Abstract
We have previously reported sodium is stored in skin and muscle. The amounts stored in hemodialysis (HD) patients are unknown. We determined whether 23Na magnetic resonance imaging (sodium-MRI) allows assessment of tissue sodium and its removal in 24 HD patients, and 27 age-matched healthy controls. We also studied 20 HD patients before and shortly after HD with a batch dialysis system with direct measurement of sodium in dialysate and ultrafiltrate. Age was associated with higher tissue sodium content in controls. This increase was paralleled by an age-dependent decrease of circulating levels of vascular endothelial growth factor-C (VEGF-C). Older (over 60 years) HD patients showed increased sodium and water in skin and muscle, and lower VEGF-C levels than age-matched controls. After HD, patients with low VEGF-C levels had significantly higher skin sodium content than patients with high VEGF-C levels (low VEGF-C: 2.3 ng/ml and skin sodium: 24.3 mmol/L; high VEGF-C: 4.1ng/ml and skin sodium: 18.2mmol/L). Thus, sodium-MRI quantitatively detects sodium stored in skin and muscle in humans and allows studying sodium storage reduction in ESRD patients. Age and VEGF-C-related local tissue-specific clearance mechanisms may determine the efficacy of tissue sodium removal with HD. Prospective trials on the relationship between tissue sodium content and hard endpoints could provide new insights into sodium homeostasis, and clarify whether increased sodium storage is a cardiovascular risk factor.
Introduction
Salt intake and renal excretion are related to hypertension.1 In hemodialysis (HD) patients, the dialyzer must serve the salt-excretory function. Interdialytic salt restriction and/or removal of salt and water during HD evidently lower blood pressure.2–6 However, normal blood pressure is rare in patients with end stage renal disease (ESRD) undergoing HD despite counseling dietary salt reduction and effective body fluid management.7–9 Recent evidence suggests that a high salt intake is associated with greater mortality in HD patients.10 Because high interdialytic weight gain is associated with poor survival in HD patients,11 attainment of “normal body fluid status” expressed as “dry weight”,12 may improve excessive mortality in HD patients and reduce cardiovascular events.2 Despite the high profile that sodium (Na+) and salt intake receive,2, 13, 14 nephrologists are limited to monitoring serum Na+ and dialysate Na+ concentrations, interdialytic weight gain, and crude estimates of salt intake.
We observed that Na+ is stored without commensurate water retention in skin and muscle.15–17 The resulting local hypertonicity from Na+ storage in skin leads to immune-cell-driven induction of local tissue electrolyte clearance via modulation of cutaneous lymph capillary density.18–20 We found that disturbance of local tissue Na+ clearance from these stores is associated with salt-sensitive increases in blood pressure. Recent findings support the idea that skin electrolyte control by lymph capillaries is relevant for blood pressure homeostasis,20 suggesting that besides renal compensatory mechanisms, local clearance mechanisms at the tissue level are important for tissue electrolyte homeostasis. We recently implemented 23Na magnetic resonance imaging (Na-MRI) for non-invasive detection and quantification of Na+ reservoir metabolism in normal and hypertensive humans21, 22 and in hypernatremia.23 We have now investigated tissue-Na+ contents in patients with ESRD and tested the hypothesis that HD can mobilize Na+ from tissue stores. Our results in HD patients conform to our earlier observations on multi-compartment Na+ balance.24
Results
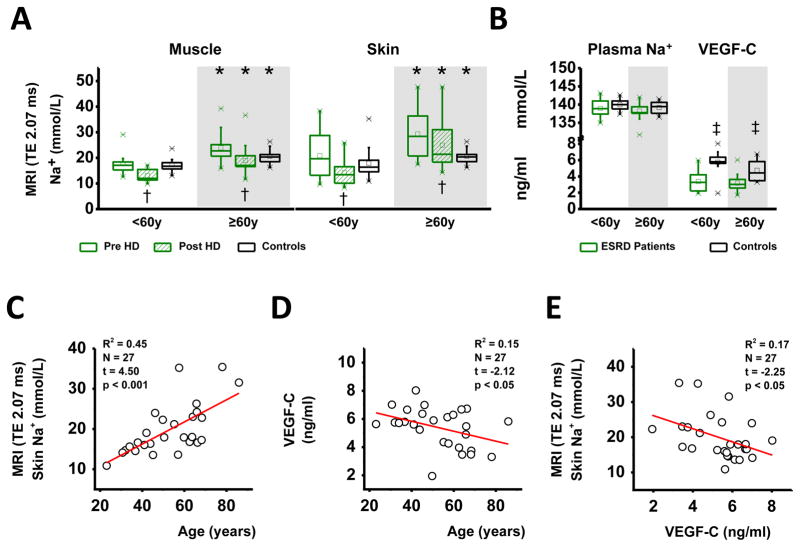
We initially studied Na+ and water content by Na-MRI and conventional H-MRI imaging in muscle and in skin (Study 1) in 24 HD patients and in 27 age-matched healthy controls (Table 1a). Because we had shown earlier that tissue Na+ content is primarily dependent on age in normal subjects, we separated patients and controls in two age groups (Group 1 <60 years; Group 2 ≥60 years).21 Dialysis session length, ultrafiltration (UF) volumes, residual renal function as assessed by daily urine volume, and dialysate Na+ and bicarbonate concentrations were not different between the two HD-age groups (Table 1b). Age was associated with increased muscle Na+ and skin Na+ content in HD patients and in controls (Figure 1A). In younger patients, we found no differences in muscle (p=0.76) or skin (p=0.31) Na+ content before HD treatment if compared with age-matched controls. HD treatment then reduced muscle Na+ and skin Na+ in these patients, resulting in lower muscle Na+ content (p<0.01) and a tendency to lower skin Na+ (p=0.1) compared with controls. In older patients, HD treatment reduced tissue Na+ content to levels that were not different from controls (muscle: p=0.37; skin: p=0.62). While plasma Na+ levels were not different between HD patients and controls (Figure 1B), VEGF-C levels were significantly lower in ESRD patients than in age-matched controls. We found that older age tended to decrease VEGF-C levels in controls (p=0.08), but not in ESRD patients. Skin Na+ content continuously increased with age in our 27 control subjects with normal creatinine levels (Figure 1C). Age explained 15% of the variability in serum VEGF-C levels. The age-dependent increase in skin Na+ content paralleled reduced tissue VEGF-C levels in the same subjects (Figure 1D). Serum VEGF-C levels furthermore explained 17% of the variability in skin Na+ content. Higher serum VEGF-C levels paralleled lower skin Na+ content (Figure 1E). This finding suggests that lower VEGF-C levels occur with age, which could reduce tissue Na+ clearance and may lead to Na+ accumulation in the skin. Furthermore, HD patients are characterized by an anti-lymphangiogenic serum profile that may predispose the patients to tissue Na+ accumulation.
Table 1.
Study 1
| (A) Anthropometric data of ESRD patients and controls (mean±SD) | ||||
|---|---|---|---|---|
| Control | ESRD | |||
| < 60 yrs (n=17) | ≥ 60 yrs (n=10) | < 60 yrs (n=10) | ≥ 60 yrs (n=14) | |
| Men / Women | 11/6 | 7/3 | 5/5 | 10/4 |
| Age (years) | 43.7 ± 10.5 | 68.9 ± 7.4* | 42.2 ± 10.4 | 69.5 ± 5.6* |
| Body Mass Index (kg/m2) | 22.7 ± 3.5 | 23.8 ± 2.9 | 26.2 ± 5.5 | 25.8 ± 4.9 |
| (B) ESRD patients and HD treatment characteristics (median, interquartile range IQR) | ||
|---|---|---|
| < 60 yrs (n=10) | ≥ 60 yrs (n=14) | |
| Diuresis (ml/day) | 0, IQR 750 | 150, IQR 500 |
| Dialysis vintage (years) | 2.0, IQR 5 | 3, IQR 5 |
| Dialysis session length (h) | 4.5, IQR 1 | 4.5, IQR 1 |
| Antihypertensive drugs (n) | 2.0, IQR 3 | 3.0, IQR 2 |
| Dialysate Na+ (mmol/l) | 138.0, IQR 3 | 138.0, IQR 1 |
| Dialysate HCO3− (mmol/l) | 32.0, IQR 4 | 32.0, IQR 1 |
| Interdialytic weight gain (kg) | 2.05, IQR 2.8 | 2.15, IQR 1.7 |
| Ultrafiltration volume (ml) | 2300, IQR 2000 | 2750, IQR 1050 |
P(age) < 0.001
Figure 1.
A. (Study 1) Muscle and skin Na+ content in younger (<60 years) and older (≥ 60years) ESRD patients before (green box plot) and after HD treatment (striped green box plot) and in aged-matched normal controls (white box plot). B. Plasma Na+ and serum VEGF-C levels in the patients (before HD treatment) and the same controls. C. Relationship between circulating VEGF-C levels and skin Na+ content in the controls with normal creatinine levels. Older age was associated with lower VEGF-C levels and with increased tissue Na+ content, suggesting that a reduction in VEGF-C levels may predispose to tissue Na+ accumulation with advancing age. * p(age) <0.05; † p(HD treatment) <0.05; ‡ p(controls versus ESRD patients) <0.05.
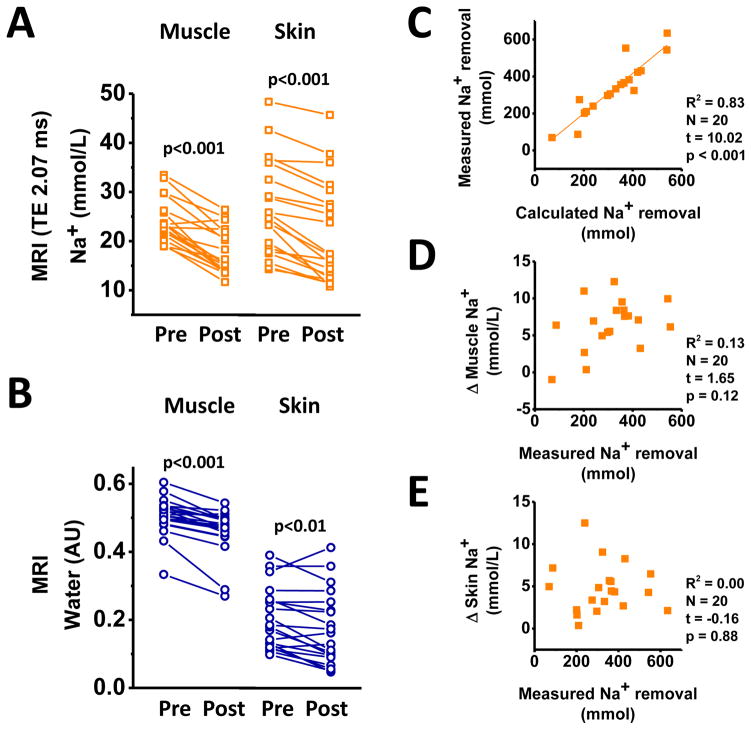
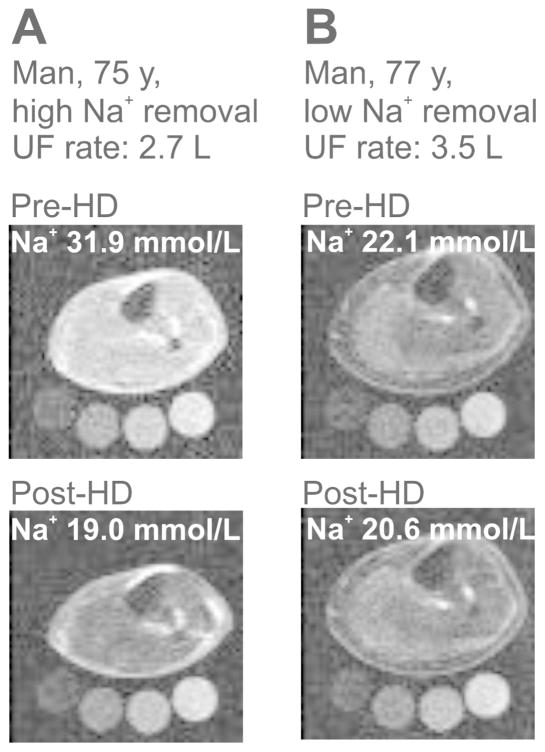
To investigate the relationship between HD-enforced Na+ removal from plasma and subsequent tissue Na+ release, we studied Na+ removal from skin, muscle, and blood plasma in a second study (Study 2) in 20 of our patients (Table 2), who underwent HD with a batch dialysis system (Genius®).25 This system allows the monitoring of Na+ elimination directly in ultrafiltrate and dialysate. Plasma [K+] and plasma osmolality were higher in serum than in dialysate (Table 2b), supporting clearance of blood electrolytes by diffusion. Dialysate [Na+] and plasma [Na+] were not different. HD reduced muscle Na+ content by 27% and skin Na+ content by 19% in the patients (Figure 2A). Na+ removal from muscle and skin was paralleled by a reduction in water content quantified by additional H-MRI (Figure 2B). We next investigated Na+ removal from the plasma space by HD. We calculated Na+ removal by the dialyzer and found that calculated Na+ removal highly correlated with Na+ retrieval in UF and dialysate (Figure 2C). Plasma [K+] levels were successfully reduced by HD treatment (Table 2). Na+ removal was achieved by UF, while higher dialysate Na+ tended to decrease plasma Na+ removal (Online Supplemental Figure S1). Although plasma Na+ removal by UF was achieved as predicted (Figure 2C), we did not find a similar linear relationship between measured Na+ removal from the vascular space and tissue Na+ removal, neither from muscle (Figure 2D), nor from skin (Figure 2E). We also found no relationship between dialysate/plasma [Na+] gradients and tissue Na+ removal (Online Supplemental Figure S2). These findings suggest that despite successful Na+ removal from the blood space, the secondary clearance of Na+ from tissue is quantitatively less predictable. The Na-MRI detection coil detects immediately below the knee joint so that skin, muscle, fat, vessels, and bone are scanned.23 Muscle and skin Na+ content at the lower limb were visualized and quantified relative to 1 L tissue volume by Na-MRI. Two representative examples of higher (Figure 3A) and lower (Figure 3B) Na+ removal in two HD patients with comparable ultrafiltration rate are shown. We next tested the hypothesis that age and circulating VEGF-C levels might determine Na+ release from tissue in HD patients.
Table 2.
Study 2. Electrolyte concentrations and osmolality in plasma and dialysate before and after HD treatment with the Genius system.
| (A) Anthropometric data and HD treatment characteristics (median, interquartile range IQR) | |
|---|---|
| Men/Women | 15/5 |
| Age (years) | 60.5, IQR 13 |
| Body mass index (kg/m2) | 29.9, IQR 5.8 |
| Diuresis (ml/d) | 200, IQR 1350 |
| Dialysate sodium (mmol/l) | 140.0, IQR 5 |
| Ultrafiltration volume (ml) | 2525, IQR 1500 |
| Dialysis session length (h/week) | 14.25, IQR 3 |
| Changes in bodyweight (kg) | 2.15, IQR 1.48 |
| (B) Effect of HD on [Na+], [K+], and osmolality in plasma and dialysate (mean±SD). | ||||||
|---|---|---|---|---|---|---|
| Plasma [Na+] mmol/L | Dialysate [Na+] mmol/L | Plasma [K+] mmol/L | Dialysate [K+] mmol/L | Plasma Osmolality mosm/kg | Dialysate Osmolality mosm/kg | |
| Pre-HD | 137.4±2.7 | 137.5±2.4 | 5.9±0.6 | 3.1±0.2 | 306.7±8.0 | 271.0±4.9 |
| Post-HD | 136.7±2.3 | 137.2±2.5 | 4.3±0.3 | 3.7±0.2 | 291.1±11.0 | 279.9±5.5 |
| Pairs (n) | 19 | 19 | 19 | 19 | 13 | 13 |
| P(pre- vs. post HD) | 0.04 | 0.38 | <0.001 | <0.001 | <0.001 | <0.001 |
| (C) Differences between [Na+], [K+], and osmolality in plasma, dialysate and ultrafiltrate volume (mean±SD). | |||
|---|---|---|---|
| Plasma | Dialysate | Ultrafiltrate volume | |
| Pre-HD | |||
| [Na+] (mmol/L) | 137.7±2.8 | 137.5±2.4 | |
| [K+] (mmol/L) | 5.8±0.7 | 3.1±0.2* | |
| Osmolality (mosm/kg) | 305.6±7.1 | 271.0±4.9* | |
| Post HD | |||
| [Na+] (mmol/L) | 136.7±2.3 | 137.2±2.6 | 138.3±2.3† |
| [K+] (mmol/L) | 4.3±0.3 | 3.8±0.2* | 3.8±0.2† |
| Osmolality (mosm/kg) | 288.8±5.4 | 279.9±5.5* | 281.2±3.2† |
P(plasma vs dialysate) < 0.001;
P(plasma vs separated ultrafiltrate volume) < 0.01.
Figure 2.
A. (Study 2) Na+ content in muscle and in skin in 20 HD patients before and after HD with the batch dialysis (Genius®) system. B. MRI-determined muscle and skin water (arbitrary units) in the same 20 patients. C. Relationship between calculated Na+ removal and measured Na+ removal from plasma in the same 20 patients. D. Relationship between measured Na+ removal from plasma and measured muscle Na+ removal in the same patients. E. Relationship between measured Na+ removal from plasma and measured skin Na+ removal in the same patients.
Fig. 3.
(Study 2) Representative lower limb Na-MRI images from two ESRD patients before and after HD. A. Patient with high Na+ removal after HD, ultrafiltration rate 2.7 liter. B. Patient with low Na+ removal, ultrafiltration rate 3.5 liter. Standards contain 10, 20, 30, 40 mmol/L Na+.
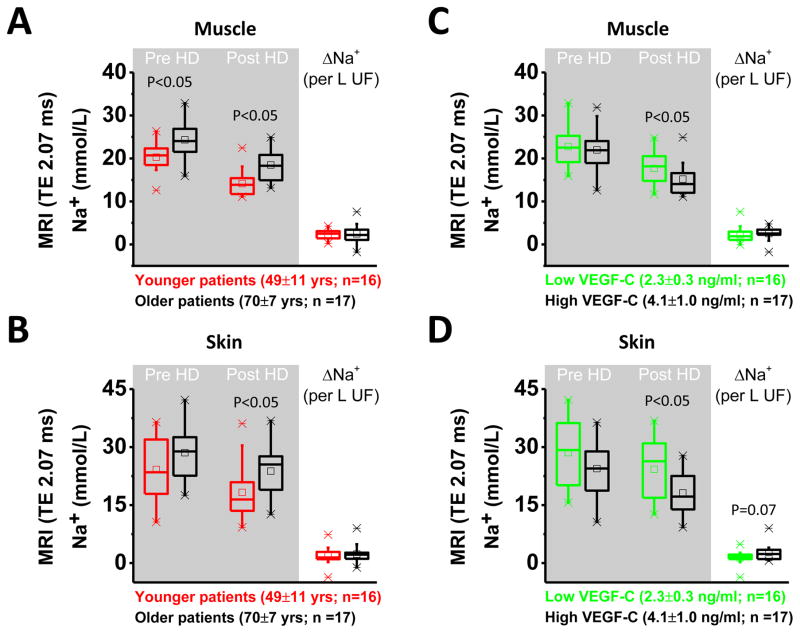
We analyzed the effect of age and circulating VEGF-C levels in 33 male HD patients in whom we had measured VEGF-C levels before HD treatment, and tissue Na+ levels before and after HD treatment (Study 1+2). We first stratified the patients by median age in groups with 16 younger or 17 older patients (Figure 4A and 4B). We found that increased age was a predictor for higher muscle Na+ content before and after HD treatment and a predictor for higher skin Na+ content after dialysis treatment. Age had no effect on acute tissue Na+ removal with HD therapy. We then stratified patients by median in two groups with either lower (n=16) or higher (n=17) circulating VEGF-C levels. Patients with higher VEGF-C levels showed lower Na+ content in muscle and in skin after HD treatment (Figure 4C and 4D), and tended to have improved Na+ removal from their skin with HD therapy.
Fig. 4.
Effect of age and circulating serum VEGF-C levels on tissue Na+ content before and after HD and on tissue Na+ removal with HD in 33 male ESRD patients from studies 1 and 2. A. and B. Effect of younger (red) or older (black) age on Na+ content in muscle and in skin and effect of age on Na+ removal (ΔNa+). Younger age leads to lower muscle Na+ content before and after HD therapy and to lower skin Na+ content post HD. C. and D. Effect of lower (green) or higher (black) VEGF-C levels on Na+ content in muscle and in skin and effect of VEGF-C on Na+ removal (ΔNa+). Higher VEGF-C levels are associated with lower tissue Na+ content after dialysis and tend to improve skin Na+ removal with HD. TE = 2.07 ms refers to echo time.
Discussion
The important finding in our study is that Na-MRI detects tissue Na+ stores and that Na+ in patients can be reliably quantified. The method allows rapid transfer of the tissue Na+ storage concept from the basic research arena15–20, 26–30 to applied clinical investigation.21–23 In this clinical study, we introduce Na-MRI as a feasible diagnostic tool to assess tissue Na+ content in ESRD patients, and to monitor their tissue Na+ removal by HD treatments. We believe that our findings are novel for several reasons.
First, we show that HD treatment mobilizes Na+ and water from these stores, indicating that tissue Na+ and water content can be modulated by a therapeutic intervention. Similar to subjects without renal disease,21 we show that tissue Na+ content increases with age in HD patients. However, tissue Na+ content increases more progressively in HD patients, compared with age-matched controls. HD treatment in most patients corrected tissue Na+ content to the level of age-matched controls. In contrast, muscle Na+ content in younger HD patients was not different from controls, so that ultrafiltration with HD actually reduced muscle Na+ content below the control level. This finding suggests that Na+ and water removal by ultrafitration volumes, determined by the “dry-weight method”, may overestimate electrolyte and volume status at the tissue level, resulting in Na+ deficit and dehydration in younger ESRD patients. MRI-based quantification of tissue electrolyte and water content will detect such states and could facilitate determination of the correct ultrafiltration volume in HD patients.
Second, we show that isotonic Na+ and water removal from plasma by HD is not directly related to similar removal of Na+ from tissue. Na-MRI-based measurement of tissue Na+ and water content thus allows investigation of extrarenal local control of tissue electrolyte homeostasis and thereby facilitates clinical study of tissue Na+. Earlier, this parameter could only be measured in animal models.18–20 We found that age determined tissue Na+ content in HD patients, but had no influence on Na+ removal by HD therapy in the same individuals. Age is characterized by a chronic pro-inflammatory state.31, 32 A greater HD vintage is associated with decreases in body weight and body cell mass in ESRD patients.33 Inflammatory processes can contribute to syndromes of malnutrition, inflammation, cachexia, and protein wasting.34, 35 We hypothesize that such pro-inflammatory processes may reduce tissue Na+ clearance and will predispose HD patients to excess Na+ storage. We will test this hypothesis in prospective trials on the relationship between tissue Na+ content, inflammatory serum markers, and dialysis vintage.
Third, our findings further support the hypothesis derived from animal experiments that immune cells regulate electrolyte homeostasis in humans. We have shown earlier that skin macrophages harbor the tonicity-enhancer binding protein (TonEBP; NFAT5), a transcription factor regulating the expression of osmoprotective genes. In macrophages entering Na+-dense skin storage sites, TonEBP binds to the promoter of VEGF-C, resulting in increased VEGF-C secretion into the skin interstitium. As a result, lymph capillary density increases and skin electrolyte clearance improves. The macrophage-driven regulatory response can be inhibited experimentally, either by blockade of macrophage infiltration,18, 19 and by systemic19 or local20 trapping of VEGF-C by overexpression of sFLT4. Experimental and skin-specific depletion of sFLT4 in a transgenic mouse model overexpressing sFLT4 in keratinocytes, in which sFLT4 functionally eliminates skin VEGF-C, disrupts cutaneous lymph capillary function.20 These experimental interventions reduce the bioavailability of VEGF-C in the skin and result in enhanced skin electrolyte accumulation and salt-sensitive hypertension.18–20 In line with the idea that VEGF-C improves tissue Na+ clearance, we found that the age-dependent increase in skin tissue Na+ content21 in controls with normal serum creatinine was associated with lower circulating VEGF-C levels. Compared to controls, we observed lower VEGF-C levels, and increased sFLT4 levels, in our HD patients. These findings suggest that an anti-lymphangiogenic serum profile is present in ESRD patients, which may predispose them to reduced tissue Na+ clearance and increased tissue Na+ content. Measurement of tissue Na+ content before and after HD treatment further supports this hypothesis. We found that patients with higher VEGF-C levels had lower skin Na+ content after therapy than patients with low VEGF-C. Similar to our findings in animals, VEGF-C thus seems to enhance skin electrolyte clearance in humans. The role of cutaneous lymph capillary transport for skin Na+ clearance in humans remains to be investigated. In line with our findings, other investigators have reported an association between VEGF-C levels and cardiovascular disease. Maynard at el reported that patients with preeclampsia, a disease with low VEGF-A and high sFLT1 levels,36 are characterized by high VEGF-C levels and, according to Lely et al, reduced sFLT4 levels.37 The authors suggested a pro-lymphangiogenic state in preeclampsia that might be a compensatory response in an effort to mobilize edema, electrolyte overload, and could buffer hypertension in preeclamptic patients.
Na+ is generally assumed to be present almost exclusively in the extracellular space where it readily equilibrates with water,38 except for Na+ storage in bone39, 40 and cartilage,41, 42 which has been regarded as osmotically inactive.43 Our earlier animal studies indicated that the bone-Na+ storage in response to high-salt intake, although present, is not very exchangeable, and that instead large quantities of Na+ are stored in muscle and skin.15, 16 Our work suggests substantial amounts of Na+ are stored in muscle and skin in humans and can be mobilized by therapeutic interventions. These possibilities make our findings germane for clinicians. Comparison between non-invasive Na-MRI measurements and directly measured tissue Na+ concentrations in humans provided us with a calibration method that we used for non-invasive quantification of tissue Na+ content in our earlier study.22 We were also able to study hypertensive patients with primary aldosteronism and found that tissue Na+ decreased after operative or spironolactone treatment. The results caused us to conclude that the tissue-stored Na+ is exchangeable. However, an opportunity to make controlled and repetitive measurements of Na+ removal was first provided by our study of ESRD patients, before and after HD. Our findings in HD patients confirmed our observations that age increases stored Na+ in tissue.21 Older HD patients had more Na+ compartmentalized in muscle and skin than younger HD patients. We hypothesize that the accelerated increase in skin and muscle Na+ content, which we did observe in the ESRD patients, might be associated with inflammation and premature aging.
A limitation of our study is the cross-sectional nature. Future prospective trials will provide with time-course information on the development of tissue Na+ overload in HD patients with age. Prospective and randomized intervention trials with different HD-treatment regimes, such as differences in HD duration, dialysate composition, efforts to reduce interdialytic weight gain by dietary salt restriction, and others, are necessary to test the hypothesis that therapeutic reduction of tissue Na+ content may improve healthspan in dialysis patients. We have shown earlier that Na+ stores in muscle can be mobilized within days or weeks in patients with primary or secondary aldosteronism, independent of volume status.22, 23 Furthermore, we observed that patients with spironolactone treatment have lower Na+ content in muscle.21 Chronic MR-activation in experimental animals leads to muscle Na+ storage by cellular Na+/K+ exchange, even without parallel changes in muscle water content.15, 17 We therefore hypothesize that aldosterone and/or mineralocorticoid receptors are important regulators of tissue Na+ stores. Here, we performed the first short-term intervention trial on acute mobilization of tissue Na+ in humans. We were surprised how rapidly tissue Na+ can be mobilized in response to intravascular volume reduction by HD therapy. The findings support the idea that an adequate dialysis-dose could prevent excess Na+ storage in HD patients. While the mechanisms by which Na+ is rapidly removed from skin and muscle remain unclear, our data suggest that a pro-lymphangiogenic serum profile does facilitate this process. The number of subjects in our study was relatively small. Larger prospective trials will be necessary to detect true differences in tissue Na+ removal in HD patients and to identify endogenous factors inside and outside the lymphatic network that enhance or block local tissue Na+ clearance. We have demonstrated good precision for non-invasive detection of true differences in tissue Na+ content by Na-MRI methodology earlier.22 We therefore conclude that trials on the relationship between tissue Na+ storage and cardiovascular outcome are possible by Na-MRI visualization. Such studies will provide answers to whether or not tissue Na+ storage is an independent cardiovascular risk factor in ESRD patients.
We conclude that quantifying Na+ tissue removal could improve strategies for managing HD patients.44 Cardiovascular mortality is markedly elevated in HD patients. However, whether or not tissue Na+ excess contributes to cardiovascular morbidity or mortality is unclear. We are optimistic that “seeing” and quantifying Na+ redistribution at the tissue level could shed light on the relevance of Na+ metabolism in HD patients and plan further studies to determine the justification for this optimism.
Materials and Methods
Studies in HD patients and controls
The University of Erlangen Committee on Human Subjects (Ethics committee) reviewed and approved this prospective observational study (Re.-No. 3948). Healthy volunteers and HD patients from local outpatient dialysis centers were recruited by solicitation. Written informed consent was obtained from all participants. HD practices in Erlangen and surrounding areas followed European Renal Association guidelines.
Comparison of tissue Na+ content in ESRD patients and age-matched controls
In our first study, we measured tissue Na+ content in 24 HD patients before and after HD treatment. We have shown earlier that tissue Na+ content is age-dependent and increases with age, even in healthy subjects.21 To test whether or not tissue Na+ content was higher in ESRD patients than in subjects without renal failure, we enrolled 27 healthy control persons, in whom we measured Na+ content in muscle and in skin. We then divided each cohort in two age groups resulting in four subgroups: i) controls <60 years (n=17; age: 43.7±10.5 years), ii) controls ≥60 years (n=10; age: 68.9±7.4 years), iii) ESRD patients <60 years (n=10; age: 42.2±10.4 years) and iiii) ESRD patients ≥60 years (n=14; age: 69.5±5.6 years). The established HD regimens, which employed modern dialysis machines from several manufacturers and high-flux dialyzers, were not changed and UF rates were adjusted with respect to interdialytic weight gain (IDWG) to achieve targeted dry weight in each patient. All but one patient dialyzed via an arteriovenous-fistula. Venous blood sampling accompanied pre-dialytic Na-MRI and H-MRI measurements.
Relationship between plasma Na+ and tissue Na+ removal by HD treatment
In our second study, we compared the amount of intravascular Na+ removal by HD therapy with secondary tissue Na+ removal. We treated 20 HD patients with a special batch hemodialysis machine (Genius®, Fresenius Corp), a technique that allows sampling of all dialysate/filtrate fluids accumulating during the treatment.25, 45 K+ concentrations and osmolality were higher in blood than in dialysate, while dialysate Na+ concentrations (either 135 or 140 mmol/l) was selected close to the patient’s plasma [Na+] to favor sodium removal solely via ultrafiltration. The [Na+] and [K+] in plasma and dialysate were determined using ion-selective electrodes. We measured plasma Na+ removal with HD by directly analyzing Na+ content in the 90 L dialysate before and after HD treatment, Na+ content in the spent dialysate, separated UF, urinary Na+ excretion (UNaV), and the standardized amount of fluid infused during termination of the HD treatment (50 mmol). We then compared measured plasma Na+ removal with calculated plasma Na+ removal:
| (1) |
Pre-HD plasma Na+ values were not statistically significantly different from dialysate (Table 2). We also directly measured Na+ and water content in muscle and skin by Na- and H-MRI in the same patients and compared measured plasma Na+ removal with tissue Na+ removal in all patients by linear regression.
Na-MRI measurements
Conventional MRI relies on detecting a radio frequency signal emitted by excited H+ atoms in the body (present in any tissue containing water molecules). However, any nucleus with a net nuclear spin (odd atomic number) could potentially be imaged with MRI. 23Na+ is naturally abundant in the body, so it can be imaged directly. We implemented Na-MRI for quantitative analysis in man. The methods were validated and published recently.21,22Na+ content was measured in lower leg muscle and skin with a 23Na+ knee-coil (Stark-Contrast, Erlangen, Germany) at 3.0 Tesla with a magnetic resonance imaging scanner (Magnetom-Verio, Siemens Healthcare, Erlangen, Germany) using a 2D-FLASH sequence (total acquisition time TA = 13.7 minutes, echo time TE = 2.07 ms, repetition time TR = 100 ms, flip angle FA = 90°, 128 averages, resolution: 3 × 3 × 30 mm3). Four tubes containing aqueous solutions with 10, 20, 30, and 40 mmol/l NaCl were included to the MR-imaging. They served as calibration standards for Na-MRI by relating intensity to a concentration in a linear trend analysis. In parallel, water content was quantified in tissue by H-MRI, using a fat saturated inversion recovery sequence with spin density contrast (inversion time TI = 210 ms, TA = 6.29 minutes, TE = 12 ms, TR = 3 s, FA1/2 = 90°/180°, 128 averages, resolution: 1.5 × 1.5 × 5 mm3). Here, the 10 mmol/l NaCl tube served as a calibration standard for tissue water in a linear trend analysis defining a water content of approximately 1 liter water per liter volume.21 Because removal of tissue Na+ content with HD treatment could be dependent on the UF rate with HD treatment, we expressed tissue Na+ removal (ΔNa+) in muscle and skin after normalization for the UF rate:
| (2) |
Clinical variables
Medical history was taken, followed by collection of blood samples. In the Na+ balance studies, we used the Genius® single-pass dialysis machines (Fresenius). The batch system permitted direct analysis of all spent dialysate and ultrafiltrate separately.25 VEGF-C was measured by Enzyme-linked immune-sorbent assays (ELISA).
Statistical analysis
Comparison of means of data from all experiments with more than one effector was calculated by multivariate or univariate analysis using the General Linear Measurements (GLM) procedure when data were normally distributed. Group differences in data series that were not normally distributed were analyzed by Mann-Whitney-U-Test. We tested the efficacy of HD therapy to remove tissue Na+ by paired T-Test. We tested the effect of age and serum VEGF-C on tissue Na+ removal in HD patients by stratifying the available data into two groups, defining 50% of the lower values for each variable as “low”, and the other 50% higher values of the data series as “high” (group separation at median). Statistical analysis was performed with the SPSS software (version 22.0). Original output data and Syntax files used for analysis will be provided on request.
Supplementary Material
Acknowledgments
Grants from the German Federal Ministry for Economics and Technology/DLR Forschung unter Weltraumbedingungen (50WB0920), the Interdisciplinary Centre for Clinical Research (IZKF Junior Research Group 2), and the NIH (R01 HL118579-01) support JT. We thank the Imaging Science Institute (Erlangen, Germany) for providing us with measurement time at the 3T MRI scanner and for the technical support and Daniela Amslinger for her assistance in conducting the clinical trial. We also thank the team of the dialysis core unit for supporting us with the Genius dialysis system. The method to determine sodium values describing the content of 23Na+ and local coil for use in such a method have been published as US patent application No: 2013/0096,415.
Footnotes
Disclosure statement
The authors have no interest conflicts and nothing to disclose.
References
- 1.Guyton AC, Coleman TC, Cowley AW, Jr, et al. A systems analysis approach to understanding long-range arterial blood pressure control and hypertension. Circ Res. 1974;35:159–176. [Google Scholar]
- 2.Levin NW, Kotanko P, Eckardt KU, et al. Blood pressure in chronic kidney disease stage 5D-report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney international. 2010;77:273–284. doi: 10.1038/ki.2009.469. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Alborzi P, Satyan S, et al. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hilali N, Al-Humoud H, Ninan VT, et al. Blood pressure control in haemodialysis patients: an audit. Nephrology. 2006;11:100–104. doi: 10.1111/j.1440-1797.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 5.Davenport A. Audit of the effect of dialysate sodium concentration on inter-dialytic weight gains and blood pressure control in chronic haemodialysis patients. Nephron Clinical practice. 2006;104:c120–125. doi: 10.1159/000094544. [DOI] [PubMed] [Google Scholar]
- 6.Krautzig S, Janssen U, Koch KM, et al. Dietary salt restriction and reduction of dialysate sodium to control hypertension in maintenance haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998;13:552–553. doi: 10.1093/ndt/13.3.552. [DOI] [PubMed] [Google Scholar]
- 7.Chazot C. Can chronic volume overload be recognized and prevented in hemodialysis patients? Use of a restricted-salt diet. Seminars in dialysis. 2009;22:482–486. doi: 10.1111/j.1525-139X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- 8.Hur E, Usta M, Toz H, et al. Effect of Fluid Management Guided by Bioimpedance Spectroscopy on Cardiovascular Parameters in Hemodialysis Patients: A Randomized Controlled Trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 doi: 10.1053/j.ajkd.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Kayikcioglu M, Tumuklu M, Ozkahya M, et al. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:956–962. doi: 10.1093/ndt/gfn599. [DOI] [PubMed] [Google Scholar]
- 10.Mc Causland FR, Waikar SS, Brunelli SM. Increased dietary sodium is independently associated with greater mortality among prevalent hemodialysis patients. Kidney international. 2012;82:204–211. doi: 10.1038/ki.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foley RN, Herzog CA, Collins AJ, et al. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney international. 2002;62:1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 12.Wabel P, Moissl U, Chamney P, et al. Towards improved cardiovascular management: the necessity of combining blood pressure and fluid overload. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:2965–2971. doi: 10.1093/ndt/gfn228. [DOI] [PubMed] [Google Scholar]
- 13.Heerspink HL, Ritz E. Sodium chloride intake: is lower always better? Journal of the American Society of Nephrology : JASN. 2012;23:1136–1139. doi: 10.1681/ASN.2012010099. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler DC, Becker GJ. Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney international. 2013;83:377–383. doi: 10.1038/ki.2012.425. [DOI] [PubMed] [Google Scholar]
- 15.Titze J, Bauer K, Schafflhuber M, et al. Internal sodium balance in DOCA-salt rats: a body composition study. Am J Physiol Renal Physiol. 2005;289:F793–802. doi: 10.1152/ajprenal.00096.2005. [DOI] [PubMed] [Google Scholar]
- 16.Titze J, Lang R, Ilies C, et al. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285:F1108–1117. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]
- 17.Ziomber A, Machnik A, Dahlmann A, et al. Sodium-, potassium-, chloride-, and bicarbonate-related effects on blood pressure and electrolyte homeostasis in deoxycorticosterone acetate-treated rats. Am J Physiol Renal Physiol. 2008;295:F1752–1763. doi: 10.1152/ajprenal.00531.2007. [DOI] [PubMed] [Google Scholar]
- 18.Machnik A, Dahlmann A, Kopp C, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55:755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 19.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 20.Wiig H, Schroder A, Neuhofer W, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp C, Linz P, Dahlmann A, et al. 23Na Magnetic Resonance Imaging-Determined Tissue Sodium in Healthy Subjects and Hypertensive Patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 22.Kopp C, Linz P, Wachsmuth L, et al. (23)Na magnetic resonance imaging of tissue sodium. Hypertension. 2012;59:167–172. doi: 10.1161/HYPERTENSIONAHA.111.183517. [DOI] [PubMed] [Google Scholar]
- 23.Kopp C, Linz P, Hammon M, et al. Seeing the sodium in a patient with hypernatremia. Kidney international. 2012;82:1343–1344. doi: 10.1038/ki.2012.314. [DOI] [PubMed] [Google Scholar]
- 24.Titze J, Dahlmann A, Lerchl K, et al. Spooky sodium balance. Kidney international; 2013. [DOI] [PubMed] [Google Scholar]
- 25.Dhondt AW, Vanholder RC, De Smet RV, et al. Studies on dialysate mixing in the Genius single-pass batch system for hemodialysis therapy. Kidney international. 2003;63:1540–1547. doi: 10.1046/j.1523-1755.2003.00862.x. [DOI] [PubMed] [Google Scholar]
- 26.Titze J, Shakibaei M, Schafflhuber M, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol. 2004;287:H203–208. doi: 10.1152/ajpheart.01237.2003. [DOI] [PubMed] [Google Scholar]
- 27.Schafflhuber M, Volpi N, Dahlmann A, et al. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol. 2007;292:F1490–1500. doi: 10.1152/ajprenal.00300.2006. [DOI] [PubMed] [Google Scholar]
- 28.Helle F, Karlsen TV, Tenstad O, et al. High-salt diet increases hormonal sensitivity in skin pre-capillary resistance vessels. Acta physiologica. 2013;207:577–581. doi: 10.1111/apha.12049. [DOI] [PubMed] [Google Scholar]
- 29.Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic T17 cells. Nature. 2013 doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanova LN, Archibasova VK, Shterental I. Sodium-depositing function of the skin in white rats. Fiziol Zh SSSR Im I M Sechenova. 1978;64:358–363. [PubMed] [Google Scholar]
- 31.Campisi J. Aging, Cellular Senescence, and Cancer. Annual review of physiology. 2012 doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Current opinion in immunology. 2012 doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chertow GM, Johansen KL, Lew N, et al. Vintage, nutritional status, and survival in hemodialysis patients. Kidney international. 2000;57:1176–1181. doi: 10.1046/j.1523-1755.2000.00945.x. [DOI] [PubMed] [Google Scholar]
- 34.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney international. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 35.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney international. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 36.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lely AT, Salahuddin S, Holwerda KM, et al. Circulating Lymphangiogenic Factors in Preeclampsia. Hypertens Pregnancy. 2013;32:42–49. doi: 10.3109/10641955.2012.697953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitts RF. Volume and composition of the body fluids. Year Book; Chicago, IL: 1974. [Google Scholar]
- 39.Bergstrom WH. The participation of bone in total body sodium metabolism in the rat. J Clin Invest. 1955;34:997–1004. doi: 10.1172/JCI103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edelman IS, Leibman J. Anatomy of body water and electrolytes. Am J Med. 1959:27. doi: 10.1016/0002-9343(59)90346-8. [DOI] [PubMed] [Google Scholar]
- 41.Farber SJ. Mucopolysaccharides and sodium metabolism. Circulation. 1960;21:941–947. doi: 10.1161/01.cir.21.5.941. [DOI] [PubMed] [Google Scholar]
- 42.Farber SJ, Schubert M, Schuster N. The binding of cations by chondroitin sulfate. J Clin Invest. 1957;36:1715–1722. doi: 10.1172/JCI103573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37:1236–1256. doi: 10.1172/JCI103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart IJ, Henrich WL. Is there any role for sodium modeling in the prevention of intradialytic hypotension in patients with large interdialytic fluid gains? Seminars in dialysis. 2011;24:422–423. doi: 10.1111/j.1525-139X.2011.00909.x. [DOI] [PubMed] [Google Scholar]
- 45.Schwenger V, Weigand MA, Hoffmann O, et al. Sustained low efficiency dialysis using a single-pass batch system in acute kidney injury - a randomized interventional trial: the REnal Replacement Therapy Study in Intensive Care Unit PatiEnts. Crit Care. 2012;16:R140. doi: 10.1186/cc11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.