Highlights
-
•
Age differences are found in hippocampal activity for source retrieval.
-
•
Performance differences are found in hippocampal activity for source retrieval.
-
•
8- to 9-year-olds do not show performance-related differences in activity.
-
•
In 10- to 11-year-olds, only high performers engaged hippocampus for source retrieval.
-
•
High performing adults engage the hippocampal head selectively.
Keywords: Episodic memory, Childhood, Development, Hippocampus, Head, Individual-differences
Abstract
The goal of the present study was to investigate whether hippocampal contribution to episodic memory retrieval varies as a function of age (8–9 versus 10–11 versus adults), performance levels (high versus low) and hippocampal sub-region (head, body, tail). We examined fMRI data collected during episodic retrieval from a large sample (N = 126). Participants judged whether a stimulus had been encoded previously, and, if so, which of three scenes it had been paired with (i.e., source judgment). For 8- to 9-years-olds as well as low-performing 10- to 11-year-olds, hippocampal activations did not reliably differentiate between trials on which item-scene associations were correctly recalled (correct source), incorrectly recalled (incorrect source), or trials on which the item was forgotten (miss trials). For high-performing 10–11-year olds and low-performing adults, selective hippocampal activation was observed for correct source relative to incorrect source and miss trials; this effect was observed across the entire hippocampus. For high-performing adults, hippocampal activation also distinguished between correct and incorrect source trialsl, but only in the hippocampal head, suggesting that good performance in adults is associated with more focal hippocampal recruitment. Thus, both age and performance are important factors for understanding the development of memory and hippocampal function.
1. Introduction
Episodic memory, or the ability to remember specific past events in the context in which they occurred, improves considerably during middle and late childhood (Brainerd et al., 2004, Ghetti and Angelini, 2008, Shing and Lindenberger, 2011). The hippocampus has been implicated in the encoding and integrating the contextual features of our experiences into bound representations and the retrieval of these representations (Diana et al., 2007, Eichenbaum et al., 2007). Thus, understanding developmental changes in hippocampal function in critical for understanding the development of episodic memory.
Although several studies have compared hippocampal function in children and adults in an attempt to explain age-related differences in episodic memory, these studies have yielded varying results. The majority of studies have found age-related differences in hippocampal activation profiles between children and adults (DeMaster and Ghetti, 2013, DeMaster et al., 2013, Ghetti et al., 2010, Paz-Alonso et al., 2008); however, the apparent nature of these developmental differences has not been consistent. For example, children have been found to show decreased memory-related hippocampal selectivity for episodic retrieval compared to adults across the entire hippocampus (DeMaster et al., 2013). However, in other studies developmental differences were restricted to the hippocampal head or tail (e.g., DeMaster and Ghetti, 2013, Paz-Alonso et al., 2008). Finally, other studies have failed to find age-related differences in hippocampal function altogether (Güler and Thomas, 2013, Ofen et al., 2012).
Part of this inconsistency may be due to the relatively small child sample sizes in previous studies.1 It is critical to examine age-related differences with large enough sample sizes to differentiate among children of different ages, not just between children and adults, when investigating a developmental period in which behavioral change is robust. Middle childhood is one such period of rapid change in memory performance. For example, several studies have shown consistent age-related improvements among children in this age range in assessments of episodic recollection (Ghetti and Angelini, 2008), source memory (Riggins, 2014) and recall (Schwenk et al., 2007). Developmental differences in hippocampal structure have similarly been reported during this period (DeMaster et al., 2014, Gogtay et al., 2006). Given these robust changes, studies that examine age differences among children are more likely to successfully characterize developmental processes than those which consider children as a single group. Results from one of the few studies of memory development in which children of multiple ages were examined separately (Ghetti et al., 2010) illustrate this possibility. In that study, there was evidence of non-linear change from middle childhood into adolescence, such that subsequent item-memory effects were observed in 8-year-olds, no subsequent memory effects were observed in 10- to 11 year-olds and subsequence source-memory effects were evident in 14-year-old and adults. This non-linear pattern underscores the importance of differentiating among children, and the risk of attenuating or misrepresenting age differences when children of different ages are examined together.
Age is not the sole source of potential variation in hippocampal activation. Individual differences in task performance may also yield differences in activation. For example, there is evidence from previous research that differences in activation profiles may be more strongly associated with individual differences in performance than with age (Paz-Alonso et al., 2013). In this study examining the neural substrates of memory suppression, some participants were able to suppress memory retrieval and some participants were not within each age group, despite overall better performance in 10–12 year old children compared to 8–9 year old children. Individual differences in performance were more strongly predictive of differences in neural activation than were age differences. Notably, these performance-related activation differences were examined in regions associated with cognitive control, and not within the hippocampus. Thus, the extent to which age and performance differences affect hippocampal activation remains a key question for investigation. A large sample allows for the examination of potential relations between hippocampal activations during retrieval and task performance, which together may paint a more coherent picture how hippocampally-mediated binding processes contribute to the development of episodic memory.
In addition to examining age- and performance-related differences in hippocampal activation, we aimed to further investigate whether this activation would differ as a function of position along the hippocampal axis. The discussion about whether there are differences in function along the longitudinal axis of the hippocampus has gained momentum in recent years (Poppenk and Moscovitch, 2011, Poppenk et al., 2013) and, although there is no consensus about the exact meaning of these differences, initial evidence from developmental dissociations has bolstered the claim in favor of functional distinctions. This initial evidence has come both from structural and functional developmental studies. Functional differences have been reported with children failing to recruit the hippocampal head to the same extent as adults (DeMaster and Ghetti, 2013, Paz-Alonso et al., 2008), and with children, but not adults, engaging the hippocampal tail (DeMaster and Ghetti, 2013). In these studies, children ages 8–11 years were examined as one group, reducing the possibility of fully appreciating development during this period. Furthermore, in other studies, age differences in hippocampal activation extended throughout the entire structure (DeMaster et al., 2013), possibly suggesting that functional distinctions along the longitudinal axis are subtle and may depend on specific aspects of the task. The present study offers the opportunity to examine these regional differences while accounting for both age- and task-related performance.
Consistent with this functional evidence, a study of hippocampal structure has shown that in children, source retrieval was positively associated with volume in the hippocampal tail, whereas in adults it was associated with volumes of more anterior regions (i.e., negatively with the hippocampal head and positively with the hippocampal body; DeMaster et al., 2014). Interestingly, the direction of the volume–behavior associations in adults reflected the direction of development differences: indeed, adults compared to children had a smaller hippocampal head, and adults with smaller hippocampal heads exhibited better source retrieval. Thus, there is strong evidence from anatomical as well as functional investigations for developmental differences along the longitudinal axis of the hippocampus, and consideration of these differences may be critical for understanding hippocampal contributions to improvements in episodic retrieval during childhood.
2. The present study
The present study was aimed at examining age- and performance-related differences in hippocampal function during episodic retrieval. In addition, we were interested in investigating whether these differences varied as a function of location along the longitudinal axis of the hippocampus. To achieve these goals, we assessed a sample of 8- to 11-year-olds and young adults with a source memory task that required participants to remember which of three scenes was initially paired with individual objects. The task was designed to: (1) reflect the basic structure of source memory tasks used in prior studies (Ghetti et al., 2010); (2) be appropriate for a wide age range; and (3) incorporate meaningful item-context pairings. In particular, contextual information in this task (i.e., the scenes) is visually and semantically richer than contextual information used in previous studies such as position (DeMaster et al., 2013), colored borders (DeMaster and Ghetti, 2013), or another paired item (Güler and Thomas, 2013).
We predicted that, compared with adults, children would not show as strong hippocampal selectivity for correct source trials compared to incorrect source trials and forgotten trials. Furthermore, we predicted differences in hippocampal activation profiles among children of different ages, given the consistent age-related behavioral differences (Ghetti and Angelini, 2008, Ghetti et al., 2010) and some initial evidence of differences in hippocampal activation observed during this period (DeMaster et al., 2013, Ghetti et al., 2010, Paz-Alonso et al., 2008). For example, in a study examining encoding-related activity (Ghetti et al., 2010), 8-year-olds recruited the hippocampus successfully, but this activation did not differ as a function of subsequent source accuracy (i.e., whether the item was subsequently remembered with the correct or incorrect source). By contrast, 10- to 11 year-olds showed a tendency for increased activation for subsequently correct source, but this difference was not as strong as in adults.
Recent work has highlighted the importance of matching participants for behavioral performance when analyzing functional results (Ghetti et al., 2010, Güler and Thomas, 2013). Given the high cognitive demand of the tasks and these previous findings, we predicted large individual differences in episodic memory among both children and adults. Thus, we sought to further characterize age-related differences in hippocampal contribution to episodic retrieval by contrasting task-related activations of higher and lower performing individuals within each age group.
Finally, we hypothesized that activation patterns and age-related differences would vary as a function of location along the longitudinal axis of the hippocampus (DeMaster and Ghetti, 2013, DeMaster et al., 2014, Gogtay et al., 2006, Poppenk et al., 2013). Based on this prior work, we predicted that adults would recruit more anterior regions during episodic retrieval compared to younger groups, possibly reflecting flexible retrieval processes involved in recalling the source of a memory (Giovanello et al., 2009).
3. Methods
3.1. Participants
Participants included 51 8- to 9-year-old children (8.02–9.98 years, mean = 9.03 years, SD = 0.66 years, 30 females) and 44 10- to 11-year-old children (10.02–11.99 years, mean = 10.67 years, SD = 0.54 years, 22 females) and 31 young adults (18.20–25.44 years old, mean = 19.18, SD = 1.34, 17 females), for a total sample size of 126. We selected the age groups based on previous studies which examined similar ages, both in overall range (DeMaster and Ghetti, 2013, DeMaster et al., 2013), and specific division into two child groups (DeMaster et al., 2014) that have previously shown developmental differences between them. Of the total analyzed sample, 60.3% identified as white or Caucasian, 17.5% as black or African American, 17.5% as Asian American, 2.4% as Native Hawaiian or Pacific Islander, and 2.3% as American Indian or Alaska Native. Nine additional children were excluded from analyses due to chance behavioral performance, and an additional 22 were excluded as a result of excessive head motion during the scans. Specifically, scans with more than 25% bad volumes (see fMRI Data Analysis) were excluded from analysis. Participants with at least two out of three usable retrieval scans based on this criterion were included. In addition, participants with only one usable retrieval scan were included if that scan had no more than 10% bad volumes. Overall, 12.4% of possible volumes were excluded, either due to motion or non-completion. Fewer volumes were excluded for adults (2.6%) compared to children (15.4%; p < .001).
Child participants were recruited using flier distributions to elementary schools in the Sacramento City Unified School District in Sacramento, California, and the Washington Unified School District in West Sacramento, California. Children received $70 for their participation, whereas young adults recruited from the University of California, Davis subject pool received course credit. Approval for study of human subjects was granted by the UC Davis Institutional Review Board.
3.2. Materials and procedure
Behavioral and functional MRI (fMRI) data were collected as part of a larger longitudinal study on memory development. Data were collected at the UC Davis Imaging Research Center in Sacramento, California. All participants completed a brief training protocol using a mock scanner located in the same facility.
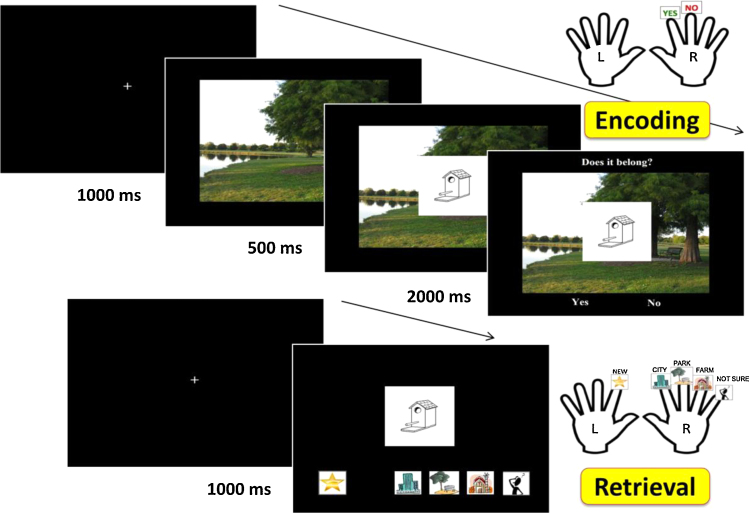
While in the scanner, participants completed a source memory task (Fig. 1). The task was subdivided into 3 alternating encoding and retrieval scans. Each encoding scan consisted of 48 item-scene pairs. On each trial, one of three scenes (city, park, or farm) was presented for 1000 ms, followed by the appearance of a superimposed line-drawn item (an object or an animal; Cycowicz et al., 1997) for 500 ms. Participants were asked to assess whether or not that object “belonged” in that scene and had 2000 ms to respond following the appearance of a “Does it belong?” prompt. Each scene presentation was separated by a jittered fixation cross, ranging from 500 to 8000 ms in duration.
Fig. 1.
Participants completed encoding (top) and retrieval (bottom) phases of a source memory task in the scanner.
For each retrieval scan, participants were shown 64 line-drawn items, of which 16 were novel and 48 were items previously viewed in the preceding encoding scan. Participants were asked to either identify the item as novel, or identify the scene with which it had been presented in the encoding scan (city, park, or farm). If participants remembered the object as previously viewed, but could not remember the scene with which it had been paired, they were instructed to select the “Don’t Know” button. This task was designed to be similar to source tasks used in previous studies (e.g., DeMaster et al., 2013, DeMaster and Ghetti, 2013, Güler and Thomas, 2013), but the use of scene was thought to enrich the contextual information.
Participants completed the task inside the scanner using two 5-button LumiTouch button boxes, using the left-hand box for “new” responses and the right-hand box for all four “old” responses (i.e., city, park, farm, and “Don’t Know”). All participants were given a 5-min break outside the scanner after the first set of encoding and retrieval scans in order to reduce fatigue.
3.3. fMRI data acquisition
Each encoding and retrieval scan was completed inside the Siemens 3T MRI scanner using a 32-channel head coil. Functional MRI data were acquired with a gradient EPI sequence (repetition time (TR) = 2000 ms, echo time (TE) = 23 ms, no interstice gap, flip angle = 90°, field of view (FOV) = 204 mm, 148 volumes per scan for encoding and 196 volumes per scan for retrieval). Each volume consisted of 37 contiguous 3 mm axial slices. Voxel size was 3 mm × 3 mm × 3 mm. Foam padding was positioned in between each participant's head and the coil to both ensure comfort and reduce head motion during the scan. All participants wore 29 db resistant earplugs to minimize scanner noise and facilitate communication with the experimenter.
3.4. Behavioral analysis
Analysis of behavior included consideration of three separate measures: source accuracy, recognition (d-prime), and response time. Source accuracy was calculated as the number of hits with correctly identified source divided by the number of hits with any identified source. This calculation excludes “Don’t Know” trials from both the numerator and denominator. Recognition performance was calculated using the d-prime sensitivity index: Z (hit rate) − Z (false alarm rate) (MacMillan and Creelman, 2005). Average response times were calculated for correct source responses, incorrect source responses and miss responses. Because we were interested in testing for the effects of performance in hippocampal activation, we identified better and worse-performing individuals within each age group by conducting a median split on source accuracy scores based on median performance for each group.
3.5. fMRI data analysis
Data were preprocessed and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Functional images were corrected for differences in slice acquisition timing and were realigned to the first volume by means of rigid body motion correction with sinc interpolation. Structural images were co-registered to the functional images and then spatially normalized to the T1 template in SPM. The normalization parameters were then applied to the functional images. Functional images were spatially smoothed with a 6-mm full-width half-maximum isotropic Gaussian kernel. To correct for effects of subject motion, bad volumes – those with motion in excess of 1 mm or signal change in excess of 2% – were detected and replaced with interpolated values using ArtRepair (Mazaika et al., 2009). Interpolated values were calculated as the average signal from the nearest usable volumes before and after the deleted volume. The data were then high-pass filtered with a limit of 120 s and submitted to statistical analyses.
The primary goal of our data analyses was to examine differences in levels in BOLD response when participants: (1) correctly identified an item with its previously paired scene (Correct source); (2) recalled the item but failed to pair it with the correct scene (Incorrect source); and (3) forgot a previously seen item, and identified as novel (Miss). Incorrect source trials included “Don’t Know” responses in addition to selection of incorrect scenes. Activation associated with correct rejections or false alarms to novel items were included in our task model but are not examined in the current report.
Whole-brain exploratory analyses were performed using a general linear model (GLM) that incorporated task effects (i.e., the trial types described above), session effects, and a general linear trend. The model also included six motion parameters, describing scan-to-scan translation and rotation, as covariates of non-interest. Task effects were modeled via epoch regressors aligned to the onset of each retrieval trial, with the epoch duration equal to the response time for that trial. Including response time in the model in this manner, helps to minimize the extent to which increased response times can drive increase BOLD signal (Grinband et al., 2008). The GLM was used to compute the least-squares parameter estimate of the height of the best-fitting synthetic response function for each trial type at each voxel. Parameter estimates associated with each trial type were combined to produce contrast images for target contrasts. Group-level t-tests were performed on these contrast images to produce group activation maps.
Region-of-interest (ROI) analyses were performed using MarsBar (Brett et al., 2002). ROIs were defined using left and right hippocampal templates from the AAL atlas (Tzourio-Mazoyer et al., 2002), further subdivided into head, body, and tail based on the coordinates described in DeMaster and Ghetti (2013). Specifically, a plane at y = −20 in MNI space separated head from body, and a plane at y = −35 separated body from tail.
The mean signal across all voxels in a defined region was submitted to the GLM analysis as described above to produce an ROI parameter estimate for each trial type for each subject. These ROI parameter estimates were then submitted to statistical analysis.
3.6. Statistical analyses
Behavioral and fMRI data were submitted to repeated measures ANOVAs in SPSS. The factors that we considered included trial type (source correct, source incorrect, or miss), age group (8- to 9-year-olds, 10- to 11-year-olds, or adults), and performance group (low or high). In addition, for hippocampal activation, we included side (left or right) and sub-region (head, body, or tail) as factors of interest. We conducted our analyses on discrete age groups, rather than considering age as a continuous variable, because our participants covered a discontinuous age range. The specific age breakdown was motivated by prior studies that have shown hippocampal differences for 8- to 9-year-olds compared to 10- to 11-year-olds (e.g., DeMaster et al., 2015, Ghetti et al., 2010), thereby facilitating the comparison between the current results and those previously reported in the literature. In preliminary analyses of both behavioral and fMRI data, we observed no effect of gender. Thus, gender was excluded as a factor from the analyses that we present below. Post hoc tests comparing specific age groups, trial types, or hippocampal sub-regions were corrected for multiple comparisons using Bonferroni correction.
4. Results
4.1. Behavioral results
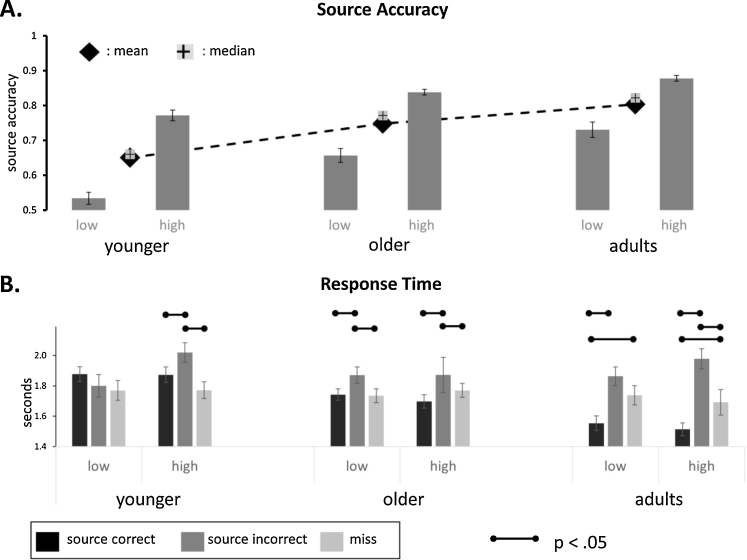
To examine age-related differences in episodic retrieval, we conducted a one-way ANOVA with age group (3 groups: 8- to 9-year-olds, 10- to 11-year-olds, adults) as the single between-subjects factor and source accuracy as the dependent variable (Fig. 2A). There was a significant effect of age group (F3,125 = 15.24, p < .001, ), such that 8- to 9-year-olds demonstrated significantly reduced source accuracy performance compared to 10- to 11-year-olds (p = .001), and adults (p < .001).
Fig. 2.
Behavioral performance on the source memory task as a function of age group and performance group, including: (A) source accuracy (see text for definition) and (B) response times. Error bars depict standard error of the mean (source accuracy) or within-subjects standard error (response time).
Age-related differences were also found for item recognition. When we conducted a similar ANOVA with d-prime as the dependent variable, we found a significant main effect of age (F3,125 = 13.24, p < .001, ; 8- to 9-year-olds, M = 1.86, SD = .84; 10- to 11-year-olds, M = 2.37, SD = .79; adults, M = 2.77, SD = .76), such that 8- to 9-year-olds had smaller d-prime values than 10- to 11-year-olds (p = .007) and adults (p < .001); the latter two groups did not statistically differ from one another (p = .10).
Analysis of response times (Fig. 2B) included trial type (Correct source, Incorrect source, or Miss) as an additional within-subjects factor. There was a main effect of age group (F3,125 = 4.88, p = .009, ), such that adults responded significantly faster than younger children (p = .007). There was a main effect of trial type (F2,252 = 42.96, p < .001, ), such that response times for incorrect hits were longer than response times for either correct hits or misses (ps < .001). There was also an interaction between age group and trial type (F4,252 = 10.08, p < .001, ), driven by a lack of differentiation in response times between correct and incorrect source judgments in 8- to 9-year-olds.
We identified high- and low-performing individuals by conducting a median split on source accuracy separately for each age group. The median corresponds to .66, .77, and .82 for 8- to 9-year-olds, 10- to 11-year-olds and young adults, respectively. Median splits resulted in 26 low-performing and 25 high-performing 8- to 9-year-olds, 22 low-performing and 22 high-performing 10- to 11-year-olds, and 16 low-performing and 15 high-performing young adults. Notably, high-performing younger children, as well as high-performing older children, had higher average source accuracy scores than low-performing adults (Fig. 2A).
4.2. Hippocampal engagement during episodic retrieval
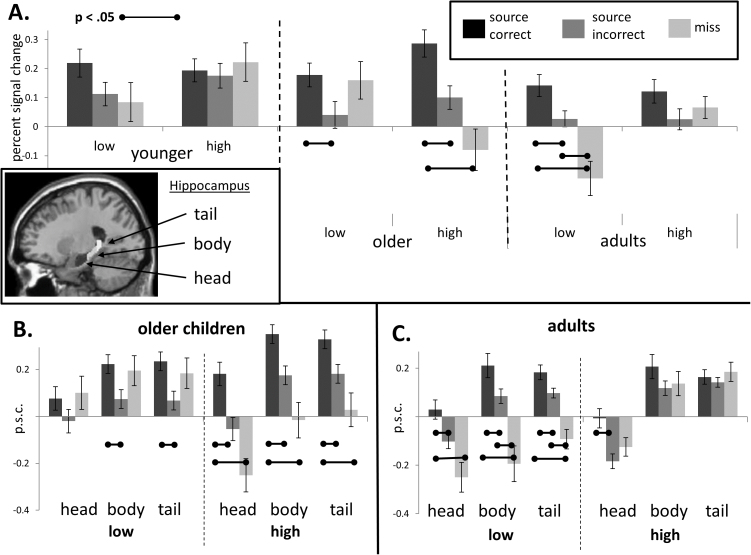
To test for age-related and performance-related differences in hippocampal activation, we submitted activation levels from the six hippocampal ROIs (left and right head, body, and tail sub-regions) to a 3 (age group) × 2 (performance group: higher vs. lower) × 3 (sub-region: head, body, tail) × 2 (hemisphere: left vs. right) × 3 (trial type: correct source, incorrect source, miss) mixed ANOVA. This analysis revealed a main effect of trial type, F2,240 = 7.75, p = .001, , with overall greater activity for correct source than both incorrect source (p < .001) and miss (p = .005) trials. There was also a main effect of hippocampal sub-region, F2,240 = 79.5, p < .001, , with greater activation of hippocampal body and tail relative to the head (ps < .001). A main effect of hemisphere approached statistical significance, F1,120 = 3.20, p = .08, , such that the left hemisphere showed a tendency toward greater activation, but none of the additional results were qualified by hemisphere.
The main effects of trial type and age group were fully qualified by a three-way interaction involving trial type, age group, and performance group (F4,240 = 3.62, p = .007, ), further qualified by a four-way interaction that also involved hippocampal sub-region (F8,240 = 2.91, p = .003, ). To understand the 3-way interaction, we examined the effects of performance level and trial type separately for each age group. Then, we explored whether/how these effects differed among hippocampal sub-regions to address the 4-way interaction.
4.2.1. Age, performance, and trial-related differences in hippocampal activation
In 8- to 9-year-olds, there was no main effect of trial type or performance level on hippocampal activation, Fs2,98 ≤ .78, ps ≥ .46, . This age group demonstrated a relatively undifferentiated pattern of activation across trial types (Fig. 3A).
Fig. 3.
(A) Hippocampal activation during memory retrieval as a function of trial type, age group and performance level; (B) hippocampal activation in older children as a function of trial type, performance group, and hippocampal region (i.e., head, body, and tail as shown in figure); (C) hippocampal activation in adults as a function of trial type, performance group, and hippocampal region. Error bars depict within-subjects standard error.
In 10- to 11-year-olds, in contrast, the significant main effect of trial type, F2,84 = 4.84, p = .01, , was qualified by an interaction with performance group (F2,84 = 4.22, p = .02, ). Specifically, as evident in Fig. 3A, high-performing 10- to 11-year-olds demonstrated a reliable effect of trial type (p = .002), driven by greater hippocampal activation for source correct trials compared to both source incorrect (p = .005) and miss trials (p = .01). The main effect of trial was not statistically significant in low-performing 10- to 11-year-olds (p = .27).
Notably, the hippocampal activation profile in low-performing 10- to 11-year-olds was statistically indistinguishable from that observed in younger children, as demonstrated by a non-significant interaction between participant group (i.e., low-performing 10- to 11-year-olds vs. all 8- to 9-year-olds) and trial type (p = .63). In contrast, the activation profile in high-performing 10- to 11-year-olds was distinct from that observed in 8- to 9-year-olds (high-performing 10- to 11-year-olds vs. all 8- to 9-year-olds by trial type: F2,156 = 4.6, p = .03, ).
In adults, there was an interaction between trial type and performance group (F2,30 = 4.01, p = .02, ), such that low-performing adults demonstrated an overall effect of trial type (p = .001 with source correct significantly higher than source incorrect, p = .008, and miss trials, p = .02), but high-performing adults did not (p = .38). One might find surprising the absence of trial effects in high-performing adults, given its clear presence in low-performing adults. However, the examination of hippocampal sub-regions clarifies this finding.
4.2.2. Effects of hippocampal region in older children and adults
As stated previously, the three-way interaction between age group, performance group, and trial type was further qualified by a four-way interaction that also involved hippocampal sub-region. This four-way interaction was driven, to a large extent, by the fact that hippocampal region further qualified patterns of activation in older children and adults.
Low-performing 10- to 11-year-olds exhibited a tendency toward elevated activation for miss trials, similarly to younger children. However, the comparison of most critical interest concerned activations in the source correct versus source incorrect trials. Thus, we focused on the critical difference between source correct and source incorrect trials in 10- to 11-year-olds (Fig. 3B), and found a significant item type X performance group X region interaction, F2,84 = 3.23, p = .045, . In high-performing 10- to 11-year-olds, the difference between correct source and incorrect source trials was significant in each sub-region (ps < .02). In contrast, in low-performing 10- to 11-year-olds this difference was not evident in the hippocampal head (p = .21), but only in the body and the tail (ps ≤ .01).
In adults, there were interactions between trial type and sub-region (F4,61 = 3.80, p = .006, ) and between performance group and region (F2,30 = 3.81, p = .03, ; Fig. 3C), such that, low-performing adults demonstrated increased activation of source correct compared to source incorrect in each of the hippocampal sub regions (ps < .001), but high-performing adults showed this effect only in the hippocampal head (p = .03), and not in the more posterior regions (ps ≥ .29).
4.3. Cortical engagement during episodic retrieval
Although we sought to focus on the hippocampus in the present manuscript, we also conducted whole-brain voxel-wise analysis to provide a more complete picture of the brain regions involved in episodic retrieval. These regions are described in the supplementary materials.
5. Discussion
The goal of the present study was to further our understanding of the developing contribution of the hippocampus to episodic retrieval in a study of sufficient scale to consider effects of both age and performance levels. The current results show age-related and performance-related differences in hippocampal selectivity for correct source retrieval, consistent with the prediction that hippocampal function continues to develop into late middle childhood. Furthermore, we present evidence that developmental differences are not homogeneous along the hippocampal axis.
5.1. Age and performance-related differences in hippocampal activation
As expected, our task probed hippocampal function. The hippocampal activation profile that we observed for adults, namely greater activation for correct source compared to incorrect source and miss trials, is broadly consistent with data from other source memory fMRI paradigms (Cansino et al., 2002, Duarte et al., 2011, Park et al., 2014). These results confirm that our paradigm is sensitive to source memory effects, and establish a benchmark against which to compare hippocampal activation patterns in children.
Previous studies reported age-related differences in hippocampal contribution during episodic retrieval (DeMaster and Ghetti, 2013, DeMaster et al., 2013, Ghetti et al., 2010, Paz-Alonso et al., 2008), which survived when performance levels were controlled for, but the additional effect of performance levels was not examined in its own right. Here, we investigated the effects of both, which yielded interesting developmental findings. Performance level did not moderate activation patterns in 8- to 9-year-olds, but it did in the 10- to 11-year-olds and adults.
The failure to reliably differentiate between trial types in the youngest age group cannot be due to the ability to perform the task given that this pattern was found in high as well as low performing children in this group. It is possible that the contribution of monitoring and decision processes engaged during retrieval alter the activation pattern observed in the hippocampus. If these additional processes fail to be recruited in response to memory signals from the hippocampus, hippocampal activation itself could manifest as less clear or differentiated. However, if this were the case, we should expect behavioral performance to track hippocampal activation in this age group, which was not the case. Furthermore, this result is not due to an overall failure to recruit the hippocampus, because – as in previous retrieval research (DeMaster et al., 2013) – 8- to 9- year-olds recruited this region above baseline levels. Future research should examine whether limits in the resolution or precision of hippocampal memory representations explain this undifferentiated pattern in this youngest group. Future research should also examine whether the combination of processes supporting episodic retrieval and processes supporting responses to novelty (e.g., see Kim, 2013 for a meta-analysis) may also contribute to this failure to differentiate between trials types.
In contrast, in 10- to 11-year-olds both item trial and performance contributed to differences in hippocampal activation profile. Low-performing children in this age group exhibited elevated source correct and miss trials, which did not differ from each other. This result made them more similar to 8- to 9-year-olds. In contrast, high-performing 10- to 11-year-olds demonstrated increased hippocampal activation for correct source trials compared to both incorrect source trials and miss trials, which was similar to low-performing adults. This reduction in activation for miss trials may reflect a change in the hippocampal response to novelty. There is considerable discussion in the field about how the hippocampus may support both encoding of new material and retrieval of old material (see Kim, 2013 for a meta-analysis) and some have proposed an anatomical dissociation between novelty and recollective processes (e.g., Ben-Yakov et al., 2014, Poppenk et al., 2010). These changes in novelty processes may contribute to the apparent changes in selectivity for correct source retrieval: to the extent that the hippocampus exhibits stronger novelty effects in children for forgotten or partially remembered trials, selectivity for correct source trials may result attenuated.
Of course, cross-sectional data cannot provide direct evidence that these distinct activation patterns reflect a developmental transition. Nevertheless, prior work has provided converging evidence suggesting a transition period in 10- to 11-year-olds and adult-like activation patterns by age 14 at least during memory encoding (Ghetti et al., 2010). There are a number of possible explanations for this apparent transition in hippocampal function at age 10–11. In addition to the interplay of processes supported by the hippocampus highlighted in the preceding paragraph, one possibility pertains to local changes in hippocampal structure resulting in functional changes. In recent years, several studies have documented changes in hippocampal structure into adolescence (e.g., DeMaster et al., 2014, Gogtay et al., 2006, Lee et al., 2014) and there is some evidence that these structural changes may be associated with the transition to puberty (Bramen et al., 2011, Goddings et al., 2014, Hu et al., 2013, Satterthwaite et al., 2015). The implications of this transition for hippocampal activation and memory performance are not clear, and longitudinal data will ultimately be critical to determine whether changes occurring during puberty, including associated hormonal changes, may explain hippocampal development and its contribution to behavioral change. It is particularly important to consider individual differences in behavioral performance during periods of substantial developmental change. These periods may be associated with increased variability in how the task is approached, ranging from the type of strategies used to increase performance (Siegler, 2000) to possible differences in motivation (Lockl and Schneider, 2004).
5.2. Role of hippocampal sub-regions
Given the growing body of evidence that recruitment of the hippocampus varies by sub-region (see Poppenk et al., 2013 for review), we included activation along the longitudinal axis as an additional variable in our analyses. In general, our results are consistent with previous literature that has highlighted the role of anterior hippocampal activation over posterior in adults, but not in children (DeMaster and Ghetti, 2013, Paz-Alonso et al., 2008). However, our results extend previous findings by demonstrating that, at least in young adults, the specialization of anterior hippocampus for episodic retrieval is associated with retrieval performance: it was not present in low-performing young adults. This anterior specialization does not solely depend on performance, however, as it was not found in high-performing older children, whose average performance was superior to that of low performing adults. In low-performing 10- to 11-year-olds, unlike 8- to 9-year-olds, there was evidence of initial differentiation between source correct and incorrect trials in the posterior regions, the body and the tail, despite the elevated activation for miss trials as in younger children. These results overall suggest a possible developmental course of hippocampal activation progressing from undifferentiated hippocampal activation to initial selectivity in posterior regions. The activation patterns in high performing 10- to 11-year-olds suggest that once the hippocampus exhibits selective activation for correct detail retrieval, it might proceed from activation extending to the entire structure to more specific activation in anterior regions as shown in high performing young adults. Future longitudinal research should examine whether within-individual change is consistent with this possibility.
Furthermore, it will be important to establish the functional significance and underlying mechanisms of these developmental differences. Previous studies have shown that the activation in the anterior hippocampus may reflect retrieval flexibility demands that are present when the retrieval probe does not fully reinstate the encoding episode (Giovanello et al., 2009). Our source task required retrieval flexibility because participants ought to recall the correct scene associated with items upon being presented these items on black background; original scenes were not presented during the test.
Although our results are consistent with developmental differences occurring along the longitudinal axis culminating in selective activation in the hippocampal head in high performing adults, we note that they do not imply stronger reliance in children on the hippocampal tail for source retrieval as found in previous work (DeMaster and Ghetti, 2013). It is possible that this difference in hippocampal activation is due to task differences; specifically, the stronger semantic associations involved in the item-scene pairs here may contribute to engagement of the more anterior parts of hippocampus in children. Furthermore, failure to show specific recruitment of the hippocampal tail in children was also evident when the task involved the retrieval of spatial elements (DeMaster et al., 2013), suggesting that the type of information being retrieved might contribute to patterns of activation along the hippocampal axis.
Our findings contribute to the growing body of literature that supports the idea of region-dependent functionality in hippocampus. Functional results like those presented here provide complementary and converging evidence with results from volumetric analyses. For example, previous work by Gogtay et al. (2006) found differences in volumes of individual sub-regions in hippocampus, one of which was a decrease in the size of anterior hippocampus into adulthood. DeMaster et al. (2014) found a link between stronger memory performance and decreased volume in the head in adults, but not in children. In children, positive associations with posterior regions were found instead. When assessed in tandem with our current study, these findings support the idea that both structure and function of the hippocampus change during childhood, and in particular that development of the hippocampal head allows this region to become central to memory retrieval in adults.
5.3. Conclusions
Overall, this investigation contributes to the growing literature observing the neural underpinnings of episodic memory development in children by confirming the presence of age-related differences in hippocampal activation. In particular, our results suggest that the hippocampal contribution to episodic retrieval undergoes change into late childhood. Further, they reinforce the idea, suggested previously, that in the path to adulthood, anterior hippocampus may become most strongly associated with high performance in tasks that require flexible retrieval. We present these conclusions in consideration of several caveats. First, given the structural changes to hippocampus over development, it is possible that analysis using a child template, or in native space, could result in different estimates of hippocampal activation. However, we note that the structural changes are small relative to the spatial resolution of the functional analysis, so differences in findings here are likely to be negligible. Second, the apparent transition in hippocampal function occurring around 10–11 years of age merits further investigation. For example, it would be important to extend the age range of the current study to include older adolescents and fill in the age gap between 11-year-olds and young adults, and then examine within-individual changes. Third, consideration of puberty may be critical for understanding this transition. Future work that examines within-individual longitudinal changes, that includes older adolescents, and that considers pubertal status, is called for as a next step to more fully characterize the trajectory of observed changes in the hippocampal contribution to memory retrieval.
Conflict of interest statement
None of the authors have any conflict of interest to declare.
Acknowledgements
Support for this research was provided by a research grant from the National Institute of Mental Health (NIMH) to S. G. and S.A.B. (MH091109). The authors wish to thank Junior Specialists Julia Ross and Jacqueline Pospisil and the research assistants at the lab for their help with data collection.
Footnotes
Functional imaging studies (e.g., DeMaster and Ghetti, 2013, DeMaster et al., 2013, Ghetti et al., 2010, Güler and Thomas, 2013, Ofen et al., 2012, Paz-Alonso et al., 2008) that have directly attempted to characterize the relation between episodic memory and hippocampal activation, overall sample sizes (including all age groups) averaged to 58 participants, less than half the sample of the current study, ranging from 30 participants to 80 participants.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2016.01.003.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Ben-Yakov A., Rubinson M., Dudai Y. Shifting gears in hippocampus: temporal dissociation between familiarity and novelty signatures in a single event. J. Neurosci. 2014;34:12973–12981. doi: 10.1523/JNEUROSCI.1892-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd C.J., Holliday R.E., Reyna V.F. Behavioral measurement ofremembering phenomenologies: so simple a child can do it. Child Dev. 2004;75:505–522. doi: 10.1111/j.1467-8624.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- Bramen J.E., Hranilovich J.A., Dahl R.E., Forbes E.E., Chen J., Toga A.W., Dinov I.D., Wortham C.M., Sowell E.R. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb. Cortex. 2011;21(3):636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16(2) http://marsbar.sourceforge.net [Google Scholar]
- Cansino S., Maquet P., Dolan R.J., Rugg M.D. Brain activity underlying encoding and retrieval of source memory. Cereb. Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D., Rothstein M., Snodgrass J.G. Picture naming by young children: norms for name agreement, familiarity, and visual complexity. J. Exp. Child Psychol. 1997;65(2):171–237. doi: 10.1006/jecp.1996.2356. [DOI] [PubMed] [Google Scholar]
- DeMaster D., Coughlin C., Ghetti S. Retrieval flexibility and reinstatement in the developing hippocampus. Hippocampus. 2015 doi: 10.1002/hipo.22538. [DOI] [PubMed] [Google Scholar]
- DeMaster D.M., Pathman T., Ghetti S. Development of memory for spatial context: hippocampal and cortical contributions. Neuropsychologia. 2013;51(12):2415–2425. doi: 10.1016/j.neuropsychologia.2013.05.026. [DOI] [PubMed] [Google Scholar]
- DeMaster D.M., Ghetti S. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex. 2013;49(6):1482–1493. doi: 10.1016/j.cortex.2012.08.004. [DOI] [PubMed] [Google Scholar]
- DeMaster D.M., Pathman T., Lee J.K., Ghetti S. Structural development of he hippocampus and episodic memory: developmental dissociations along the anterior/posterior axis. Cereb. Cortex. 2014;24(11):3036–3045. doi: 10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- Diana R.A., Yonelinas A.P., Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Duarte A., Henson R.N., Graham K.S. The effect of stimulus content on the neuralcorrelates of source recollection. Brain Res. 2011;1373:110–123. doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Yonelinas A.P., Ranganath C. The medial temporal lobe and recognition memory. Ann. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S., Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from dual process signal detection. Child Dev. 2008;79:339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S., DeMaster D.M., Yonelinas A.P., Bunge S.A. Developmental differences in medial temporal lobe function during memory encoding. J. Neurosci. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello K.S., Schnyer D., Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Geidd J.N., Viner R.M., Blakemore S.J. The influence of puberty on subcortical brain development. NeuroImage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Nugent T.F., III, Herman D.H., Ordonez A., Greenstein D., Hayashi K.M., Clasen L., Toga A.W., Giedd J.N., Rapoport J.L., Thompson P.M. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Grinband J., Wager T.D., Lindquist M., Ferrera V.P., Hirsch J. Detection of time varying signals in event-related fMRI designs. NeuroImage. 2008;43:509–520. doi: 10.1016/j.neuroimage.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler O.E., Thomas K.M. Developmental differences in the neural correlates of relational encoding and recall in children: an event-related fMRI study. Dev. Cogn. Neurosci. 2013;3:106–116. doi: 10.1016/j.dcn.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Pruessner J.C., Coupé P., Collins D.L. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. NeuroImage. 2013;74:276–287. doi: 10.1016/j.neuroimage.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Kim H. Differential neural activity in the recognition of old versus new events: an activation likelihood estimation meta-analysis. Hum. Brain Mapp. 2013;34:814–836. doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Ekstrom A.D., Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage. 2014;94C:162–171. doi: 10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Lockl K., Schneider W. The effects of incentives and instructions on children's allocation of study time. Eur. J. Dev. Psychol. 2004;1(2):153–169. [Google Scholar]
- MacMillan N.A., Creelman D.C. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. Detection Theory: A User's Guide. [Google Scholar]
- Mazaika P., Hoeft F., Glover G.H., Reiss A.L. Methods and software for fMRI analysis for clinical subjects. Presented at the 15th Annual Meeting of the Organization for Human Brain Mapping; June, San Francisco, CA; 2009. [Google Scholar]
- Ofen N., Chai X.J., Schuil K.D.I., Whitfield-Gabrieli S., Gabrieli J.D.E. The development of brain systems associated with successful memory retrieval of scenes. J. Neurosci. 2012;32(29):10012–10020. doi: 10.1523/JNEUROSCI.1082-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Abellanoza C., Schaeffer J., Gandy K. Source recognition by stimulus content in the MTL. Brain Res. 2014 doi: 10.1016/j.brainres.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Paz-Alonso P.M., Ghetti S., Donohue S.E., Goodman G.S., Bunge S.A. Neurodevelopmental correlates of true and false recognition. Cereb. Cortex. 2008;18:2208–2216. doi: 10.1093/cercor/bhm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Alonso P.M., Gallego P., Ghetti S. Age differences in hippocampus cortex connectivity during true and false memory retrieval. J. Int. Neuropsychol. Soc. 2013;19(10):1031–1041. doi: 10.1017/S1355617713001069. [DOI] [PubMed] [Google Scholar]
- Poppenk J., McIntosh A.R., Craik F.I.M., Moscovitch M. Past experience modulates the neural mechanisms of episodic memory formation. J. Neurosci. 2010;30:4707–4716. doi: 10.1523/JNEUROSCI.5466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H.R., Moscovitch M., Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Poppenk J., Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011;6:931–937. doi: 10.1016/j.neuron.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Dev. Psychol. 2014;50(2):449–459. doi: 10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Vandekar S., Wolf D.H., Ruparel K., Roalf D.R., Jackson C., Elliott M.A., Bilker W.B., Calkins M.E., Prabhakaran K., Davatzikos C., Hakonarson H., Gur R.E., Gur R.C. Sex differences in the effect of puberty on hippocampal morphology. J. Am. Acad. Child Adolesc. Psychiatry. 2015;53(3):341–350. doi: 10.1016/j.jaac.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk C., Bjorklund D.F., Schneider W. Factors influencing the incidence of utilization deficiencies and other patterns of recall/strategy-use relations in a strategic memory task. Child Dev. 2007;78:1771–1787. doi: 10.1111/j.1467-8624.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- Shing Y.L., Lindenberger U. The development of episodic memory: lifespan lessons. Child Dev. Perspect. 2011;5(2):148–155. [Google Scholar]
- Siegler R.S. Unconscious insights. Curr. Dir. Psychol. Sci. 2000;9(3):79–83. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Deleorix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.