Supplemental digital contents are available in the text.
Key Words: schizoaffective disorder, paliperidone palmitate, acute exacerbation
Abstract
The optimal treatment for schizoaffective disorder (SCA) is not well established. In this initial 6-month open-label treatment period of a large, multiphase, relapse-prevention study, the efficacy and safety of paliperidone palmitate once-monthly (PP1M) injectable were evaluated in subjects with symptomatic SCA. Subjects with acute exacerbation of SCA (ie, with psychotic and either depressive and/or manic symptoms) were enrolled and treated with PP1M either as monotherapy or in combination with antidepressants or mood stabilizers (combination therapy group). After flexible-dose treatment with PP1M for 13 weeks, stabilized subjects continued into a 12-week fixed-dose PP1M treatment period. A total of 667 subjects were enrolled; 320 received monotherapy and 347 received PP1M as combination therapy; 334 subjects completed the entire 25-week treatment. Statistically significant and clinically meaningful improvements from baseline were observed for all efficacy measures in psychosis (per Positive and Negative Syndrome Scale), mood symptoms (per Young Mania Rating Scale and Hamilton Depression Rating Scale—21 items), and functioning (per Personal and Social Performance Scale) from week 1 to all time points during the 25-week treatment period (P < 0.001). Similar improvements in efficacy measures were observed between subjects receiving monotherapy or combination therapy. Efficacy benefits persisted throughout the 25-week period. The most common adverse events were akathisia (11.1%), injection-site pain (10.6%), and insomnia (10.0%). Paliperidone palmitate once-monthly administered as monotherapy or in combination with mood stabilizers or antidepressants in patients with an acute exacerbation of SCA provided rapid, broad, and persistent reduction in psychotic, depressive, and manic symptoms, as well as improved functioning.
Schizoaffective disorder (SCA), a chronic mental illness characterized by concurrent symptoms of both schizophrenia and affective disorder,1 is estimated to affect 0.3% of the general population2,3 and may account for up to 25% of inpatient admissions to mental health care facilities.4 However, SCA-specific treatment is comparatively underexplored; its psychotic, depressive, and manic symptoms are often managed as distinct targets, resulting in complex pharmacologic regimens of antipsychotics, antidepressants, and mood stabilizers.5–7
For patients with acute exacerbation of SCA, the primary goal of pharmacotherapy is rapid, robust symptom control of both psychosis and affective symptoms,8 in conjunction with acceptable safety and tolerability, to minimize disruptions to the patient's life and improve long-term outcomes.
Oral paliperidone, which is given as monotherapy or in combination with mood stabilizers and/or antidepressants, is the only broad spectrum oral medication approved by regulatory authorities for the acute treatment of SCA.9–12 However, long-term adherence to daily oral treatment regimens is often difficult to achieve.13–17 In contrast, long-acting injectable therapies, such as paliperidone palmitate once-monthly (PP1M), eliminate the burden of daily oral medication intake18,19 and have been demonstrated to improve long-term outcomes in schizophrenic patients compared with placebo or oral antipsychotics.20–22
To demonstrate that PP1M is effective for the maintenance treatment of SCA, a long-term, multiphase, relapse-prevention study was conducted. This study comprised an initial 25-week open-label (OL) treatment period that was designed to achieve stable symptom control of acute exacerbations of SCA with PP1M treatment without oral supplementation, followed by a 15-month double-blind (DB) period during which stabilized subjects were randomly assigned to continue PP1M treatment or to placebo injections. Results of the DB period have been previously reported; subjects who continued treatment with PP1M experienced a significant delay in time to relapse and reduced risk to relapse compared with those who received placebo.23 This article describes results of the initial 25-week OL period of the study.
MATERIALS AND METHODS
Study Design and Population
This multiphase, international study comprised an initial 13-week lead-in and a 12-week stabilization period of PP1M OL treatment followed by a 15-month, randomized, DB, placebo-controlled relapse-prevention period in SCA (NCT01193153) conducted between September 20, 2010, and October 22, 2013 (Supplementary Figure 1, Supplemental Digital Content 1, http://links.lww.com/JCP/A372).23 Inclusion and exclusion criteria and results of the DB phase of the study have been previously described.23 Subjects were required to have had a lifetime and current diagnosis of SCA, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Axis I Disorders (SCID) (Clinician Version) with an acute exacerbation of psychotic and either depressive and/or manic symptoms before screening.
Treatment
All subjects were treated with PP1M either as monotherapy or in combination with prestudy doses of mood stabilizers or antidepressants. The first dose of PP1M was 234 mg (150 mg eq) given intramuscularly (IM) on day 1 and the second dose was 156 mg (100 mg eq) IM on day 8. The first 2 injections were administered in the deltoid muscle; later injections could be given in either the deltoid or gluteal muscle. Doses on days 36, 64, and 92 (lead-in period) were maintained, increased, or decreased in stepwise changes within the range of 78 to 234 mg (50–150 mg eq) IM, as clinically indicated. Subjects who completed the lead-in period and who met predefined stabilization criteria (Positive and Negative Syndrome Scale [PANSS] total score ≤70; Young Mania Rating Scale [YMRS] and 21-item Hamilton Depression Rating Scale [HAM-D-21] score ≤12 at the end of the 13-week lead-in period) entered a 12-week stabilization period.
Assessments
Assessments in the OL period included the following scales: PANSS,24 Clinical Global Impression of Severity for Schizoaffective Disorder (CGI-S-SCA),25 HAM-D-21,26 YMRS,27 and Personal and Social Performance (PSP).28,29 Based on the literature, clinically meaningful improvements in these assessments were defined as a greater than or equal to 10-point decrease30 in the PANSS total score, greater than or equal to 4-point decrease in the HAM-D-21 score,31 and greater than or equal to 6-point decrease in the YMRS score.31 Protocol-defined stabilization criteria were PANSS total score less than or equal to 70 and YMRS and HAM-D-21 scores less than or equal to 12. On the basis of the literature, remission criteria were defined as PANSS total score less than or equal to 60 and YMRS and HAM-D-21 scores less than or equal to 7.25,31,32 Safety assessments included reporting of treatment-emergent adverse events (TEAEs), clinical laboratory testing, vital signs, and movement disorder assessment with the Extrapyramidal Symptoms Rating Scale—Abbreviated.33
Statistics
Efficacy and safety summaries were based on the OL intent-to-treat analysis set of all subjects who received greater than or equal to 1 dose of PP1M. Changes in efficacy variables occurring during the lead-in and stabilization periods of the OL treatment period are summarized descriptively at each time point, including week 25, using a last observation carried forward (LOCF) approach. Differences from baseline were evaluated using paired t tests within each group. Additional summaries are provided for subjects who were receiving monotherapy and combination therapy, and for subjects who entered the stabilization period. A further analysis was conducted in subjects who received prior risperidone or paliperidone within 2 weeks before study entry. Subgroup differences were examined using the Cochran–Mantel–Haenszel test stratified by concomitant medication use for categorical values and by analysis of covariance models with concomitant medication use and corresponding baseline scores as covariates for continuous end points. No adjustments were made for multiplicity, as the OL period was used to determine acceptability for entry to the DB period. Treatment-emergent adverse events and clinical laboratory results were summarized for the monotherapy and combination therapy groups using descriptive statistics and listed for each subject at each measurement time point.
RESULTS
Subjects and Disposition
A total of 921 subjects were screened for entry into the study and 667 were enrolled; 320 (48.0%) and 347 (52.0%) subjects received PP1M as monotherapy and as combination therapy, respectively (Supplementary Figure 1, Supplemental Digital Content 1, http://links.lww.com/JCP/A372 and Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JCP/A373). Overall, 333/667 (49.9%) of enrolled subjects discontinued treatment during the OL period of the study: 235 (35.2%) during the lead-in period and 98 (14.7%) during the stabilization period. The most common reasons for discontinuation were withdrawal of consent (n = 98; 14.7%), failure to meet the predetermined stabilization criteria (n = 82; 12.3%), adverse events (AEs; n = 50; 7.5%), lost to follow-up (n = 42; 6.3%), or lack of efficacy (n = 31; 4.6%). The remaining 334 subjects were randomly assigned into the DB relapse-prevention period, which has been described elsewhere.23
Demographic and disease characteristics in the OL period appeared similar for subjects in the monotherapy and combination therapy groups (Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JCP/A373). At study entry, the mean age of subjects was 39.5 years, 53.1% were white, 46.5% were women, 24.3% had a history of prior suicide attempt, and 37.0% had a history of substance use.
Of the enrolled subjects, 200/667 (30.0%) were hospitalized at screening or before the first injection of study drug. All subjects had psychotic and mood symptoms at baseline per inclusion criteria, experiencing either depressive episodes (48.0%), manic episodes (26.8%), or mixed episodes (25.2%). Mean (SD) baseline symptom scores in this acute population were PANSS, 85.8 (12.8); overall CGI-S-SCA, 4.4 (0.6); HAM-D-21, 20.4 (7.8); and YMRS, 18.6 (9.5). Based on the CGI-S-SCA overall score, most subjects were moderately ill (57.7%) or markedly ill (37.6%) at baseline. The mean (SD) PSP total score at baseline was 51.4 (11.0) with most subjects having variable (93.3% with a score of 31–70) or poor (4.5% with a score of ≤30) functioning. A total of 563 (84.4%) subjects had taken 1 or more psychotropic medications within the 7 days before enrollment (atypical antipsychotics [57.1%], mood stabilizers and antiepileptics [28.8%], antidepressants [24.6%], benzodiazepines [22.8%], nonbenzodiazepine hypnotics and anxiolytics [12.9%], typical antipsychotics [12.6%], anti–extrapyramidal symptoms [EPS] medications [12.3%], β-blockers [6.7%], antihistamines [4.9%], and stimulants [0.3%]); 204 (30.6%) subjects received risperidone or paliperidone within 2 weeks before study entry. Adequate previous exposure to any formulations of paliperidone or risperidone before the study was documented for 350 (52.5%) subjects. The remaining 317 (47.5%) subjects required tolerability testing with oral paliperidone before first PP1M injection.
Most subjects (54.9%) received all 7 injections of PP1M during the 25-week treatment period, including 50.6% and 58.8% of subjects in the monotherapy and combination therapy groups, respectively. The median duration of PP1M exposure of subjects in the 25-week period was 147.0 days (range, 1–166 days). Of the 667 enrolled subjects, the distribution of the last injection dose of PP1M was 78 mg (50 mg eq), 2.7%; 117 mg (75 mg eq), 7.3%; 156 mg (100 mg eq), 52.8%; and 234 mg (150 mg eq), 37.2%.
Efficacy
Changes in Symptom Scores and Stabilization
Significant improvement in psychotic, depressive, and manic symptoms in acutely ill subjects was observed after treatment with PP1M throughout the OL period beginning by week 1 of treatment. Results of the PANSS total score, HAM-D-21 score, and YMRS score changes over time from baseline to the 25-week end point are depicted in Figures 1A–C. Similar improvements were observed in the monotherapy and combination therapy subgroups.
FIGURE 1.
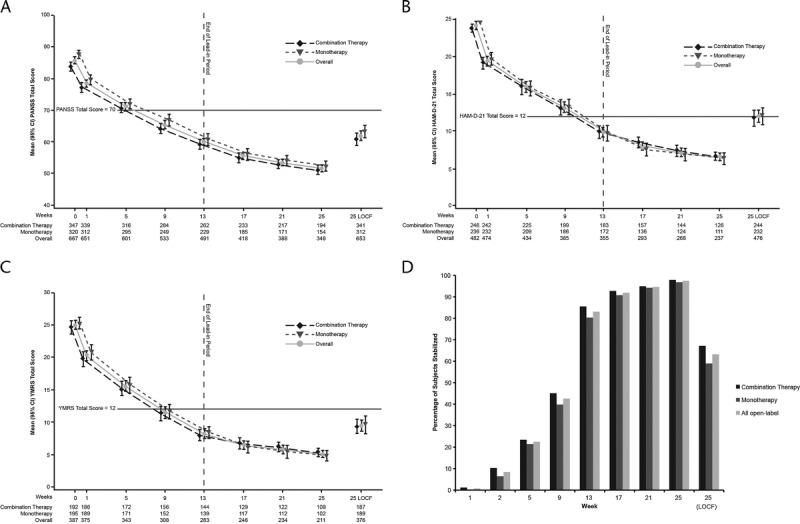
Efficacy during the 25-week OL PP1M treatment phase.* A, Positive and Negative Syndrome Scale (PANSS).† B, Hamilton Rating Scale for Depression, 21-item version (HAM-D-21),† in subjects with baseline HAM-D-21 score ≥16. C, Young Mania Rating Scale (YMRS)† in subjects with baseline YMRS score ≥16. D, Proportion of subjects stabilized on paliperidone palmitate once-monthly injection.‡ CI, confidence interval; HAM-D-21, Hamilton Rating Scale for Depression, 21-item version; LOCF, last observation carried forward; PANSS, Positive and Negative Syndrome Scale; PP1M, paliperidone palmitate once-monthly. *P < 0.001 for change from baseline to end point. †Values are the mean (95% confidence interval). ‡PANSS score ≤70; YMRS and HAM-D-21 scores ≤12. The horizontal line in A–C indicates stabilization criteria.
The proportion of subjects meeting stabilization criteria in all 3 symptom domains (PANSS total score ≤70 and HAM-D-21 and YMRS scores ≤12) increased over time during the 13 weeks of acute treatment (Fig. 1D). Stabilized subjects continued for an additional 12 weeks of treatment. At the end of the 25 weeks of treatment, for those who had a postbaseline efficacy assessment carried forward (LOCF), 63.2% (413/653), 67.2% (229/341), and 59.0% (184/312) in the overall population, combination therapy group, and monotherapy group, respectively, were stabilized in all 3 symptom domains (Fig. 1D).
No significant differences were observed between subjects who had received risperidone or paliperidone within the 2 weeks before the first PP1M injection (n = 204) versus those who did not (n = 463) at either week 13 or week 25 (Supplementary Table 2, Supplemental Digital Content 3, http://links.lww.com/JCP/A374).
Subjects With Clinically Meaningful Improvement in Symptom Domains
Beginning from week 1 and at each time point during the 13 weeks of acute treatment, an increasing proportion of subjects experienced clinically meaningful symptom improvements (≥10-point decrease in the PANSS total score, ≥4-point decrease in the HAM-D-21 score, and ≥6-point decrease in the YMRS score) (Supplementary Table 3, Supplemental Digital Content 4, http://links.lww.com/JCP/A375). This trend was observed in the overall population, and in both the combination therapy and monotherapy groups.
Subjects Meeting Remission Criteria
By week 25, for those subjects who completed the entire 25-week treatment, the proportion of subjects who met remission criteria (PANSS total score ≤60, HAM-D-21 score ≤7, and YMRS score ≤7) was 171/348 (49.1%), 93/194 (47.9%), and 78/154 (50.6 %) in the overall, combination therapy, and monotherapy populations, respectively. These proportions were not significantly different between groups. For those who had a postbaseline efficacy assessment carried forward to week 25 (LOCF), the corresponding values were 198/653 (30.3%), 107/341 (31.4%), and 91/312 (29.2%), respectively (no significant differences between groups).
Changes in Subject Functioning
Significant improvement in subject functioning in acutely ill subjects was observed after treatment with PP1M throughout the OL period beginning by week 1 of treatment. Results of the PSP total score change over time from baseline to the 25-week end point are depicted in Supplementary Figure 2, Supplemental Digital Content 5, http://links.lww.com/JCP/A376. Similar improvements were observed in the monotherapy and combination therapy subgroups.
Safety
Overall, 62.5% of the 667 subjects reported 1 or more TEAEs during a 25-week period. A total of 50 (7.5%) subjects reported TEAEs that led to treatment discontinuation and 54 (8.1%) experienced 1 or more serious AEs (Supplementary Table 4, Supplemental Digital Content 6, http://links.lww.com/JCP/A377). Most TEAEs were mild to moderate in intensity. The most frequently reported TEAEs were nervous system events and psychiatric disorders in 29.8% and 21.9% of subjects, respectively. The most commonly occurring TEAEs (≥5% of subjects) were akathisia (11.1%), injection-site pain (10.6%), insomnia (10.0%), weight increase (8.5%), parkinsonism (6.4%), and headache (5.4%). No differences in TEAEs were identified between subjects in the combination therapy and monotherapy groups. Treatment-emergent adverse events related to EPS, prolactin, and glucose are reported in Supplementary Table 4, Supplemental Digital Content 6, http://links.lww.com/JCP/A377.
DISCUSSION
The findings from the 25-week OL period of this study in subjects with acute exacerbation of SCA demonstrated that treatment with PP1M (without oral supplementation) produced a rapid beneficial effect in subject functioning and the 3 major symptom domains of SCA: psychosis, depression, and mania. This response was significant from week 1 onward. Paliperidone palmitate once-monthly was rapidly effective when used as monotherapy and in combination with mood stabilizers or antidepressants.
For those subjects who were stabilized during the lead-in period (65% of the enrolled subjects), continued improvement in these symptoms was observed with treatment during the 12-week stabilization period. A notable proportion of subjects who completed the entire 25 weeks of treatment (49.1%) met remission criteria for all 3 symptom domains at end point, providing further evidence of PP1M efficacy on all SCA symptom domains with continued therapy after acute treatment. Future analyses are needed to identify predictors of remission to determine which individuals benefit most from PP1M treatment.
Fifty percent of enrolled subjects discontinued treatment during the 25-week OL portion of this study, with 35% discontinuing during the 13-week lead-in period and 15% discontinuing in the subsequent 12-week stabilization period. Overall, 12.2% of subjects discontinued during this phase (7% during the lead-in and 5.2% during the stabilization periods) because they failed to meet the strict, predetermined stabilization criteria for both psychotic and mood symptoms (Supplementary Figure 1, Supplemental Digital Content 1, http://links.lww.com/JCP/A372). Similarly low discontinuation rates (ie, <10%) due to failure to meet stabilization criteria have also been previously reported for studies of both oral and injectable paliperidone that incorporated a lead-in/stabilization period.34,35 However, variations in illness severity among patient populations must be considered when comparing discontinuation rates between studies. The additional reasons for discontinuation—mainly withdrawal of consent, loss to follow-up, lack of efficacy, or AEs—and their rates were similar to those reported in previous studies of PP1M in schizophrenia.18,19 Of the 7.5% subjects who discontinued the study due to AEs, most withdrawals (82%, 41 of 50) occurred during the 13-week lead-in period.
The safety findings in this study are consistent with those of PP1M when used for the acute treatment of schizophrenia18,19 and with those of oral paliperidone when used for the acute treatment of SCA.9,10 The most frequently reported TEAEs (≥10% of subjects) were nervous system–related and psychiatric disorder–related AEs and EPS, and incidence rates were within previously reported ranges.18,19
The limitations of this analysis include its relatively short period of observation for persons with a chronic lifetime illness and the lack of placebo or an active comparator in this portion of the larger study to provide a reference perspective. However, observations in this study regarding efficacy and safety for acute treatment of psychosis and mood symptoms are similar to those observed with oral paliperidone in 2 randomized, placebo-controlled, 6-week studies of patients with acute SCA exacerbation.9,10 Another limitation of this study is that the stabilization criteria restricted the number of participants that could continue for the additional 12-week period of the study. Additionally, because subjects in this study agreed to use long-acting injectable therapy, these results may not be generalizable to all patients with SCA, whose personal preference for therapy options for treatment of their acute SCA symptoms may exclude PP1M.
Currently, antipsychotics form the basis of treatment for SCA, with antidepressants also used for patients with the depressive SCA subtype who do not respond adequately to antipsychotics alone. Patients with a bipolar subtype are frequently prescribed mood stabilizers.16 However, subjects in the current study who received PP1M combination therapy showed improvements in symptoms and functioning that were similar to those observed in patients receiving monotherapy, with no differences in safety and efficacy. These data support a role for PP1M as a monotherapy or in combination with mood stabilizers or antidepressants for the treatment of SCA. Furthermore, the comparable efficacy and safety of PP1M observed when used as either monotherapy or in combination therapy suggests that PP1M therapy alone may be sufficient to manage the acute exacerbation of SCA symptoms without defaulting immediately to the complexities of polypharmacy. Nonetheless, further investigation is required.
Prior results23 and those of this study suggest that PP1M is safe and effective for the acute and maintenance treatment of the psychotic, depressive, and manic symptoms of SCA when used as monotherapy or in combination with mood stabilizers or antidepressants. Paliperidone palmitate once-monthly may be a treatment option of monthly dosing for patients in the acute phase of SCA.
ACKNOWLEDGMENTS
The authors thank Matthew Grzywacz, PhD (ApotheCom, Yardley, PA), for providing writing and editorial assistance; and Cynthia A. Bossie, PhD (Janssen Scientific Affairs, LLC), for her scientific input and discussions.
AUTHOR DISCLOSURE INFORMATION
D.-J. Fu and L. Alphs are employees of Janssen Scientific Affairs, LLC, and are Johnson & Johnson stockholders. I. Turkoz, R. B. Simonson, and C. Canuso are employees of Janssen Research & Development, LLC, and are Johnson & Johnson stockholders. D. Walling has received grant/research support from Janssen Research Foundation, Otsuka, Lundbeck, Sunovion, Forum, Alkermes, and Pfizer. N. Schooler receives grant support from Otsuka and has served on advisory boards/consultant to Allergan, Alkermes, Forum, and Sunovion. J.-P. Lindenmayer has received grant/research support from Janssen, Eli Lilly, Pfizer, Otsuka, Dainippon Sumitomo, Sunovion, Neurocrine, EnVivo, and Roche and is a consultant for Janssen. This study was funded by Janssen Scientific Affairs, LLC.
Footnotes
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the Journal's Web site (www.psychopharmacology.com).
REFERENCES
- 1.Malaspina D, Owen MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150:21–25. [DOI] [PubMed] [Google Scholar]
- 2.Malhi GS, Green M, Fagiolini A, et al. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10:215–230. [DOI] [PubMed] [Google Scholar]
- 3.Perala J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. [DOI] [PubMed] [Google Scholar]
- 4.Kent S, Fogarty M, Yellowlees P. Heavy utilization of inpatient and outpatient services in a public mental health service. Psychiatr Serv. 1995;46:1254–1257. [DOI] [PubMed] [Google Scholar]
- 5.McElroy SL, Keck PE, Jr, Strakowski SM. An overview of the treatment of schizoaffective disorder. J Clin Psychiatry. 1999;60:16–21. [PubMed] [Google Scholar]
- 6.Flynn J, Grieger TA, Benedek DM. Pharmacologic treatment of hospitalized patients with schizoaffective disorder. Psychiatr Serv. 2002;53:94–96. [DOI] [PubMed] [Google Scholar]
- 7.Lerner V, Libov I, Kotler M, et al. Combination of “atypical” antipsychotic medication in the management of treatment-resistant schizophrenia and schizoaffective disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:89–98. [DOI] [PubMed] [Google Scholar]
- 8.Nasrallah HA, Goldberg JF, Correll CU. Differential diagnosis and therapeutic management of schizoaffective disorder. Ann Clin Psychiatry. 2010;22:S1–S12. [PubMed] [Google Scholar]
- 9.Canuso CM, Schooler N, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized, controlled study comparing a flexible dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30:487–495. [DOI] [PubMed] [Google Scholar]
- 10.Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo-controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587–598. [DOI] [PubMed] [Google Scholar]
- 11.Canuso CM, Turkoz I, Fu DJ, et al. Role of paliperidone extended-release in treatment of schizoaffective disorder. Neuropsychiatr Dis Treat. 2010;6:667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Invega (paliperidone) extended-release tablets [package insert]. Titusville, NJ: Ortho-McNeil-Janssen Pharmaceuticals Inc; 2014. http://www.invega.com/prescribing-information. Accessed March 8, 2016. [Google Scholar]
- 13.Karve S, Markowitz M, Fu DJ, et al. Assessing medication adherence and health care utilization and cost patterns among hospital discharged patients with schizoaffective disorder. Appl Health Econ Health Policy. 2014;12:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry. 2002;159:103–108. [DOI] [PubMed] [Google Scholar]
- 15.Murru A, Pacchiarotti I, Nivoli AM, et al. Rates and clinical correlates of treatment non-adherence in schizoaffective bipolar patients. Acta Psychiatr Scand. 2012;125:412–418. [DOI] [PubMed] [Google Scholar]
- 16.Murru A, Pacchiarotti I, Amann BL, et al. Treatment adherence in bipolar I and schizoaffective disorder, bipolar type. J Affect Disord. 2013;151:1003–1008. [DOI] [PubMed] [Google Scholar]
- 17.Perkins DO, Johnson JL, Hamer RM, et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83:53–63. [DOI] [PubMed] [Google Scholar]
- 18.Pandina GJ, Lindenmayer JP, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30:235–244. [DOI] [PubMed] [Google Scholar]
- 19.Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:218–226. [DOI] [PubMed] [Google Scholar]
- 20.Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160:1125–1132. [DOI] [PubMed] [Google Scholar]
- 21.Nasrallah HA. The case for long-acting antipsychotic agents in the post-CATIE era. Acta Psychiatr Scand. 2007;115:260–267. [DOI] [PubMed] [Google Scholar]
- 22.Alphs L, Benson C, Cheshire-Kenny K, et al. Real-world outcomes of -paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76:554–561. [DOI] [PubMed] [Google Scholar]
- 23.Fu DJ, Turkoz I, Simonson RB, et al. Paliperidone palmitate once-monthly reduces risk of relapse of psychotic, depressive, and manic symptoms and maintains functioning in a double-blind, randomized study of schizoaffective disorder. J Clin Psychiatry. 2015;76:253–262. [DOI] [PubMed] [Google Scholar]
- 24.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 25.Allen MH, Daniel DG, Revicki DA, et al. Development and psychometric evaluation of a clinical global impression for schizoaffective disorder scale. Innov Clin Neurosci. 2012;9:15–24. [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 28.Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV social and occupational functioning assessment scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–329. [PubMed] [Google Scholar]
- 29.Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res. 2008;161:213–224. [DOI] [PubMed] [Google Scholar]
- 30.Leucht S, Kane JM, Etschel E, et al. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31:2318–2325. [DOI] [PubMed] [Google Scholar]
- 31.Turkoz I, Fu DJ, Bossie CA, et al. Relationship between the clinical global impression of severity for schizoaffective disorder scale and established mood scales for mania and depression. J Affect Disord. 2013;150:17–22. [DOI] [PubMed] [Google Scholar]
- 32.Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res. 2005;79:231–238. [DOI] [PubMed] [Google Scholar]
- 33.Chouinard G, Margolese HC. Manual for the extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res. 2005;76:247–265. [DOI] [PubMed] [Google Scholar]
- 34.Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2007;27:6–14. [DOI] [PubMed] [Google Scholar]
- 35.Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116:107–117. [DOI] [PubMed] [Google Scholar]


