Abstract
Background
Several different interventions have been examined to alleviate pain and reduce frequency of trigeminal neuralgia (TN) paroxysms. However, some patients continue to have persistent or recurrent painful attacks. Using a systematic review and meta-analysis approach, we aimed to synthesize evidence from published randomized controlled trials (RCTs) regarding safety and efficacy of botulinum toxin type A (BTX-A) as a possible emerging choice of treatment for TN.
Methods
We conducted an electronic search in 10 databases/electronic search engines to access relevant publications. All articles in all languages reporting RCTs on the efficacy and safety of BTX-A in the treatment of TN were included for systematic review and meta-analysis.
Results
A total of four RCTs (n = 178) were identified for final meta-analysis. The overall effect favored BTX-A versus placebo in terms of proportion of responders (risk ratio RR = 2.87, 95 % confidence interval CI [1.76, 4.69], p <0.0001) with no significant detected heterogeneity (p = 0.31; I2 = 4 %). Paroxysms frequency per day was significantly lower for BTX-A group (mean difference MD = -29.79, 95 % CI [-38.50,–21.08], p <0.00001) with no significant heterogeneity (p = 0.21; I2 = 36 %).
Conclusion
Despite limited data, our results suggest that BTX-A may be an effective and safe treatment option for patients with TN. Further larger and well-designed RCTs are encouraged to translate these findings into better clinical outcome and better quality of life for TN patients.
Electronic supplementary material
The online version of this article (doi:10.1186/s10194-016-0651-8) contains supplementary material, which is available to authorized users.
Keywords: Botulinum, BTX-A, Trigeminal neuralgia, Clinical trials, Systematic review, Meta-analysis
Introduction
Trigeminal neuralgia (TN) is a characteristic pain along the distribution of one or more branches of the trigeminal nerve. The pain is usually unilateral and described as severe, sharp, and stabbing electric shock-like pain. Consequently, quality of life of TN patients is profoundly worsened due to impairment of daily life activities, thus patients are at a high risk of depression and other psychiatric disorders [1–3]. Epidemiological studies showed that approximately 4 to 28.9/100,000 persons worldwide experience TN [4, 5]. Moreover, a recent large retrospective cohort study reported increased risk of depression and anxiety disorders in TN patients [6]. To date, the mechanisms underlying the pathogenesis of TN are still controversial; however, the microvascular compression is the most common hypothesis [7, 8].
Recently, therapeutic and surgical approaches have been evolved to alleviate the neuropathic pain and improve quality of life in TN patients. Oral antiepileptic drugs, including carbamazepine, remain the first line of treatment [9]. Yet, 25–50 % of cases became refractory to the drug therapy [10]. Invasive operations such as neurovascular decompression [11], gamma knife radiosurgery [12], partial sensory rhizotomy, and percutaneous radiofrequency thermo-coagulation [13] may be required in some intractable cases. Then again, Surgical interventions occasionally effect severe and often untreatable complications that might be even worse than the primary TN [14]. Moreover, a study reported recurrence of the pain in about half of the patients within 2 years of percutaneous radiofrequency rhizotomy [15]. Recently, a cross specialty management program for TN patients was implemented in a tertiary referral center for headache and pain [16]. The program involved collaboration between neurologists, neuroradiologists, and neurosurgeons and showed promising in terms of feasibility and utility in management of TN [16].
In light of the evidence currently available, it seems fair to argue that there is an overwhelming need for developing much safer, better tolerated, and efficacious treatment for TN. Botulinum toxin type A (BTX-A) is a neurotoxin derived from Clostridium botulinum [17]. It acts via inhibiting release of acetylcholine at neuromuscular junctions, causing relaxation of the muscle [18, 19]. BTX-A has been shown to be a promising option for treatment of headache [20, 21]. It was further approved by the US Food and Drug Administration (FDA) for prevention of headache in adults suffering from chronic migraine.
In 2002, Micheli and colleagues [22] reported successful relief of twitching and pain in a patient with TN-associated hemifacial spasm. Since then, several clinical trials have been investigating the safety and efficacy of BTX-A in TN. The drug showed favorable effects in many reports. Having said that, the majority of studies were at a high risk of bias, aside from the small sample size included in each trial. This necessitates a confirmatory evidence to be released asserting the notion that BTX-A may be safe and effective option for management of TN.
The aim of this study is to provide a clear-cut evidence regarding safety and efficacy of botulinum toxin type A in trigeminal neuralgia from published randomized controlled trials in a systematic review and meta-analysis approach.
Methods
Search Strategy
The study strictly followed the recommendation of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Additional file 1: Table S1). During September and October, we established the protocol and registered our study with PROSPERO, University of York (CRD42015026861). Ten electronic search engines/libraries were systematically searched for relevant publications, including PubMed, Scopus, Web of Science, Google Scholar, Virtual Health Library (VHL), WHO Global Health Library (GHL), ClinicalTrials, POPLINE, System for Information on Grey Literature in Europe (SIGLE), and the New York Academy of Medicine (NYAM). Except for Google Scholar, the search term for all other libraries was as follows: (botulinum OR onabotulinumtoxinA OR onabotulinum OR botox OR botulinus) AND ((trigeminal neuralgia) OR prosopalgia OR (Fothergill's disease) OR (tic doloureux)). For Google Scholar, we used the advanced setting in which “trigeminal neuralgia” was filled in “with all of the words” and “botulinum botulinus botox onabotulinum toxin A onabotulinum” for “with at least one of the words”. Additionally, we conducted a manual search by reviewing the citations within the included publications and reviewing the related references presented in PubMed.
Selection criteria and title and abstract screening
Search results from the ten aforementioned search engines/libraries were imported into Endnote X7 (Thompson Reuter, CA, USA) for duplicates deletion. Two reviewers independently screened the references using the predetermined eligibility criteria. The inclusion criteria were: (i) randomized-controlled trials (RCTs) reporting efficacy and safety of botulinum toxin in treatment of trigeminal neuralgia and (ii) no restriction on language, area, publication year, and age of patients. The exclusion criteria were: (i) unreliably extracted data; (ii) over-lapped data sets; (iii) book chapters, abstract articles only, conference papers, reviews, theses, and posters; and (iv) in vitro or animal studies. Any different decision in screening step was discussed between two reviewers to reach a consensus. Consultation from supervisor (NTH and KH) was acquired if necessary. The full texts of included references were then retrieved through the Library of Nagasaki University, and full text screening was subsequently conducted to identify relevant references for data extraction.
Outcome measures
All patients’ outcomes were considered to analyze the efficacy and safety of botulinum toxin in the treatment of trigeminal neuralgia: (1) proportion of respondents – defined as patients with >50 % reduction in mean pain score from baseline to endpoint; (2) mean paroxysms frequency per day; (3) mean visual analog scale (VAS) score at the end of follow up; and (4) adverse events and complications of BTX A treatment.
Data extraction
The standardized template was developed through a pilot extraction with the two most relevant references. Two researchers then independently extracted the data into the template. Extracted data included: authors, publication year, journal, country of authors, country of patients, source of BTX-A, active drug types and their doses, control types and their doses, number and site of injection, characteristics of patients (gender and age), duration of treatment, duration of follow up, year of neuralgia, affected branch, surgical procedures, efficacy point, proportion of responders, relapse, visual analog scale, number of paroxysms per day, quality of life, anxiety scale, and depression scale .
Statistical method
Statistical analysis was carried out using RevMan version 5.3 (The Cochrane Collaboration, Oxford, United Kingdom). Continuous data were pooled as mean difference (MD), while pooled risk ratio (RR) was combined for dichotomous data using Mantel-Haenszel (M-H) method. Fixed effect model was adopted in all analyses. We performed a sensitivity analysis to: (1) investigate the effect of the model assumed on the overall effect size, by running the analysis under the random effects model and observing if a difference exists (2) explore the effect of the study quality on the summary effect estimate, by omitting studies rated as "high risk of bias" for both random sequence generation and allocation concealment. Heterogeneity was assessed by visual inspection of the forest plots and measured by I-square and Chi-square tests. P-values reported in this paper are not adjusted for multiplicity; however, adequate randomization and blinding was achieved by most included studies, ensuring appropriate control for confounders. P-value < 0.05 was considered statistically significant.
Quality assessment
The methodological quality of each RCT was independently assessed by two reviewers using "The Cochrane Collaboration's tool for assessing risk of bias". It is a two-part tool, addressing seven specific domains, including: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. The judgment of each reviewer on each domain was categorized as ‘low risk,’ ‘high risk,’ or ‘unclear risk’ of bias. Any disagreement was resolved by discussion between two reviewers and by consultation from a supervisor to reach a consensus.
Results
Literature search
A total number of 267 articles were retrieved from six search engines/libraries (Fig. 1). SIGLE, POPLINE, NYAM, and ClinicalTrials generated no results. After the initial title and abstract screening of the 267 articles, 28 articles were selected for full-text reading. Two independent reviewers performed the full-text screening after which 24 articles were excluded due to: (1) inappropriate study design; (2) unreliably extracted data; (3) in vitro or animal study; and (4) posters. Finally, a total of four unique RCTs, with a total of 178 TN patients, were included for data extraction and final analysis.
Fig. 1.

PRISMA flow diagram of studies’ screening and selection
Study characteristics
The characteristics of four RCTs included in the final analysis were summarized in Table 1. A total of 178 patients (99 in the BTX-A group and 79 in the placebo control group) were included from four trials. The mean age for both case and control groups in three RCTs [23–25] was ranging from 58.1 to 64.5 for case group and from 58 to 66 for control group. There was no significant difference between BTX-A and placebo groups in terms of frequency of attacks and pain severity before treatment as reported in each included study. The follow-up period varied from 8 to 12 weeks except for no report from study of Zhang et al. The mean duration of the effect was also ranging between 8 to 12 weeks in two trials [23, 25].
Table 1.
Baseline characteristics of included RCTs
| Author | No. of patients | Mean age (year) | Mean duration before treatment (year) | Frequency of attacks per day before treatment (SD) | Pain severity before treatment, VAS Mean (SD) | Mean follow up (week) | Mean duration of effect (week) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cases | control | cases | control | cases | control | cases | control | cases | control | cases | control | cases | control | ||||
| Zhang et al. [24] | 25 Group1: 25U | 28 Group2: 75U | 27 | 58.16 | 62.64 | 58.41 | ND | ND | ND | ND | 6.24 (2.13) | 7.18 (2.21) | 6.96 (1.97) | ND | ND | ND | ND |
| Shehata et al. [26] | 10 | 10 | ND | ND | ND | ND | 36.7 (3.13) | 39.2 (3.05) | 8.3 | 8.5 | 12 | 12 | ND | ND | |||
| Wu et al. [23] | 20 | 22 | 59.14 | 58 | ND | ND | 21.71 (22.68) | 20.53 (10.38) | 7.05 (2.03) | 6.88 (2.25) | 12 | 12 | 12 | 12 | |||
| Zúñiga et al. [25] | 16 | 20 | 64.5 | 66.06 | 6.2 | 5.2 | 29.1 (31.36) | 31.06 (31.62) | 8.85 | 8.19 | 8 | 8 | 8 | 8 | |||
ND: no data
RCT: randomized controlled trials
The amount of BTX-A injected, the route of injection, the number of injections, and the injection site varied among studies. The amount of BTX-A injected ranged from a minimum of 25 U in study of Zhang et al. [24] to a maximum of 100 U in study of Shehata et al. [26]. In addition, 50 U and 75 U of BTX-A were used in studies of Zúñiga et al. [25] and Wu et al. [23], respectively. The administration of BTX-A included subcutaneous or intradermal routes. The details of the injection protocol in each trial are summarized in Table 2.
Table 2.
Injection protocol of BTX-A
| Author | Type of BTX-A | Source of BTX-A | Amount of BTX-A | Injection sites | No. of injection | Rout of injection | Blinding |
|---|---|---|---|---|---|---|---|
| Zhang et al. [24] | Botulinum toxin type A (BTX-A) | Lanzhou Bioproduction Institute, Lanzhou, China | Group 1:25u Group 2:75u | Dermatome and/or mucosa | 20 | Intradermal and submucosal | Double blind |
| Shehata et al. [26] | Botulinum toxin type A (Botox®) | Not reported | 100u | Trigger zones | 1 | SC | Double blind |
| Wu et al. [23] | BTX-A (100U of Clostridium botulinum type-A neurotoxin complex, 5 mg gelatin, 25 mg dextran, and 25 mg saccharose) | Lanzhou Biological Products Institute, China | 75u | Trigger zones | 15 | Intradermal | Double blind |
| Zúñiga et al. [25] | Onabotulinum toxin A (Botox) | Not reported | 50u | Various sites, 1 cm apart from one another | 1 | SC | Double blind |
SC Subcutaneous
Quality assessment
The quality of included studies ranged from medium to high as illustrated in Fig. 2. Authors’ judgments with justifications are shown in (Additional file 2: Table S2).
Fig. 2.
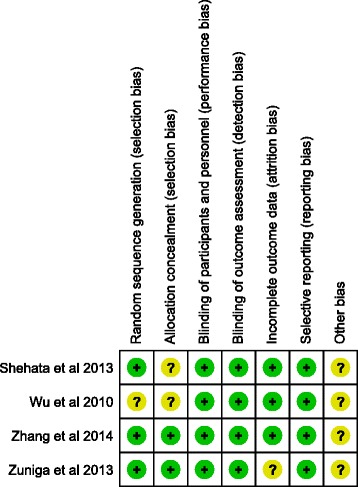
Cochrane bias assessment of included studies
Therapeutic efficacy
Three patients’ therapeutic outcomes include: (1) proportion of respondents – defined as patients with >50 % reduction in mean pain score from baseline to endpoint; (2) mean paroxysms frequency per day; and (3) mean visual analog scale (VAS) score at the end of follow up.
The overall effect favored BTX-A compared with placebo in terms of proportion of responders (RR = 2.87, 95 % CI [1.76, 4.69], P < 0.0001) with no significant heterogeneity detected (P = 0.31; I2 = 4 %) (Fig. 3a). Mean VAS score was significantly lower for BTX-A group at the end of the first month (MD = -2.89, 95 % CI [-4.66,–1.12], P = 0.001), at the end of the 2nd month (MD = -2.47, 95 % CI [-3.96,–0.99], P = 0.001), and at the end of the 3rd month of follow up (MD = -3.43, 95 % CI [-5.21,–1.64], P = 0.0002) with no significant heterogeneity detected for all endpoints (Fig. 3b). Mean paroxysms frequency per day was significantly lower for BTX-A group (MD = -29.79, 95 % CI [-38.50,–21.08], P < 0.00001) with no significant heterogeneity (P = 0.21; I2 = 36 %) (Fig. 3c).
Fig. 3.
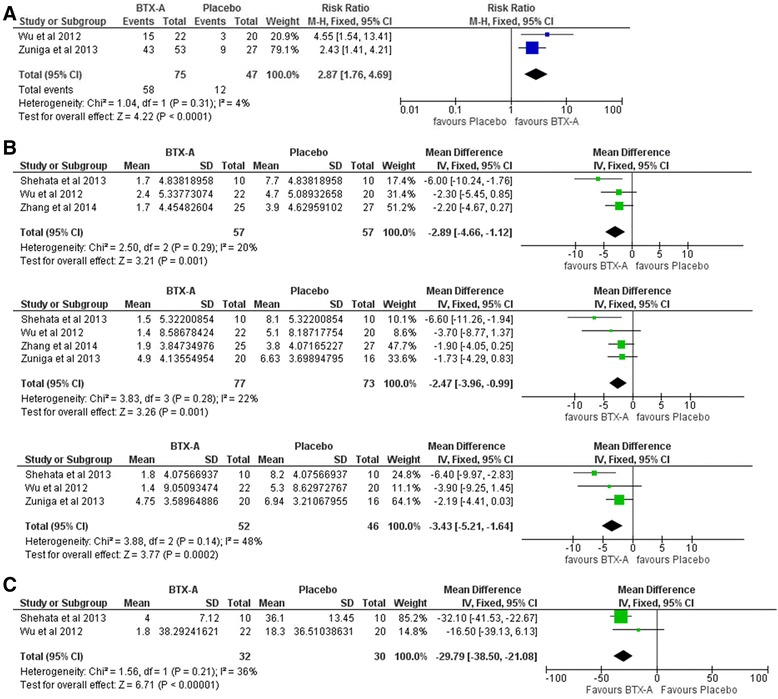
A Forest plots of relative risk (RR) for proportion of respondents comparing BTX A with placebo. M-H: Mantel-Haenzel, CI: confidence interval. B Forest plots of mean difference in VAS score comparing BTX A with placebo at the end of follow up (A), 1 month (B), 2 month (C), and 3 month (D). VAS: Visual analogue scale, MD: Mean difference, M-H: Mantel-Haenzel, CI: confidence interval. C Forest plots of mean difference in number of paroxysm comparing BTX A with placebo. MD: Mean difference, M-H: Mantel-Haenzel, CI: confidence interval
Adverse events
Regarding safety of BTX-A, there were two reported injection-related side effects: facial asymmetry and edema/hematoma at the site of injection, and both of which were generally tolerated and transitory in nature. The overall occurrence of facial asymmetry in BTX-A group ranged from 2-5 patients while it ranged between 1–2 for edema/hematoma. Facial asymmetry recovered within 5–7 weeks while edema/hematoma resolved within 5–6 days. None of the controlled patients developed facial asymmetry or edema/hematoma except two patients in Shehata et al. [26] and one patient in Wu et al. [23]. The adverse events were fully summarized in Table 3.
Table 3.
Adverse events for all patients in included RCTs
| Author/reference | Facial asymmetry | Edema/hematoma at injection area | ||||
|---|---|---|---|---|---|---|
| Exposed | Control | Disappearance | Exposed | Control | Disappearance | |
| Zhang et al. [24] | 3 | 0 | 6 weeks | 2 | 0 | 5 days |
| Shehata et al. [26] | 4 | 0 | not reported | 1 | 2 | not reported |
| Wu et al [23] | 5 | 0 | 7 weeks | 2 | 1 | 7 days |
| Zúñiga et al [25] | 2 | 0 | not reported | 2 | 0 | not reported |
Additional analysis
Only one study [23] had both sequence generation and allocation concealment rating of "high risk of bias". Omitting this study from the analysis, slightly changed the effect estimate and CI but did not have a serious impact on the findings (Fig. 4). Moreover, the overall effect estimate did not differ significantly for any of the outcomes on using the random effects model (Fig. 5). This in turn adds to the robustness of the results.
Fig. 4.
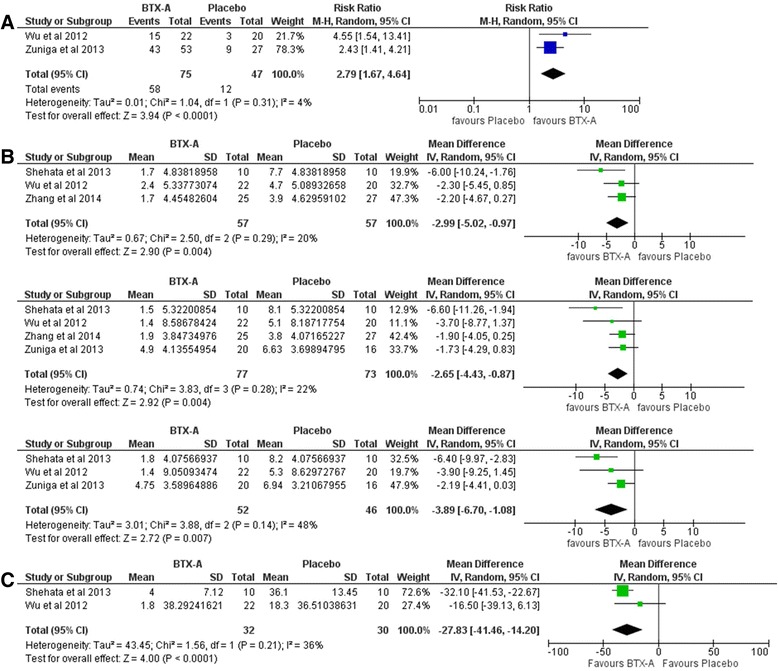
Forest plots of Sensitivity analysis (random effects model)
Fig. 5.
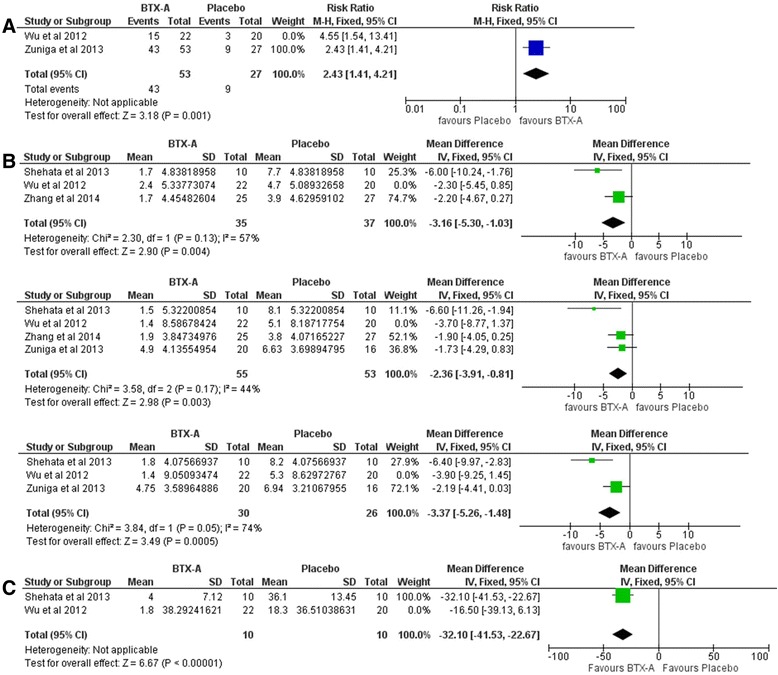
Forest plots of Sensitivity analysis (removing one study at a time)
Discussion
Treatment of TN continues to present a clinical appeal to both patients and health care providers. Medications (such as antiepileptic drugs) were poorly tolerated. They effected central nervous system adverse events in a substantial proportion of patients [27]. As such, refractory cases were approached by alternative surgical interventions, which yielded marked improvement in about 63–94 % of cases [28]. Nonetheless, serious complications of invasive surgery (including aseptic meningitis and hearing loss) rendered further sufferings to TN patients [28].
Thus safer, well tolerated, and more effective alternative is imperative for TN. The evidence from this systematic review suggests that botulinum toxin type A (BTX-A) has a clinically significant benefit in treatment of trigeminal neuralgia when compared to placebo in terms of proportion of responders, the mean paroxysms frequency per day, and the VAS score at the end of follow up. These overall outcomes consistently favored BTX-A compared with placebo across studies.
The duration of effect for BTX-A seems to be of relatively long duration (at least 3 months). In Wu et al. [23] trial, BTX-A was effective within a median follow-up duration of 90 days. Also, Zuniga et al. [25] showed that BTX-A was effective during the 3 months follow-up period. However, a conclusion cannot be made on how long the effect of BTX-A lasts, and how often recurrent injections are needed. This raises the need for further studies with extended follow-up duration that implement chronic pain measurement scales. A point of variation is the dosage of BTX-A; the used dosage of BTX-A in the analyzed RCTs ranged from 25 to 100 U. Zhang et al. [24] found that lower dose (25 U) and higher dose (75 U) were similar in efficacy on short-term assessment. Moreover, a dramatic response to BTX-A was also reported in an open-label trial [29] at a much lower dose of BTX-A (6.45–9.11 U).
The treatment mechanism of BTX-A in TN remains unclear. Previous preclinical studies suggested that it acts locally or on the trigeminal ganglia [30, 31]. Conversely, Matak et al. reported a central antinociceptive effect of BTX-A in a rat model of TN [32, 33]. Wu et al. recently asserted this notion and proved that peripherally applied BTX-A exerts antinociceptive function via reducing central sensitization and inhibiting high expression of nociceptors [34].
It is noteworthy to mention that Yong Hu et al. [35] approached the same topic with our study, but they mostly included open-label trials, which potentially weakened their conclusion. Furthermore, by combining one randomized, double-blind, placebo control trial with five open-label trials, the previous study possibly introduced some heterogeneity into their review.
One limitation of the current systematic review was the small number of trials that investigated the safety and efficacy of BTX-A in trigeminal neuralgia. Therefore, we cannot explore the publication bias using Egger's test for funnel plot asymmetry, we rather searched clinical trial registries and grey literature and found only two protocols of ongoing studies. No completed unpublished studies were retrieved. Missing data (mean baseline age in Shehata et al. and length of follow up in Zhang et al.) could not be used to adjust for possible biases in the aforementioned studies. More double-blinded RCTs are required to elaborate more on (1) optimal dose of BTX-A, (2) optimal duration of treatment, and (3) optimal route of administration. In addition, further research is needed to explore more on the mechanism of action of BTX-A to relieve pain in trigeminal neuralgia.
Conclusions
In conclusion, our systematic review suggests that BTX-A is a promising alternative treatment option that might spare the need for surgical interventions for refractory cases of trigeminal neuralgia in the future. Evidence from larger and well-designed RCTs is still required to assert upon these findings.
Article highlights
➢ RCTs reporting the efficacy and safety of BTX-A in the treatment of TN were included for systematic review and meta-analysis.
➢ BTX-A is an effective and safe treatment option for patients with trigeminal neuralgia.
➢ No serious adverse events or systemic reaction of BTX-A have been reported.
Acknowledgments
This study was supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Additional files
Table S1. The PRISMA checklist. (DOCX 28 kb)
Table S2. Risk of bias with author judgments with justifications. (DOCX 17 kb)
Footnotes
Competing interest
The authors declare that they have no competing interest.
Author contributions
NTH, MEM, KH participated in the design of the study. MEM, AE, AA, AMK, AMAA, TLHV, MRM, KH, NTH carried out in the data collection and analysis. All authors wrote the manuscript, read and approved the final manuscript.
Contributor Information
Mostafa Ebraheem Morra, Email: mostafamorra430@gmail.com.
Ahmed Elgebaly, Email: ahmedelgebaly94@gmail.com.
Ahmed Elmaraezy, Email: ahmadalmraezy@azhar.edu.eg.
Adham M. Khalil, Email: adham19.hazem91@gmail.com
Ahmed M. A. Altibi, Email: ahmed.mt@outlook.com
Tran Le-Huy Vu, Email: vtran138@ucla.edu.
Mostafa Reda Mostafa, Email: moustafazaalouk33@gmail.com.
Nguyen Tien Huy, Phone: +81-95-819-7805, FAX: +81-95-819-7821, Email: tienhuy@nagasaki-u.ac.jp.
Kenji Hirayama, Email: hiraken@nagasaki-u.ac.jp.
References
- 1.Devor M, Wood I, Sharav Y, Zakrzewska JM (2008) Trigeminal neuralgia during sleep. Pain Pract 8(4):263–8. http://onlinelibrary.wiley.com/doi/10.1111/j.1533-2500.2008.00214.x/full [DOI] [PubMed]
- 2.Hals EKB, Stubhaug A. Mental and somatic co-morbidities in chronic orofacial pain conditions: Pain patients in need of multiprofessional team approach. Scand. J. Pain. 2011;2(4):153–154. doi: 10.1016/j.sjpain.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Zakrzewska JM. Multi-dimensionality of chronic pain of the oral cavity and face. J. Headache Pain. 2013;14:37. doi: 10.1186/1129-2377-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos WK, Linhares MN. A prospective study of 39 patients with trigeminal neuralgia treated with percutaneous balloon compression. Arq. Neuropsiquiatr. 2011;69(2A):221–6. doi: 10.1590/S0004-282X2011000200016. [DOI] [PubMed] [Google Scholar]
- 5.Zakrzewska JM, McMillan R. Trigeminal neuralgia: the diagnosis and management of this excruciating and poorly understood facial pain. Postgrad. Med. J. 2011;87(1028):410–6. doi: 10.1136/pgmj.2009.080473. [DOI] [PubMed] [Google Scholar]
- 6.Wu T-H, Hu L-Y, Lu T, Chen P-M, Chen H-J, Shen C-C, Wen C-H. Risk of psychiatric disorders following trigeminal neuralgia: a nationwide population-based retrospective cohort study. J. Headache Pain. 2015;16:64. doi: 10.1186/s10194-015-0548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia D, Li G. Bioresonance hypothesis: a new mechanism on the pathogenesis of trigeminal neuralgia. Med. Hypotheses. 2010;74(3):505–7. doi: 10.1016/j.mehy.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Obermann M, Holle D, Katsarava Z. Trigeminal neuralgia and persistent idiopathic facial pain. Expert Rev. Neurother. 2011;11(11):1619–29. doi: 10.1586/ern.11.156. [DOI] [PubMed] [Google Scholar]
- 9.Cruccu G, Gronseth G, Alksne J, Argoff C, Brainin M, Burchiel K, Nurmikko T, Zakrzewska JM. AAN-EFNS guidelines on trigeminal neuralgia management. Eur. J. Neurol. 2008;15(10):1013–28. doi: 10.1111/j.1468-1331.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 10.Cruccu G, Truini A. Refractory trigeminal neuralgia. Non-surgical treatment options. CNS Drugs. 2013;27(2):91–6. doi: 10.1007/s40263-012-0023-0. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarthi PS, Ghanta R, Kattimani V. Microvascular decompression treatment for trigeminal neuralgia. J. Craniofac. Surg. 2011;22(3):894–898. doi: 10.1097/SCS.0b013e31821a07b7. [DOI] [PubMed] [Google Scholar]
- 12.Park K-J, Kano H, Berkowitz O, Awan NR, Flickinger JC, Lunsford LD, Kondziolka D. Computed tomography-guided γ knife stereotactic radiosurgery for trigeminal neuralgia. Acta Neurochir (Wien) 2011;153(8):1601–1609. doi: 10.1007/s00701-011-1026-1. [DOI] [PubMed] [Google Scholar]
- 13.Koopman JSHA, De Vries LM, Dieleman JP, Huygen FJ, Stricker BHC, Sturkenboom MCJM. A nationwide study of three invasive treatments for trigeminal neuralgia. Pain. 2011;152(3):507–513. doi: 10.1016/j.pain.2010.10.049. [DOI] [PubMed] [Google Scholar]
- 14.Taylor JC, Brauer S, Espir ML. Long-term treatment of trigeminal neuralgia with carbamazepine. Postgrad. Med. J. 1981;57(663):16–8. doi: 10.1136/pgmj.57.663.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taha JM, Tew JM. Treatment of trigeminal neuralgia by percutaneous radiofrequency rhizotomy. Neurosurg. Clin. N. Am. 1997;8(1):31–9. [PubMed] [Google Scholar]
- 16.Heinskou T, Maarbjerg S, Rochat P, Wolfram F, Jensen RH, Bendtsen L. Trigeminal neuralgia--a coherent cross-specialty management program. J. Headache Pain. 2015;16:66. doi: 10.1186/s10194-015-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oguma K, Fujinaga Y, Inoue K. Clostridium Botulinum Toxin. Toxin Rev. 1997;16(4):253–266. [Google Scholar]
- 18.Humeau Y, Doussau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82(5):427–446. doi: 10.1016/S0300-9084(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 19.Pearce LB, First ER, Maccallum RD, Gupta A. Pharmacologic characterization of Botulinum toxin for basic science and medicine. Toxicon. 1997;35(9):1373–1412. doi: 10.1016/S0041-0101(96)00180-8. [DOI] [PubMed] [Google Scholar]
- 20.Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, Diener HC, Brin MF. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793–803. doi: 10.1177/0333102410364676. [DOI] [PubMed] [Google Scholar]
- 21.Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, Silberstein SD, Brin MF. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–14. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 22.Micheli F, Scorticati MC, Raina G (2002) Beneficial effects of botulinum toxin type a for patients with painful tic convulsif. Clin Neuropharmacol 25(5):260–2. http://journals.lww.com/clinicalneuropharm/Abstract/2002/09000/Beneficial_Effects_of_Botulinum_Toxin_Type_A_for.6.asp [DOI] [PubMed]
- 23.Wu C-J, Lian Y-J, Zheng Y-K, Zhang H-F, Chen Y, Xie N-C, Wang L-J. Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 2012;32(6):443–50. doi: 10.1177/0333102412441721. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Lian Y, Ma Y, Chen Y, He C, Xie N, Wu C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J. Headache Pain. 2014;15:65. doi: 10.1186/1129-2377-15-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zúñiga C, Piedimonte F, Díaz S, Micheli F. Acute Treatment of Trigeminal Neuralgia With Onabotulinum Toxin A. Clin. Neuropharmacol. 2013;36(5):146–150. doi: 10.1097/WNF.0b013e31829cb60e. [DOI] [PubMed] [Google Scholar]
- 26.Shehata HS, El-Tamawy MS, Shalaby NM, Ramzy G. Botulinum toxin-type A: could it be an effective treatment option in intractable trigeminal neuralgia? J. Headache Pain. 2013;14:92. doi: 10.1186/1129-2377-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakrzewska JM. Medical management of trigeminal neuropathic pains. Expert Opin. Pharmacother. 2010;11(8):1239–54. doi: 10.1517/14656561003767449. [DOI] [PubMed] [Google Scholar]
- 28.Bond AE, Zada G, Gonzalez AA, Hansen C, Giannotta SL. Operative strategies for minimizing hearing loss and other major complications associated with microvascular decompression for trigeminal neuralgia. World Neurosurg. 2010;74(1):172–7. doi: 10.1016/j.wneu.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Piovesan EJ, Teive HG, Kowacs PA, Della Coletta MV, Werneck LC, Silberstein SD. An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology. 2005;65(8):1306–1308. doi: 10.1212/01.wnl.0000180940.98815.74. [DOI] [PubMed] [Google Scholar]
- 30.Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin a reduces formalin-induced pain. Pain. 2004;107(1–2):125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Xiao L, Cheng J, Zhuang Y, Qu W, Muir J, Liang H, Zhang D. Botulinum toxin type A reduces hyperalgesia and TRPV1 expression in rats with neuropathic pain. Pain Med. 2013;14(2):276–86. doi: 10.1111/pme.12017. [DOI] [PubMed] [Google Scholar]
- 32.Matak I, Bach-Rojecky L, Filipović B, Lacković Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–7. doi: 10.1016/j.neuroscience.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Matak I, Riederer P, Lacković Z. Botulinum toxin’s axonal transport from periphery to the spinal cord. Neurochem. Int. 2012;61(2):236–9. doi: 10.1016/j.neuint.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Wu C, Xie N, Lian Y, Xu H, Chen C, Zheng Y, Chen Y, Zhang H. Central antinociceptive activity of peripherally applied botulinum toxin type A in lab rat model of trigeminal neuralgia. Springerplus. 2016;5:431. doi: 10.1186/s40064-016-2071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Guan X, Fan L, Li M, Liao Y, Nie Z, Jin L. Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: a systematic review. J. Headache Pain. 2013;14(1):72. doi: 10.1186/1129-2377-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]


