Abstract
[Purpose] Several action observation/imagery training studies have been conducted in patients with limited physical activity showing improvements in motor function. However, most studies compared effects of action observation and imagery, so little is known about the changes caused by subsequent observation of target objects. Moreover, few studies analyzed brain wave changes in the EEG mu rhythm. [Subjects and Methods] Eighteen healthy female adults participated in this study, and were divided into two groups: ‘Visual Stimuli’ and ‘Non-Visual Stimuli’. EEG amplitude in the 8–13 Hz frequency band over the sensorimotor cortex was evaluated. [Results] Significant mu suppression was obtained in the action observation trials. Mu power showed a main effect of visual stimuli, with decreased power during action observation, and increased power post-observation in both conditions. Comparing the ‘Visual Stimuli’ and ‘Non-Visual Stimuli’ conditions during the post-observation period, mu power demonstrated a greater increase in the ‘Non-Visual Stimuli’ condition. Furthermore, mu power was lower post-observation than pre-observation. [Conclusion] These results show the effects of visual input between maintaining target objects and no visual input, and their relevance to modulations of the mirror neuron system. It also suggests that greater visual input may be more effective for cognitive rehabilitation.
Key words: Mirror neuron system, Mu rhythm, Visual stimuli
INTRODUCTION
Mirror neurons are cortical single-cells, first discovered in the macaque’s motor representation area (F5 area: ventral premotor cortex)1,2,3), that are activated when the monkey performs particular object-directed actions, when it observes another individual performing the same action, or when the monkey merely sees graspable objects4, 5). There may be a similar system in humans, with increasing evidence that a putative human mirror neuron system exists2, 5,6,7,8,9,10,11), with paradigms, like those used in monkeys, demonstrating cells which discharge both when a movement is imagined and when the same movement is observed.
A growing number of EEG studies have investigated changes in the sensorimotor cortex during action observation or imagery12,13,14). Most previous studies showed that the brain wave was changed, especially during observation, and suggested that action observation would be more effective than action imagery for patients15).
However, there is a large body of evidence showing that the action system becomes activated when viewing objects that afford actions16, 17). According to these studies, viewing graspable tools activates motor regions. In other words, viewing a manipulable object linked automatically into its motoric properties, which are associated with motor representations, and therefore the mirror neuron system (MNS) was activated.
The mu rhythm is the EEG wave in the 8–13 Hz frequency band seen over sensorimotor cortex3, 18). It is most pronounced (i.e., incereased power) when a person is at rest, and becomes suppressed (i.e., decreased power) both when observing actions of another person and during action imagery16). Therefore, the mu rhythm is thought to reflect downstream modulation of MNS activity. Suppression has been successfully used to measure mirror neuron activity in human adults13, 16), whereas increasing mu range reflects inhibition of the MNS19).
Recently, several action observation/imagery training studies have been conducted in patients with limited physical activity20,21,22), with results demonstrating a clinical improvement in motor function20,21,22). In addition, previous studies have investigated brain wave changes such as relative spectral power15), but there are few studies which analyzed the mu rhythm. Moreover, most studies compared the effect of action observation and action imagery, and little is known about changes caused by subsequent action observation of the target objects.
In patients with limited physical activity, visual stimuli from the environment are very important in the recovery of motor learning. Thus, we investigated the effects of action observation and compared different visual objects on the mu rhythm.
SUBJECTS AND METHODS
Due to gender differences during action observation23), only female subjects participated in this study; 18 healthy adults took part. Mean age ± standard deviation was 27.52 ± 3.4 years. All subjects were physically healthy, had no neurological diseases, had not taken any medicines for the purpose of treatment, had normal or corrected normal vision, and were right-handed based on the Edinburgh Handedness Inventory. All volunteers were informed of the sequence of the research procedure, understood its details, and provided signed, informed consent prior to participation. The study was approved by the ethics committee of Kyungnam University, Republic of Korea.
Scalp EEG was recorded using a multi-channel recorder (Neurofax EEG-1200, Nihon Kohden, Tokyo, Japan). Electrodes were attached according to the international 10–20 electrode system. Ag/AgCl ring electrodes filled with electro-conductive gel were used. The reference electrodes were set to A1 and A2 (ear lobes) and recorded in the mono-polar mode. Electrode impedances was kept below 10 kΩ. The lower filter was set at 0.53 Hz and the upper filter at 35 Hz, with a 256 Hz sampling rate. All signals were A/D digitized and transferred onto a PC for analysis with sampling frequency. Fast Fourier Transform (FFT) was used in order to separate the mu band from the whole EEG trace.
The experiment took place in a sound-isolated booth with a 22-inch monitor approximately 100 cm in front of the subject. Subjects were sat comfortably in a chair with their hands on the armrests. They were asked to minimize any movements (e.g., eye blink, head and limb movements) while fixated on a computer monitor, in order to eliminate artifacts in the row EEG trace during presentation of visual stimuli. They were given approximately 10 minutes to adapt to the test environment.
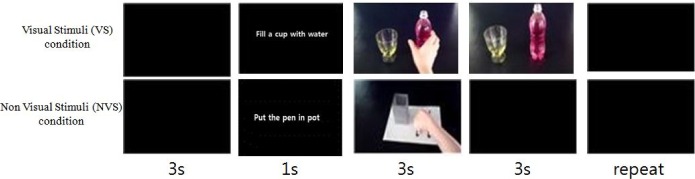
Stimuli consisted of different video clips of actions performed by hands and objects (3 s duration) that were recorded using a high-resolution digital camcorder and edited (Fig. 1). All subjects were divided into one of two groups: ‘Visual Stimuli’ (VS) and ‘Non-Visual Stimuli’ (NVS). Each test condition lasted 130 s including breaks, comprising randomized 13 s videos, and thus all subjects were provided with 10 trials of visual stimuli in total.
Fig. 1.
Examples of the experimental stimuli and trial structure. Participants watched short videos of actions performed by a hand. Each trial started with a blank screen. After 3 s, the action cue appeared for 1 s and then the hand action for 3 s. In the NVS condition, the screen was then replaced with a blank black screen; in the VS condition, the target object remained on the screen for 3 s and thereafter was replaced with a blank screen. In total, a trial length was 13 s, all subjects were provided with 10 trials.
EEG artifacts during visual stimuli were removed prior to analysis using a cosine window. Eye blink, and eye and head movements were inspected visually to eliminate epochs with artifacts. Segments with artifacts were excluded from further analysis.
The mu amplitude at 8–13 Hz was computed across trials for each condition using an FFT that was performed for each electrode. Resulting power magnitudes (μV2) were averaged for each time-locked block. For the statistical analysis of the action observation trials, mu suppression was calculated by the difference in power ratios between the sub-blocks (e.g., hand action observation) and baseline blocks (e.g., black screen)24, 25). These were computed separately for the ‘VS’ and ‘NVS’ conditions. A ratio was used to control for the variability in mu power as a result of individual differences as opposed to mirror neuron activity. However, ratio data are inherently non-normal, due to factors such as scalp thickness and electrode impedance, therefore a log transformation was applied prior to statistical analyses. A log ratio of less than zero indicates mu suppression. Although data were obtained from electrodes across the scalp, mu rhythm is defined as oscillations measured over the sensorimotor cortex. Activity of the sensorimotor cortex during action observation is thought to reflect downstream motor modulations1, 26), thus only data from C3 and C4 are presented24, 27).
A one-sample t-test to examine suppression, as indicated by a log ratio significantly less than 0 in left and right hemisphere electrodes during hand observation with objects, and an independent-samples t-test of the difference between the two observation conditions, were computed. A two-way ANOVA was conducted with block (pre/observation/post) and condition (VS/NVS) as factors. Post-hoc testing was based on estimates of marginal means using LSD.
All statistical analyses were performed using SPSS for Windows 18.0. The statistical significance level was set at p=0.05.
RESULTS
Significant mu suppression was obtained in the action observation trials, in the C3 (t(17)= −9.682, p<0.001, two tailed) and C4 areas (t(17)= −9.134, p<0.001, two tailed). There was no significant difference in the amount of mu suppression in either region during action observation blocks (C3, t(17)= −0.346, p<0.734, two tailed; C4, t(17)= −0.524, p<0.607, two tailed).
Mu power was compared using a repeated-measures two-way ANOVA with ‘block’ and ‘condition’ as factors. Sphericity of the data was verified prior to performing the ANOVA (Mauchly’s test, p>0.05). The ANOVA revealed a main effect of ‘block’ in both areas (C3, F(2,34)=11.525, p<0.001; C4, F(2,34)=10.723, p<0.001). Post-hoc testing showed that differed significantly between blocks. Mu power was desynchronized during action observation (C3: −1.011 μV2 ± 0.246, p<0.001; C4: −0.955 μV2 ± 0.249, p<0.001), and synchronized post-observation (C3: 0.507 μV2 ± 0.169, p<0.009; C4: 0.485 μV2 ± 0.145, p<0.004). Further, the mu power was lower post-observation than pre-observation (C3: −0.504 μV2 ± 0.209, p<0.028; C4: −0.470 μV2 ± 0.210, p<0.040) (Table 1). No other main effect or interaction was significant (all p>0.05).
Table 1. Grand-averaged mu power amplitudes obtained across all trials .
| Pre-Observation | Observation* | Post-observation†# | ||
|---|---|---|---|---|
| C3 | VS | 3.50 ± 2.91 | 2.59 ± 2.83 | 2.89 ± 2.42 |
| NVS | 3.21 ± 2.83 | 2.10 ± 2.10 | 2.81 ± 2.56 | |
| C4 | VS | 3.46 ± 3.01 | 2.59 ± 2.83 | 2.92 ± 2.41 |
| NVS | 3.13 ± 2.80 | 2.09 ± 2.13 | 2.73 ± 2.43 |
Values shown are mean ± SD. *p≤0.05 indicates significant difference between Pre-observation and Observation; †p≤0.05 indicates significant difference between Observation and Post-observation; #p≤0.05 indicates significant difference between Pre-observation and Post-observation. EEG mu power is significantly decreased by observing objects manipulated by a hand in both conditions. When the action visual inputs are terminated, the mu power was significantly increased. However, mu power was more increased in ‘NVS’ condition during the post-observation period.
DISCUSSION
Brain imaging studies have shown that the human mirror neuron systems are activated both when performing and observing actions, with a number of EEG studies having investigated the oscillatory activity of the cortex28,29,30). In this study, visual stimuli that would modulate mirror neuron activity were examined. The results show that the EEG mu rhythm is suppressed by the observation of actions on objects the hands in both conditions, reflecting activation of the MNS and consistent with previous studies of the mu rhythm13, 21, 24, 31). These results suggest that mu suppression involves a range of structures that modulate motor preparation activities and are sensitive to visual input13). In other studies comparing brain waves in stroke patients and healthy subjects during action observation15), both groups showed significant differences in relative alpha power, suggesting action observation may be used as a therapeutic method for cognitive intervention. In a clinical setting, based on these brain wave changes, Kawasaki et al.31) showed that observational learning increased motor performance involving a ball rotation task, and Kim et al.32) suggested that action observation can enhance the walking performance of patients with post-stroke hemiparesis.
When the action visual inputs were terminated, the mu power was significantly increased during the post-observation period in both conditions, notwithstanding differences in visual input. However, the applied analysis is imprecise in identifying between the conditions during the post-observation period. Focusing on comparing the ‘VS’ and ‘NVS’ groups showed that mu power was more greatly increased in the ‘NVS’ group (Table 1). We suggest that this demonstrates the effects of visual input between observed objects and disappeared objects, and is particularly relevant to MNS modulations. Tucker and Ellis33) showed that the premotor cortex and related areas become activated when manipulable object appeared and suggested that an active object representation is sufficient to generate affordance compatibility effects based on execution. Proverbio16) showed that the motor cortex was activated during visual perception of tools with the actual possibility.
These previous studies reflect the visuosomatomotor integration of the cerebral cortex and that an observed object is automatically encoded30, 33). Vision and action systems evolved together to rapidly gather information from the environment and produce the appropriate motor response17), assuming that vision flows fluently and automatically into action. Therefore, we suggest arranging target objects around patients with limited physical activity to help facilitate motor recovery.
However some researchers have not found a difference in the magnitude of mirror neuron activity between object and no object conditions27). Similarly, studies have observed that no suppression was found while participants observed still objects3). However, these studies differ with ours, as these were not performed subsequent to action observation. We suggest that more visual input with action and object observation may be more effective for cognitive rehabilitation purposes.
We conclude that if visual stimuli (i.e., target objects) are sufficiently provided in the daily living environment during an observation training program, the program will be more effective, especially in patients with cerebral damage of the nervous system.
REFERENCES
- 1.Enticott PG, Kennedy HA, Bradshaw JL, et al. : Understanding mirror neurons: evidence for enhanced corticospinal excitability during the observation of transitive but not intransitive hand gestures. Neuropsychologia, 2010, 48: 2675–2680. [DOI] [PubMed] [Google Scholar]
- 2.Muthukumaraswamy SD, Johnson BW, McNair NA: Mu rhythm modulation during observation of an object-directed grasp. Brain Res Cogn Brain Res, 2004, 19: 195–201. [DOI] [PubMed] [Google Scholar]
- 3.Perry A, Bentin S: Mirror activity in the human brain while observing hand movements: a comparison between EEG desynchronization in the mu-range and previous fMRI results. Brain Res, 2009, 1282: 126–132. [DOI] [PubMed] [Google Scholar]
- 4.Buccino G, Binkofski F, Fink GR, et al. : Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci, 2001, 13: 400–404. [PubMed] [Google Scholar]
- 5.Buccino G, Riggio L: The role of the mirror neuron system in motor learning. Kinesiology, 2006, 38: 5–15. [Google Scholar]
- 6.Baldissera F, Cavallari P, Craighero L, et al. : Modulation of spinal excitability during observation of hand actions in humans. Eur J Neurosci, 2001, 13: 190–194. [DOI] [PubMed] [Google Scholar]
- 7.Maeda F, Kleiner-Fisman G, Pascual-Leone A: Motor facilitation while observing hand actions: specificity of the effect and role of observer’s orientation. J Neurophysiol, 2002, 87: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 8.Fadiga L, Craighero L, Olivier E: Human motor cortex excitability during the perception of others’ action. Curr Opin Neurobiol, 2005, 15: 213–218. [DOI] [PubMed] [Google Scholar]
- 9.Lacourse MG, Orr EL, Cramer SC, et al. : Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage, 2005, 27: 505–519. [DOI] [PubMed] [Google Scholar]
- 10.Francuz P, Zapała D: The suppression of the μ rhythm during the creation of imagery representation of movement. Neurosci Lett, 2011, 495: 39–43. [DOI] [PubMed] [Google Scholar]
- 11.Nishitani N, Hari R: Temporal dynamics of cortical representation for action. Proc Natl Acad Sci USA, 2000, 97: 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthukumaraswamy SD, Johnson BW: Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology, 2004, 41: 152–156. [DOI] [PubMed] [Google Scholar]
- 13.Braadbaart L, Williams JH, Waiter GD: Do mirror neuron areas mediate mu rhythm suppression during imitation and action observation? Int J Psychophysiol, 2013, 89: 99–105. [DOI] [PubMed] [Google Scholar]
- 14.Ohno K, Higashi T, Sugawara K, et al. : Excitability changes in the human primary motor cortex during observation with motor imagery of chopstick use. J Phys Ther Sci, 2011, 23: 703–706. [Google Scholar]
- 15.Kim J, Lee B, Lee HS, et al. : Differences in brain waves of normal persons and stroke patients during action observation and motor imagery. J Phys Ther Sci, 2014, 26: 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proverbio AM: Tool perception suppresses 10–12Hz μ rhythm of EEG over the somatosensory area. Biol Psychol, 2012, 91: 1–7. [DOI] [PubMed] [Google Scholar]
- 17.Pfurtscheller G, Brunner C, Schlögl A, et al. : Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage, 2006, 31: 153–159. [DOI] [PubMed] [Google Scholar]
- 18.Gastaut HJ, Bert J: EEG changes during cinematographic presentation; moving picture activation of the EEG. Electroencephalogr Clin Neurophysiol, 1954, 6: 433–444. [DOI] [PubMed] [Google Scholar]
- 19.Klimesch W, Sauseng P, Hanslmayr S: EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Brain Res Rev, 2007, 53: 63–88. [DOI] [PubMed] [Google Scholar]
- 20.Kim E, Kim K: Effect of purposeful action observation on upper extremity function in stroke patients. J Phys Ther Sci, 2015, 27: 2867–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SS, Lee BH: Motor imagery training improves upper extremity performance in stroke patients. J Phys Ther Sci, 2015, 27: 2289–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds C, Osuagwu BA, Vuckovic A: Influence of motor imagination on cortical activation during functional electrical stimulation. Clin Neurophysiol, 2015, 126: 1360–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, Lee PL, Yang CY, et al. : Gender differences in the mu rhythm of the human mirror-neuron system. PLoS ONE, 2008, 3: e2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernier R, Dawson G, Webb S, et al. : EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain Cogn, 2007, 64: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pineda JA, Hecht E: Mirroring and mu rhythm involvement in social cognition: are there dissociable subcomponents of theory of mind? Biol Psychol, 2009, 80: 306–314. [DOI] [PubMed] [Google Scholar]
- 26.Lepage JF, Tremblay S, Théoret H: Early non-specific modulation of corticospinal excitability during action observation. Eur J Neurosci, 2010, 31: 931–937. [DOI] [PubMed] [Google Scholar]
- 27.Oberman LM, McCleery JP, Ramachandran VS, et al. : EEG evidence for mirror neuron activity during the observation of human and robot actions: toward an analysis of the human qualities of interactive robots. Neurocomputing, 2007, 70: 2194–2203. [Google Scholar]
- 28.Alegre M, Guridi J, Artieda J: The mirror system, theory of mind and Parkinson’s disease. J Neurol Sci, 2011, 310: 194–196. [DOI] [PubMed] [Google Scholar]
- 29.Babiloni C, Babiloni F, Carducci F, et al. : Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. Neuroimage, 2002, 17: 559–572. [PubMed] [Google Scholar]
- 30.Cochin S, Barthelemy C, Roux S, et al. : Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur J Neurosci, 1999, 11: 1839–1842. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki T, Aramaki H, Tozawa R: An effective model for observational learning to improve novel motor performance. J Phys Ther Sci, 2015, 27: 3829–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JS, Kim K: Clinical feasibility of action observation based on mirror neuron system on walking performance in post stroke patients. J Phys Ther Sci, 2012, 24: 597–599. [Google Scholar]
- 33.Tucker M, Ellis R: Action priming by briefly presented objects. Acta Psychol (Amst), 2004, 116: 185–203. [DOI] [PubMed] [Google Scholar]