histologic features in a large cohort of children with biopsy-proven NAFLD[18]. Importantly, serum CK18 fragment levels decreased significantly with improvement in liver histologies in response to treatment in two large randomized controlled trials that included both adults and children [19]. These findings indicate that serum CK18 fragments could become an attractive biomarker for monitoring response to different therapeutic agents [20]. Other cell death biomarkers that have been evaluated to diagnose NASH include uncleaved CK18 (released from hepatocyte during both necrosis and apoptosis and measured using the M65 antigen) and soluble Fas and Fas ligand (markers of the extrinsic apoptosis pathway) [21–24]. However, the available data are limited and require further validation before integration into clinical practice.
2.1.2. Biomarkers of Oxidative Stress and Inflammation
Oxidative stress (OS) plays a central role in hepatocyte injury and disease progression to NASH [25–29], yet precise molecular species have not yet been identified. Several oxidation pathways contribute to lipid peroxidation in NASH including enzymatic and non-enzymatic free radical mediated processes. Each of these pathways generates different oxidation products that can be quantified. Chalasani et al. measured systemic lipid peroxidation in patients with biopsy-proven NASH and age-, gender-, and BMI-matched controls and showed that both oxidized LDL and thiobarbituric acid-reacting substances were significantly higher in the NASH group [30]. By using mass spectrometry approach, our group has demonstrated that products of free radical-mediated oxidation of linoleic acid (9- and 13-HODEs and 9- and 13-oxoODEs) measured in the plasma, were significantly elevated in patients with NASH compared to those with SS and normal liver biopsy [31]. Based on these findings, we developed the oxNASH score which is calculated from the ratio of 13-HODE to linoleic acid, age, BMI, and AST. Patients with oxNASH score > 72 were 10 times more likely to have NASH compared to those with oxNASH score < 47 (18) and the score correlated with each of the histological features that define NASH including steatosis, ballooning, and inflammation [32].
Levels of pro-inflammatory cytokines such as tumor necrosis factor α a (TNFα) and interleukin 6 [33, 34] have been shown to be higher in NASH compared to NAFL but the differences have not been significant enough to allow the use of these cytokines as noninvasive markers. Many other cytokines (IL-1B, macrophage inflammatory proteins) and adipokines (resistin, visfatin, retinol binding protein-4) have been studied as potential biomarkers with conflicting results. Blood neutrophil to lymphocyte (N/L) ratio is a simple indicator of the overall inflammatory status of the body that has been used to predict outcomes in patients with cancer. Our group studied N/L ratio as a noninvasive marker of NAFLD severity and demonstrated that this ratio was higher in patients with NASH compared to those with NAFL [35]. Recently, Kowdley et al. have demonstrated that elevated ferritin > 1.5 the upper limit of normal was associated with the diagnosis of NASH and advanced fibrosis in a large cohort of biopsy-proven NAFLD patients enrolled in the NASH Clinical Research Network [36].
2.2. Predictive Models of NASH
Multiple predictive models that combine routinely assessed clinical variables with laboratory tests and different biomarkers have been developed to predict the presence of NASH. Examples of predictive models that include the combination of clinical and laboratory data include the HAIR score [Hypertension, Aspartate aminotransferase (ALT), Insulin Resistance] [37] and the NASH predictive index or NPI which includes age, female gender, body mass index (BMI), homeostatic model assessment (HOMA) of insulin resistance, and log [aspartate aminotransferase (AST) × ALT] [38]. The accuracy of these models for predicting the presence of NASH is promising (AUROC of 0.87 to 0.90), but they lack external validation. The NASHTest was developed in a set of 160 patients using the combination of 13 clinical and biochemical variables including age, gender, weight, height, and serum levels of cholesterol, triglycerides, α2 macroglobulin, apolipoprotein A1, haptoglobin, gamma glutamyl transferase (GGT), ALT, AST and bilirubin [39]. The AUROC for diagnosing NASH as determined by liver biopsy was 0.78. The NASHTest has been validated in a cohort of 97 patients from different centers. Recent data from genome wide association studies have provided information on major genetic determinant of NAFLD and its severity, with the PNPLA3 genotype being a strong noninvasive biomarker that can predict the presence of histological NASH [40]. The PNPLA3 genotype was utilized to develop the “NASH score” which also includes insulin and ASLT levels and has shown good accuracy in two independent European cohorts [41]. Future work is needed to evaluate these predictive models in different populations and to establish their usefulness in predicting clinical outcomes and response to therapy before they can replace liver biopsy as the gold standard for diagnosing NASH.
2.3. Novel Approaches to Diagnosing NASH
Microparticles (MPs) are small extracellular vesicles that are released through controlled blebbing of the plasma membrane from activated or dying cells [42]. They are essential for cell-to-cell communications and carry signatures from the original cells including lipids, proteins, receptors, and RNAs. Importantly, MPs are released from the tissue of origin into the blood stream which makes ideal candidates as noninvasive biomarkers. Recent pilot studies demonstrated that patients with NAFLD had increased levels of MPs from macrophages/monocytes (CD14+) and invariant natural killer cells ant that the levels of these MPs correlated with serum ALT and the histologic severity of NASH [43]. It should be noted that MPs derived from inflammatory cells are not liver specific and could be elevated in other extrahepatic immune and inflammatory conditions. Therefore, we studies hepatocyte-derived MPs that are released in response to free-fatty acid induced lipotoxicity. By using a proteomic approach, we identified a potential signature in blood MPs that can discriminate between NASH and NAFL [44]. MPs released in NASH were associated with higher number of proteins involved in cell death, angiogenesis, and inflammation.
Another novel approach to diagnosing NASH is the analysis of volatile organic compounds (VOCs) in the exhaled breath. Breath testing is becoming an increasingly important noninvasive diagnostic method that can be used in the evaluation of health and disease states [45, 46]. More recent technological advancements in breath testing and analysis, such as gas and liquid chromatography and mass spectrometry, have made it possible to identify thousands of VOCs in the breath. Some of these compounds are considered as markers of oxidative stress and can indicate the presence of reactive oxygen species that are derived from peroxidation of polyunsaturated fatty acids [47]. A recent study by Verdam et al analyzed VOCs in the exhaled breath of 65 obese subjects undergoing bariatric surgery and liver biopsy [48]. They found that three VOCs (n-tridecane, 3-methyl-butanonitrile, and 1-propanol) were sufficient to distinguish patients with NASH and without NASH with AUROC of 0.77 (95% CI 0.64–0.89). Further work is needed to determine the exact origin of these VOCs and to validate these data in other groups of patients. Given its simplicity and safety, analysis of the exhaled breath could become a first line screening tool for NASH.
3. Noninvasive Diagnosis of Liver Fibrosis
NASH-associated fibrosis has different stages ranging from absent (stage F0) to cirrhosis (stage 4) with fibrosis stage F2–F4 considered clinically significant and fibrosis stages F3–F4 considered advanced fibrosis. When interpreting studies on noninvasive tests for hepatic fibrosis, it is important to determine their primary objective whether it is the identification of any fibrosis, clinically significant fibrosis, or advanced fibrosis. Risk factors that have been shown to predict the development of progressive fibrosis and cirrhosis include: older age, severe obesity, type 2 diabetes, elevated AST-to-ALT ratio, hypertension, dyslipidemia, and the presence of the metabolic syndrome, [37, 49, 50]. The stage of liver fibrosis is potentially the most important factor in determining the prognosis of NAFLD and predicting the risk of progression to cirrhosis and its complications [51]. In fact, two recent landmark studies have clearly established liver fibrosis as the strongest predictor of long-term outcomes in patients with NAFLD including liver-related and overall mortality [52, 53].
Therefore, many noninvasive strategies have been developed to predict the stage of liver fibrosis in this patient population. Non-radiological tests can be divided into simple bedside models using combination of clinical variables [50, 54] and more complex models that use serum markers of fibrosis such as the enhanced liver fibrosis (ELF) test [55]. Imaging studies are based on the idea of measuring liver stiffness to assess for the presence of liver fibrosis.
3.1. Simple Predictive Models for Fibrosis
The AST-to-ALT ratio (AAR) is the simplest predictive model for fibrosis. ALT is typically higher than AST in NAFLD; however, having an AAR > 1 is suggestive of the presence of advanced fibrosis. AAR has a good negative predictive value to rule out advanced fibrosis [56] as shown in Table 1. The BARD score is derived from the weighted sum of 3 variables (BMI ≥ 28 = 1 point, AST-to-ALT Ratio ≥ 0.8 = 2 points, the presence of Diabetes = 1 point) with scores of two or more being associated with advanced fibrosis (AUROC ranging from 0.70 to 0.81) [54, 56, 57].
Table 1.
The Use of Simple Predictive Models to Rule out the Presence of Advanced Fibrosis
| Model | Calculation Method | Cut-Off | NPV |
|---|---|---|---|
| AAR | AST/ALT | < 0.8 | 93% |
| BARD Score | Weighted sum of BMI ≥ 28 = 1 point, AAR ≥ 0.8 = 2 points, Diabetes = 1 point |
< 2 | 95% |
| FIB4 Index | http://gihep.com/calculators/hepatology/fibrosis-4-score/ | < 1.30 | 95% |
| NFS | http://nafldscore.com/ | < −1.455 | 92% |
AAR, AST-to-ALT ratio; NFS, NAFLD fibrosis score, NPV, negative predictive value.
Reference: McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–1269.
The FIB4 index includes age, platelet count, ALT and AST [58]. It can be calculated using the following online calculator: http://gihep.com/calculators/hepatology/fibrosis-4-score/. Although the FIB4 index was originally developed to stage liver fibrosis in patients with hepatitis C infection, it has shown promising results in patients with NAFLD with a cut-off value of < 1.3 having a negative predictive value of 90–95% for ruling out advanced fibrosis [56, 57].
Perhaps the most validated score to date is the NAFLD fibrosis score (NFS) which was developed by Angulo et al in a large cohort of patients with NAFLD confirmed by biopsy to predict advanced fibrosis [59]. NFS includes age, impaired fasting glucose/diabetes, BMI, platelets, albumin and AST-to-ALT ratio with two cut-off values: < − 1.455 to predict the absence of advanced fibrosis (F0–F2) and > 0.675 to predict the presence of advanced fibrosis (F3–F4). This score has been validated in multiple studies with an estimated AUROC of 0.85 (95% CI 0.81–0.90) [14] and has been acknowledged by the current NAFLD guidelines as a clinically useful tool for identifying advanced fibrosis in NAFLD patients [8] (http://nafldscore.com/). Another advantage of the NFS is its ability to provide prognostic information and identify patients with NAFLD who are at increased risk for liver-related complications (such as ascites and gastroesophageal varices) or death [60]. A major limitation of this score is that a significant percentage (20–58%) of patients fall between the two proposed cutoff values and will have an indeterminate score. Overall, these simple predictive models perform best at excluding advanced fibrosis/cirrhosis and could be used as a first line test to identify individuals at low risk for advanced disease [61].
3.2. Complex Predictive Models for Fibrosis
The European Liver Fibrosis (ELF) panel was developed based on the concept that liver fibrosis is a dynamic process that result in increased serum levels of extra-cellular matrix turnover markers. ELF includes three biomarkers of fibrosis: hyaluronic acid, tissue inhibitor of metalloproteinase 1, and aminoterminal peptide of procollagen III with an excellent performance for predicting advanced fibrosis [AUROC of 0.90 (95%CI 0.84–0.96)] [55]. Similar to the NFS, the ELF panel has been shown to be a good predictor of clinical outcomes (liver-related morbidity/liver-related death) in a group of patients with chronic liver disease including those with NAFLD making it a promising prognostic tool [62].
The FibroTest is another panel that predicts the presence of fibrosis by using five biomarkers (haptoglobin, α2-macroglobulin, apolipoprotein A1, total bilirubin, and GGT). The diagnostic value of FibroTest was assessed in a large cohort of NAFLD patients and demonstrated that it can reliably predict advanced fibrosis with an AUROC of 0.88 (95% CI 0.82–0.92) [63]. However, caution should be exercised when interpreting the results of this test in those with Gilbert’s syndrome, cholestasis and acute inflammation as these conditions will increase bilirubin and haptoglobin, respectively. Fibrotest is available commercially in the United States as part of the NASH-FibroSURE® (LabCorp, NC, USA).
3.3. Imaging Studies for Fibrosis
Over the past decade, advances in imaging studies have revolutionized the management of liver disease by enhancing our ability to noninvasively quantify liver fibrosis. These studies estimate liver stiffness measurement (LSM), or elastography, by creating an elastic shear wave through liver tissue and then measuring its velocity which is directly proportional to tissue stiffness. Vibration controlled transient elastography (VCTE) or FibroScan ® (EchoSens, Paris, France) was the first imaging technology to make it to the hepatology clinic as a simple point of care way to assess hepatic fibrosis. The clinician induces a mild amplitude and low frequency shear wave into liver tissue from a small mechanical vibrator at the end of the FibroScan probe (Figure 1). VCTE evaluates a representative volume of the liver that is 100-fold greater than needle biopsy and the LSM is expressed in kilopascals (kPa) with values > 10.5 kPa being consistent with the presence of advanced fibrosis/cirrhosis [64]. Typically, 10 successful VCTE measurements with a median interquartile range/median ration of less than 30% are needed to have a reliable LSM. Unfortunately, the VCTE regular probe or the M probe is less reliable in severely obese patients with NAFLD given the effect of BMI on its performance [65]. Therefore, a new probe called the XL probe was developed to overcome this issue in patients with BMI > 30 kg/m2 with the explored region of interest being deeper from the skin surface to decrease the effect of thick subcutaneous fat. It is important to note that the cutoff values to diagnose advanced fibrosis for the XL probe may be lower than those for the M probe. Another important issue to be aware of is the risk of overestimating liver stiffness with VCTE due to other confounding factors such as congestive heart failure, extrahepatic cholestasis, ALT flares, and recent food intake [66].
Figure 1. Different Imaging Studies for Liver Fibrosis in Patients with NAFLD.
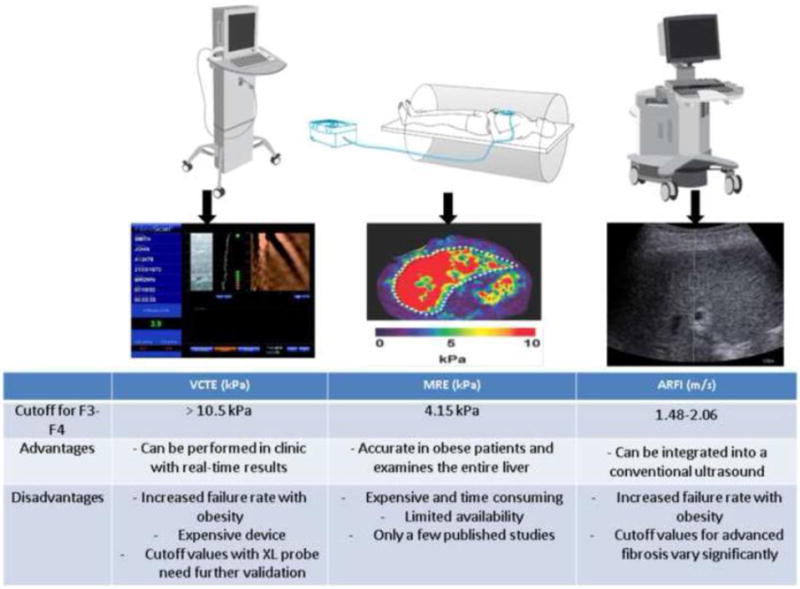
VCTE, Vibration controlled transient elastography; MRE, Magnetic resonance elastography; ARFI, Acoustic radiation force impulse.
An alternative modality to estimate liver stiffness is acoustic radiation force impulse (ARFI) which can be integrated on a conventional ultrasound probe thus providing LSM during routine ultrasonography. While performing B-mode imaging, a region of interest in the liver is targeted to be mechanically excited using acoustic push pulses. Liver stiffness is expressed as shear wave velocity in meter per second (m/s) after calculating the median for 10 successful acquisitions. Several studies have demonstrated similar diagnostic performance of ARFI to VCTE [67, 68]; however, the cutoff values for ARFI to diagnose different fibrotic stages vary significantly ranging from 1.48–2.06 m/s for predicting advanced fibrosis [69].
Magnetic resonance elastography (MRE) is another useful noninvasive modality to diagnose fibrosis in patients with NAFLD. The device is composed of an active acoustic driver system located outside the magnet room that produces low frequency vibrations that are transmitted to a drum-like acoustic passive driver positioned over the liver (Figure 1). Only a few studies have been published on the utility of MRE in NAFLD and further studies are needed to determine the cutoff values to be used to predict different fibrosis stages. In a retrospective study that included 142 NAFLD patients who underwent liver biopsy within 1 year of MRE, Kim et al. showed that the best cutoff for advanced fibrosis was 4.15 kPa (AUROC = 0.954, sensitivity = 0.85, specificity = 0.929) [70]. In a recent prospective study by Loomba et al, MRE showed promising results for discriminating advanced fibrosis (F3–F4) from stage 0–2 fibrosis with an AUROC of 0.924 [71]. A cutoff value of > 3.63 kPa provided a sensitivity of 86%, NPV of 97%, specificity of 91%, and PPV of 68%. A recent study demonstrated the superiority of MRI in comparison to simple predictive model for diagnosing advanced fibrosis in patients with biopsy proven NAFLD [72]. More importantly, data on the potential for magnetic resonance imaging to predict liver-related complications are emerging [73], which may make the staging of liver fibrosis with biopsy obsolete.
4. Conclusion and Perspectives
Accurate noninvasive diagnosis of NASH and advanced fibrosis within the spectrum of NAFLD is of utmost importance to identify patients who are likely to develop liver-related morbidity and mortality. Despite the growing understanding of pathophysiologic mechanisms involved in disease progression to NASH and the discovery of several mechanism-based biomarkers, we still lack a validated non-invasive test that can accurately predict the presence of NASH. Novel diagnostic tests for NASH such as MPs and breath testing for VOCs are promising. On the other hand, recent advances in serology-based predictive models and imaging studies now allow clinicians to diagnose the stage of fibrosis in patients with NAFLD. Our approach in the hepatology clinic is to use a combination of the NFS and VCTE to determine the presence of advanced fibrosis as illustrated in figure 2. This approach has been validated by Petta and colleagues in two separate Italian cohorts that included 321 patients yielding a 0% false-positive rate and 7.3% false-negative rate [74]. More recently, Tapper et al. demonstrated the cost-effectiveness of this approach compared to the current standard of liver biopsy [75]. We envision a future where liver biopsy becomes obsolete for the purpose of determining the severity of NAFLD and clinicians can rely solely on noninvasive tests to determine disease progression and response to novel therapeutic options.
Figure 2. Algorithm to Diagnose Advanced Fibrosis in NAFLD.
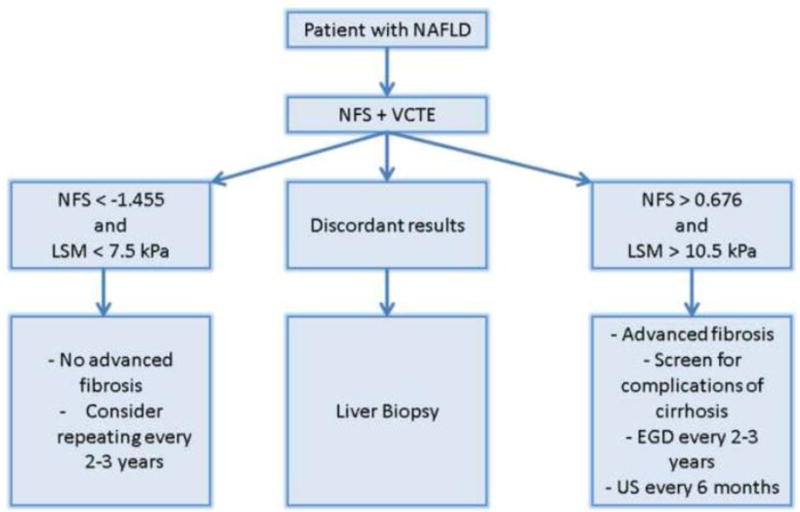
The algorithm is based on using the combination of liver stiffness measurement (LSM) by vibration controlled transient elastography (VCTE) plus the NAFLD fibrosis score (NFS). Having concordant low values for both LSM and NFS indicates the absence of advanced fibrosis and both tests can be repeated in 2–3 years. Having concordant high values for both tests indicates the presence of advanced fibrosis and the need to screen for cirrhosis complications including hepatocellular carcinoma and varices. Having discordant results indicates the need for liver biopsy to determine the fibrosis stage.
Highlights.
Nonalcoholic fatty liver disease (NAFLD) is the dominant liver disease in the USA.
Nonalcoholic steatohepatitis (NASH) is the aggressive form of NAFLD.
Liver biopsy remains the gold standard to diagnose NASH and liver fibrosis.
Biomarkers of hepatocyte apoptosis and inflammation are used to diagnose NASH.
Predictive models and imaging studies that measure liver stiffness can stage fibrosis.
Acknowledgments
Funding: Supported by the ACG Junior Faculty Development Award to NA and by NIH grants DK076852 and (DK082451) to AEF.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NAFL
nonalcoholic fatty liver
- NASH
nonalcoholic steatohepatitis
- CK 18
Cytokeratin 18
- OS
Oxidative stress
- AUROC
area under the receiver operating characteristic curve
- ALT
alanine aminotransferase
- BMI
body mass index
- ALT
aspartate aminotransferase
- GGT
gamma glutamyl transferase
- VOCs
volatile organic compounds
- AAR
AST-to-ALT ratio
- NFS
NAFLD fibrosis score
- VCTE
vibration controlled transient elastography
- ARFI
acoustic radiation force impulse
- MRE
magnetic resonance elastography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: AEF reports that he is named as co-inventor on pending and issued patents filed by the Cleveland Clinic and UCSD that refer to the use of biomarkers in fatty liver disorders. NA has no conflict of interest.
Contributions of authors: NA and AEF drafted the article and revised it critically for important intellectual content.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Boza C, Riquelme A, Ibanez L, Duarte I, Norero E, Viviani P, et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 2005;15(8):1148–53. doi: 10.1381/0960892055002347. [DOI] [PubMed] [Google Scholar]
- 5.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Alimentary pharmacology & therapeutics. 2011;34(3):274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 7.Charlton M. Evolving aspects of liver transplantation for nonalcoholic steatohepatitis. Curr Opin Organ Transplant. 2013;18(3):251–8. doi: 10.1097/MOT.0b013e3283615d30. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 9.de Ledinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. Journal of hepatology. 2014;60(5):1026–31. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. Journal of magnetic resonance imaging : JMRI. 2011;34(4):729–49. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 14.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of nonalcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 15.Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert review of gastroenterology & hepatology. 2011;5(2):201–12. doi: 10.1586/egh.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–8. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44(1):27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 18.Feldstein AE, Alkhouri N, De Vito R, Alisi A, Lopez R, Nobili V. Serum Cytokeratin-18 Fragment Levels Are Useful Biomarkers for Nonalcoholic Steatohepatitis in Children. The American journal of gastroenterology. 2013 doi: 10.1038/ajg.2013.168. [DOI] [PubMed] [Google Scholar]
- 19.Vuppalanchi R, Jain AK, Deppe R, Yates K, Comerford M, Masuoka HC, et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(12):2121-30 e1–2. doi: 10.1016/j.cgh.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L, American Association for the Study of Liver D, United States F et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61(4):1392–405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkhouri N, Alisi A, Okwu V, Matloob A, Ferrari F, Crudele A, et al. Circulating Soluble Fas and Fas Ligand Levels Are Elevated in Children with Nonalcoholic Steatohepatitis. Digestive diseases and sciences. 2015;60(8):2353–9. doi: 10.1007/s10620-015-3614-z. [DOI] [PubMed] [Google Scholar]
- 22.Tamimi TI, Elgouhari HM, Alkhouri N, Yerian LM, Berk MP, Lopez R, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. Journal of hepatology. 2011;54(6):1224–9. doi: 10.1016/j.jhep.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz Y, Dolar E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, et al. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World journal of gastroenterology : WJG. 2007;13(6):837–44. doi: 10.3748/wjg.v13.i6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younossi ZM, Page S, Rafiq N, Birerdinc A, Stepanova M, Hossain N, et al. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg. 2011;21(4):431–9. doi: 10.1007/s11695-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 25.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16(5):663–78. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira CP, da Costa Gayotto LC, Tatai C, Della Bina BI, Janiszewski M, Lima ES, et al. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J Cell Mol Med. 2002;6(3):399–406. doi: 10.1111/j.1582-4934.2002.tb00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163(4):1301–11. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med. 2004;37(9):1499–507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 29.Gao D, Wei C, Chen L, Huang J, Yang S, Diehl AM. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287(5):G1070–7. doi: 10.1152/ajpgi.00228.2004. [DOI] [PubMed] [Google Scholar]
- 30.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. The American journal of gastroenterology. 2004;99(8):1497–502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 31.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51(10):3046–54. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkhouri N, Berk M, Yerian L, Lopez R, Chung YM, Zhang R, et al. OxNASH score correlates with histologic features and severity of nonalcoholic fatty liver disease. Digestive diseases and sciences. 2014;59(7):1617–24. doi: 10.1007/s10620-014-3031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, et al. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver international : official journal of the International Association for the Study of the Liver. 2006;26(1):39–45. doi: 10.1111/j.1478-3231.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 34.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. The American journal of gastroenterology. 2008;103(6):1372–9. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 35.Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2011 doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 36.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(1):77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 38.Zein CO, Edmison JM, Schluchter M, Feldstein AE, Zein NN, McCullough A. A NASH Predictive Index (NPI) for use in patients with nonalcoholic fatty liver disease [abstract] Hepatology. 2007;46(4):747A. [Google Scholar]
- 39.Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dongiovanni P, Valenti L. Genetics of nonalcoholic fatty liver disease. Metabolism. 2015 doi: 10.1016/j.metabol.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Hyysalo J, Mannisto VT, Zhou Y, Arola J, Karja V, Leivonen M, et al. A population-based study on the prevalence of NASH using scores validated against liver histology. Journal of hepatology. 2014;60(4):839–46. doi: 10.1016/j.jhep.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143(2):448–58. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PloS one. 2014;9(12):e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mashir A, Dweik RA. Exhaled breath analysis: The new interface between medicine and engineering. Adv Powder Technol. 2009;20(5):420–5. doi: 10.1016/j.apt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paschke KM, Mashir A, Dweik RA. Clinical applications of breath testing. F1000 Med Rep. 2010;2:56. doi: 10.3410/M2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paredi P, Kharitonov SA, Barnes PJ. Analysis of expired air for oxidation products. Am J Respir Crit Care Med. 2002;166(12 Pt 2):S31–7. doi: 10.1164/rccm.2206012. [DOI] [PubMed] [Google Scholar]
- 48.Verdam FJ, Dallinga JW, Driessen A, de Jonge C, Moonen EJ, van Berkel JB, et al. Nonalcoholic steatohepatitis: a non-invasive diagnosis by analysis of exhaled breath. Journal of hepatology. 2013;58(3):543–8. doi: 10.1016/j.jhep.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 49.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30(6):1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 50.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118(6):1117–23. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 51.Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51(2):373–5. doi: 10.1002/hep.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Associates With Long-Term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 54.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441–7. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 55.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47(2):455–60. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 56.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–9. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 57.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(10):1104–12. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 59.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 60.Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782–9 e4. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castera L. Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35(3):291–303. doi: 10.1055/s-0035-1562948. [DOI] [PubMed] [Google Scholar]
- 62.Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59(9):1245–51. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 63.Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilder J, Patel K. The clinical utility of FibroScan((R)) as a noninvasive diagnostic test for liver disease. Med Devices (Auckl) 2014;7:107–14. doi: 10.2147/MDER.S46943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonder A, Tapper EB, Afdhal NH. Contemporary assessment of hepatic fibrosis. Clin Liver Dis. 2015;19(1):123–34. doi: 10.1016/j.cld.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 66.European Association for Study of L, Asociacion Latinoamericana para el Estudio del H. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. Journal of hepatology. 2015;63(1):237–64. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Ebinuma H, Saito H, Komuta M, Ojiro K, Wakabayashi K, Usui S, et al. Evaluation of liver fibrosis by transient elastography using acoustic radiation force impulse: comparison with Fibroscan((R)) J Gastroenterol. 2011;46(10):1238–48. doi: 10.1007/s00535-011-0437-3. [DOI] [PubMed] [Google Scholar]
- 68.Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, et al. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81(3):e325–31. doi: 10.1016/j.ejrad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 69.Yoshioka K, Hashimoto S, Kawabe N. Measurement of liver stiffness as a non-invasive method for diagnosis of non-alcoholic fatty liver disease. Hepatol Res. 2015;45(2):142–51. doi: 10.1111/hepr.12388. [DOI] [PubMed] [Google Scholar]
- 70.Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268(2):411–9. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loomba R, Wolfson T, Ang B, Booker J, Behling C, Peterson M, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: A prospective study. Hepatology. 2014 doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui J, Ang B, Haufe W, Hernandez C, Verna EC, Sirlin CB, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for noninvasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Alimentary pharmacology & therapeutics. 2015;41(12):1271–80. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pavlides M, Banerjee R, Sellwood J, Kelly CJ, Robson MD, Booth JC, et al. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. Journal of hepatology. 2015 doi: 10.1016/j.jhep.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petta S, Vanni E, Bugianesi E, Di Marco V, Camma C, Cabibi D, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver international : official journal of the International Association for the Study of the Liver. 2015;35(5):1566–73. doi: 10.1111/liv.12584. [DOI] [PubMed] [Google Scholar]
- 75.Tapper EB, Sengupta N, Hunink MG, Afdhal NH, Lai M. Cost-Effective Evaluation of Nonalcoholic Fatty Liver Disease With NAFLD Fibrosis Score and Vibration Controlled Transient Elastography. The American journal of gastroenterology. 2015;110(9):1298–304. doi: 10.1038/ajg.2015.241. [DOI] [PubMed] [Google Scholar]


