Abstract
Objective
To compare the effectiveness of different approaches to nutrition education in diabetes self-management education and support (DSME/S).
Methods
We randomized 150 adults with type 2 diabetes to either certified diabetes educator (CDE)-delivered DSME/S with carbohydrate gram counting or the modified plate method versus general health education. The primary outcome was change in HbA1C over 6 months.
Results
At 6 months, HbA1C improved within the plate method [−0.83% (−1.29, −0.33), P<0.001] and carbohydrate counting groups [−0.63% (−1.03, −0.18), P=0.04] but not the control group [P = 0.34]. Change in HbA1C from baseline between the control and intervention groups was not significant at 6 months (carbohydrate counting, P=0.36; modified plate method, P=0.08). In a pre-specified subgroup analysis of patients with a baseline HbA1C 7–10%, change in HbA1C from baseline improved in the carbohydrate counting [−0.86% (−1.47, −0.26), P=0.006] and plate method groups [−0.76% (−1.33, −0.19), P=0.01] compared to controls.
Conclusion
CDE-delivered DSME/S focused on carbohydrate counting or the modified plate method improved glycemic control in patients with an initial HbA1C between 7–10%.
Practice Implications
Both carbohydrate counting and the modified plate method improve glycemic control as part of DSME/S.
Key terms: diabetes education, nutrition, numeracy, type 2 diabetes
1. Introduction
Successful diabetes management requires daily self-management activities [1–3]. Diabetes self-management education and support (DSME/S), which is the ongoing process of facilitating the knowledge, skill, and abilities necessary for diabetes self-care [2], provides the foundation upon which patients and providers build individualized, patient-centered diabetes care plans [4]. Because nearly all diabetes care is performed by patients outside of a healthcare setting [5], patient-centered approaches to diabetes care that empower and equip patients to take responsibility for managing their diabetes are critical [6].
Although DSME/S improves diabetes knowledge, self-care behaviors, quality of life, and glycemic control [7–11], approaches to DSME/S vary widely. Nutrition counseling is a critical component of DSME/S [12] and improves glycemic control similar to many glucose-lowering medications [13]. DSME/S allows for multiple approaches to carbohydrate monitoring, including gram counting, exchanges, and experience-based estimation [14]. However, the comparative effectiveness of these approaches and the characteristics of patients who benefit from each approach are unknown. Although carbohydrate gram counting may improve the accuracy of carbohydrate monitoring and allow dietary flexibility, it is computationally intensive and may intimidate some patients [15]. The modified plate method [16] – which divides serving plates into sections designated for specific food types and uses cups and bowls to assist with measurement – may be easier to learn and implement.
Individualization of DSME/S based on an individual’s cultural preferences, health beliefs, psychosocial status, self-management skills, literacy, and numeracy skills is important to facilitating behavior change [2, 17]. Health literacy and numeracy are associated with diabetes knowledge, self-care, and glycemic control, and addressing literacy and numeracy can improve glycemic control [18, 19]. Individualizing nutrition education according to literacy and numeracy skills may be especially important because individuals with low health literacy and numeracy have difficulty understanding food labels and estimating portion sizes [20, 21].
This study was designed to evaluate the role of two approaches to nutrition education as part of DSME/S and evaluate the impact of certified diabetes educator (CDE)-delivered DSME/S on glycemic control in patients with type 2 diabetes. We hypothesized that patients receiving CDE–delivered DSME/S including a nutrition focus on either carbohydrate counting or the modified plate method would have improved glycemic outcomes compared with those in an attention control group. Furthermore, we hypothesized that patients with lower diabetes numeracy would have greater improvements in glycated hemoglobin (HbA1C) with the modified plate method approach than with carbohydrate counting.
2. Research Design and Methods
2.1 Design, Setting, and Participants
The Diabetes Nutrition Education Study (DINES) was a three-arm, randomized controlled trial designed to examine the impact of CDE-delivered DSME/S and the role of different approaches to nutrition education on glycemic control in patients with type 2 diabetes. We also examined the interaction between diabetes numeracy and nutrition education on glycemic control. Participants were recruited from the Vanderbilt Primary Care Clinics, the Vanderbilt Heart Institute, and the Nashville Veterans Affairs Medical Center via patient flyers and direct recruitment. Eligible patients were English speaking adults age 18–85 with type 2 diabetes, a most recent HbA1C ≥7.0% (53 mmol/mol) indicating uncontrolled diabetes, and no formal diabetes or nutrition education in the past year. Patients actively counting carbohydrate grams or using a plate method were excluded. Additional exclusion criteria included: use of flexible dose insulin based on carbohydrate gram counting, poor visual acuity, dementia or psychosis, and life expectancy less than 1 year. All participants provided written informed consent, and study procedures were approved by the Vanderbilt Institutional Review Board. Participants received $50 as compensation.
One hundred fifty patients were enrolled and randomized using computer-generated random number assignment (Stata 9.0; StataCorp LP, College Station, TX) [22]. The allocation sequence was concealed from the research assistant responsible for recruitment and consent. Following randomization, group assignment was revealed to the patient and research team.
2.2 Intervention
All patients received usual diabetes care from their primary care provider throughout the study. Each patient also received three 30–60 minute face-to-face education visits over a 3 month period, with the focus of the education differing by study assignment. Patients randomized to the attention control group received general health education visits with a health educator focusing on fall prevention, vaccinations, osteoporosis, and oral hygiene. Patients randomized to the two intervention arms received individualized DSME/S based on current standards [8] from one of two clinic-based registered dietician-certified diabetes educators (RD-CDEs). One group focused on carbohydrate gram counting with instruction on reading food labels, correct serving sizes, using online carbohydrate-counting resources, and negotiated individualized carbohydrate gram goals. The other group focused on a modified plate method approach. The plate method [16, 23, 24] was originally developed in Sweden to teach meal planning to patients with diabetes and later modified to meet American Diabetes Association nutritional guidelines. The plate method provides patients with sized plates (9-inch) and bowls (4 and 8 ounces) and instructs them on how much of different types of foods they can consume. For lunch and dinner, patients are typically instructed that they can use ½ the plate “free” foods such as low-carbohydrate, non-starchy vegetables, ¼ of the plate for their protein such as meat or eggs, and ¼ of the plate for carbohydrates. This approach allows patients to restrict their carbohydrate intake without calculating specific portions with measuring cups, food labels, or other devices. Since the traditional plate method [16, 23, 24] can vary widely in the amount of carbohydrate consumed, we provided patients with the carbohydrate content of common foods and appropriate portion sizes to improve the precision of carbohydrate intake. We also marked individualized portion-sizes of carbohydrates on plates (9-inch), bowls, and cups (4 ounce and 8 ounce) to assist in meal preparation. Portions of high carbohydrate and starch containing foods were negotiated with patients and individualized based on blood glucose readings. All DSME/S utilized literacy and numeracy sensitive educational materials from the Diabetes Literacy and Numeracy Education Toolkit (https://www.mc.vanderbilt.edu/root/vumc.php?site=CDTR&doc=37816) [19, 25].
2.3 Measures and Outcomes
Patient characteristics were collected at enrollment and follow-up visits by self-report and review of electronic medical records by trained research assistants. Health literacy and numeracy were assessed using the Rapid Estimation of Adult Literacy in Medicine (REALM) [26, 27] and the 15-item Diabetes Numeracy Test (DNT) [28]. HbA1C was measured at enrollment, 3 and 6 months follow-up. At each time point, HbA1C values collected during routine clinical care were accepted if available in the electronic medical record. If HbA1C was not available, testing was obtained through the Vanderbilt University General Clinical Research Center. Additional outcomes included: weight, treatment satisfaction assessed with the Diabetes Treatment Satisfaction Questionnaire (DTSQ) [29], and self-efficacy assessed with the Perceived Diabetes Self-Management Scale (PDSMS) [30]. The validated DTSQ includes 8 items, with 6 of the items scored for treatment satisfaction. Each item is scored on a 0–6 scale with a total score of 0–36. Previous studies that modified insulin regimens suggest that a 1–2 point improvement in DTSQ score may be clinically important [31]. The PDSMS scale includes 8 items that are each scored from 1–5, giving a total score of 8–40. Higher scores are correlated with improved glycemic control as measured by A1C (r=−0.30, p<0.001) [30]. In a previous intervention study, we found that a health literacy focused intervention increased self-efficacy by 5 points at 6 month follow-up, compared with a 2 point increase in an active control group [19].
2.4 Statistical Analyses
Baseline measures were reported as proportions for categorical variables and medians and interquartile ranges for continuous and ordinal variables. Baseline characteristics were compared using Wilcoxon rank-sum, Kruskal-Wallis, and Pearson Chi-square tests, as appropriate. All enrolled patients and data were analyzed using the intention to treat principle.
The primary outcome was change in HbA1C at 6 months, which was 3 months after completion of the intervention, between the control group and each of the intervention groups (modified plate method or carbohydrate counting). To examine CDE-delivered care overall, we also analyzed change in HbA1C by combining the two intervention groups. Secondary outcomes included the change in patient weight, self-efficacy, and treatment satisfaction at 6 months between the control and intervention groups.
For unadjusted within-group comparisons, linear or proportional odds (PO) logistic regression was used depending on the normality of linear regression residuals. Although, we compared baseline to 3 months and baseline to 6 months separately, the analysis was performed from a single regression model that included observations from all 3 time points. For linear regression we reported model based change and 95% confidence intervals. For PO logistic regression, we report mean change and 95% bootstrapped confidence intervals.
Adjusted between-group comparisons were performed using linear regression models. All models adjusted for age, gender, race, income, duration of diabetes, baseline value of the corresponding outcome variable, and an interaction term of treatment arm and time (3 or 6 months). Age and duration of diabetes were modeled as non-linear terms using restricted cubic splines with three knots [32]. Multivariable regression analysis was performed with inclusion of cross product terms between treatment arms and time (both treated as categorical variables) with adjustment for the above listed covariates. For HbA1C, analyses were performed with and without log transformations and results were similar, so we report analyses without transformation. Proportional odds logistic regression and linear regression were used to analyze self-efficacy and treatment satisfaction. Results were similar, so results of linear regression analyses are presented for ease of understanding. We also conducted a pre-specified subgroup analysis of patients with a baseline HbA1C 7.0–10% (53–86 mmol/mol) to examine outcomes in patients most likely to benefit from education interventions [13].
In additional pre-specified analyses, we explored the effect modification of the patient’s numeracy skill level on change in HbA1C. In unadjusted analyses, we stratified numeracy into low [DNT score in the lowest quartile of the distribution (score <47%)] and high (DNT score≥47%) [18] numeracy groups and examined the within-group change in HbA1C between baseline and 6 months using paired t-tests. We used linear regression to adjust for baseline HbA1C, treatment arm (plate method, carbohydrate counting, and control), study time point (3 months, and/or 6 months), numeracy score (as a continuous variable), and a three-way interaction term of treatment arm, time, and numeracy.
For all regression analyses, the Huber White robust sandwich variance-covariance estimator was used to account for repeated observations. Two-sided p-values of 0.05 or less were considered statistically significant for all findings. Statistical analyses were performed with R statistical software version 2.15 (http://www.r-project.org).
2.5 Sample Size Analysis
The study was designed with 80% power to detect a mean 1.0% difference in HbA1C with standard deviation of 1.5% between each intervention group and control at 6 months. Power calculations were based on a sample size of 44 participants per arm completing the study with Bonferroni adjusted 2-sided significance level of 0.025.
3. Results
3.1. Participant Characteristics
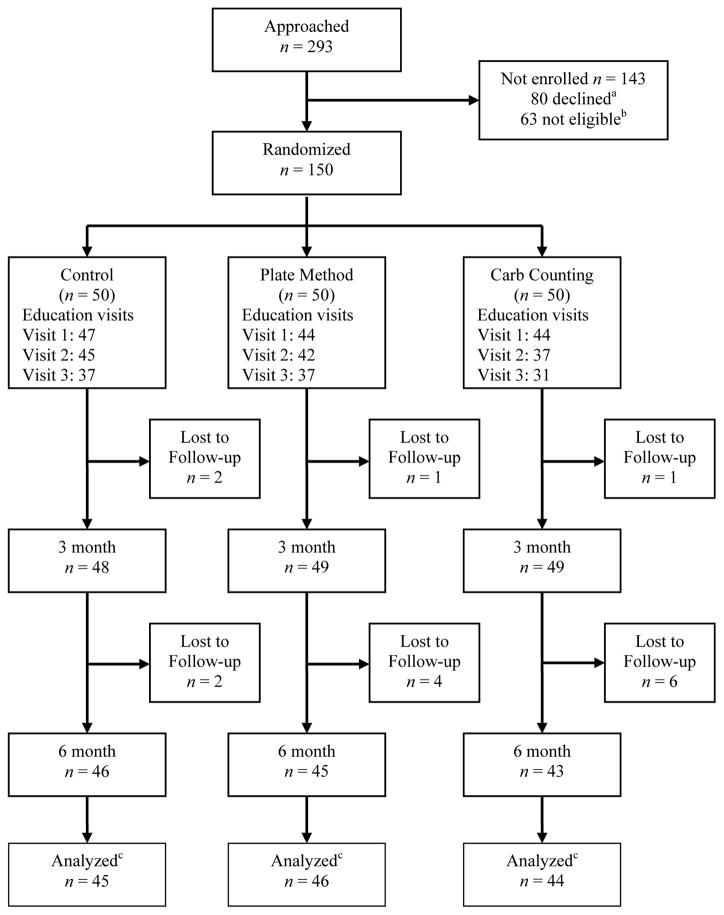
A total of 293 patients were approached for enrollment between May 2008 and March 2010 of whom 230 met eligibility criteria. Eighty individuals declined participation (Figure 1). A total of 150 patients were enrolled and randomized; 135 participants had baseline and follow-up HbA1C data (at 3 and/or 6 months). The a priori subgroup analysis of participants with a baseline HbA1c 7.0–10.0% included 70% (n=105) of participants.
Figure 1. Study flow diagram.
aReasons for declining participation included time constraints (n = 33), lack of interest (n = 29), transportation (n = 12), and deferred participation (n = 9). Some individuals reported more than one reason for not participating.
bReasons for not meeting eligibility criteria (n = 63) included pre-enrollment HbA1C < 7.0% (53 mmol/mol) (n = 47), cognitive or visual impairment (n = 7), adjusting insulin for carbohydrate intake (n = 3), not established patients (n= 3), enrolled in diabetes education program (n = 2), type 1 diabetes (n = 1).
cPatients were included in analysis if they had a baseline and either 3 or 6 month HbA1C available
Table 1 summarizes patient characteristics, which were similar across study groups. Overall, patients were a median (interquartile range) of 55 (47, 62) years old with a BMI of 34 (30, 38) kg/m2. Patients were 53% female, with 34% being non-white race. Twenty-five percent of patients had less than a high school education, and 11% had less than a 9th grade literacy level. The median score on the DNT-15 was 67% (47%, 87%). The median duration of diabetes was 8 (3, 11) years, 58% of patients had previously received diabetes education, and 26% reported prior knowledge of carbohydrate grams. At baseline, 35% of patients were using insulin, and the median HbA1C was 8.2% (7.5%, 9.9%) [63 mmol/mol (58–73)].
Table 1.
Baseline characteristics of DINES participants
| Control | Carbohydrate Counting | Modified Plate Method | Combined CDEa | All Patients | Subgroup with HbA1C 7–10% | |
|---|---|---|---|---|---|---|
| N | 50 | 50 | 50 | 100 | 150 | 105 |
| Age, median (IQR), years | 57 (48, 62) | 54 (47, 68) | 55 (45, 60) | 54 (47, 62) | 55 (47, 62) | 58 (48, 66) |
| Female (%) | 52 | 62 | 46 | 54 | 53 | 51 |
| Non-white race (%) | 34 | 42 | 27 | 34 | 34 | 30 |
| Income <$20,000/year (%) | 22 | 22 | 26 | 24 | 23 | 20 |
| Education <12 years (%) | 22 | 18 | 36 | 27 | 25 | 23 |
| Private insurance (%) | 62 | 64 | 64 | 64 | 63 | 62 |
| REALMc score<9th grade level (%) | 10 | 8 | 14 | 11 | 11 | 9 |
| DNT-15d, median (IQR), (% correct) | 67 (47, 80) | 73 (40, 93) | 67 (40, 85) | 70 (40, 87) | 67 (47, 87) | 67 (47, 87) |
| Weight, median (IQR), pounds | 216 (180, 243) | 218 (190, 252) | 224 (189, 259) | 220 (189, 258) | 220 (188, 251) | 212 (183, 240) |
| BMI, median (IQR), (kg/m2) | 34 (30, 39) | 34 (30, 37) | 34 (30, 39) | 34 (30, 38) | 34 (30, 38) | 33 (29, 38) |
| Prior diabetes education (%) | 68 | 52 | 54 | 53 | 58 | 59 |
| Prior carb count education (%) | 24 | 22 | 32 | 27 | 26 | 29 |
| Current insulin use (%) | 34 | 28 | 42 | 35 | 35 | 30 |
| >1 insulin shot/day (%)b | 47 | 57 | 77 | 79 | 62 | 56 |
| Diabetes duration, median (IQR), years | 8 (3, 13) | 8 (4, 10) | 7 (3, 10) | 8 (3, 11) | 8 (3, 11) | 8 (4, 12) |
| HbA1C, median (IQR), % | 8.0 (7.5, 9.7) | 8.4 (7.6, 9.7) | 8.3 (7.5, 10.4) | 8.3 (7.6, 9.9) | 8.2 (7.5, 9.9) | 7.9 (7.5, 8.8) |
| Treatment satisfaction (DTSQ)e, median (IQR) | 26 (19, 31) | 24 (17, 30) | 22 (17, 29) | 23 (17, 29) | 24 (18, 30) | 24 (18, 30) |
| Self-efficacy (PDSMS)f, median (IQR) | 24 (21, 29) | 24 (18, 29) | 24 (21, 27) | 24 (20, 28) | 24 (20, 29) | 24 (21, 28) |
SI conversion factors: mmol/mol = [10.93* HbA1c (%)] − 23.50
p>0.05 for all comparisons in the control, carbohydrate counting, and modified plate method groups
CDE, certified diabetes educator. Combined CDE group represents combined carbohydrate counting and modified plate method groups.
Restricted to patients prescribed insulin (n = 53)
REALM, Rapid Estimation Adult Literacy in Medicine
DNT-15, Diabetes Numeracy Test-15
DTSQ, Diabetes Treatment Satisfaction Questionnaire (DTSQ), scoring range 0–36
PDSMS, Perceived Diabetes Self-Management Scale (PDSMS), scoring range 8–40
3.2 Glycemic control
The results of unadjusted within group comparisons are shown in Table 2. Immediately after completion of the intervention (3 months after enrollment), HbA1C decreased compared to baseline in all groups: carbohydrate counting: [−0.99% (−1.42, −0.56); −10.8 mmol/mol (−15.5, −6.1), P < 0.001]; modified plate method: [−1.04% (−1.52, −0.56); −11.4 mmol/mol (−16.6, −6.1), P < 0.001]; combined CDE: [−1.01% (−1.33, −0.69); −11.0 mmol/mol (−14.5, −7.5), P < 0.001]; and control: [−0.98% (−1.39, −0.57); −10.7 mmol/mol (−15.2, −6.2), P < 0.001]. At the 6 month follow-up, which was 3 months after completion of the intervention, the mean (95% CI) decrease in HbA1C compared to baseline remained significant in the carbohydrate [−0.45% (−0.88, −0.01); −4.9 mmol/mol (−9.6, −0.1), P = 0.04]), modified plate method [−1.13% (−1.65, −0.6); −12.4 mmol/mol (−18.0, −6.6), P < 0.001], and the combined CDE group [−0.79% (−1.13, −0.45); −8.6 mmol/mol (−12.4, −4.9), P < 0.001]. The decrease in the attention control group was no longer statistically significant [−0.25% (−0.78, 0.27); −2.7 mmol/mol (−8.5, 3.0), P = 0.34].
Table 2.
Within-group change in HbA1C, weight, treatment satisfaction, and self-efficacy
| Control | Carbohydrate Counting | Modified Plate Method | Combined CDEf | |
|---|---|---|---|---|
| Change in HbA1C, % | ||||
| Baseline to 3 months | −0.98 (−1.39, −0.57)a | −0.99 (−1.42, −0.56)a | −1.04 (−1.52, −0.56)a | −1.01 (−1.33, −0.69)a |
| Baseline to 6 months | −0.25 (−0.78, 0.27) | −0.45 (−0.88, −0.01)b | −1.13 (−1.65, −0.6)a | −0.79 (−1.13, −0.45)a |
| Change in Weight, pounds | ||||
| Baseline to 3 months | 1.09 (−3.75, 5.93) | 0.9 (−7.76, 9.55) | −3.92 (−9.53, 1.69) | −1.60 (−6.68, 3.48) |
| Baseline to 6 months | 0.66 (−4.66, 5.98) | −2.07 (−9.34, 5.20) | − 8.00 (−13.90, −2.10)b | −5.14 (−9.71, −0.58)b |
| Change in Treatment Satisfaction (DTSQ)d | ||||
| Baseline to 3 months | 2.93 (0.8, 5.04)b | 5.21 (2.82, 7.61)a | 6.88 (4.38, 9.21)a | 6.09 (4.36, 7.85)a |
| Baseline to 6 months | 3.86 (0.98, 6.16)b | 4.49 (1.98, 7.15)b | 7.33 (5.07, 9.91)a | 5.98 (4.12, 7.81)a |
| Change in Self-efficacy (PDSMS)e | ||||
| Baseline to 3 months | 2.87 (0.82, 5.13)b | 3.79 (2.03, 5.55)a | 4.17 (2.19, 5.98)a | 3.99 (2.67, 5.36)a |
| Baseline to 6 months | 3.16 (1.61, 4.89)a | 4.44 (2.15, 6.54)a | 4.49 (2.36, 6.53)a | 4.47 (2.99, 5.93)a |
| Subgroup Analysis c: Baseline HbA1C 7–10% (53–86 mmol/mol) | ||||
| Change in HbA1C, % | ||||
| Baseline to 3 months | −0.65 (−0.95, −0.36)a | −0.89 (−1.23, −0.56)a | −0.62 (−0.94, −0.29)a | −0.76 (−1.0, −0.53)a |
| Baseline to 6 months | 0.20 (−0.35, 0.75) | −0.51 (−0.92, −0.10)b | −0.48 (−0.78, −0.17)b | −0.51 (−0.77, −0.26)a |
| Change in Weight, pounds | ||||
| Baseline to 3 months | 2.77 (− 3.0, 8.53) | 1.24 (−5.26, 7.75) | − 1.05 (−5.75, 3.64) | 0.04 (−3.91, 3.98) |
| Baseline to 6 months | 0.73 (−5.85, 7.31) | −2.81 (−12.64, 7.03) | − 6.25 (−11.77, −0.74)b | − 4.95 (−10.39, −0.49) |
| Change in Treatment Satisfaction (DTSQ)d | ||||
| Baseline to 3 months | 1.69 (−0.66, 4.17) | 5.17 (2.57, 7.53)a | 6.55 (3.14, 9.69)a | 5.85 (3.80, 8.10)a |
| Baseline to 6 months | 2.67 (−0.48, 5.74) | 5.07 (1.79, 8.07)b | 8.25 (5.31, 11.28)a | 6.74 (4.56, 8.93)a |
| Change in Self-efficacy (PDSMS)e | ||||
| Baseline to 3 months | 3.17 (−0.10, 6.17)b | 2.83 (0.83, 4.73)b | 3.76 (1.55, 6.07)b | 3.29 (1.85, 4.78)* |
| Baseline to 6 months | 3.59 (1.11, 6.00) b | 3.72 (1.38, 5.79)b | 5.16 (2.88, 7.44)a | 4.48 (2.93, 6.00)a |
Data presented as mean change from baseline (95% bootstrap confidence intervals). P-values computed using contrasts in unadjusted model.
SI conversion factors: mmol/mol = [10.93* HbA1c (%)] − 23.50
P < 0.001
P < 0.05
n = 105
Diabetes Treatment Satisfaction Questionnaire (DTSQ), scoring range 0–36
Perceived Diabetes Self-management Scale (PDSMS), scoring range 8–40
CDE, certified diabetes educator. Combined CDE group represents combined carbohydrate counting and modified plate method groups.
Table 3 summarizes the adjusted comparisons between the control and intervention groups. At 6 months, the change in HbA1C from baseline between the control and two nutrition education groups was not statistically significant in adjusted analyses (carbohydrate counting, P = 0.36; modified plate method, P = 0.08; combined CDE, P = 0.12). However, in the adjusted subgroup analysis of patients with a baseline HbA1C between 7–10% (53–86 mmol/mol), the decrease in HbA1C between baseline and 6 months was significant in the carbohydrate counting group [mean difference from control HbA1C −0.86% (95% CI −1.47, −0.26); −9.4 mmol/mol (−16.1, −2.8), P = 0.006], modified plate method group [−0.76% (95% CI −1.33, −0.19); −8.3 mmol/mol (−14.5, −2.1), P = 0.01] and the combined CDE group [−0.80% (95% CI −1.33, −0.27); −8.7 mmol/mol (−14.5, −3.0), P = 0.004] compared with the control group.
Table 3.
Adjusted change in HbA1C, weight, treatment satisfaction, and self-efficacy at 6 months
| Carbohydrate Counting vs. Control | Modified Plate Method vs. Control | Combined CDEc vs. Control | |
|---|---|---|---|
| Primary Outcome, Mean (95% CI) Change in HbA1C | |||
| Baseline to 6 months, % | −0.28 (−0.87, 0.31) | −0.52 (−1.10, 0.06) | −0.41 (−0.91, 0.10) |
| Secondary Outcomes, Mean (95% CI) Change in Weight, Satisfaction, Self-efficacy | |||
| Change in weight, pounds | |||
| Baseline to 6 months | −3.55 (−7.05, −0.05)a | −3.65 (−7.47, 0.17) | −3.59 (−6.66, −0.52)a |
| Change in Treatment Satisfaction (DTSQ) | |||
| Baseline to 6 months | −0.01(−2.90, 2.89) | 2.27 (−0.028, 4.57) | 1.20 (−1.03, 3.43) |
| Change in Self-efficacy (PDSMS) | |||
| Baseline to 6 months | 0.88 (−1.37, 3.11) | 1.36 (−0.73, 3.45) | 1.14 (−0.68, 2.96) |
| Subgroup Analysis b: Baseline HbA1C 7–10% | |||
| Primary Outcome, Mean (95% CI) Change in HbA1C | |||
| Baseline to 6 months, % | −0.86 (−1.47, −0.26)a | −0.76 (−1.33, −0.19)a | −0.80 (−1.33, −0.27)a |
| Secondary Outcomes, Mean (95% CI) Change in Weight, Satisfaction, Self-efficacy | |||
| Change in weight, pounds | |||
| Baseline to 6 months | −3.54 (−8.05, 0.97) | −3.58 (−8.10, 0.93) | −3.52 (−7.30, 0.25) |
| Change in Treatment Satisfaction (DTSQ) | |||
| Baseline to 6 months | 2.93 (−0.59, 6.44) | 4.64 (1.72, 7.56)a | 3.87 (1.06, 6.68)a |
| Change in Self-efficacy (PDSMS) | |||
| Baseline to 6 months | 1.45 (−1.13, 4.02) | 2.12 (−0.17, 4.41) | 1.82 (−0.26, 3.90) |
SI conversion factors: mmol/mol = [10.93* HbA1c (%)] − 23.50
P < 0.05
n = 105
CDE, certified diabetes educator. Combined CDE group represents combined carbohydrate counting and modified plate method groups
3.3 Weight, treatment satisfaction, and self-efficacy
In unadjusted within group comparison analyses (Table 2), no significant decrease in weight was observed at 3 months; however, at 6 months, patients in the modified plate method [−8.00 pounds (−13.90, −2.10), P = 0.008] and combined CDE groups [−5.14 pounds (−9.71, −0.58), P = 0.03] had modest, statistically significant weight loss compared to their own baseline.
In adjusted analyses, the carbohydrate counting [mean (95% CI) difference −3.55 pounds (−7.05, −0.05), P = 0.05] and combined CDE [−3.59 pounds (−6.66, −0.52), P = 0.023] groups had significantly greater weight loss compared with the control group at 6 months (Table 3).
At 3 months and 6 months, diabetes treatment satisfaction improved significantly within all groups relative to their own baseline (Table 2). However, at 6 months, no statistically significant differences in treatment satisfaction were observed between the modified plate method [2.3 (−0.03, 4.6), P = 0.05], carbohydrate counting [−0.01 (−2.9, 2.9), P = 0.99], and combined CDE [1.2 (−1.0, 3.4), P = 0.29] groups relative to the control group (Table 3). In patients with baseline HbA1C 7–10% (53–86 mmol/mol), statistically significant increases in diabetes treatment satisfaction at 6 months were observed in the modified plate method group [4.6 (1.7, 7.6), P = 0.002] and combined CDE group [3.9 (1.1, 6.7), P = 0.008] compared with the control group (Table 3).
Diabetes self-efficacy improved significantly from baseline in all groups at both 3 and 6 months (Table 2). However, no differential change was observed between the control and intervention groups at 6 months (Table 3).
3.4 Diabetes numeracy
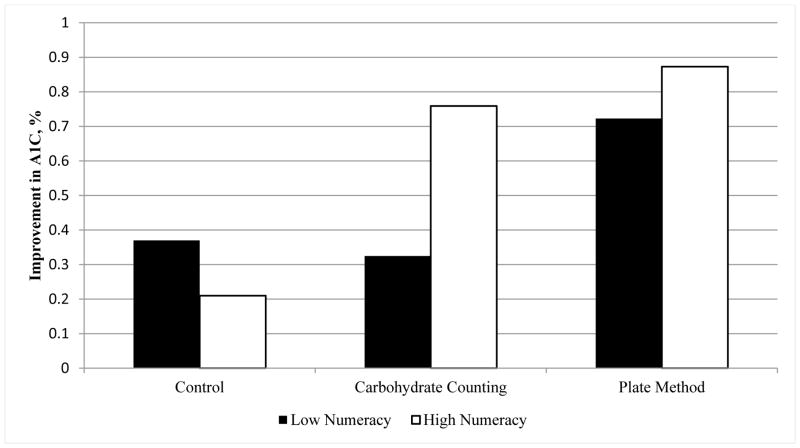
In exploratory, unadjusted analyses stratified by numeracy status (Figure 2), no significant change in HbA1C from baseline to 6 months was observed for low numeracy patients [n=14; −0.37% (−1.72, 0.97); −4.0 mmol/mol (−18.8, 10.6), P = 0.56] or for high numeracy patients [n=28; −0.21%, (−0.79, 0.36); −2.3 mmol/mol (−8.6, 3.9), P = 0.45] in the control arm. Among patients in the carbohydrate counting arm, those with low numeracy did not have a significant improvement in HbA1C at 6 months [n=12; −0.33%, (−0.83, 0.18); −3.6 mmol/mol (−9.1, 2.0), P = 0.18] but patients with higher numeracy did have significant improvement [n=29; −0.76%, (−1.37, −0.15); −8.3 mmol/mol (−15.0, −1.6), P = 0.02]. In the plate method arm, patients with both low numeracy [n=13; −0.72%, (−1.50, 0.05); −7.9 mmol/mol (−16.4, 1.4), P = 0.06] and high numeracy [n= 30; −0.88%, (−1.54, −0.21); −9.6 (−16.8, −6.0), P = 0.01] had important improvements. However, sample size in each subgroup was small, and adjusted regression analyses did not reveal significant differences between subgroups (P = 0.37 for interaction effect).
Figure 2. Within group Percent Change in HbA1C from Baseline by Numeracy Status.
Numeracy assessed using the Diabetes Numeracy Test (DNT). Low numeracy: DNT score in the lowest quartile of the distribution (score <47%); High numeracy (DNT score≥47%). SI conversion factors: mmol/mol = [10.93* HbA1c (%)] − 23.50
4. Discussion and Conclusion
4.1 Discussion
Delivery of DSME/S by CDEs improved glycemic control in patients with type 2 diabetes compared with an attention control group receiving general health education. By including an attention control group to account for increased contact with the healthcare system resulting from study participation, our study addresses an important limitation in prior DSME/S studies. Although improvements in glycemic control were observed in both carbohydrate counting and modified plate method groups compared with the control group, these findings did not reach statistical significance overall. However, in patients with a baseline HbA1C 7.0–10.0% (53–86 mmol/mol), the modified plate method, carbohydrate counting, and the combined CDE-delivered nutrition intervention significantly improved glycemic control compared with the attention control group. Patients in the modified plate method group also reported significantly higher diabetes treatment satisfaction compared to the control group. Our exploratory analyses suggest that all patients, regardless of numeracy skill level, may have significant improvements in HbA1C using the modified plate method, but those patients with lower numeracy may not be as successful applying carbohydrate counting. All patients may benefit from simplified approaches to nutrition education.
Few studies have examined the effectiveness of different approaches to nutrition education in type 2 diabetes within the context of DSME/S. Ziemer et al [33] examined a quantitative, exchange-based meal plan versus a qualitative, healthy food choices plan and observed no differences in HbA1C between the two groups. Our study expands upon this work by comparing carbohydrate gram counting, which is one of the most frequently taught nutrition approaches in DSME/S, with a less computationally intensive modified plate-based method. Even though glycemic improvement in the carbohydrate counting and modified plate method groups relative to control did not reach statistical significance, both groups demonstrated similar, significant improvements in glycemic control in the a priori subgroup analysis of patients with a HbA1C between 7–10% (53–86 mmol/mol). Our findings suggest that selecting patients with moderately uncontrolled diabetes (HbA1C between 7–10%) for DSME/S may allow improved targeting of DSME/S in settings with limited resources and a shortage of CDEs and nutritionists. Our inability to demonstrate improved HbA1c in the full analytic sample may be relate to difficulties improving glycemic control in patients that are poorly controlled (HbA1C >10%, 86 mmol/mol), non-adherent to treatment plans, have socioeconomic barriers to optimal control, or other factors. Although the National Standards for DSME/S [2] do not endorse specific approaches to nutrition education, our findings suggest that both carbohydrate counting and the modified plate method are effective. Larger studies are needed to determine ways to make DSME/S more effective for patients with HbA1C >10% (86 mmol/mol) and directly compare the effectiveness of carbohydrate counting and the modified plate method.
Although contact time is an important predictor of intervention effects in DSME/S [9], many previous studies examining the impact of DSME/S have failed to adequately control for increased contact with the healthcare system resulting from DSME/S participation. Improved study outcomes in control groups can be influenced by study participation, attention, and the intensity of contact with the healthcare team [34, 35]. Thus, in previous studies, it is unclear if observed improvements are related to DSME/S or increased interactions. [7, 9]. Our study included a comparison group receiving general health education visits to control for increased attention resulting from participation in the study. During the first 3 months of the study, participants in the attention control group had significant improvements in HbA1c that were similar to participants in the intervention groups. However, this effect diminished after intervention completion. Our findings suggest that DSME/S improves glycemic control independent of increased contact with the healthcare system.
Individualized DSME/S strategies that are sensitive to patient preferences, willingness to change, beliefs, knowledge, literacy, and numeracy skills are an important part of patient-centered diabetes care [17]. Low numeracy, which is the ability to use numbers in daily life [36], is associated with worse diabetes knowledge [37], poorer diabetes self-management skills [18], difficulty reading food labels [20] and quantifying dietary intake [21], and glycemic control [18]. Although our exploratory analyses suggest the plate method, but not carbohydrate counting, may improve HbA1C in patients with both high and low numeracy, numeracy did not significantly moderate the response to nutrition education in adjusted analyses. Additional, sufficiently powered studies are needed to further evaluate numeracy as an effect modifier of the response to diabetes nutrition education. It is possible that the modified plate method may be easier for participants of all skill levels to understand and execute because it relies on visual cues and assessments to guide dietary choices. In contrast, carbohydrate gram counting requires more advanced numeracy skills that may be difficult for a wide range of patients to integrate into daily self-care. Patients randomized to the modified plate method also reported statistically significant increases in patient satisfaction and diabetes self-efficacy supporting prior research findings that the modified plate method is highly accessible, easy to understand, and facilitates individual meal planning in the home, supermarket, and restaurant settings [38].
Patients participating in DSME/S interventions achieve modest weight loss that can contribute to improvements in glycemic control and long term sustainability of glycemic improvements. However, participants in behavioral and educational interventions for type 2 diabetes can improve glycemic control without significant weight loss [39]. Although weight loss is a desired outcome of nutrition interventions, improved glycemic control can decrease rates of macrovascular and microvascular diabetes complications without weight loss or in the presence of weight gain [40]. The magnitude of weight loss observed in our study is similar to that reported in a meta-analysis of DSME/S interventions [39]. Although patients randomized to the plate method achieved greater weight loss at 6 months, no difference in weight loss was observed relative to the control group. However, patients in the carbohydrate counting group achieved statistically significant weight loss at 6 months relative to the control group. Because carbohydrate counting teaches patients to quantitatively track carbohydrate intake at each meal, patients skilled in carbohydrate counting may be more successful in meeting defined dietary targets to promote weight loss.
There are several limitations to this study. First, this study was conducted in an academic setting with English speaking patients, and may not generalize to other settings or populations. Second, because only two CDEs were available for this study, each CDE provided instruction on both the carbohydrate counting and modified plate methods during the study, which may contaminate the intervention arms. Although CDEs were not blinded to group assignment, they were trained to deliver only the assigned approach to limit potential bias. Additionally, we utilized highly experienced CDEs and our findings may not generalize to all CDEs. Third, since the duration of the study was only 6 months, we were unable to assess the long term impact and sustainability of the interventions on glycemic outcomes. Fourth, our study was not designed to evaluate the role of weight loss and other mechanisms by which DSME/S interventions improve glycemic control. Finally, while our exploration of the role of numeracy was pre-specified, this study was not adequately powered to examine differences in intervention effectiveness by diabetes numeracy.
4.2 Conclusion
Our study suggests that CDE-delivered DSME/S utilizing carbohydrate counting or the modified plate method of nutrition can significantly improve glycemic control independent of increased healthcare interactions for individuals with HbA1Cs between 7–10% (53–86 mmol/mol). Consistent with national DSME/S guidelines, our study also suggests that approaches to diabetes education may need to be customized to specific patient characteristics – including numeracy level.
4.3 Practice Implications
DSME/S has an important role in improving glycemic control in patients with type 2 diabetes, especially those with a HbA1C between 7–10% (53–86 mmol/mol). Increased awareness and understanding of patient characteristics may be important for tailoring approaches to nutrition education. Additional studies are needed to identify the key attributes of DSME/S programs that are most effective in improving outcomes for patients with type 2 diabetes.
Highlights.
HbA1c improved with both carbohydrate counting and plate method of nutrition.
HbA1C improved independently of increased contact time from diabetes education.
In those with a HbA1C 7–10%, both nutrition methods improved diabetes control.
The plate method may improve control for both higher and lower numeracy patients.
Acknowledgments
This study was funded by the American Association of Diabetes Educators (AADE) Education and Research Foundation (RFA 0100-06, Building the Evidence), the Vanderbilt Diabetes Research and Training Center (NIDDK P60DK020593), Vanderbilt Center for Diabetes Translational Research (NIDDK P30DK092986) and the Vanderbilt Institute for Clinical and Translational Research (NCATS 2UL1TR000445).
Dr. Cavanaugh was supported by NIH/NIDDK K23 DK080952.
Dr. Bowen was supported by the VA National Quality Scholars Fellowship, the National Center for Advancing Translational Sciences of the NIH (KL2TR001103) and the NIH/NIDDK K23 DK104065.
M.B participated in analysis, data interpretation, and drafted the manuscript. K.C. participated in design, analysis, and interpretation, and manuscript revision. Ka.W., D.D., and R.G. developed nutrition education materials, delivered study interventions, and revised the manuscript. A.S. and S.E. participated in study design, conducted analyses, interpreted data, and revised the manuscript. T.E. and Ke.W. participated in study design and manuscript revision. R.R. designed the study, participated in analysis, data interpretation, and manuscript revision. R.R. had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Preliminary data from this study was presented at the 2010 American Association of Diabetes Educator’s national meeting (San Antonio, TX).
The authors would like to thank Shari Barto, MBA for her efforts as study coordinator and Duff Green, BA, M. Div for serving as the patient educator in the control arm.
Footnotes
Clinical Trials Registration: NCT00715585, ClinicalTrials.gov
Conflict of Interest Statement: No potential conflicts of interest relevant to this article are reported
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson RM, Funnell MM. Compliance and adherence are dysfunctional concepts in diabetes care. Diabetes Educ. 2000;26:597–604. doi: 10.1177/014572170002600405. [DOI] [PubMed] [Google Scholar]
- 2.Haas L, Maryniuk M, Beck J, Cox CE, Duker P, Edwards L, Fisher EB, Hanson L, Kent D, Kolb L, McLaughlin S, Orzeck E, Piette JD, Rhinehart AS, Rothman R, Sklaroff S, Tomky D, Youssef G. National standards for diabetes self-management education and support. Diabetes Care. 2013;36(Suppl 1):S100–8. doi: 10.2337/dc13-S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Accessed August 10, 2011];Block Brief 2000 Food Frequency Questionnaire. Available from www.nutritionquest.com.
- 4.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funnell MM, Anderson RM. Patient empowerment: a look back, a look ahead. Diabetes Educ. 2003;29:454–8. doi: 10.1177/014572170302900310. [DOI] [PubMed] [Google Scholar]
- 6.Funnell MM, Anderson RM. Empowerment and Self-Management of Diabetes. Clinical Diabetes. 2004;22:123–7. [Google Scholar]
- 7.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561–87. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 8.Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, Maryniuk M, Peyrot M, Piette JD, Reader D, Siminerio LM, Weinger K, Weiss MA. National standards for diabetes self-management education. Diabetes Care. 2010;33(Suppl 1):S89–96. doi: 10.2337/dc10-S089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–71. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 10.Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient education: a meta-analysis and meta-regression. Patient Educ Couns. 2004;52:97–105. doi: 10.1016/s0738-3991(03)00016-8. [DOI] [PubMed] [Google Scholar]
- 11.Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly A, Michael P, Johnson EQ, Harrington CC, Patrick S, Bender T. Diabetes white paper: Defining the delivery of nutrition services in Medicare medical nutrition therapy vs Medicare diabetes self-management training programs. J Am Diet Assoc. 2009;109:528–39. doi: 10.1016/j.jada.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Franz MJ, Powers MA, Leontos C, Holzmeister LA, Kulkarni K, Monk A, Wedel N, Gradwell E. The evidence for medical nutrition therapy for Type 1 and Type 2 diabetes in adults. J Am Diet Assoc. 2010;110:1852–1889. doi: 10.1016/j.jada.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 15.Davis NJ, Wylie-Rosett J. Death to carbohydrate counting? Diabetes Care. 2008;31:1467–8. doi: 10.2337/dc08-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camelon KM, Hadell K, Jamsen PT, Ketonen KJ, Kohtamaki HM, Makimatilla S, Tormala ML, Valve RH. The Plate Model: a visual method of teaching meal planning. DAIS Project Group. Diabetes Atherosclerosis Intervention Study. J Am Diet Assoc. 1998;98:1155–8. doi: 10.1016/s0002-8223(98)00267-3. [DOI] [PubMed] [Google Scholar]
- 17.American Association of Diabetes Educators. AADE Position Statement: Individualization of Diabetes Self-management Education. The Diabetes Educator. 2007;33:45–9. doi: 10.1177/0145721706298308. [DOI] [PubMed] [Google Scholar]
- 18.Cavanaugh K, Huizinga MM, Wallston KA, Gebretsadik T, Shintani A, Davis D, Gregory RP, Fuchs L, Malone R, Cherrington A, Pignone M, DeWalt DA, Elasy TA, Rothman RL. Association of numeracy and diabetes control. Ann Intern Med. 2008;148:737–46. doi: 10.7326/0003-4819-148-10-200805200-00006. [DOI] [PubMed] [Google Scholar]
- 19.Cavanaugh K, Wallston KA, Gebretsadik T, Shintani A, Huizinga MM, Davis D, Gregory RP, Malone R, Pignone M, DeWalt D, Elasy TA, Rothman RL. Addressing literacy and numeracy to improve diabetes care: two randomized controlled trials. Diabetes Care. 2009;32:2149–55. doi: 10.2337/dc09-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman RL, Housam R, Weiss H, Davis D, Gregory R, Gebretsadik T, Shintani A, Elasy TA. Patient understanding of food labels: the role of literacy and numeracy. Am J Prev Med. 2006;31:391–8. doi: 10.1016/j.amepre.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Huizinga MM, Carlisle AJ, Cavanaugh KL, Davis DL, Gregory RP, Schlundt DG, Rothman RL. Literacy, numeracy, and portion-size estimation skills. Am J Prev Med. 2009;36:324–8. doi: 10.1016/j.amepre.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StataCorp. Stata Statistical Software Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 23. [Accessed November 16, 2011];Idaho Plate Method. 2011 Available from www.platemethod.com/about.html.
- 24.Karlstrom BVB, Eliasson M. Diet -- A balanced approach. In: Larkins RZP, Chisholm D, editors. Diabetes. Elsevier; Amsterdam: 1988. pp. 923–5. [Google Scholar]
- 25.Wolff K, Cavanaugh K, Malone R, Hawk V, Gregory BP, Davis D, Wallston K, Rothman RL. The Diabetes Literacy and Numeracy Education Toolkit (DLNET): materials to facilitate diabetes education and management in patients with low literacy and numeracy skills. Diabetes Educ. 2009;35:233–45. doi: 10.1177/0145721709331945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis TC, Crouch MA, Long SW, Jackson RH, Bates P, George RB, Bairnsfather LE. Rapid assessment of literacy levels of adult primary care patients. Fam Med. 1991;23:433–5. [PubMed] [Google Scholar]
- 27.Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, Crouch MA. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25:391–5. [PubMed] [Google Scholar]
- 28.Huizinga MM, Elasy TA, Wallston KA, Cavanaugh K, Davis D, Gregory RP, Fuchs LS, Malone R, Cherrington A, DeWalt DA, Buse J, Pignone M, Rothman RL. Development and validation of the Diabetes Numeracy Test (DNT) BMC Health Serv Res. 2008;8:96. doi: 10.1186/1472-6963-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley C. Handbook of Psychology and Diabetes: a guide to psychological measurement in diabetes research and practice. Harwood Academic Publishers; Switzerland: 1994. [Google Scholar]
- 30.Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the Perceived Diabetes Self-Management Scale (PDSMS) Journal of behavioral medicine. 2007;30:395–401. doi: 10.1007/s10865-007-9110-y. [DOI] [PubMed] [Google Scholar]
- 31.Ashwell SG, Bradley C, Stephens JW, Witthaus E, Home PD. Treatment satisfaction and quality of life with insulin glargine plus insulin lispro compared with NPH insulin plus unmodified human insulin in individuals with type 1 diabetes. Diabetes Care. 2008;31:1112–7. doi: 10.2337/dc07-1183. [DOI] [PubMed] [Google Scholar]
- 32.Harrell FE. Regression Modeling Strategies. Springer; New York: 2001. [Google Scholar]
- 33.Ziemer DC, Berkowitz KJ, Panayioto RM, El-Kebbi IM, Musey VC, Anderson LA, Wanko NS, Fowke ML, Brazier CW, Dunbar VG, Slocum W, Bacha GM, Gallina DL, Cook CB, Phillips LS. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719–24. doi: 10.2337/diacare.26.6.1719. [DOI] [PubMed] [Google Scholar]
- 34.Adair JG. The Hawthorne Effect: A Reconsideration of the Methodological Artifact. Journal of Applied Psychology. 1984;69:334–45. [Google Scholar]
- 35.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC medical research methodology. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothman RL, Montori VM, Cherrington A, Pignone MP. Perspective: the role of numeracy in health care. J Health Commun. 2008;13:583–95. doi: 10.1080/10810730802281791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothman RL, Malone R, Bryant B, Wolfe C, Padgett P, DeWalt DA, Weinberger M, Pignone M. The Spoken Knowledge in Low Literacy in Diabetes scale: a diabetes knowledge scale for vulnerable patients. Diabetes Educ. 2005;31:215–24. doi: 10.1177/0145721705275002. [DOI] [PubMed] [Google Scholar]
- 38.Raidl M, Spain K, Lanting R, Lockard M, Johnson S, Spencer M, Sant L, Welch J, Liddil A, Hartman-Cunningham M. The healthy diabetes plate. Preventing chronic disease. 2007;4:A12. [PMC free article] [PubMed] [Google Scholar]
- 39.Gary TL, Genkinger JM, Guallar E, Peyrot M, Brancati FL. Meta-analysis of randomized educational and behavioral interventions in type 2 diabetes. Diabetes Educ. 2003;29:488–501. doi: 10.1177/014572170302900313. [DOI] [PubMed] [Google Scholar]
- 40.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. [PubMed] [Google Scholar]