Abstract
[Purpose] The Hybrid Assistive Limb® (HAL®) robot suit is a powered exoskeleton that can assist a user’s lower limb movement. The purpose of this study was to assess the effectiveness of HAL® in stroke rehabilitation, focusing on the change of the sit-to-stand (STS) movement pattern and standing posture. [Subjects and Methods] Five stroke patients participated in this study. Single leg HAL® was attached to each subject’s paretic lower limb. The subjects performed STS three times both with and without HAL® use. A tri-axial accelerometer was used to assess the STS movement pattern. Forward-tilt angle (FTA) and the time required for STS were measured with and without HAL® use. Surface electromyography (EMG) of STS and standing were recorded to assess the vastus medialis muscle activities of the paretic limb. [Results] The average FTA without HAL® use was 35° and it improved to 43° with HAL® use. The time required for STS was longer for all subjects with HAL® use (without HAL® use: 3.42 s, with HAL® use: 5.11 s). The integrated EMGs of HAL® use compared to those without HAL®, were 83.6% and 66.3% for STS and standing, respectively. [Conclusion] HAL® may be effective in improving STS and standing patterns of stroke patients.
Key words: HAL®, Robot, Stroke rehabilitation
INTRODUCTION
Since the late 1990s, robot-assisted gait rehabilitation has become popular around the world. Robot-assisted rehabilitation is often used in the treatment of central nervous system disorders such as stroke, spinal cord injury, multiple sclerosis, and cerebral palsy. The Hybrid Assistive Limb® (HAL®) robot suit is a powered exoskeleton that was developed by Cyberdyne Cooperation to assist user’s lower limb movements. HAL® estimates the user’s intended motion by detecting slight bioelectrical signals, such as myoelectricity, that are produced by the user’s voluntary muscle contraction. This allows HAL® to work as the user’s muscle. HAL® also has an autonomous control system that performs processing of signals emitted by built-in sensors1,2,3,4). The combination of these control systems allows HAL® to assist a user in performing movements smoothly and stably.
More than 185 HAL® have been introduced to Japanese medical institutions for the purpose of rehabilitation5). Several studies have reported on the effectiveness of robot-assisted gait training in improving walking speed, step length, muscle strength, balance and patient’s motivation for treatment5,6,7,8,9,10,11,12). However, there has been minimal research13) of the effectiveness of robot-assisted rehabilitation for sit-to-stand (STS) and standing movements. Performing STS smoothly and stably is important to prevent patients from being bedridden. Previous studies have reported that the difficulty of STS is a main cause of falls, with 20% of falls caused by standing from a wheelchair, and 22% caused by standing from a bed14,15,16). Furthermore, the main reason patients give up living in their home and enter a nursing home is the loss of the ability to perform STS17). Hence, rehabilitation including STS and standing is important for maintaining activities of daily living abilities of patients and for reducing health care costs. HAL® is able to assist hip and knee flexion-extension movements. We hypothesized that this function of HAL® may be useful for improving STS and standing movements in stroke patients. The purpose of the present study was to assess the effectiveness of HAL® in rehabilitation, focusing on the change of the STS movement pattern and standing posture of stroke patients.
SUBJECTS AND METHODS
Subjects who were able to attach HAL® to their lower limb and perform movements such as STS and walking with HAL® were selected. In accordance with the HAL® instruction manual18), subjects were excluded if they weighed over 80 kg; were taller than 185 cm or shorter than 145 cm; had remarkable deformity and/or movement restrictions in their hip, knee, or ankle joint; had remarkable spasticity, ataxia, or severe dementia; were unable to attach electrodes to their skin due to skin diseases; had an embedded active implantation device such as a pacemaker; were in an unstable physical condition, or experienced pain with HAL® use. Five stroke patients, who had experienced stroke onset within 3 months of the study, participated in the study. The characteristics of the subjects are described in Table 1. This study was approved by Tokyo University of Technology Ethics Committee (Approval no. E12HS-015), and implemented in accordance with the ethical principles of the Declaration of Helsinki. Informed consent was obtained from each subject.
Table 1. Characteristics of the subjects (n=5).
| Subject | Gender (M/F) | Age (years) | Paretic lower limb | Brs | Orthosis | Need upper limb support |
|---|---|---|---|---|---|---|
| A | M | 52 | Rt | III | - | yes |
| B | F | 67 | Rt | IV | AFO | yes |
| C | F | 76 | Lt | IV | AFO | yes |
| D | M | 28 | Rt | IV | AFO | no |
| E | M | 84 | Lt | IV | - | no |
Brs: Brunnstrom stage, AFO: ankle foot orthosis
A single leg HAL®, weighing 10 kg, was attached to each subject’s paretic lower limb. Before the measurement, HAL® adjustment was conducted by qualified physical therapists who had completed a HAL® safety training course. The positions of the HAL® hip and knee joint were adjusted to match those of each subject. Then, the HAL®’s assistance level was adjusted. Subjects were asked to perform flexion and extension of the hip and knee joint, STS, and walking with HAL® in order to decide the assistance level. Due to safety concerns, the minimum or a low assistance level was adopted for all subjects. After all the adjustments had been completed, the subjects performed one or two short training sessions (30 min) to practice moving with HAL®.
A rehabilitation couch 45 cm high was used for the measurements. The use of a leg brace and arm support were allowed so that the subjects could perform STS in their usual manner. The subjects performed STS three times both with and without HAL® use. In order to minimize the risk, the patients’ preferred movement speed was adopted and they could take a break at anytime during the measurements. Physical therapists monitored the subjects throughout the measurements.
A tri-axial accelerometer (ACC) (35 g, 40 × 55 × 20 mm; frequency range: DC up to 50 Hz; Logical Product Inc. Hiroshima, Japan) was used for the measurements. One ACC was attached at the position of the seventh cervical spinous process (C7) of the subjects. Adhesive surgical tape was used to attach the ACC. The anterior-posterior direction of acceleration during STS was measured to assess the postural change of C7. Figure 1 shows the initial angle. The forward-tilt angle (FTA) was measured with and without HAL® use. The FTA was calculated as the difference between the maximum forward tilt angle and the initial angle during STS (Fig. 2).
Fig. 1.
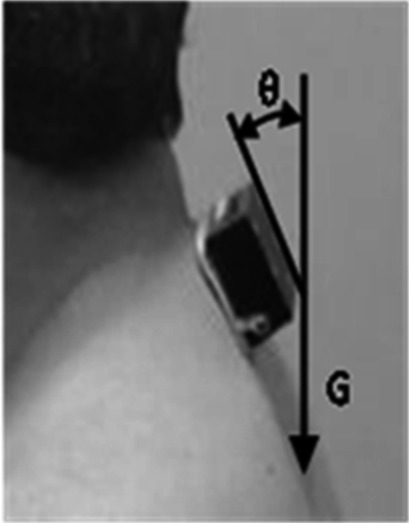
Attachment of the tri-axial accelerometer (ACC) at C7, and the initial angle
Fig. 2.
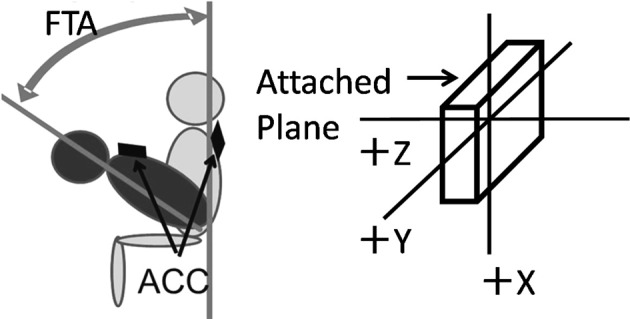
Schema of the measurement of the forward-tilt angle (FTA) by the tri-axial accelerometer (ACC)
The time required for STS was assessed with and without HAL® use. Two-dimensional STS movement was recorded by video camera at 30 frames/s. Video images were synchronized to ACC signals using Pixel Runner G software (Pixel Gate, Tokyo, Japan). STS was divided into two phases. The first phase (P1) started with the forward movement of the head, and ended with buttocks lift-off. The timing of the forward head movement was identified by video images. The second phase (P2) started with hip and knee extension, and ended with standing19, 20). The times required for P1 and P2 were compared with and without HAL® use.
Surface electromyograms (EMG) of STS and standing were recorded to assess muscle activity with and without HAL® use. As the rectus femoris muscle, gluteus maximus, vastus lateralis, and biceps femoris of the paretic limb were engaged in operating HAL®, the EMG activity of the vastus medialis (VM) of paretic limb was measured. An EMG recording system (EMG Logger LP-MS1002, Logical Product Inc., Hiroshima, Japan) was used. Before the electrodes were attached, the skin over the muscle was cleansed with alcohol and a specific gel to reduce the impedance at the skin-electrode interface. Two disposable electrodes (Vitrode M; Nihon Kohden, Tokyo, Japan) were attached at the top of the VM and approximately 2 cm medial to the patella along the run of the VM muscle fibers21). EMG signals were filtered with a low cut-off time constant of 0.03 s and a 422 Hz low-pass Butterworth filter, and amplified 500 times. The EMG sampling rate was 1 KHz. The EMG signals were fully rectified and the integrated EMG (IEMG) of STS and IEMG of standing were computed. Because the time required for STS was different in each measurement, the value of IEMG was time-normalized. A one-second segment of IEMG standing data was also analyzed. The IEMG with HAL® use was divided by IEMG without HAL® use to compare the differences between the VMO muscle activities of each condition.
RESULTS
The FTAs of with and without HAL® use are shown in Table 2. FTA with HAL® use was improved in 4 of the 5 subjects. The average FTA without HAL® use was 35° and it improved to 43° with HAL® use. The time required for STS was longer for all subjects with HAL® use. The average time with HAL® use was 5.11 s, and the average time without HAL® use was 3.42 s. The time for STS with HAL® use was approximately 1.5 times longer than that without HAL® use (Table 3). The IEMG with and without HAL® use are shown in Table 4. IEMG values with HAL® were decreased in all but one subject. The average IEMG values with HAL® compared to those without HAL® were 83.6% and 66.3% for STS and standing, respectively.
Table 2. FTA with HAL and without HAL (deg).
| Subject | Without HAL | With HAL |
|---|---|---|
| A | 26.4 | 37.3 |
| B | 25.7 | 34 |
| C | 29.5 | 45.9 |
| D | 48 | 44.1 |
| E | 44.8 | 52.6 |
FTA: forward-tilt angle
Table 3. Times required for STS with and without HAL.
| Subject | Phase | Without HAL (s) | With HAL (s) |
|---|---|---|---|
| A | P1 | 0.59 | 1.24 |
| P2 | 1.47 | 2.57 | |
| total time | 2.05 | 3.81 | |
| B | P1 | 0.99 | 1.74 |
| P2 | 1.65 | 2.18 | |
| total time | 2.64 | 3.92 | |
| C | P1 | 1.53 | 2.04 |
| P2 | 2.55 | 3.69 | |
| total time | 4.08 | 5.73 | |
| D | P1 | 0.87 | 0.71 |
| P2 | 1.49 | 2.19 | |
| total time | 2.37 | 2.90 | |
| E | P1 | 2.58 | 3.54 |
| P2 | 3.41 | 5.64 | |
| total time | 6.00 | 9.18 |
P1: first phase of STS, P2: second phase of STS
Table 4. % IEMG of STS and standing.
| Subject | STS | Standing |
|---|---|---|
| A | 144.0 | 54.5 |
| B | 54.5 | 31.3 |
| C | 87.3 | 76.9 |
| D | 59.6 | 109.8 |
| E | 72.5 | 58.8 |
| Average | 83.6 | 66.3 |
%IEMG: percentage of integrated electromyography. All variables are shown with HAL/ without HAL (%). The decreased %IEMG suggests involuntary muscle activities like spasticity were well controlled with HAL use.
DISCUSSION
STS is one of the most difficult movements, because large hip range of motion, and hip and knee joint torque are required. As age increases, lower extremity muscle strength decreases, and forward shift of the center of pressure also decrease22). Muscle strength, balance, and segmental motor control are necessary to perform STS appropriately23). Limited hip range of motion is often observed in stroke patients. Insufficient hip flexion limits the forward shift of the body’s center of gravity (COG) during STS. The present study demonstrated that FTA was improved with HAL® use. HAL® facilitates the trunk’s forward movement by assisting hip flexion, which enables the COG to be shifted forward. HAL® also facilitates upright standing by assisting in hip and knee extension. Hence, HAL® may be useful for making STS and standing movements more efficient and stable. However, the FTA of subject D slightly decreased with HAL® use. In healthy controls, STS is performed with 60° of forward trunk movement16). The average FTA of subject D without HAL® use was 48°, which is close to that of a healthy person. For subject D, HAL® might not have provided assistance because STS was already being performed in a normal manner.
In the present study, the average time of STS with HAL® use was 5.1 s. In particular, the P2 period was remarkably prolonged. According to Ada9), the time required for STS by stroke patients is approximately 2.3 s. The prolonged time observed in the present study might be attributable to a time lag between the recognition of the wearer’s intended motion by HAL® and the initiation of assistance by HAL®. It might also be that rapidly raising the COG makes the body unstable during the P2 period. Therefore, the autonomous control of HAL® might have worked to make STS movements occur in a safer manner. Further investigation is necessary to identify the reason for the prolonged STS time.
EMG activity of stroke patients is variable and unstable due to pathological symptoms such as spasticity, muscle weakness, and motor-control deficiency. Coenen et al. reported that robot-assisted gait decreased the activities of the quadriceps and hamstrings, and contributed to stabilization of the posture of patients with spinal cord injuries24). In our present study, IEMG of standing of four of the five subjects decreased with HAL® use. This suggests involuntary muscle activities like spasticity were well controlled by the assistance of HAL®. However, IEMG of subject D increased with HAL® use. As subject D could perform both STS and standing smoothly, HAL® might have stopped providing assistance. In addition, IEMG of subject A increased during STS with HAL® use. Subject A had the most severe motor paralysis among all subjects, and the IEMG of subject A without HAL® use was remarkably less than those of the other subjects during the buttocks lift-off period. This suggests that the increase in muscle activity with HAL® use might have been a positive effect.
In summary, the present study suggests that HAL® can effectively change the STS and standing patterns of the stroke patients, including range of motion and muscle activity. This study succeeded in demonstrating the immediate effect of HAL®. We speculate that if patients were to continue appropriate training with HAL®, more benefits would be seen. Therefore, the long-term effect of HAL® is an issue that needs to be investigated.
This study had some study limitations. The small number of subjects made it impossible to carry out a statistical analysis. The measurement methods should also be considered. Hip range of motion and other muscle activities, including those on the non-paralyzed side, could not be assessed with HAL® use. Because HAL® is an exoskeleton robot, it is difficult to attach devices that measure biological signals. Additionally, the mechanism by which HAL® operates is unclear. If the mechanism is disclosed, more analyses would be possible.
Lastly, there is a serious issue in terms of using HAL® for the purpose of rehabilitation. Nishio demonstrated that the time required to set up HAL® was approximately 30 min, which is almost the same amount time spent on the rehabilitation25). In our study, the same time was necessary to set up HAL®. Therefore, shortening this time is necessary to reduce the burden on the subjects and the rehabilitation staff. This was the main obstacle to our study. Improvements in these aspects will be necessary for the widespread adoption of HAL® by rehabilitation facilities.
Acknowledgments
This study was partially supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant-in-Aid for Scientific Research (C).
REFERENCES
- 1.Kasaoka K, Sankai Y: Predictive control estimating operator’s intention for stepping-up motion by exo-sckeleton type power assist system HAL. IEEE/RSJ, International Conference on Intelligent Robots and Systems (IROS 2001), 2001, 1578–1583.
- 2.Suzuki K, Kawamura Y, Hayashi T, et al. : Intention-based walking support for paraplegia patient. IEEE International Conference on Systems, Man and Cybernetics. Vol.3, 2001, 2707–2713.
- 3.Hayashi T, Kawamoto H, Sankai Y: Control method of robot suit HAL working as operator’s muscle using biological and dynamical information. IEEE/RSJ, International Conference on Intelligent Robots and Systems (IROS 2005), 2005, 3063−3068.
- 4.Nakajima T: Clinical trial of robot suit HAL technology for neuromuscular intractable rare diseases. J Natl Inst Public Health, 2011, 60: 130–137. [Google Scholar]
- 5.Watanabe H, Tanaka N, Kanamori T, et al. : Clinical application of ROBOT SUIT HAL for rehabilitation case study. J Phys Ther Sci, 2012, 27: 723–729. [Google Scholar]
- 6.Taketomi T, Sankai Y: Walking assistance for cerebral palsy with robot suit HAL. Med Biol Eng, 2012, 50: 105–110. [Google Scholar]
- 7.Maeshima S, Osawa A, Nishio D, et al. : Clinical application of hybrid assistive limb for stroke patients with hemiplegia. Bull Jpn Soc Prosthet Orthot, 2013, 29: 46–49. [Google Scholar]
- 8.Kamibayashi K: Short –term adaptation elicited by robotic assistant to unilateral lug during locomotion: toward gait recovery for hemiplegic patients. Research Report of Grants-in–Aid for Scientific Research. 2011. http://hdl.handle.net/2241/115065(Accessed Sep. 10. 2015)
- 9.Huang Q, Zhou Y, Yu L, et al. : The reliability of evaluation of hip muscle strength in rehabilitation robot walking training. J Phys Ther Sci, 2015, 27: 3073–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang CG, Chun MH, Jang MC, et al. : Views of physiatrists and physical therapists on the use of gait-training robots for stroke patients. J Phys Ther Sci, 2016, 28: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho DY, Park SW, Lee MJ, et al. : Effects of robot-assisted gait training on the balance and gait of chronic stroke patients: focus on dependent ambulators. J Phys Ther Sci, 2015, 27: 3053–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae YH, Ko YJ, Chang WH, et al. : Effects of robot-assisted gait training combined with functional electrical stimulation on recovery of locomotor mobility in chronic stroke patients: a randomized controlled trial. J Phys Ther Sci, 2014, 26: 1949–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ada L, Westwood P: A kinematic analysis of recovery of the ability to stand up following stroke. Aust J Physiother, 1992, 38: 135–142. [DOI] [PubMed] [Google Scholar]
- 14.Sorock G, Pomerantz R: A case-control study of falling episodes among hospitalized elderly. Gerontologist, 1980, 20: 240. [Google Scholar]
- 15.Yoshida K, Iwakura H, Inoue F: Motion analysis in the movements of standing up from and sitting down on a chair. A comparison of normal and hemiparetic subjects and the differences of sex and age among the normals. Scand J Rehabil Med, 1983, 15: 133–140. [PubMed] [Google Scholar]
- 16.Tinetti ME, Speechley M, Ginter SF: Risk factors for falls among elderly persons living in the community. N Engl J Med, 1988, 319: 1701–1707. [DOI] [PubMed] [Google Scholar]
- 17.Branch LG, Meyers AR: Assessing physical function in the elderly. Clin Geriatr Med, 1987, 3: 29–51. [PubMed] [Google Scholar]
- 18.CYBERDYNE Inc: HAL Instruction manual Ver.3.3:WHL01-JP, 16–17.
- 19.Carr JH, Gentile AM: The effect of arm movement on the biomechanics of standing up. Hum Mov Sci, 1994, 13: 175–193. [Google Scholar]
- 20.Shepherd C, Lord SR: Sit-to-stand: functional relationships between upper body and lower limb segments. Hum Mov Sci, 1994, 13: 817–840. [Google Scholar]
- 21.Shimono T: Surface Electromyography: Clinical Application Manual. Tokyo: SAKAI Medical, 2010, pp 130–167. [Google Scholar]
- 22.Miyoshi K, Kimura T, Yokokawa Y, et al. : Effect of ageing on quadriceps muscle strength and on the forward shift of center of pressure during sit-to-stand movement from a chair. J Phys Ther Sci, 2005, 17: 23–28. [Google Scholar]
- 23.Carr JH, Shepherd RB: Authorized translation of the original English language edition “Stroke Rehabilitation Guidelines for Exercise and Training to Optimized Motor Skill”. Tokyo: Igaku-Shoin, 2004, pp 106–113. [Google Scholar]
- 24.Coenen P, van Werven G, van Nunen MP, et al. : Robot-assisted walking vs overground walking in stroke patients: an evaluation of muscle activity. J Rehabil Med, 2012, 44: 331–337. [DOI] [PubMed] [Google Scholar]
- 25.Nishio D, Maejima S, Osawa A, et al. : Study on a practical use of both-legs type Robot Suits HAL for wellbeing in stroke patients with hemiplegia. Bull Jpn Soc Prosthet Orthot, 2012, 28: 53–56. [Google Scholar]


