Abstract
Background
During IgE-mediated immediate hypersensitivity reactions, vascular endothelial cells permeabilize in response to mast cell mediators. We have previously demonstrated that patients and mice with STAT3 mutations (autosomal dominant-hyper IgE syndrome; AD-HIES) are partially protected from anaphylaxis.
Objectives
To further study the mechanism by which STAT3 contributes to anaphylaxis, and determine whether small molecule inhibition of STAT3 can prevent anaphylaxis.
Methods
Using unaffected and STAT3-inhibited or genetic loss of function samples, we performed histamine skin prick testing, investigated the contribution of STAT3 to animal models of anaphylaxis, measured endothelial cell permeability, gene and protein expression, and histamine receptor-mediated signaling.
Results
While mouse mast cell degranulation was minimally affected by STAT3 blockade, mast cell mediator-induced anaphylaxis was blunted in Stat3 mutant AD-HIES mice and in wild-type mice subjected to small molecule STAT3 inhibition. Histamine skin prick responses were diminished in AD-HIES patients. Human umbilical vein vascular endothelial cells (HUVECs) derived from patients with AD-HIES or treated with a STAT3 inhibitor failed to properly signal through Src or to appropriately dissolute the adherens junctions made up of the proteins vascular endothelial (VE)-cadherin and β-catenin. Further, we found that diminished STAT3-target mir17–92 expression in AD-HIES HUVECS is associated with increased PTEN expression, which inhibits Src, and increased E2F1 expression, which regulates β-catenin cellular dynamics.
Conclusions
These data demonstrate that STAT3-dependent transcriptional activity regulates critical components for the architecture and functional dynamics of endothelial junctions thus permitting vascular permeability.
Keywords: Allergy, immunology, innate immunity, STAT3, AD-HIES
INTRODUCTION
The signal transducer and activator of transcription (STAT) family of transcription factors are integral components of many cytokine receptor signaling systems 1–3. Multiple clinical phenotypes result from both germline gain- and loss-of-function mutations in STATs 4–7. Dominant negative STAT3 mutations in humans result in dermatitis, elevated serum IgE, enhanced susceptibility to staphylococcal skin and respiratory infection, mucocutaneous candidiasis, and connective tissue and skeletal abnormalities 8. Despite a significant burden of eczematous skin disease and associated elevations in both total and allergen-specific serum IgE, clinical food allergy and anaphylaxis are markedly diminished in patients with AD-HIES 9.. One potential mechanism contributing to this phenomenon may involve STAT3-mediated regulation of mast cell degranulation9 and mitochondrial activity10
Following mast cell degranulation, mediators such as histamine, platelet activating factor (PAF), and thrombin act on target vascular endothelium to induce nitric oxide synthesis, a potent vasodilator11, 12, intracellular calcium release13, 14, and vascular leak, resulting in symptoms of immediate hypersensitivity allergic reactions including flushing and hypotension 15, 16. Factors such as histamine, PAF, or vascular endothelial growth factor (VEGF) result in destabilization of VE-Cadherin in the adherens junctions 17 by uncoupling VE-cadherin from β-catenin anchors via a Src/Yes kinase-dependent mechanism 18–20.
STAT3 is activated following adheren junction formation21 and STAT3 signaling has been implicated in gap junction intercellular communication, IL-6-induced vascular leakage, downregulation of VE-cadherin, and mir17–92/E2F1 dependent regulation of β-catenin nuclear translocation and transcriptional activity22–28. However, the specific role of STAT3 in endovascular permeability, in particular to mast cell mediators, has not previously been explored. Here we demonstrate that intact STAT3 signaling is essential for mast cell mediator-induced vascular endothelial permeability, and that small molecule inhibition of STAT3 prevents endothelial permeability in vitro and anaphylaxis in vivo.
METHODS
A full description of the methods used in this study can be found in the Methods section in this article’s Online Repository at www.jacionline.org.
Histamine skin prick test
Reactions to histamine skin prick testing were measured at 15 min and recorded as per standard of care. Data are reported as the total area calculated by the length equating to the widest point of a wheal/flare multiplied by the width measuring perpendicular to the widest point.
Mice and STAT3 inhibition in vivo
Wild type (WT) C57Bl/6, mice were obtained from Jackson Laboratory (Bar Harbor, ME) and mut-Stat3 (AD-HIES) mice with their corresponding WT littermate controls were kindly provided by Dr. J. O’Shea 29. Mice received daily i.p. injections with either C188–9 at 50 mg/kg or vehicle for 1, 4 or 7 days. Anaphylaxis was induced 24 h after the last C188–9 injection.
Mouse model of systemic anaphylaxis
Systemic anaphylaxisis was measured as previously described 30. For IgE-induced passive systemic anaphylaxis (PSA), WT mice were sensitized intravenously (i.v.) with 3 µg of DNP-specific IgE (200 µl, clone H1-DNP-ε-26.82) and challenged 24 h later with 200 µg of DNP-HSA (i.v.; Sigma-Aldrich). Alternatively, systemic anaphylaxis was induced in WT or AD- HIES mice by an intravenous bolus of histamine dihydrochloride (5 µmol in 200 µl PBS, Sigma Aldrich) or by 0.3 µg of platelet activating factor (PAF; Tocris Bioscience, Bristol, UK).
For measurements of histamine and mast cell protease MCPT-1 released into circulation, C188–9- and vehicle-treated mice were euthanized by CO2 inhalation 90 s or 30 min after the induction of anaphylaxis. Blood was collected in EDTA-containing tubes (BD Biosciences, San Jose, CA) and histamine and MCPT-1 levels in plasma were measured with specific ELISA kits (Beckman Coulter, Fullerton, CA and eBioscience, San Diego, CA, respectively).
Assessment of murine vascular permeability in vivo
Hematocrit levels were determined by collecting blood from the retro-orbital plexus 2 d before (baseline) and 2 h after the induction of anaphylaxis in C188–9 and vehicle treated mice, with heparinized micro-hematocrit tubes (Jorvet, Loveland, CO) and the volume percentage of red blood cells determined with a hematocrit reader.
To investigate peripheral vascular leakage in response to IgE/Ag-induced anaphylaxis in the skin, following sensitization, mice were challenged 24 h later i.v. with 200 µg of DNP-HSA in 200 µl PBS containing 1% Evans Blue (Sigma Aldrich). Evans Blue dye extravasated into the ear tissue was extracted using 700 µl of formamide at 55°C for 2 h and quantified by absorbance at 620 nm after clearing the extracts by centrifugation.
Cell culture
Cell culture conditions used in this study can be found in the Methods section in this article’s Online Repository at www.jacionline.org.
In vitro inhibitor treatments
For in vitro treatments, C188–9 was added to the cells at a concentration of 1µM unless otherwise stated. 1 µM was determined to be the optimal dose after performing a dose response assay of 500nm, 1 µM, and 10 µM. For PTEN inhibitor treatment, VO-OHpic (Echelon Biosciences, Salt Lake City, UT) was added to the cells at a concentration of 250–500nM. All inhibitors were compared to DMSO treated controls.
Mast cell degranulation assays
LAD2 cells, 7–10 week-old primary HuMCs, 4–6 week-old BMMCs or PDMCs were incubated with different concentrations of C188–9 (0.3–10.0 µM) or DMSO for 1 week. Degranulation was measured as β-hexosaminidase release into the media 30 min after Ag-challenge (100 ng/ml DNP-HSA (Sigma-Aldrich) for DNP-IgE sensitized murine mast cells or after 10 ng/ml streptavidin (Sigma-Aldrich) for biotinylated-IgE coated human mast cells as described 31
Flow cytometry
Following 7 injections of C188–9 (or vehicle), mice were euthanized and peritoneal lavage obtained using 10 ml of RPMI media and stained with an antibody specific for phosphorylated STAT3-Serine727-PE (Cat No. 558557; BD Biosciences, San Jose, CA).
LAD2 cells were serum starved in RPMI for 30 minutes at 37°C and then treated with C188–9 (3, 30, and 100 uM) for 1 h at 37°C. Samples were stimulated with 100 ng/ml IL-6 for 15 minutes or 500ng/ml IFN-γ for 15 minutes and then fixed and permeabilized (as described in the online methods). After washing with FACS buffer, cells were stained with phosphorylated STAT1-AF488 (Cat No. 612596; BD Biosciences) and phosphorylated STAT3-tyrosine705-AF647 (Cat No. 557815; BD Biosciences) for 30 minutes.
Immunofluorescence
Primary antibodies used were total β-catenin (Cat No. 2677; 1:100), active (non-phosphorylated) β-catenin (Cat No. 8814; 1:100), VE-Cadherin (Cat No.2500; 1:100) (Cell Signaling, Danvers, MA). For full details see online methods.
Real-Time PCR
Predesigned Taqman primers and probes were purchased from Life Technologies and real time PCR was performed using a 7500 real time PCR system (Life Technologies).
Transfections
HUVECs were transfected using the Nucleofector transfection system (Lonza, Allendale, NJ) following the manufactures recommended protocol. Briefly, 5 × 10 cells were re-suspended in nucleofector solution, combined with 200nM of either a non-targeting microRNA mimic, a mir19a microRNA mimic, a non-targeting antagomir, or a mir19a antagomir (Dharmacon, Lafayette, CO), and delivered to the intracellular environment by electroporation. Cells were then added to pre-equilibrated culture media for 48hrs and then underwent a second transfection. Finally, 24 hours later cells were harvested for Western analysis or seeded for permeability assays.
Cytoplasmic/Nuclear Extraction
Cytoplasmic and nuclear cell fractions were isolated using the NE-PER kit (Thermo Fisher) following the manufactures protocol and then analyzed by SDS-PAGE and western blotting.
SDS-PAGE and Western blotting
A measure of 50 µg of protein was isolated and subjected to SDS-PAGE and transferred onto PVDF. Primary antibodies (see online methods) were added at appropriate dilutions in 5% milk/TBST and incubated overnight at 4 °C with rotation. Membranes were subsequently washed three times in PBS and horseradish peroxidase conjugated secondary antibodies were added at 1:2000 for 1 h at room temperature (Santa Cruz Biotech, Santa Cruz, CA). Bands were visualized using ECL Plus (Thermo Fisher, Rockford, IL).
Calcium Assays
WT HUVECs, AD-HIES HUVECs, and MLE cells were seeded at 20,000/well in a 96 well plate (Corning, Tewksbury, MA) and incubated overnight. A Fluo-8 calcium assay was then performed (Abcam, Cambridge, MA). Cells were washed in PBS and 100ul of Fluo-8 dye-loading solution was added to each well. Plates were incubated at 37°C for 30 minutes and then 30 minutes at room temperature. Histamine was added (1 µM, 10 µM, 100 µM, and 1000 µM) and fluorescent intensity was monitored at Ex/Em=490/525 nm using a Flexstation II (Molecular Devices, Sunnyvale CA).
In vitro permeability assays
In vitro permeability assays were performed as previously described 32,33 and in the online methods. Cells were exposed to 1mg FITC-Dextran (Sigma) and histamine, PAF, or IL11. After collecting all time points, fluorescent intensity was monitored at Ex/Em=490/525 nm.
STAT3 reporter assays
HUVECs were transfected with a 1ug Cignal STAT3 reporter (Qiagen) as described above. Next, 24 hours following transfection cells were treated with increasing amounts of the STAT3 agonist IL-11 for 20 hours and subjected to a Dual Luciferase reporter assay (Promega, Madison, WI) following the manufactures protocol.
Statistics
For statistical analysis information see online methods.
RESULTS
Repeated injections with C188–9 reduce anaphylaxis severity in mice
In order to establish a model of systemic STAT3 inhibition we pretreated wild-type (WT) mice with STAT3 inhibitor, C188–934. Treatment for 5–7 days profoundly diminished the duration and severity of IgE mediated anaphylaxis, while treatment for 1 day or 4 days were ineffective or mildly effective, respectively (see Figure 1A and Figure E1A and B in this article’s Online Repository at www.jacionline.org). C188–9 injections were well tolerated and resulted in efficient reduction of STAT3 phosphorylation (Serine 727, the residue phosphorylated by vascular mediators, such as histamine) in peritoneal cells (see Figure E1C in this article’s Online Repository at www.jacionline.org).
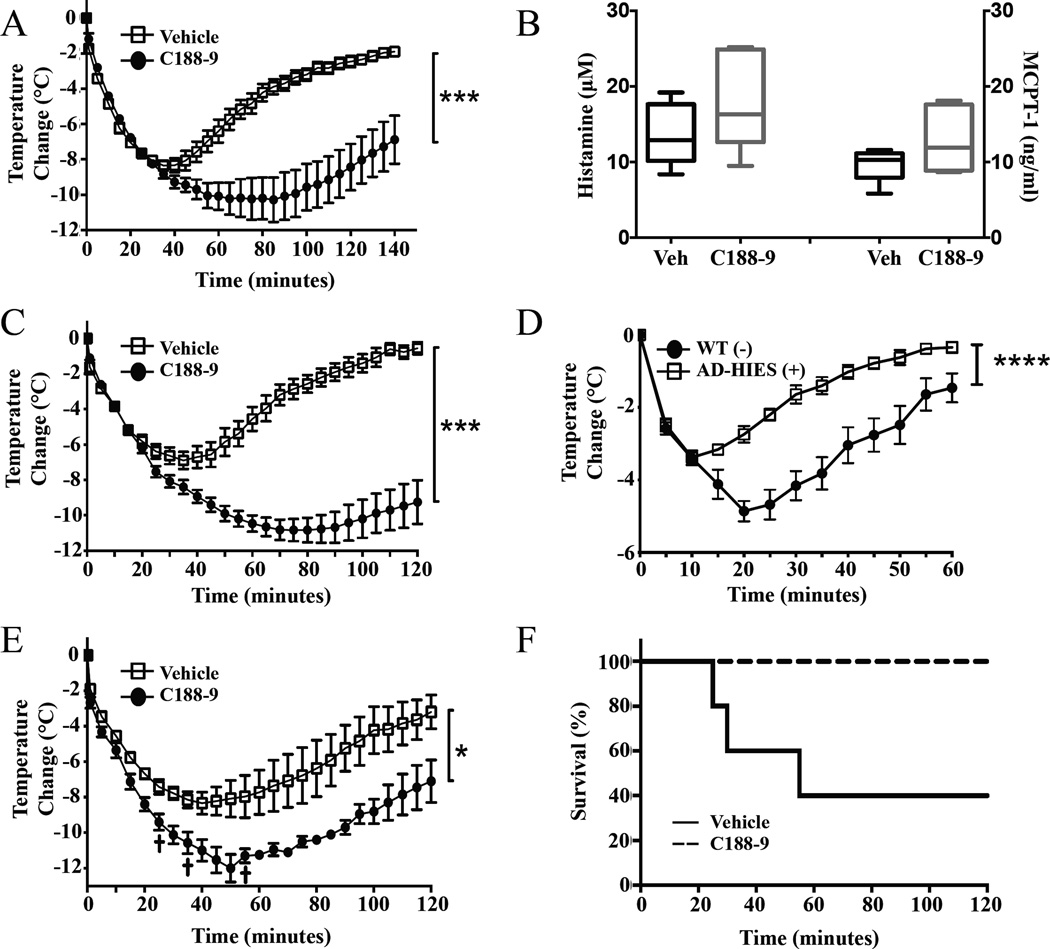
Figure 1. Reduction in STAT3 confers resistance to anaphylaxis.
(A) Effect of C188–9 pretreatment (7 days) on body temperature changes in male C57Bl/6 mice during anaphylactic shock. Mice (n=5–6/group) were sensitized with 3 µg DNP-specific IgE and challenged 24 h later with 200 µg DNP-HSA. (B) Histamine and MCPT-1 released into circulation (90 sec) after challenging sensitized C188–9 or vehicle treated mice with Ag (n=6/group). Results are presented as boxplots with min/max range. Temperature changes during anaphylaxis induced by histamine (5 µmol) in C188–9 and vehicle treated C57BL/6 (C) or AD-HIES mice (D) (n=6/group). Temperature changes during anaphylaxis induced by PAF (0.3 mg) in C188–9 and vehicle treated mice (E). (F) Survival curve of mice after PAF treatment (n=6/group in B and n=5/group in C).
Degranulation in mouse mast cells, but not human mast cells, is STAT3 independent
Surprisingly, the reduced IgE-mediated anaphylactic responses in C188–9 treated mice did not correspond with decreases in the early release (90 sec) of the markers of mast cell activation histamine and mast cell protease-1 (MCPT-1) (see Figure 1B), or alterations in their subsequent metabolism (30 min) (see Figure E1D in this article’s Online Repository at www.jacionline.org). Similarly, the reduced anaphylaxis observed in a Stat3 mutated mouse model of AD-HIES 9 did not correspond with significant differences in the release of histamine after challenge (see Figure E1E in this article’s Online Repository at www.jacionline.org). In order to resolve the discrepancy between the mouse data and in vitro data showing STAT3 to be important for human mast cell degranulation 9, we examined the effect of C188–9 inhibition on mouse BMMC and PDMC. Unlike human-derived mast cells, which showed a 30–40 % reduction in degranulation when pretreated with C188–9 for 5 days (see Figure E2A and B in this article’s Online Repository at www.jacionline.org), IgE/Ag-induced degranulation in BMMCs or PDMCs (see Figure E2C and 2D in this article’s Online Repository at www.jacionline.org) was unaltered by C188–9 treatment. Only when PDMCs were cultured in the presence of IL-6 did the degranulation of these cells become mildly sensitive to C188–9 treatment. Short-term treatment with C188–9 for 24h had no effect on degranulation in human mast cells (see Figure E2E and F in this article’s Online Repository at www.jacionline.org).
The effects of C188–9 in human mast cells required long-term treatment (5–7 days). This was not due to slow uptake or action of the inhibitor because incubation of LAD2 with C188–9 for just 1 h effectively prevented STAT3 phosphorylation (Tyr-705) induced by IL-6 stimulation (see Figure E3A–B in this article’s Online Repository at www.jacionline.org). The reduced pSTAT1 response under IL-6 stimulating conditions (see Figure E3A–B in this article’s Online Repository at www.jacionline.org) was likely due to lower levels of IL-6 induced STAT1/STAT3 heterodimers since no inhibition of STAT1 phosphorylation was seen following IFN-γ stimulation—a more pure STAT1 activator, in C188–9 treated LAD2 cells (see Figure E3C–D in this article’s Online Repository at www.jacionline.org).
These data suggest that the mechanism by which STAT3 inhibition prevents anaphylactic responses is not solely dependent upon effects on mast cell degranulation, and in the mouse, mast cell degranulation is not as dependent on STAT3.
STAT3 inhibition reduces vascular permeability in response to mast cell mediators
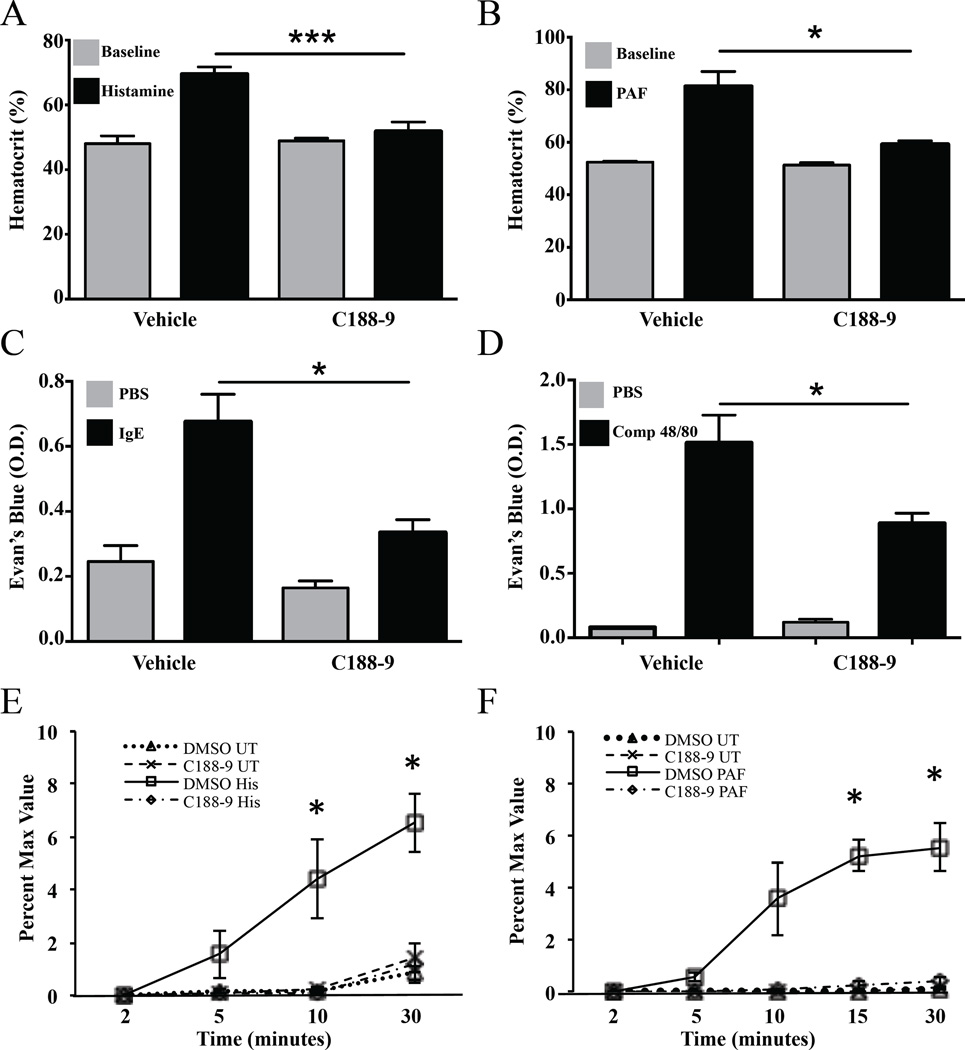
In order to determine the compartment responsible for the impaired anaphylactic response in STAT3 inhibited conditions, we looked for differences in the responsiveness to mast cell mediators themselves such as histamine and PAF. C188–9 treated or Stat3 mutated mice were protected against anaphylaxis induced by a bolus of 5µ-mol histamine (see Figure 1C, D). In addition, C188–9 treatment reduced the severity and duration of passive systemic anaphylaxis induced by the potent mediator PAF (see Figure 1E) and prevented death associated with PAF administration (see Figure 1F). Correlating with the protective effect of C188–9 in the anaphylactic response, the increase in hematocrit following treatment with either histamine or PAF (reflective of increases in vascular permeability) was completely blunted in mice treated with C188–9 (see Figure 2A and B) and extravasation of Evan’s Blue dye during a cutaneous hypersensitivity reaction induced by IgE/Ag (see Figure 2C) or compound 48/80 (see Figure 2D), was significantly reduced in mice treated with C188–9 compared to those treated with vehicle alone.
Figure 2. C188–9 decreases histamine or PAF-induced vascular leakage.
Hematocrit values at baseline and after passive systemic anaphylaxis (PSA) induced by (A) histamine or (B) PAF in C188–9 (n=5/group) and vehicle treated mice (n=6/group). (C–D) Evan’s blue leakage in response to IgE/Ag-challenge (C; n=10/group) or compound 48/80 (D; n=5/group) in C188–9 and vehicle treated mice. (E–F) In vitro permeability assay of mouse lung endothelial cells exposed to histamine (E; 1 µM) or PAF (F; 10ug) following pretreatment with DMSO or C188–9 (1 µM; n=3/group). (UT: untreated; His: histamine; PAF: platelet activating factor). Data are representative of 3 independent experiments. Data are expressed as the mean ± S.E.M. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
We then examined whether resistance to anaphylaxis was afforded by reduced responsiveness of the vascular endothelium to mast cell mediators. Treatment of mouse lung endothelial cell (MLE cells) cultures with C188–9 for 5 days resulted in a pronounced decrease in cell permeability following treatment with histamine (100 µM; see Figure 2E) or PAF (10 µg/ml; see Figure 2F) indicating that STAT3 inhibition in mice protects against anaphylaxis independently of mast cell responsiveness, by impeding vascular mediator-induced endothelial permeability.
STAT3 inhibition reduces permeability in HUVECs by stabilizing VE-Cadherin/β-catenin junctions and prevents Src-mediated adheren junction dissolution
We next investigated the effect of STAT3 inhibition in human umbilical vein endothelial cells (WT HUVECs). After establishing an appropriate dose and confirming that the inhibitor led to very low STAT3 transcriptional activity (see Figured E4 and E5 in this article’s Online Repository at www.jacionline.org), treatment with C188–9 for 5 days resulted in a significant decrease in WT HUVEC permeability in vitro following exposure to histamine (100 µM) or PAF (10 µg/ml) (see Figure 3A and B).
Figure 3. C188–9 decreases vascular permeability through strengthening VE-cadherin junctions.
In vitro permeability assay of human umbilical vascular endothelial cells (HUVECs) pretreated with DMSO or C188–9 (1 µM) and subsequently exposed to (A) histamine (100 µM) or (B) PAF (10 ug/ml) (UT: untreated; His: histamine; PAF: platelet activating factor). Data are representative of 3 independent experiments. (C) Confocal microscopy of VE-cadherin (green), total β-catenin (red), and DAPI (blue) in DMSO and C188–9 (1µM) pre-treated HUVECs then subsequently exposed to histamine (100 µM). (D) Confocal microscopy of active (non-phosphorylated) β-Catenin (green) and DAPI (blue) in DMSO and C188–9 (1 µM) pre-treated HUVECs then subsequently exposed to histamine (100 µM). (E) Western analysis of Stat3, and VE-cadherin signaling in DMSO and C188–9 treated HUVECs. Data are represented as the mean ± S.E.M. *p<0.05.
Vascular endothelial cadherin (VE-cadherin) and its intracellular anchor β-catenin are junctional proteins associated with endothelial cell adhesion. Because dissociation of β-catenin from VE-cadherin is required for increased vessel permeability, we next characterized VE-cadherin/β-catenin complexes following inhibition of STAT3. Immunofluorescent analysis confirmed co-localization of VE-cadherin and β-catenin in HUVECs (see Figure E5 B–C). As expected, histamine treatment resulted in junction dissolution of VE-cadherin and β-catenin in vehicle treated WT HUVECs (see Figure 3C, left panel), with the reduction of β-catenin appearing to be a selective disruption of the active, non-phosphorylated form (see Figure 3D, left panel). The dissolution was quantitated across multiple 6.5-micron sections of the cell membrane (see Figure E6 in this article’s Online Repository at www.jacionline.org).
STAT3 inhibition with C188–9 prevented histamine-mediated dissociation of both VE-Cadherin and β-catenin (see Figure 3C, right panel), and active β-catenin dissociation (see Figure 3D, right panel). This inability to dissociate was due to a failure of VE-cadherin to become phosphorylated in the presence of the STAT3 inhibitor. In DMSO treated HUVECS, histamine treatment resulted in phosphorylation of the histamine specific residue of STAT3 (S727) but not STAT3 (Y705) and a phosphorylation of VE-cadherin (needed for dissolution of the junction). In STAT3 inhibited (C188–9 treated) HUVECs very little activity was detected for the phosphorylation of STAT3 or VE-cadherin suggesting that the mechanism regulating VE-cadherin associated junction dissolution is being constrained (see Figure 3E).
Histamine skin prick responses are reduced among autosomal dominant hyper IgE syndrome (AD-HIES) patients
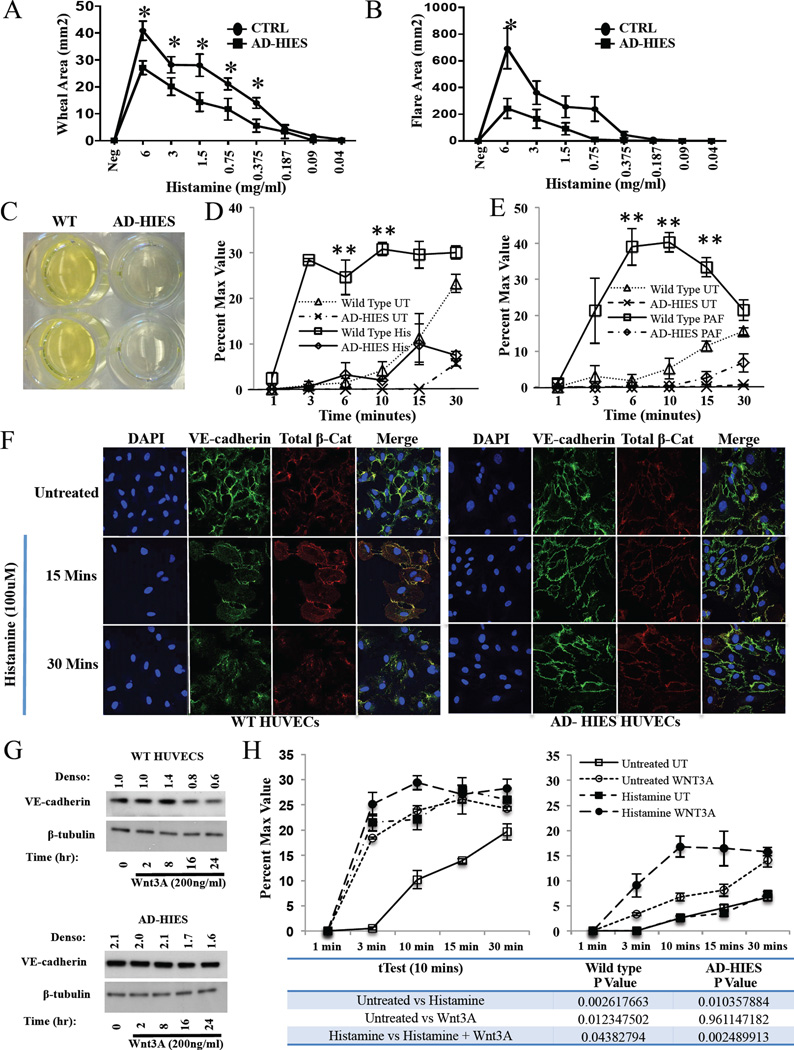
Previous data from patients with dominant negative STAT3 mutations have demonstrated preserved responses to a single, potentially saturating dose of histamine when examining only wheal diameter 9. In light of diminished systemic responsiveness to histamine in the Stat3mut mouse, we performed skin prick testing using serial dilutions of histamine and measured the areas of the elicited wheal and flare in order to more accurately measure potential differences in cutaneous responsiveness. We found that AD-HIES patients demonstrated significantly diminished histamine-induced wheal (see Figure 4A) or flare area (see Figure 4B) at several dilutions.
Figure 4. AD-HIES patient HUVECs exhibit intrinsically decreased vascular permeability through increased VE-cadherin junctions.
AD-HIES and healthy control (n=10/group) patients were skin prick tested with increasing amounts of histamine. The area of the (A) Wheal and (B) Flare was recorded. (C) Image of the bottom of a transwell chamber following wild type and AD-HIES HUVECs exposure to the fluorescent reporter FITC-Dex (1 mg/well) for 30 min. (D–E) In vitro permeability assay of wild type HUVECs and AD-HIES HUVECs exposed to histamine (D; 100 µM) or PAF (E; 10ug/ml) (UT: untreated; His: histamine; PAF: platelet activating factor; n=3/group). Data are representative of 3 independent experiments. (F) Immunofluorescent analysis of VE-cadherin (green), total β-catenin (red), and DAPI (blue) in wild type and AD-HIES HUVECs following exposure to histamine (100 µM). (G) Western analysis of VE-cadherin in wild type and AD-HIES HUVECs following exposure to Wnt3A (200 ng/ml). Data are representative of 2 independent experiments. (H) In vitro permeability assay of wild type (left panel; n=3/group) and AD-HIES (right panel; n=3/group) HUVECs pretreated with Wnt3A (200 ng/ml) and subsequently exposed to histamine. Data are representative of 3 independent experiments. Data are represented as the mean ± SEM. *p<0.05.
Intrinsic increase in VE-cadherin/β-catenin stability and reduced permeability among AD-HIES patient HUVECs
Similar to C188–9 treated WT HUVECs, HUVECs derived from two different newborns with AD-HIES were less permeable than WT HUVECs when exposed to fluorescein isothiocyanate-dextran (FITC-Dex) alone for 30 minutes (see Figure 4C) or stimulated with histamine (see Figure 4D; 100 µM) or PAF (see Figure 4E; 10µg/ml) in the presence of FITC-Dex. AD-HIES HUVECs were also resistant to the dissolution of VE-cadherin/total β-catenin junctions, and the active form of β-catenin, following histamine treatment (see Figure 4F and Figure E7A and B in this article’s Online Repository at www.jacionline.org). β-Catenin is a well-established downstream target of the canonical Wnt pathway. In order to determine the level at which the dissolution defect exists, we found that while WNT3A RNA and protein levels were undetectable (data not shown), AD-HIES HUVECs treated with exogenous Wnt3A (200 ng/ml), led to decreased VE-cadherin and active β-catenin expression at 24 h in WT and AD-HIES HUVECs (see Figure 4G and Figure E7C in this article’s Online Repository at www.jacionline.org), partially restoring AD-HIES HUVEC permeability in response to histamine (see Figure 4H).
Reduced adherin junction dissolution is due to inhibited Src signaling
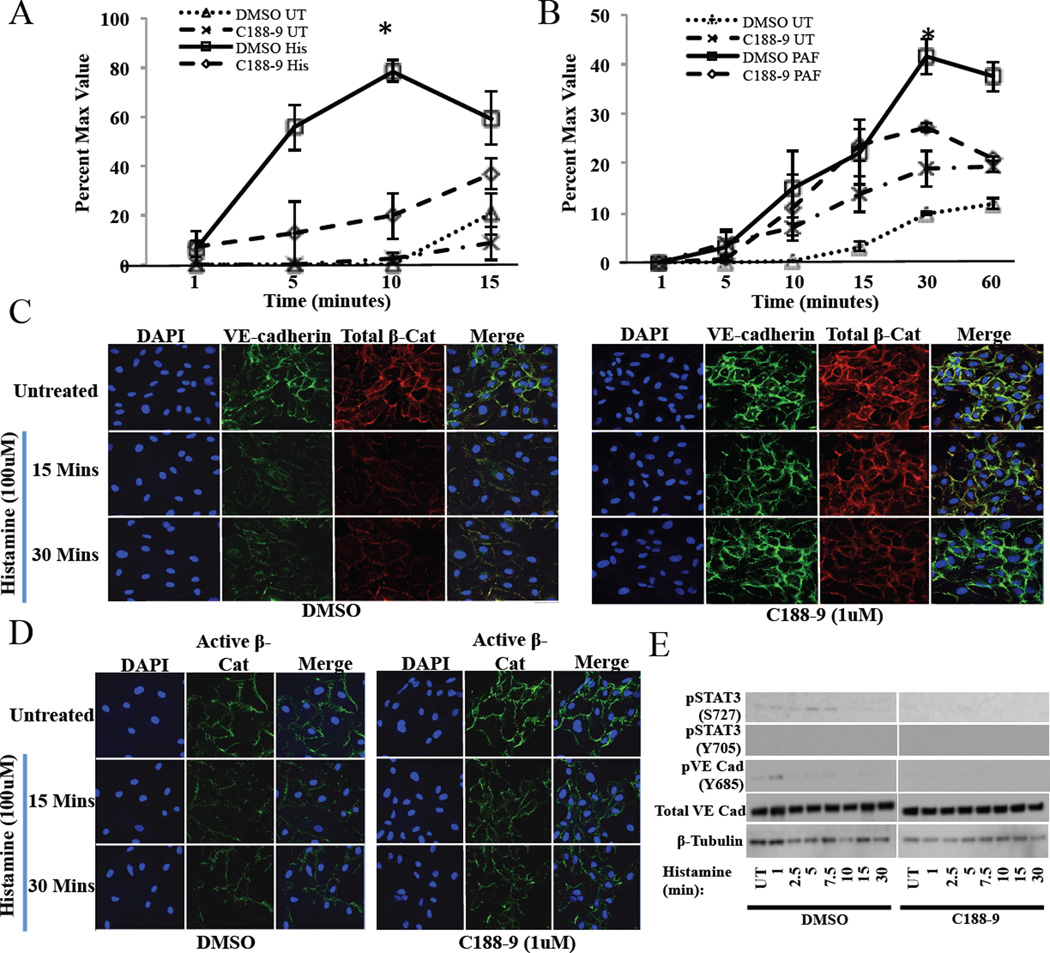
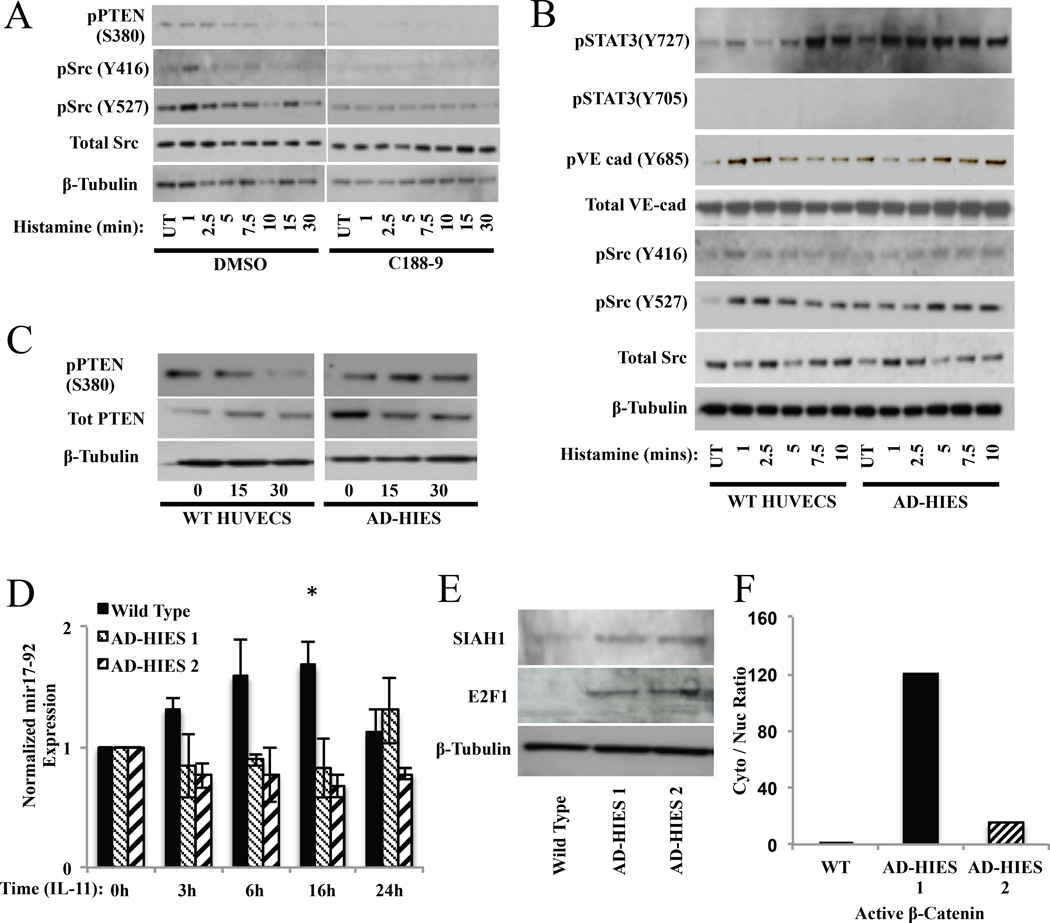
Since calcium signaling was normal after the addition of histamine to STAT3 attenuated HUVECs (see Figure E8A and B in this article’s Online Repository at www.jacionline.org; also seen in MLE cells Figure E6C in this article’s Online Repository at www.jacionline.org) and the failure of the adherins junctions to dissociate could be rescued by exogenous WNT3a, we hypothesized that other upstream signaling defects might exist in STAT3 inhibited or mutated HUVECs, as was seen in STAT3 inhibited mast cells 9. Histamine receptor signaling through proximal signaling Src molecules is at least in part responsible for VE-Cadherin responses 35. In DMSO treated HUVECS, histamine treatment resulted in an increase in the phosphorylation of SrcY416 (activating Src), which was mirrored by phosphorylation of SrcY527 (inactivating Src). However, in C188–9 treated HUVECs, very little Src phosphorylation was detected. (see Figure 5A; right). Further, at early time points, histamine-induced Src and VE-cadherin phosphorylation is delayed in AD-HIES HUVECs compared to WT (see Figure 5B; pSrc(Y416); lane 2 vs lane 10, pVE-Cad; lane 2 vs lane 10). Concomitant elevation of total PTEN (see Figure 5C), a lipid and protein phosphatase known to inhibit Src 36, and a reduction in the inactive, phosphorylated form of PTEN (see Figure 5A; left) in AD-HIES HUVECs was also observed, suggesting the failure to downregulate VE-cadherin may have been due to excessive PTEN-mediated inhibition of Src. Therefore, a failure (C188–9) or delay (AD-HIES) in activation of Src signaling, potentially due to excess PTEN expression, contributes to impaired permeability in these STAT3 inhibited or mutated cells.
Figure 5. HUVECs permeability is regulated by STAT3-induced mir17–92 and can be restored in AD-HIES patient cells through the canonical Wnt signaling pathway.
(A) Western blot analysis of phosphorylated Src, total Src, and phosphorylated PTEN, in DMSO and C188–9 HUVECs following exposure to histamine (100 µM). (B–C) Western blot analysis of phosphorylated VE-cadherin, total VE-cadherin, active (non-phosphorylated) β-catenin, total β-catenin, phosphorylated Src, total Src, phosphorylated PTEN, total PTEN, and phosphorylated STAT3, in wild type and AD-HIES HUVECs following exposure to histamine (100 µM). Data are represented as the mean ± S.E.M. *p<0.05. (D) Real time PCR analysis of mir17–95 RNA expression in IL-11 (100ng/ml) treated wild type and AD-HIES HUVECs. Data are representative of 3 independent experiments. (E) Western analysis of SIAH1 and E2F1 expression in wild type and AD-HIES. Data are representative of 2 independent experiments. (F) Analysis of cytoplasmic verses nuclear active β-catenin in wild type and AD-HIES HUVECs.
Mutated STAT3 leads to decreased expression of transcriptional target mir-17–92 that regulates elements of the endothelial response to histamine
To demonstrate that the direct phosphorylation of STAT3 was not responsible for the effects on vascular permeability, we treated HUVECs with IL-11, which acts upon endothelial cell specific IL-11 receptors, which induced STAT phosphorylation in WT and AD-HIES HUVECs within 10 minutes. However, short-term IL-11 treatment (see Figure E9A in this article’s Online Repository at www.jacionline.org) did not affect vascular permeability of WT or AD-HIES HUVECs alone or in combination with histamine treatment (see Figure E7B, C, D in this article’s Online Repository at www.jacionline.org).
The micro RNA cluster mir17–92 is upregulated by STAT325 and strongly inhibits PTEN, a phosphatase that regulates Src signaling, under normal conditions36. We therefore found that the STAT3 agonist IL-1137 induced mir17–92 expression in wild type HUVECS within 3 h and was sustained over 24 h (see Figure 5D) but not in AD-HIES HUVECs. In agreement with reduced mir17–92 expression in STAT3 mutated cells, E2F1 and SIAH1 (regulators of β-catenin signaling) expression was increased (see Figure 5E), which may contribute to the increased basal cytoplasmic to nuclear active β-catenin observed in AD-HIES HUVECs (see Figure 5F)
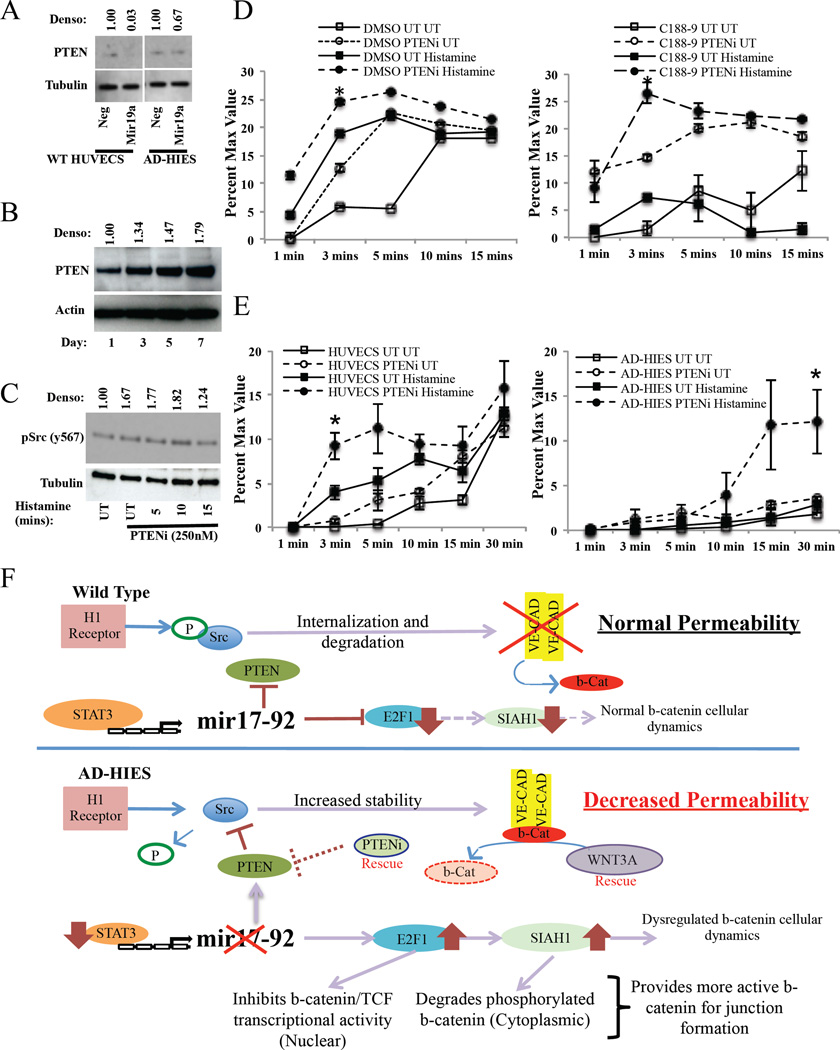
Because AD-HIES HUVECs have impaired Src signaling and mir17–92 cluster induction, and enhanced PTEN expression, we asked if exogenous expression of mir17–92 or inhibition of PTEN could rescue the permeability defect. Transfection with a mir1 9a mimic led to a decrease in total PTEN expression in wild type (nearly 100%) and AD-HIES HUVECs (33%) (see Figure 6A) and rescued the vascular permeability defect in AD-HIES HUVECs (see Figure E10 in this article’s Online Repository at www.jacionline.org). After transfection, total PTEN levels were 1.21 fold increased in AD-HIES compared to WT HUVECs. In addition, transfection of a mir19a antagomir in WT HUVECs under STAT3 stimulating conditions lead to increased total PTEN levels (see Figure E10B in this article’s Online Repository at www.jacionline.org).
Figure 6. PTEN inhibition restores STAT3-inhibited or mutated HUVECs permeability.
(A) PTEN expression in wild-type and AD-HIES HUVECs following transfection with a mir19 mimic. (B) PTEN expression in STAT3 inhibited wild-type HUVECs over 7 days. (C) Expression of phosphorylated Src in PTEN inhibitor pre-treated HUVECs (1h) that were subsequently exposed to histamine (100µM). (D–E) In vitro permeability assay of (D) DMSO or C188–9 treated HUVECs or (E) wild-type and AD-HIES HUVECs treated with a PTEN inhibitor (1h) and subsequently exposed to histamine (100µM). (F) Model of STAT3-regulation of vascular permeability in wild type and AD-HIES HUVECs. In wild type cells, histamine treatment leads to Src phosphorylation resulting in the dissociation of β-catenin and the internalization and degradation of VE-cadherin. Under conditions of normal STAT3 signaling, STAT3 induces expression of the mir17–92 microRNA cluster that suppresses PTEN, E2F1 and SIAH1 levels. This allows for the normal regulation of Src signaling and normal distribution of β-catenin cellular dynamics and thus, directly, regulates the amount of VE-cadherin and β-catenin. In STAT3-mutated cells, histamine treatment leads to no Src phosphorylation, due to decreased expression of mir17–92 resulting in increased PTEN. This prevents the internalization and degradation of VE-cadherin, ultimately increasing its expression. Mir17–92 deficiency also allows for increased E2F1 and SIAH1. This will degrade any phosphorylated β-catenin in the cytoplasm and inhibits nuclear translocation of non-phosphorylated cytoplasmic β-catenin. This provides more non-phosphorylated β-catenin to form adherin junctions, leading to decreased vascular permeability.
In order to demonstrate STAT3 inhibition, as opposed to just STAT3-mutation, also increases total PTEN levels we treated WT HUVECS with C188–9 for up to 7 days and found increased total PTEN levels (see Figure 6B and Figure E10C in this article’s Online Repository at www.jacionline.org). Treatment with a PTEN inhibitor (VO-OHpic) increased Src activity (see Figure 6C) and rescued the permeability defect seen in C188–9 treated (see Figure 6D) and AD-HIES (see Figure 6E) HUVECs. Of note, in DMSO-treated or wild type HUVECs, treatment with the PTEN inhibitor alone was sufficient to partially rescue permeability (see Figures D and E, left panels) correlating to the 67% increase seen in basal pSrc levels in the presence of the PTEN inhibitor alone (see Figure 6C). Taken together these data demonstrate that STAT3 inhibition promotes PTEN upregulation, increased PTEN impairs Src signaling, and inhibition of PTEN via small molecule or mir17–92 mediated inhibition can restore Src signaling and overcome the decreased vascular leakiness caused by impaired STAT3 signaling.
DISCUSSION
We describe a STAT3-dependent effect in humans and mice on adherens protein stability, which leads to altered vascular permeability and protection from systemic anaphylaxis, suggesting that certain clinical responses to mast cell mediators could be controlled via the inhibition of STAT3.
In agreement with our previous finding that STAT3 mutant AD-HIES patients are less susceptible to anaphylaxis compared to other highly atopic individuals9, IgE-mediated passive systemic anaphylaxis was more attenuated in mice that were treated with the STAT3 inhibitor C188–9.
Although mitochondrial STAT3 has been shown to play a role in IgE-antigen mediated mast cell degranulation 10 in rodent models, in the present study STAT3 inhibition did not result in a detectable difference in circulating mast cell mediators (histamine, MCPT1) in an in vivo anaphylaxis model, or defective β-hexosaminidase release in vitro in a mast cell activation assay. These differences may be reconciled by the fact that Erlich et al mainly used different concentrations of inhibitor and different cell lines that may have resulted in different on- and off-target effects. We found that hematocrit levels and local plasma transudate in response to IgE/Ag, but also to histamine alone, were significantly lower in C188–9 treated mice compared to untreated mice. These data suggest that STAT3 inhibition interferes with a common pathway regulating vascular permeability, and was supported by a decrease in the mediator-induced permeability in HUVECs in vitro. As such, while human mast cell function may indeed be affected by reduced STAT3 signaling, there appears to be a significant effect on endothelial cell responsiveness to mast cell mediators that contributes to the protection from immediate hypersensitivity reactions. Of note, the proximal histamine signaling defect observed here was similar to that seen via FcεRI in STAT3 deficient human mast cells and may suggest a similar underlying etiology 9.
The inflammatory cytokine IL-6 has also been shown to contribute to vascular leakage through IL-6 –related trans-signaling using STAT3 and ERK 22. While HUVECs do not express IL-6R 38, IL-11-mediated phosphorylation of STAT3 in HUVECs was observed had no effect on vascular permeability following short-term stimulation could be detected. Coupled with the fact that mice had to be pretreated with a STAT3 inhibitor for multiple days before any effect on permeability was observed, it is surmised that STAT3-linked transcriptionally controlled events were required to affect permeability.
MicroRNA cluster, mir17–92, is a direct downstream target of STAT325. Upregulation of mir17–92 leads to the direct suppression of PTEN and E2F126, negative regulators of Src and β-catenin/TCF signaling, respectively. Mir17–92 expression leads to a concomitant reduction in E2F1-dependent expression of the E3-ubiquitin ligase, SIAH139. Our data demonstrate that this pathway is active in HUVECs that have intact STAT3 but suppressed in STAT3-mutated AD-HIES HUVECs. That suppression appears to be occurring via two routes: (1) increased PTEN preventing Src-related dissolution of VE-cadherin, and (2) increased E2F1/SIAH1 altering β-catenin cellular dynamics, increasing adherens junction anchor availability. Of note, increased E2F1 leads to p53-dependant repression of VE 40,41, which normally destabilizes VE-cadherin junctions19, which may also contribute to this phenotype. This observation is supported by the fact that E2F1 knockout mice have increased vascular permeability, rapid PTEN reduction is seen during anaphylaxis, and that PTEN-deficient mice have enhanced allergic responses and increased vascular permeability 40,42–44. While the link between STAT3, the miR17–92 complex, PTEN expression and SRC-mediated responsiveness to histamine appears intriguing, further work will be necessary to definitively describe this mechanism.
In addition to β-catenin being a major component of VE-cadherin junctions, it is also a well-established target of the canonical Wnt pathway 45–47. Studies have shown that endothelial cell contact may be required to enhance co-localization of VE-cadherin and β-catenin at the cell membrane and that signaling via the canonical Wnt pathway (Wnt3A–mediated) disrupts the VE-cadherin/β-catenin interaction 48. In addition, non-canonical Wnt signaling (Wnt5A) can repress Wnt/β-catenin signaling 49,50. Consistent with these data, activation of the canonical Wnt signaling pathway in HUVECs decreased membrane associated β-catenin, and increased permeability even in AD-HIES patient cells.
Since activation of the Wnt pathway or inhibition of PTEN can rescue permeability and normal calcium mobilization is seen in both wild type and AD-HIES HUVECs, it is likely that a focal pathway defect exists in AD-HIES as opposed to a global problem of vascular permeability. The model generated from our data is summarized in Figure 6F.
Conclusions
This study shows that impaired STAT3 signaling reduces mast cell mediator-induced vascular permeability via enhanced expression and stability of VE-cadherin/β-catenin complexes in vascular endothelial cells. Reduced STAT3 signaling protects against passive anaphylaxis in both mut-Stat3 mice and WT mice treated with a Stat3 inhibitor. Such inhibition may be useful in preventing severe immediate hypersensitivity reactions in a variety of settings. It will also be of great interest to learn in future studies how STAT3 inhibition might disrupt abnormal vascular permeability observed in other inflammatory and non-inflammatory settings—in particular because AD-HIES patients’ inflammatory responses to infection are markedly curtailed 51,52.
Clinical Implications.
Long-term functional ablation of STAT3 prevents vascular mediator-induced dissolution of adherens junctions, and suggests that clinical conditions of excess vascular permeability of all sorts may be modulated via small molecule inhibition of STAT3.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the NIAID. This project has been funded [in part] with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations used
- AD-HIES
autosomal dominant-hyper IgE syndrome
- BMMC
bone marrow-derived mast cells
- DNP-HAS
Dinitrophenyl-human serum albumin
- MCPT1
mast cell protease 1
- Mir
microRNA
- PCA
passive cutaneous anaphylaxis
- PDMC
peritoneal derived mast cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: V.H., M.P.O. J.J.L., A.O. wrote the manuscript, designed the research, performed research, analyzed and interpreted data, and provided study material. P.S. performed research, analyzed and interpreted data. T.D., N.J., C.N., performed research. M.B., S.M.H., A.F.F. provided study material and patients. D.J.T. provided study material. D.D.M., J.D.M. wrote the manuscript, designed the research, analyzed and interpreted data, and provided study material and patients.
Competing financial interests:
David J. Tweardy is President and CEO of StemMed, the company that provided the C188–9 compound.
References
- 1.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 2.Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 4.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515–528. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Lango Allen H, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46:812–814. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2014 doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haapaniemi EM, Kaustio M, Rajala HL, van Adrichem AJ, Kainulainen L, Glumoff V, et al. Autoimmunity, hypogammaglobulinemia, lymphoproliferation and mycobacterial disease in patients with dominant activating mutations in STAT3. Blood. 2014 doi: 10.1182/blood-2014-04-570101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman AF, Holland SM. Clinical manifestations of hyper IgE syndromes. Dis Markers. 2010;29:123–130. doi: 10.3233/DMA-2010-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, Jung MY, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013;132:1388–1396. doi: 10.1016/j.jaci.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erlich TH, Yagil Z, Kay G, Peretz A, Migalovich-Sheikhet H, Tshori S, et al. Mitochondrial STAT3 plays a major role in IgE-antigen-mediated mast cell exocytosis. J Allergy Clin Immunol. 2014;134:460–469. doi: 10.1016/j.jaci.2013.12.1075. [DOI] [PubMed] [Google Scholar]
- 11.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 12.Mitsuhata H, Shimizu R, Yokoyama MM. Role of nitric oxide in anaphylactic shock. J Clin Immunol. 1995;15:277–283. doi: 10.1007/BF01541317. [DOI] [PubMed] [Google Scholar]
- 13.Valone FH, Johnson B. Modulation of platelet-activating-factor-induced calcium influx and intracellular calcium release in platelets by phorbol esters. Biochem J. 1987;247:669–674. doi: 10.1042/bj2470669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotlikoff MI, Murray RK, Reynolds EE. Histamine-induced calcium release and phorbol antagonism in cultured airway smooth muscle cells. Am J Physiol. 1987;253:C561–c566. doi: 10.1152/ajpcell.1987.253.4.C561. [DOI] [PubMed] [Google Scholar]
- 15.Kaliner M, Sigler R, Summers R, Shelhamer JH. Effects of infused histamine: analysis of the effects of H-1 and H-2 histamine receptor antagonists on cardiovascular and pulmonary responses. J Allergy Clin Immunol. 1981;68:365–371. doi: 10.1016/0091-6749(81)90134-2. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch CM, Brokaw JJ, Prow DM, White GW. Mechanism of platelet activating factor-induced vascular leakage in the rat trachea. Exp Lung Res. 1992;18:447–459. doi: 10.3109/01902149209064339. [DOI] [PubMed] [Google Scholar]
- 17.Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol. 1999;19:2286–2297. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- 18.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, et al. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–1077. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 19.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deo DD, Bazan NG, Hunt JD. Activation of platelet-activating factor receptor-coupled G alpha q leads to stimulation of Src and focal adhesion kinase via two separate pathways in human umbilical vein endothelial cells. J Biol Chem. 2004;279:3497–3508. doi: 10.1074/jbc.M304497200. [DOI] [PubMed] [Google Scholar]
- 21.Geletu M, Guy S, Arulanandam R, Feracci H, Raptis L. Engaged for survival: From cadherin ligation to STAT3 activation. JAKSTAT. 2013;2:e27363. doi: 10.4161/jkst.27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei LH, Chou CH, Chen MW, Rose-John S, Kuo ML, Chen SU, et al. The role of IL-6 trans-signaling in vascular leakage: implications for ovarian hyperstimulation syndrome in a murine model. J Clin Endocrinol Metab. 2013;98:E472–E484. doi: 10.1210/jc.2012-3462. [DOI] [PubMed] [Google Scholar]
- 23.Guy S, Geletu M, Arulanandam R, Raptis L. Stat3 and gap junctions in normal and lung cancer cells. Cancers (Basel) 2014;6:646–662. doi: 10.3390/cancers6020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder-Talkington BN, Schwegler-Berry D, Castranova V, Qian Y, Guo NL. Multi-walled carbon nanotubes induce human microvascular endothelial cellular effects in an alveolar-capillary co-culture with small airway epithelial cells. Part Fibre Toxicol. 2013;10:35. doi: 10.1186/1743-8977-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai B, Meng J, Peyton M, Girard L, Bornmann WG, Ji L, et al. STAT3 mediates resistance to MEK inhibitor through microRNA miR-17. Cancer Res. 2011;71:3658–3668. doi: 10.1158/0008-5472.CAN-10-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Haaften G, Agami R. Tumorigenicity of the miR-17–92 cluster distilled. Genes Dev. 2010;24:1–4. doi: 10.1101/gad.1887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armanious H, Gelebart P, Mackey J, Ma Y, Lai R. STAT3 upregulates the protein expression and transcriptional activity of beta-catenin in breast cancer. Int J Clin Exp Pathol. 2010;3:654–664. [PMC free article] [PubMed] [Google Scholar]
- 28.Kawada M, Seno H, Uenoyama Y, Sawabu T, Kanda N, Fukui H, et al. Signal transducers and activators of transcription 3 activation is involved in nuclear accumulation of beta-catenin in colorectal cancer. Cancer Res. 2006;66:2913–2917. doi: 10.1158/0008-5472.CAN-05-3460. [DOI] [PubMed] [Google Scholar]
- 29.Steward-Tharp SM, Laurence A, Kanno Y, Kotlyar A, Villarino AV, Sciume G, et al. A mouse model of HIES reveals pro- and anti-inflammatory functions of STAT3. Blood. 2014;123:2978–2987. doi: 10.1182/blood-2013-09-523167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–1440. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cgamma and Cbeta: a novel mechanism for PI3K–independent enhancement of FcepsilonRI-induced mast cell mediator release. Cell Signal. 2008;20:625–636. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB, Tweardy DJ. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood. 2011;117:5701–5709. doi: 10.1182/blood-2010-04-280123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidibe A, Imhof BA. VE-cadherin phosphorylation decides: vascular permeability or diapedesis. Nat Immunol. 2014;15:215–217. doi: 10.1038/ni.2825. [DOI] [PubMed] [Google Scholar]
- 36.Dey N, Crosswell HE, De P, Parsons R, Peng Q, Su JD, et al. The protein phosphatase activity of PTEN regulates SRC family kinases and controls glioma migration. Cancer Res. 2008;68:1862–1871. doi: 10.1158/0008-5472.CAN-07-1182. [DOI] [PubMed] [Google Scholar]
- 37.McKinley D, Wu Q, Yang-Feng T, Yang YC. Genomic sequence and chromosomal location of human interleukin-11 gene (IL11) Genomics. 1992;13:814–819. doi: 10.1016/0888-7543(92)90158-o. [DOI] [PubMed] [Google Scholar]
- 38.Matsumiya T, Imaizumi T, Fujimoto K, Cui X, Shibata T, Tamo W, et al. Soluble interleukin-6 receptor alpha inhibits the cytokine-Induced fractalkine/CX3CL1 expression in human vascular endothelial cells in culture. Exp Cell Res. 2001;269:35–41. doi: 10.1006/excr.2001.5300. [DOI] [PubMed] [Google Scholar]
- 39.Xie W, Jin L, Mei Y, Wu M. E2F1 represses beta-catenin/TCF activity by direct up-regulation of Siah1. J Cell Mol Med. 2009;13:1719–1727. doi: 10.1111/j.1582-4934.2008.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin G, Kishore R, Dolan CM, Silver M, Wecker A, Luedemann CN, et al. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc Natl Acad Sci U S A. 2006;103:11015–11020. doi: 10.1073/pnas.0509533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61:6952–6957. [PubMed] [Google Scholar]
- 42.Kang NI, Yoon HY, Kim HA, Kim KJ, Han MK, Lee YR, et al. Protein kinase CK2/PTEN pathway plays a key role in platelet-activating factor-mediated murine anaphylactic shock. J Immunol. 2011;186:6625–6632. doi: 10.4049/jimmunol.1100007. [DOI] [PubMed] [Google Scholar]
- 43.Furumoto Y, Charles N, Olivera A, Leung WH, Dillahunt S, Sargent JL, et al. PTEN deficiency in mast cells causes a mastocytosis-like proliferative disease that heightens allergic responses and vascular permeability. Blood. 2011;118:5466–5475. doi: 10.1182/blood-2010-09-309955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee KS, Kim SR, Park SJ, Lee HK, Park HS, Min KH, et al. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) reduces vascular endothelial growth factor expression in allergen-induced airway inflammation. Mol Pharmacol. 2006;69:1829–1839. doi: 10.1124/mol.106.022228. [DOI] [PubMed] [Google Scholar]
- 45.Lien WH, Fuchs E. Wnt some lose some: transcriptional governance of stem cells by Wnt/beta-catenin signaling. Genes Dev. 2014;28:1517–1532. doi: 10.1101/gad.244772.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenbluh J, Wang X, Hahn WC. Genomic insights into WNT/beta-catenin signaling. Trends Pharmacol Sci. 2014;35:103–109. doi: 10.1016/j.tips.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Menge T, Gerber M, Wataha K, Reid W, Guha S, Cox CS, Jr, et al. Human mesenchymal stem cells inhibit endothelial proliferation and angiogenesis via cell-cell contact through modulation of the VE-Cadherin/beta-catenin signaling pathway. Stem Cells Dev. 2013;22:148–157. doi: 10.1089/scd.2012.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis SD, Schaller J, Wedgwood RJ. Job’s Syndrome Recurrent, “cold”, staphylococcal abscesses. Lancet. 1966;1:1013–1015. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- 52.Buckley RH, Wray BB, Belmaker EZ. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972;49:59–70. [PubMed] [Google Scholar]