Abstract
Purpose
To determine the value of 68Ga-DOTA-TOC and 18F-FDG PET/CT for initial and follow-up evaluation of patients with neuroendocrine tumour (NET) treated with peptide receptor radionuclide therapy (PRRT).
Methods
We evaluated 66 patients who had histologically proven NET and underwent both PRRT and three combined 68Ga-DOTA-TOC and 18F-FDG PET/CT studies. 68Ga-DOTA-TOC PET/CT was performed before PRRT, 3 months after completion of PRRT and after a further 6 – 9 months. 18F-FDG PET/CT was done within 2 months of 68Ga-DOTA-TOC PET/CT. Follow-up ranged from 11.8 to 80.0 months (mean 34.5 months).
Results
All patients were 68Ga-DOTA-TOC PET-positive initially and at follow-up after the first full PRRT cycle. Overall, 62 of the 198 18F-FDG PET studies (31 %) were true-positive in 38 of the 66 patients (58 %). Of the 66 patients, 28 (5 grade 1, 23 grade 2) were 18F-FDG-negative initially and during follow-up (group 1), 24 (5 grade 1, 13 grade 2, 6 grade 3) were 18F-FDG-positive initially and during follow-up (group 2), 9 patients (2 grade 1, 6 grade 2, 1 grade 3) were 18F-FDG-negative initially but 18F-FDG-positive during follow-up (group 3), and 5 patients (all grade 2) were 18F-FDG-positive initially but 18F-FDG-negative during follow-up (group 4).18F-FDG PET showed more and/or larger metastases than 68Ga-DOTA-TOC PET in five patients of group 2 and four patients of group 3, all with progressive disease. In three patients with progressive disease who died during follow-up tumour SUVmax increased by 41 – 82 % from the first to the last follow-up investigation.
Conclusion
In NET patients, the presence of 18F-FDG-positive tumours correlates strongly with a higher risk of progression. Initially, patients with 18F-FDG-negative NET may show 18F-FDG-positive tumours during follow-up. Also patients with grade 1 and grade 2 NET may have 18F-FDG-positive tumours. Therefore, 18F-FDG PET/CT is a complementary tool to 68Ga-DOTA-TOC PET/CT with clinical relevance for molecular investigation.
Keywords: 68Ga-DOTA-TOC, 18F-FDG, PET/CT, NET, PRRT
Introduction
Neuroendocrine tumours (NET) are a heterogeneous group of neoplasms characterized by their overexpression of somatostatin receptors (SSTR) on the cell surface [1]. Several studies have demonstrated the effectiveness of SSTR-targeted imaging for diagnosis, staging, follow-up and deciding upon the suitability of peptide receptor radionuclide therapy (PRRT) [2–5]. The standard indication for PRRT with the radiolabelled SSTR analogues 177Lu/90Y-labelled DOTA-Tyr3-octreotide (TOC)/TATE/lanreotide is metastatic and inoperable SSTR-positive NET, evaluated by SSTR imaging with 68Ga-DOTA-TOC/TATE/NOC/lanreotide PET/CT or 99mTc-HYNIC-TOC/111In-DOTA-TOC/lanreotide scintigraphy, among others [6–10]. The former approach is preferable because of its superior resolution and hence better sensitivity than that of conventional scintigraphy. PET/CT imaging has also been shown to be superior to conventional imaging (CT, MRI) for staging [2, 3, 11].
NET typically have a wide range of cellular differentiation. WHO guidelines classify NET into three grades based on cell proliferation, the number of mitoses and the expression of the nuclear antigen Ki-67. Gastroenteropancreatic NET are classified as low grade (grade 1, Ki-67 <2 %), intermediate grade (grade 2, Ki-67 3 – 20 %) and high grade (grade 3, Ki-67 >20 %) [12]. Both proliferation index and grade strongly correlate with tumour behaviour and prognosis [10, 13, 14]. High-grade, poorly differentiated NET often have limited expression of SSTR [10], what can lead to false-negative SSTR imaging results and make the molecular investigation difficult.
18F-FDG PET/CT is used to assess glycolytic metabolism, and higher uptake of 18F-FDG has been found to be associated with tumour aggressiveness [15]. 18F-FDG PET/CT has thus been used increasingly in the recent years for the evaluation of high-grade NET [15, 16]. To date, however, only a few studies have investigated the correlation between 18F-FDG and SSTR imaging and NET grade. A dichotomous behaviour has been found between these approaches in well-differentiated and poorly differentiated NET, where the former was more positive on SSTR imaging [16, 17] and the latter on 18F-FDG PET [18–20]. Hence, adopting a dual-tracer approach encompassing SSTR and 18F-FDG PET imaging, assessing SSTR expression and glycolytic metabolism, respectively, could support better individualization of therapy selection in patients with NET. High 18F-FDG uptake would suggest an aggressive behaviour and the possibility of treatment refractoriness of the cells at the site, whereas low uptake would indicate a biologically indolent lesion.
We performed an intrapatient comparison of the results of 68Ga-DOTA-TOC and 18F-FDG PET/CT in the initial and follow-up evaluation of NET patients who had received the first full treatment cycle with PRRT. We also evaluated whether possible changes in tumour 18F-FDG uptake correlate with disease course.
Materials and methods
Patients
We retrospectively evaluated a cohort of 66 patients with histological confirmation of NET (according to the ENETS criteria) [13] who underwent PRRT (after confirmation of SSTR-positive lesions with 68Ga-DOTA-TOC PET/CT) and underwent three combined studies with 68Ga-DOTA-TOC and 18F-FDG PET/CT at our institution between 2005 and 2013. The methods of tissue collection were resection of the primary tumour in 27 patients, surgical excision of a metastatic lesion in 3 patients and biopsy of a metastatic lesion in the other 36 patients. The Ki-67 index was evaluated with immunohistochemistry. Patient demographics are shown in Table 1.
Table 1.
Demographic data
| Characteristic | Value |
|---|---|
| Total number of patients | 66 |
| Age at initial diagnosis (years) | |
| Mean ± standard deviation | 57.2 ± 7.0 |
| Range | 34 – 78 |
| Gender | |
| Male | 38 |
| Female | 28 |
| Primary tumour site | |
| Pancreas | 20 |
| Stomach | 1 |
| Jejunum | 9 |
| Ileum | 15 |
| Colon | 2 |
| Rectum | 2 |
| Lung | 8 |
| Unknown | 9 |
| Sites of metastases | |
| Liver | 60 |
| Lymph nodes | 33 |
| Bone | 22 |
| Lung | 11 |
| Adrenal gland | 2 |
| Brain | 1 |
| Thyroid gland | 1 |
| Spleen | 1 |
| Grade | |
| 1 | 12 |
| 2 | 47 |
| 3 | 7 |
The data presented are number of patients, except age in years
All patients included in the study were in advanced stages requiring systemic antitumour therapy in a palliative setting. In particular, more than 65 % had metastases in more than one location, and most of them showed widespread metastases. Before undergoing PRRT, 43 patients were treated with other modalities including resection of the primary tumour (27 patients), chemotherapy (7 patients), and radiofrequency ablation or embolization of liver metastases (9 patients). The remaining 23 patients with widespread metastases were referred to our department for PRRT without previous therapy. Three patients were syndromic (two with insulinoma, one with gastrinoma). Fifteen patients had tumour progression at study entry. The average time from initial diagnosis to PRRT was 3.8 years (range 1 – 11 years, standard deviation ±1.4 years).
68Ga-DOTA-TOC PET/CT was performed at baseline (i.e. before PRRT), 3 months after completion of the first full PRRT cycle and every 6 – 9 months thereafter. All patients proceeding to PRRT underwent a 18F-FDG PET/CT scan as part of routine work-up. 68Ga-DOTA-TOC and 18F-FDG PET/CT were performed within 2 months of each other. Between these two scans no PRRT was given. A total of 198 combined 68Ga-DOTA-TOC and 18F-FDG PET/CT studies (baseline and after the first PRRT) were evaluated. Follow-up ranged from 11.8 to 80.0 months (mean 34.5 months).
Peptide receptor radionuclide therapy
90Y-DOTA-TOC was used as the radiopharmaceutical of first choice for PRRT. 177Lu-DOTA-TATE was used if tumour lesions were smaller than 2 cm in diameter or in patients undergoing retreatment. 90Y-DOTA-TOC and 177Lu-DOTA-TATE were administered intravenously.
The periods between baseline evaluation and the first PRRT administration, and between RECIST 1.1 assessment and PRRT administration were <4 weeks. Three to four therapy cycles with 3.7 GBq 90Y-DOTA-TOC or 7.4 GBq 177Lu-DOTA-TATE were administered at an interval of 10 – 14 weeks. The treatment protocol used in our department [6] included the additional administration of “cold” long-acting SST analogues. These were administered after PRRT and repeated 4 weeks later. Retreatment with PRRT was performed at least 6 weeks after the use of “cold” long-acting SST analogues.
In 23 patients treatment was solely with 90Y-DOTA-TOC, in 22 patients solely with 177Lu-DOTA-TATE and in 21 patients with both agents sequentially. Thirty-five patients were retreated with PRRT. The number of therapy cycles with 90Y-DOTA-TOC ranged from 4 to 11 (cumulative activity 10.6 – 27 GBq) and with 177Lu-DOTA-TATE ranged from 4 to 9 (cumulative activity 16.3 – 37.5 GBq).
Positron emission tomography
68Ga-DOTA-TOC
Preparation of 68Ga-DOTA-TOC was based on a fully automated synthesis, as described previously [21]. The patients received 100 – 150 MBq of 68Ga-DOTA-TOC (20 – 30 μg) intravenously. The radiation exposure related to 68Ga-DOTA-TOC was 2.3 – 3.45 mSv [22]. PET acquisition was started 60 – 90 min (median 75 min) after injection. Imaging was performed with a dedicated PET scanner (MS-Advance or MS-Discovery 450; GE Healthcare). Images were acquired from the head to the mid-thigh. Attenuation correction was performed using transmission data obtained with a 67Ge pin source at 3 min per bed position (MS-Advance) or a CT scan (MS-Discovery 450). Ordered-subsets expectation maximization was used for image reconstruction.
18F-FDG
Patients received 200 – 300 MBq of 18F-FDG intravenously after fasting for at least 8 hours. The radiation exposure related to 18F-FDG was 2.4 – 3.6 mSv [23]. PET acquisition was started 52 – 80 min (median 65 min) after injection. The settings and protocol were as described for 68Ga-DOTA-TOC.
CT
A 2.5-mm helical CT scan was performed on a HiSpeed CT/I Advantage scanner (GE Healthcare). Approximately 1.5 mL/kg body weight of Visipaque 320 contrast medium (GE Healthcare) was administered. The radiation exposure related to CT was 2 – 12 mSv [24].
Image review
68Ga-DOTA-TOC and 18F-FDG PET images were assessed by two experienced board-certified nuclear medicine physicians. Criteria for a positive finding on PET studies were focal area(s) of increased tracer uptake or diffusely increased uptake, excluding physiological uptake, in comparison with adjacent tissue on axial, coronal and sagittal images. When the PET results corresponded with those of conventional imaging or histopathology, or when a corresponding lesion appeared on conventional imaging during follow-up, the PET results were rated as true-positive. Lesions not detected on PET but seen on conventional imaging and showing progression during follow-up or confirmed by histopathology were rated as false-negative. PET results suggestive of tumour lesions without corresponding lesions found on conventional imaging during follow-up or verification by histopathology were rated as false-positive.
All PET/CT images were analysed using commercially available software (eNTEGRA; GE Healthcare), which allowed review of PET, CT and fused imaging data. Semiquantitative analysis of all pathological lesions on 18F-FDG PET, calculating the maximum standardized uptake value (SUVmax), was performed. For calculation of the SUV, regions of interest were drawn around areas with focally increased uptake on transaxial slices and automatically adapted to a 3-D volume of interest at a 70 % isocontour. The lesion with the highest SUVmax was chosen for data analysis. No SUVmax cut-off value was applied to differentiate benign from malignant lesions.
RECIST 1.1 was used for determining tumour response to treatment. Based on all imaging and histological findings as appropriated, tumour response was categorized as complete response (CR), partial remission (PR), stable disease (SD) or progressive disease (PD) [25, 26].
Statistical analysis
SPSS software (version 18.0 for Windows SPSS Inc., Chicago, IL, and LEAD Technologies, Charlotte, NC) was used for statistical evaluation of the results. Continuous variables are expressed as mean values with standard deviations. The chi-squared test and t test were used. A p value <0.05 was considered statistically significant.
Results
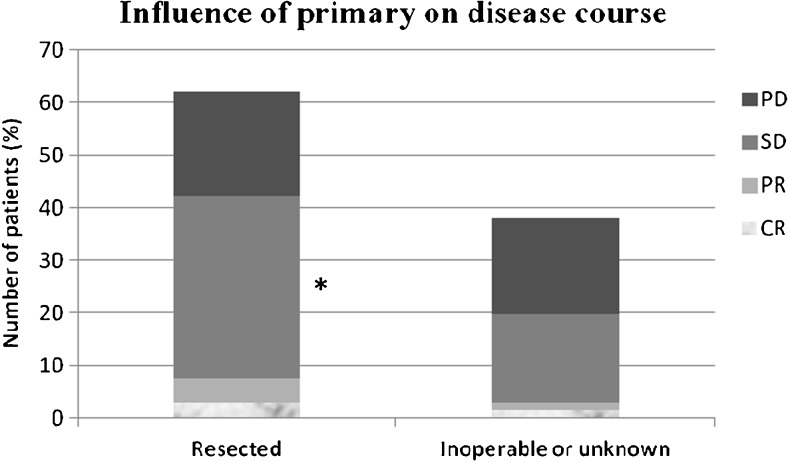
The disease course at last follow-up was CR in 3 patients (4.5 %), PR in 4 (6.1 %), SD in 35 (53.0 %) and PD in 24 (36.4 %). Figure 1 shows the disease course according to primary site.Figure 2 shows the influence of the primary tumour on disease course.
Fig. 1.
Disease course according to primary site (CR complete response, PR partial remission, SD stable disease, PD progressive disease) *p < 0.05 as compared with SD and PR; Δ p < 0.05 as compared with PD
Fig. 2.
Influence of the primary tumour on disease course (CR complete response, PR partial remission, SD stable disease, PD progressive disease) *p < 0.05 as compared with group of inoperable or unknown primaries
68Ga-DOTA-TOC PET
All patients showed SSTR-positive tumour lesions at baseline and follow-up after the first full PRRT cycle. 68Ga-DOTA-TOC-positive lesions were primary tumours in 17 patients (25.8 %; pancreas in 12, lung in 4, ileum in 1 patient) and metastatic sites in 65 patients (98.5 %; liver in 63, lymph node in 32, bone in 23, lung in 8, brain in 1, adrenal gland in 1 patient).
18F-FDG PET
Overall, 62 of the 198 18F-FDG PET/CT studies (31.3 %) were true-positive in 38 of the 66 patients (57.6 %). Two other patients showed 18F-FDG-positive lesions (lymph nodes in 2 patients, lung in 1 patient) only once during follow-up without correlating lesions on 68Ga-DOTA-TOC PET/CT and that were not seen on further follow-up investigations even though no therapeutic intervention took place. These two 18F-FDG PET/CT investigations were rated as false-positive.
Analysis on a per-patient basis
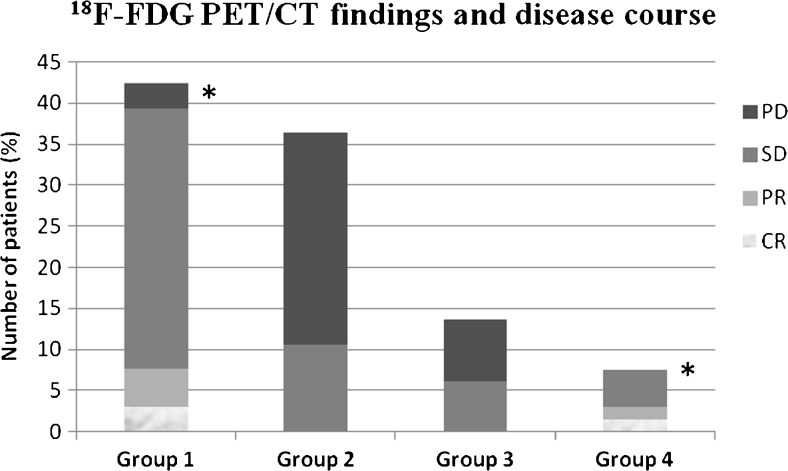
Patients were classified into four groups according to the 18F-FDG PET results as defined in the following sections. The 18F-FDG PET findings and disease course in each patient group are shown in Fig. 3.
Fig. 3.
18F-FDG PET findings and disease course in each patient group: Group 1 patients 18F-FDG-negative initially and during follow-up; Group 2 patients 18F-FDG-positive initially and during follow-up; Group 3 patients 18F-FDG-negative initially, but 18F-FDG-positive during follow-up; Group 4 patients 18F-FDG-positive initially, but 18F-FDG-negative during follow-up (CR complete response, PR partial remission, SD stable disease, PD progressive disease; *p < 0.05 as compared with group 2
Group 1
Patients 18F-FDG-negative initially and during follow-up (28 patients, 42.4 %)
68Ga-DOTA-TOC was positive in 2 primary tumours (2 patients) and in 52 metastatic sites (28 patients; Fig. 4).The tumour was grade 1 in 5 patients and grade 2 in 23 patients.
Fig. 4.
A 75-year-old male patient with grade 1 NET in the ileum. Positive 68Ga-DOTA-TOC PET (progressive disease) and negative 18F-FDG PET initially and during follow-up
Group 2
Patients 18F-FDG-positive initially and during follow-up (24 patients, 36.4 %)
18F-FDG PET was positive in 8 primary tumours (8 patients) and 44 metastatic sites (24 patients; Fig. 5). 18F-FDG PET showed more and/or larger metastases than 68Ga-DOTA-TOC PET in 5 patients (all with PD during follow-up). A ‘flip/flop’ phenomenon (with negative 18F-FDG PET but positive 68Ga-DOTA-TOC PET in some lesions, and positive 18F-FDG PET but negative 68Ga-DOTA-TOC PET in other lesions) was seen in 2 patients with PD.
Fig. 5.
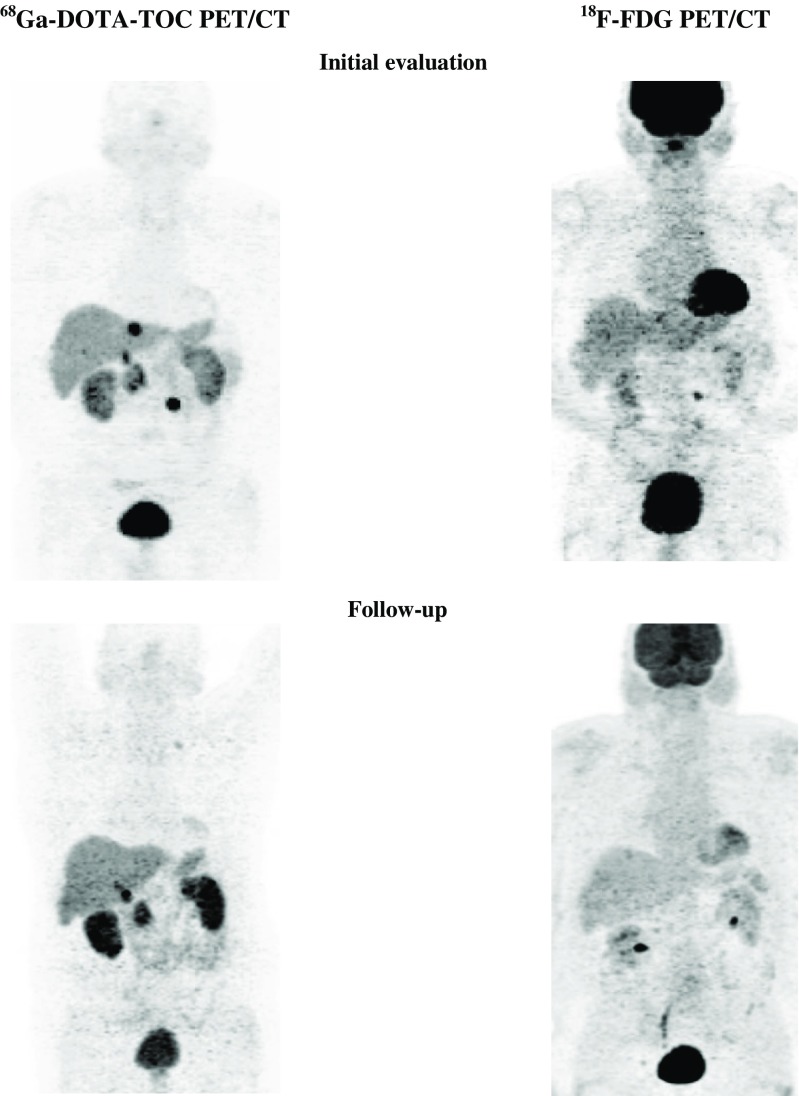
A 32-year-old male patient with grade 2 NET in the pancreatic tail. Positive 68Ga-DOTA-TOC PET and 18F-FDG PET initially and during follow-up (progressive disease)
68Ga-DOTA-TOC PET was positive in 9 primary tumours (9 patients) and 55 metastatic sites (24 patients). 68Ga-DOTA-TOC PET showed more metastases than 18F-FDG PET in 11 patients (3 patients with SD and 8 patients with PD during follow-up).
The tumour was grade 1 in 5 patients, grade 2 in 13 patients and grade 3 in 6 patients. Five patients died during follow-up (SD in 1 patient with grade 1, PD in 1 patient with grade 1, 2 patients with grade 2 and 1 patient with grade 3 tumour).
Group 3
Patients 18F-FDG-negative initially but 18F-FDG-positive during follow-up (9 patients, 13.6 %)
18F-FDG PET was positive in 3 primary tumours (3 patients) and 12 metastatic sites (9 patients; Fig. 6). 18F-FDG PET showed more and/or larger metastases than 68Ga-DOTA-TOC PET in 4 patients (all with PD during follow-up).
Fig. 6.
A 59-year-old female patient with grade 2 NET in the pancreatic head. Positive 68Ga-DOTA-TOC PET and negative 18F-FDG PET initially but positive 18F-FDG-PET/CT during follow-up (progressive disease)
Positive 68Ga-DOTA-TOC PET was seen in 3 primary tumours (three patients) and 13 metastatic sites (nine patients). The tumour was grade 1 in two patients, grade 2 in six patients and grade 3 in one patient.
Group 4
Patients 18F-FDG-positive initially but 18F-FDG-negative during follow-up (five patients, 7.6 %)
Positive 18F-FDG PET was found in three primary tumours (three patients) and eight metastatic sites (five patients). Positive 68Ga-DOTA-TOC PET was seen in three primary tumours (three patients) and eight metastatic sites (five patients). The tumour was grade 2 in all patients. The disease course was CR in one patient (who was re-treated with PRRT and had radiofrequency ablation of liver metastases), PR in one patient (who was re-treated with PRRT; Fig. 7), and SD in three patients (one with surgical resection of primary tumour and LN metastases).
Fig. 7.
A 64-year-old male patient with grade 2 NET in the pancreatic head. Positive 68Ga-DOTA-TOC PET and 18F-FDG PET initially but negative 18F-FDG-PET during follow-up (partial remission)
SUVmax in 18F-FDG PET
In patients with 18F-FDG-positive lesions the SUVmax ranged from 3.0 to 13.0. No significant differences in SUVmax were found among the tumour grades or the various disease course groups. In patients with a more favourable disease course, the SUVmax in the primary tumour and metastases remained unchanged or showed a slight variation (5 % to 13 %) from first to last follow-up. However, in 3 patients with PD who died during follow-up, the SUVmax of the primary tumour (1 patient) and in various metastases (3 patients) showed an increase of 41 % to 82 % from first to last follow-up (SUVmax ranging from 11.2 to 11.9).
Discussion
In the past few years, SSTR imaging with 68Ga-labelled peptide PET/CT has been shown to provide excellent sensitivity and specificity for diagnosing and staging NET [2, 3]. 18F-FDG PET is widely used in oncology, but its use in NET is a matter of controversy. Initial studies [20, 27], performed in a small number of patients only, cast doubt on the use of 18F-FDG PET in NET patients. The loss of SSTR expression was found to coincide with a gain in glucose utilization in tumours [28]. Consequently, it was suggested that the use of 18F-FDG PET be limited to SSTR-negative NET. More recent studies including a larger number of patients evaluated the xsensitivity of 18F-FDG PET in NET patients in comparison with that of 68Ga-DOTA-TATE PET/CT [16] and in relation to survival [29]. These studies showed that the higher the grade of NET, the higher is the prevalence of glucose hypermetabolic tumours [16], which have been linked with more aggressive tumour features including a higher risk of death [29]. This study confirmed that 18F-FDG positivity is strongly correlated with a higher risk of progression, in agreement with the findings of Garin et al. [30] showing that 18F-FDG PET has a prognostic value for early tumour progression. Furthermore, an important finding of our investigation is the evidence that patients may develop 18F-FDG-positive lesions during follow-up. This finding supports the value of repeating 18F-FDG PET in the long-term follow-up of NET patients, in particular if there are signs of progression on other imaging methods such as SSTR PET/CT.
Based on the Ki-67 proliferation index which is determined from analysis of a tumour sample taken mostly at the time of initial diagnosis, it is possible to stratify patients into different risk groups. However, a one-time collected parameter might not have the power to form the basis for therapeutic decisions at all stages and time-points during the disease course. Moreover, the collected tumour tissue might not be representative of all tumour lesions. For appropriate therapy planning it is important to detect the highest proliferative activity among all tumour lesions. SSTR imaging and 18F-FDG PET are modalities for noninvasive visualization of the whole body.
High 18F-FDG SUVs seem to be strongly correlated with short survival in NET patients. In accordance with Binderup et al. [29] who found that patients with a SUVmax higher than 9 are more likely to have a shorter overall survival, in 3 patients with progression who died during follow-up we found SUVmax in the range 11.2 to 11.9 in various tumours at the last follow-up, and importantly the values had increased 41 % to 82 % from the first follow-up. We did not perform a correlation study between 18F-FDG SUVs and Ki-67 index. Further prospective ongoing studies are necessary to establish the value of SUV in NET patients, in particular for assessing survival and progression-free survival.
The ENETS guidelines recommend that in patients with fast progression of NET classified as grade 1 or grade 2 a re-biopsy should be considered, but do not recommend 18F-FDG PET routinely during follow-up [31]. Guidelines now consider a place for 18F-FDG PET in a patient with grade 2 tumour in whom liver transplantation is planned to ascertain the absence of extrahepatic tumour lesions or other malignancies [10]. In this study, we confirm that patients with grade 1 or grade 2 NET may also have 18F-FDG-positive tumours initially and may develop 18F-FDG-positive lesions during follow-up. These findings must be taken into account, especially in individualized and optimized therapy planning.
The limitations of this retrospective study were the small number of patients in the subgroups, due in part to the heterogeneity of NET and the tumour primary sites, and the different treatments performed before and during follow-up. The treatment protocol used in our department included 90Y-DOTA-TOC as the radiopharmaceutical of first choice for PRRT. Although the possible superiority of 90Y for larger tumours has not been shown by direct comparison of 90Y-DOTA-TOC and 177Lu-DOTA-TATE, the rationale for using 90Y-DOTA-TOC in patients with tumours larger than 2 cm in diameter was based on data using a mathematical modelling approach based on the physical characteristics of therapeutic radionuclides [32]. A previous study has indicated that PRRT may lead to a reduction in glucose metabolism in NET lesions, depending on the amount of SSTR uptake as demonstrated by 68Ga-DOTA-NOC PET [9]. We evaluated mainly patients with grade 1 and grade 2 NET. Therefore, we were not able to further investigate the behaviour of grade 3 NET during the course of the disease with regard to 18F-FDG positivity of the tumour lesions.
In conclusion, our results show that investigation only of SSTR status by 68Ga-DOTA-TOC PET/CT may not reflect progression in certain NET lesions. Therefore, the decision on a change in therapeutic strategy in a patient with a poor prognosis cannot be based on this one modality alone. Hence, we recommend performing 18F-FDG PET in the initial evaluation and during follow-up of NET patients, especially when SSTR PET/CT shows progression. 18F-FDG PET/CT is a clinically relevant, complementary tool to 68Ga-DOTA-TOC PET and its use represents a step towards personalized medicine in the management of NET patients.
Acknowledgements
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Compliance with ethical standards
Conflicts of interest
None.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of our institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all patients included in this study.
References
- 1.Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 3.Putzer D, Gabriel M, Henninger B, Kendler D, Uprimny C, Dobrozemsky G, et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med. 2009;50:1214–1221. doi: 10.2967/jnumed.108.060236. [DOI] [PubMed] [Google Scholar]
- 4.Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. 2012;42:80–87. doi: 10.1007/s12020-012-9631-1. [DOI] [PubMed] [Google Scholar]
- 5.Wong KK, Waterfield RT, Marzola MC, Scarsbrook AF, Chowdhury FU, Gross MD, et al. Contemporary nuclear medicine imaging of neuroendocrine tumours. Clin Radiol. 2012;67:1035–1050. doi: 10.1016/j.crad.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel M, Andergassen U, Putzer D, Kroiss A, Waitz D, Von Guggenberg E, et al. Individualized peptide-related-radionuclide-therapy concept using different radiolabelled somatostatin analogs in advanced cancer patients. Q J Nucl Med Mol Imaging. 2010;54:92–99. [PubMed] [Google Scholar]
- 7.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA 0,Tyr3] octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 8.Öberg K, Knigge U, Kwekkeboom D, Perren A, ESMO Guidelines Working Group Neuroendocrine gastro-entero-pancreatic tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii124–vii130. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 9.Oh S, Prasad V, Lee DS, Baum RP. Effect of peptide receptor radionuclide therapy on somatostatin receptor status and glucose metabolism in neuroendocrine tumors: intraindividual comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging. 2011;2011:524130. doi: 10.1155/2011/524130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 11.Naswa N, Sharma P, Kumar A, Nazar AH, Kumar R, Chumber S, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. AJR Am J Roentgenol. 2011;197:1221–1228. doi: 10.2214/AJR.11.7298. [DOI] [PubMed] [Google Scholar]
- 12.Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise N, editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. pp. 13–14. [Google Scholar]
- 13.Rindi G. The ENETS guidelines: the new TNM classification system. Tumori. 2010;96:806–809. doi: 10.1177/030089161009600532. [DOI] [PubMed] [Google Scholar]
- 14.Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40:1262–1268. doi: 10.1016/j.humpath.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Kwee TC, Basu S, Saboury B, Ambrosini V, Torigian DA, Alavi A. A new dimension of FDG-PET interpretation: assessment of tumor biology. Eur J Nucl Med Mol Imaging. 2011;38:1158–1170. doi: 10.1007/s00259-010-1713-9. [DOI] [PubMed] [Google Scholar]
- 16.Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–2455. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Naswa N, Kc SS, Alvarado LA, Dwivedi AK, Yadav Y, et al. Comparison of the prognostic values of 68Ga-DOTANOC PET/CT and 18F-FDG PET/CT in patients with well-differentiated neuroendocrine tumor. Eur J Nucl Med Mol Imaging. 2014;41:2194–2202. doi: 10.1007/s00259-014-2850-3. [DOI] [PubMed] [Google Scholar]
- 18.Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 2011;38:987–991. doi: 10.1007/s00259-011-1787-z. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Sirohi B, Shrikhande SV. Dual tracer imaging approach in assessing tumor biology and heterogeneity in neuroendocrine tumors: its correlation with tumor proliferation index and possible multifaceted implications for personalized clinical management decisions, with focus on PRRT. Eur J Nucl Med Mol Imaging. 2014;41:1492–1496. doi: 10.1007/s00259-014-2805-8. [DOI] [PubMed] [Google Scholar]
- 20.Belhocine T, Foidart J, Rigo P, Najjar F, Thiry A, Quatresooz P, et al. Fluorodeoxyglucose positron emission tomography and somatostatin receptor scintigraphy for diagnosing and staging carcinoid tumours: correlations with the pathological indexes p53 and Ki-67. Nucl Med Commun. 2002;23:727–734. doi: 10.1097/00006231-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Decristoforo C, Knopp R, von Guggenberg E, Rupprich M, Dreger T, Hess A, et al. A fully automated synthesis for the preparation of 68Ga-labelled peptides. Nucl Med Commun. 2007;28:870–875. doi: 10.1097/MNM.0b013e3282f1753d. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann H, Zöphel K, Freudenberg R, Oehme L, Andreeff M, Wunderlich G, et al. Radiation exposure of patients during 68Ga-DOTATOC PET/CT examinations. Nuklearmedizin. 2009;48:201–207. doi: 10.3413/nukmed-0214. [DOI] [PubMed] [Google Scholar]
- 23.Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimate report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med. 2002;43:210–214. [PubMed] [Google Scholar]
- 24.Stamm G, Nagel HD. CT-expo – a novel program for dose evaluation in CT. Rofo. 2002;174:1570–1576. doi: 10.1055/s-2002-35937. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Gabriel M, Oberauer A, Dobrozemsky G, Decristoforo C, Putzer D, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50:1427–1434. doi: 10.2967/jnumed.108.053421. [DOI] [PubMed] [Google Scholar]
- 27.Adams S, Baum R, Rink T, Schumm-Drager PM, Usadel KH, Hor G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998;25:79–83. doi: 10.1007/s002590050197. [DOI] [PubMed] [Google Scholar]
- 28.Krenning EP, Valkema R, Kwekkeboom DJ, de Herder WW, van Eijck CH, de Jong M, et al. Molecular imaging as in vivo molecular pathology for gastroenteropancreatic neuroendocrine tumors: implications for follow-up after therapy. J Nucl Med. 2005;46 Suppl 1:76S–82. [PubMed]
- 29.Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–985. doi: 10.1158/1078-0432.CCR-09-1759. [DOI] [PubMed] [Google Scholar]
- 30.Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50:858–864. doi: 10.2967/jnumed.108.057505. [DOI] [PubMed] [Google Scholar]
- 31.Arnold R, Chen YJ, Costa F, Falconi M, Gross D, Grossman AB, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: follow-up and documentation. Neuroendocrinology. 2009;90:227–233. doi: 10.1159/000225952. [DOI] [PubMed] [Google Scholar]
- 32.O’Donoghue JA, Bardiès M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995;36:1902–1909. [PubMed] [Google Scholar]