Abstract
Antipsychotics are the drugs prescribed to treat psychotic disorders; however, patients often fail to adhere to their treatment, and this has a severe negative effect on prognosis in these kinds of illnesses. Among the wide range of risk factors for treatment nonadherence, this systematic review covers those that are most important from the point of view of clinicians and patients and proposes guidelines for addressing them. Analyzing 38 studies conducted in a total of 51,796 patients, including patients with schizophrenia spectrum disorders and bipolar disorder, we found that younger age, substance abuse, poor insight, cognitive impairments, low level of education, minority ethnicity, poor therapeutic alliance, experience of barriers to care, high intensity of delusional symptoms and suspiciousness, and low socioeconomic status are the main risk factors for medication nonadherence in both types of disorder. In the future, prospective studies should be conducted on the use of personalized patient-tailored treatments, taking into account risk factors that may affect each individual, to assess the ability of such approaches to improve adherence and hence prognosis in these patients.
Key Words: adherence, antipsychotic, schizophrenia, bipolar disorder
One of the greatest problems clinicians face when dealing with chronic illnesses is the effectiveness of treatment. This is determined by various different factors such as patient tolerance of the drug, the appropriateness of the regimen,1 and, above all, adherence to the treatment prescribed. The best medication at the best dose can never be effective if the patient does not take it.
Medication adherence, previously known as compliance,2 has been defined as “the extent to which a person's behavior coincides with the medical advice given.”3 This may include refusing to attend medical appointments or to start a treatment program or early discontinuation, as well as incomplete implementation of the doctor's instructions.4 Such behavior has a negative effect on the outcome of the illness and leads to higher rates of recurrence and hospitalization, worsening of signs and symptoms, and increases in hospital costs.5
At least half of patients who are prescribed long-term medication do not finish the course, this phenomenon representing a particularly serious problem in chronic psychiatric illnesses,6–10 in which treatment adherence rates are even lower than in other conditions.11,12 Specifically, considering 2 serious psychiatric disorders, bipolar disorder, and schizophrenia,13 mean rates of treatment adherence are approximately 42% in schizophrenia14 and 41% in bipolar disorder, with considerable variation between studies.15 This variation is mainly attributable to a lack of consensus on the best methodology for assessing adherence (qualitative vs quantitative research, patient self-reporting vs reports of clinicians, direct measurement of blood or urine parameters vs indirect measurements), the period of observation (from a week to several months), and the criteria for defining lack of adherence.16 Furthermore, medication adherence is a dynamic dichotomous behavior, influenced by multiple factors17 that may be related to patients (adverse effects of medication), their social relationships (family support and therapeutic alliance), cognitive problems such as impaired memory or attention,18 and the system for providing health services.19 Analysis of these factors has become a critical issue for clinicians and researchers, given that identification of specific risk factors will make it possible to carry out patient-targeted interventions.5,20 This is particularly important in early stages of severe mental illness, where it has been seen that treatment nonadherence is most critical for patient outcome.21
It has been reported that nonadherence to antipsychotic drugs in patients diagnosed with schizophrenia or schizophreniform psychosis is associated with a lower probability of a good response to treatment and significantly less improvement than in those who adhere to treatment,1 a higher rate of positive and negative psychotic symptoms,22 and a greater risk of hospital readmission.23 Similarly, it has been found that patients with bipolar disorder with good treatment adherence had less severe signs and symptoms, lower scores in the Clinical Global Impressions bipolar mania and hallucinations/delusions scales,24 and a lower risk of suicide.25 Martinez-Aran et al18 demonstrated that a history of nonadherence in adults with bipolar disorder was significantly associated with cognitive impairment.
The objectives of this systematic review are to provide a detailed and comprehensive description of the most important factors associated with lack of adherence to antipsychotic medication in patients with schizophrenia spectrum disorder and bipolar disorder and thereby to contribute to clarify our understanding of the factors underlying nonadherence.
MATERIALS AND METHODS
Literature Search
This systematic review was conducted and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.26 We performed an electronic search in the PubMed (1990–2015) database, using the following MeSH terms: medication adherence, antipsychotic agents, mood disorder, psychotic disorder, and bipolar disorder. We selected 1990 as the start date for the search because of the reintroduction of clozapine in the following decade and the approval of risperidone by the Food and Drug Administration in the same period (1993).
In addition, we used the following filters: randomized controlled trial, meta-analysis, clinical trial, systematic review, controlled clinical trial, observational study, and humans. We reviewed all the articles published in English and Spanish. Subsequently, reference lists from the studies included in our systematic review were hand searched for additional relevant publications.
Inclusion Criteria
We included all the systematic reviews, meta-analyses, clinical trials, randomized clinical trials, and observational studies in which the study population was patients diagnosed with bipolar disorder, schizophrenia, schizoaffective disorder, or schizophreniform disorder who were being treated with antipsychotics and in whom factors associated with treatment adherence were assessed. Articles were excluded if patients had a diagnosis other than those mentioned previously or medical treatment with agents other than antipsychotics (eg, lithium or mood stabilizers), as well as if there was no assessment of factors associated with adherence to treatment with antipsychotics.
Data Collection and Extraction
From the set of articles selected in the systematic review, we excluded those that did not meet all the inclusion criteria or met any of the exclusion criteria. After reading the titles and the abstracts, we selected articles related to the objective of our study. These were then summarized and assessed by 2 independent reviewers using the “Critical Reading Sheets” tool developed by the Basque Office for Health Technology Assessment,27 and the most relevant data were retrieved. In the event of disagreement, a third researcher analyzed the article independently. The Basque Office for Health Technology Assessment tool facilitated the assessment of the methodological quality of the research described, classifying it as low, moderate, or high. In this review, we only included high-quality studies.
RESULTS
From the PubMed and manual backward searches, we identified a total of 96 articles. After screening and selection processes, we included 38 articles in this systematic review (Fig. 1). These corresponded to 22 cohort studies, 8 clinical trials, 6 reviews, 1 clinical guideline, and 1 meta-analysis. The characteristics of each study are summarized in Table 1.
FIGURE 1.
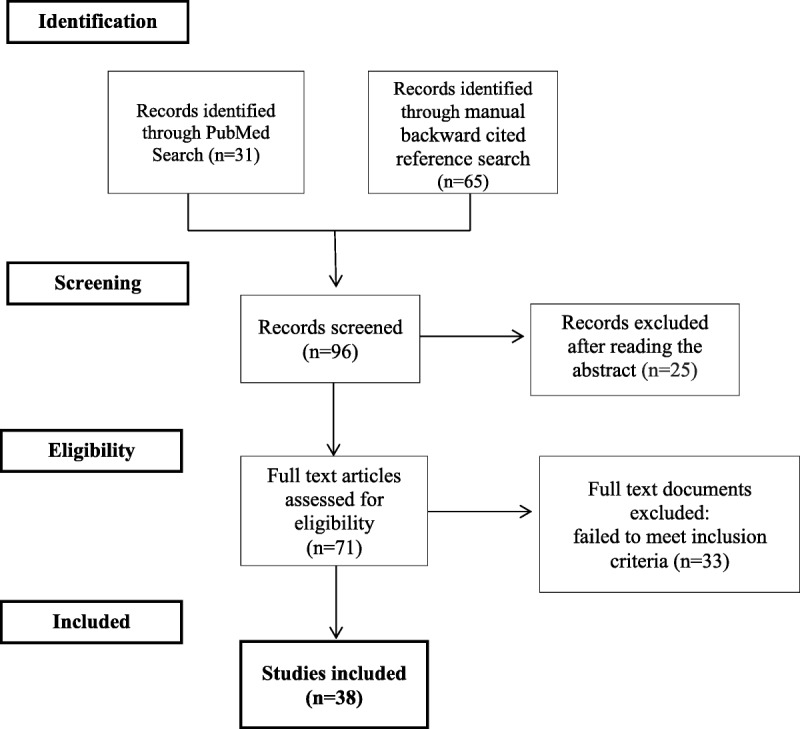
The PRISMA flow diagram figures.
TABLE 1.
Summary of the Characteristics of the 38 Articles Included in the Review
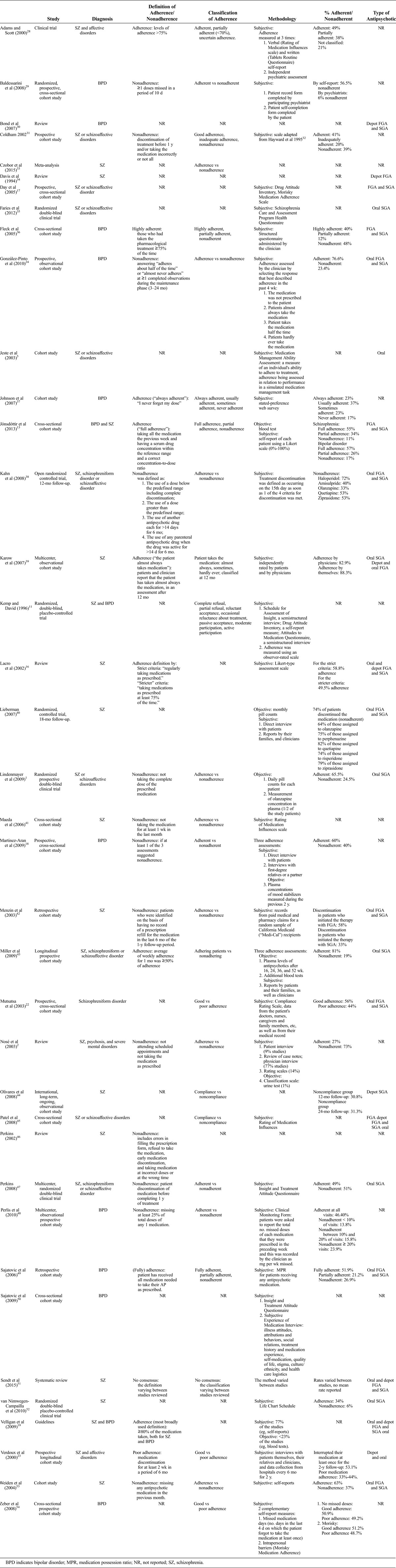
A total of 51,796 patients were included, of whom 40,298 had been diagnosed with bipolar disorder, 10,385 with schizophrenia, 544 with schizoaffective disorder, 516 with schizophreniform disorders, and 53 with psychosis not otherwise specified.
Adherence to drug treatment can be measured by subjective methods, such as self-report and physician report, or objective methods, such as pill counting, blood or urine analysis, electronic monitoring, and electronic refill records.19 Of the 38 studies included in our review, 66% of the studies used subjective measures to assess adherence, 16% also used objective measures, 2% used only objective measures, and 16% of studies did not specify the measures used. The study by Lindenmayer et al1 was the only one in which adherence was measured with objective measures only and patients had a mean adherence of 65.5%. In studies in which objective and subjective measures were combined, adherence ranged from 60%18 to 81%,43 whereas in studies in which only subjective methods were used, adherence ranged from 34%52 to approximately 80%.39 Note that the reviews, meta-analysis, and guideline are not included in this description, because they are based on multiple studies using different methods and hence could have introduced bias into the analysis.
According to our findings, factors that influence treatment nonadherence are associated with patients themselves, the drug treatment, social issues, and the health system provider.
Patient-Related Factors
This category includes attitudes and behaviors, comorbidities and the severity of signs and symptoms, demographic and environmental factors, and the cognitive functioning of patients, as well as their relationship with their medication.
As part of the EMBLEM Project, González-Pinto et al24 analyzed 1831 patients with bipolar disorder and found that the following factors were significantly positively associated with good adherence: good illness awareness (good adherence from the start of treatment) and a short duration of episodes. On the other hand, factors related to poor adherence were high scores in the Clinical Global Impressions hallucinations/delusions scale at baseline and depressive symptoms during mania. Regarding symptoms, a study including 128 patients diagnosed with schizophrenia52 observed that the time to discontinuation was significantly longer in those with an early nondysphoric response (7.3 months) than those with an early dysphoric response. In patients with schizophrenia and affective disorders, Verdoux et al23 found that the intensity of delusional symptoms predicted poor treatment adherence (P = 0.03). In contrast, Patel et al45 did not find symptom to be predictive of adherence.
Analyzing 469 patients with bipolar disorders, Johnson et al37 found differences in adherence related to demographic characteristics; these included ethnic differences, with white patients having better treatment adherence than patients from other ethnic groups. These findings are in agreement with those of Zeber et al54 and Fleck et al.36 The authors found that Afro-American patients reported significantly more missed medication days and greater barriers to adherence than white patients. They also found a higher prevalence of patient-related factors influencing adherence (fear of becoming addicted and feeling that medication is a symbol of illness) in Afro-American patients than white patients, whereas the rates of treatment- or illness-related factors were similar in the 2 ethnic groups. Perkins et al47 confirmed these findings, with black ethnicity again being associated with lower medication adherence in patients with schizophrenia. Among white patients, Perlis et al48 observed in a cohort of 3460 patients with bipolar disorder that being Hispanic was associated with poor adherence, and, moreover, this association was not confounded by differences in other predictors such as household income or education. A similar pattern was observed in the study of Sajatovic et al,49 in which patients with bipolar disorder from minority races had poorer adherence than other individuals with the same diagnosis. Education was another demographic characteristic related to adherence in the study of Johnson et al37 in bipolar disorder (adherence decreased with level of education).
Young age has also been identified as a predictor of poor adherence in many studies, both in patients with schizophrenia and those with bipolar disorder.2,11,19 For the latter diagnosis, this association was found in the studies of González-Pinto et al,24 Sajatovic et al,49 Johnson et al,37 who reported that adherence decreased to a mean age of 41 years and thereafter increased with age, and Baldessarini et al29 in which youth was a predictor of poor adherence, behind alcohol dependence and ahead of symptoms and adverse effects. In schizophrenia, Maeda et al41 noted that the age of patients was associated with increased awareness of disease prevention, older patients having more experience in the course of the disease, and possible relapses and hospital readmissions, and this led them to be more compliant with medication.
In addition to younger age, age at onset has also been cited as a risk factor for nonadherence to treatment, both in schizophrenia and bipolar disorder. Coldham et al31 found that nonadherent schizophrenic patients had an earlier age of onset, as well as being younger, and having poorer quality of life and premorbid functioning. Similarly, in a prospective study in 2010, Perlis et al48 observed that 874 of 3640 patients with bipolar disorder (24%) reported nonadherence on 20% or more study visits and the clinical features that were significantly associated with this included earlier onset of illness, as well as suicide attempts and alcohol abuse.
Nevertheless, the association of age at onset and nonadherence might be related to younger age (ie, in first-episode studies, younger age, and age at onset are equivalent), and this has not been well investigated. Furthermore, there is no consensus on this association between age and adherence within the set of studies included in the review, some authors13,16,18,22,39,47,54 having observed no significant differences between patients in different age groups.
In the study carried out by Lindenmayer et al,1 in 599 patients with schizophrenia, no baseline characteristics of patients, including demographic characteristics, initial body weight, and history of substance abuse, seem to be good predictors of adherence, whereas the severity of the depressive symptoms at baseline and a high level of hostility during the study were risk factors for nonadherence. In contrast to the aforementioned findings of Lindenmayer et al,1 alcohol and cannabis use and abuse have been found to be significantly associated with nonadherence to medication in several studies. In the 2015 meta-analysis of Czobor et al,33 in which they combined 2 studies, the European First-Episode Schizophrenia Trial (EUFEST) and the Clinical Antipsychotic Trials of Intervention Effectiveness, yielding a cohort of 1154 patients diagnosed with schizophrenia, they found that nonadherence to treatment was associated with substance abuse and hostility. This was consistent with earlier studies in schizophrenia, namely, those of van Nimwegen-Campailla et al,52 who found that patients who did not consume cannabis during treatment had a significantly longer treatment period (mean, 6.4 months) than cannabis users (mean, 4.3 months), and those of Miller et al,43 who found that the use of cannabis was associated with a 2.4-fold lower rate of adherence, independent of age, socioeconomic status, sex, and the medication prescribed. Similarly, in patients with bipolar disorder, Gonzalez-Pinto et al25 observed that the use and abuse of cannabis were key factors for nonadherence. Furthermore, Coldham et al31 found that schizophrenic patients who were nonadherent (73 of 186 patients) consumed significantly more cannabis and alcohol than an adherent group, and Verdoux et al23 described lower adherence in patients with schizophrenia and bipolar disorder who had alcohol abuse problems. Notably, in a clinical trial with 400 schizophrenic patients, ongoing substance abuse significantly predicted poor adherence,47 and Sajatovic et al49 found similar results in their study with veterans with bipolar disorder.
Regarding cognitive factors, Martinez-Aran et al18 found that nonadherent bipolar patients showed greater cognitive impairment in verbal learning tasks and some executive functions, as well as greater deterioration in spatial memory and in their ability to inhibit interference than adherent patients. Also in patients with bipolar disorder, Perlis et al48 found that cognitive impairment was the only adverse effect significantly associated with nonadherence. In line with this, in patients with schizophrenia, Jeste et al5 found that deterioration in cognitive functions, in particular conceptualization and memory, had greater predictive value of poor patient medication self-management than other factors, namely, sex, age, level of education, symptom severity, and attitudes toward medication. In contrast to these findings, in patients with schizophrenia, Perkins et al47 found the highest level of adherence to be significantly associated with lower executive functioning, and in the review by Send et al,51 neurocognitive functioning did not seem to impact medication adherence.
Some adverse effects, such as secondary extrapyramidal symptoms (akathisia, pseudoparkinsonism, dyskinesia, and acute dystonic reactions), neuroleptic dysphoria, sexual dysfunction, and weight gain are associated with nonadherence in schizophrenic patients.46 Subjective distress, weight gain, and body mass index (BMI) were found to be predictive of therapeutic nonadherence, specifically, obese individuals being twice as likely to report nonadherence as patients with a normal BMI.53 Weight gain was also a fear in patients with bipolar disorder and a better predictor of nonadherence than adverse effects such as excessive sedation and tremors.29
In both types of disorders, illness awareness and trust in the medication have been found to be predictive factors for good adherence.2,19,31,33,46,51 In schizophrenia, according to patients, the most important reasons for continuing with their medication are the beneficial effects in terms of control of positive symptoms, a perception of improvement,35,39 a reduction in the rate of hospital readmissions, and the prevention of relapses.52 With regard to the reasons for discontinuing treatment, patients have cited insufficient improvement or actual worsening of symptoms, adverse effects of the medication,19,35,37 denial of the illness, and not considering medication to be necessary.19,45 In the clinical trial carried out by Adams and Scott,28 including 39 patients with schizophrenia, it was found that perception of illness severity and benefits of the treatment explained 43% of the variance in adherence.
Administering structured interviews about concerns and expectations regarding medication to 90 patients with bipolar disorder, Sajatovic et al50 found that 39% of patients were not concerned about their medication; 29% had specific concerns (worrying about developing more health problems); 6% feared becoming addicted; and 5% were worried about the economic costs. Patients' expectations ranged from hoping that the medication would be able to decrease their symptoms and stabilize their mood (23%) to helping them to become “normal” (20%) and even curing them (20%), individuals reporting a feeling of disappointment when this did not happen.
Drug Treatment-Related Factors
First- Versus Second-Generation Antipsychotic Drugs
The Clinical Antipsychotic Trials of Intervention Effectiveness compared effectiveness of first-generation antipsychotic (FGA) and second-generation antipsychotic (SGA) drugs in patients with chronic schizophrenia. Differences in time to discontinuation of treatment due to ineffectiveness were lower with olanzapine, although there were no differences between the FGA perphenazine and SGA drugs such as risperidone or quetiapine.40 The EUFEST study also found that the risk of discontinuation was lower with olanzapine than with haloperidol (33% vs 72%). In fact, the risk of discontinuation due to any cause was higher with haloperidol than with all SGAs. With respect to discontinuation due to nonadherence, there were also no differences between first- and second-generation drugs.38
Another study compared 298 schizophrenic patients starting antipsychotic treatment with FGAs (n = 93) or SGAs (n = 205), the SGAs being associated with significantly less treatment switching and less use of concomitant medications than FGAs. On the other hand, in the 1-year follow-up, it was observed that both groups of patients took the drugs on 60% of days.42 In line with these findings, in a review of the risks of nonadherence, Lacro et al16 reported that there was inconclusive evidence of a relationship between nonadherence and the type of treatment.
In a recent systematic review, that only included studies in schizophrenia, Send et al51 found no significant differences in rates of adherence between the 2 types of antipsychotics. On the other hand, in bipolar disorder, Sajatovic et al49 observed that patients who take FGAs were more adherent than those taking SGAs.
To sum up, it seems that some SGAs give some advantages in relation to adherence versus FGA. Nevertheless, the rates of nonadherence are high, and new therapeutic approaches are required.
Depot Versus Oral
Formulation type has been found not to be a consistent predictor of nonadherence.45,51 The main reasons for changing from an oral to an intramuscular or depot antipsychotic30,34,44 are usually nonadherence and resistance to oral antipsychotics.34,44,45 Prescription of a depot medication must, however, be accompanied by discussion with the patient about personal benefits, because beliefs and attitudes have an important influence on adherence to depot medication.45
Factors Associated With Social Relationships
A good therapeutic alliance between the patient and the physician17,54 and the level of family support31,46 have been found to be significantly associated with good treatment adherence in both pathologies.19 In the multivariate analysis carried out by Zeber et al,54 with patients with bipolar disorder, the overall score on the Health Care Climate Questionnaire (a measure of therapeutic alliance) was found to be significantly positively associated with the number of days on which medication was not missed. Furthermore, in schizophrenic patients, Coldham et al31 found a higher level of family involvement in the adherent group (80%) than the nonadherent group (51%).
Factors Associated With the Health Service Provider
Barriers to or difficulties accessing treatment (lack of economic resources for buying medication or lack of transport to reach health service providers) were found to be predictive of nonadherence in schizophrenic and bipolar patients in the reviews conducted by Perkins 46 and Velligan et al,19 respectively. Patient experience with the health system was also found to be associated with subsequent adherence to drug treatments in both types of disorder.17,19
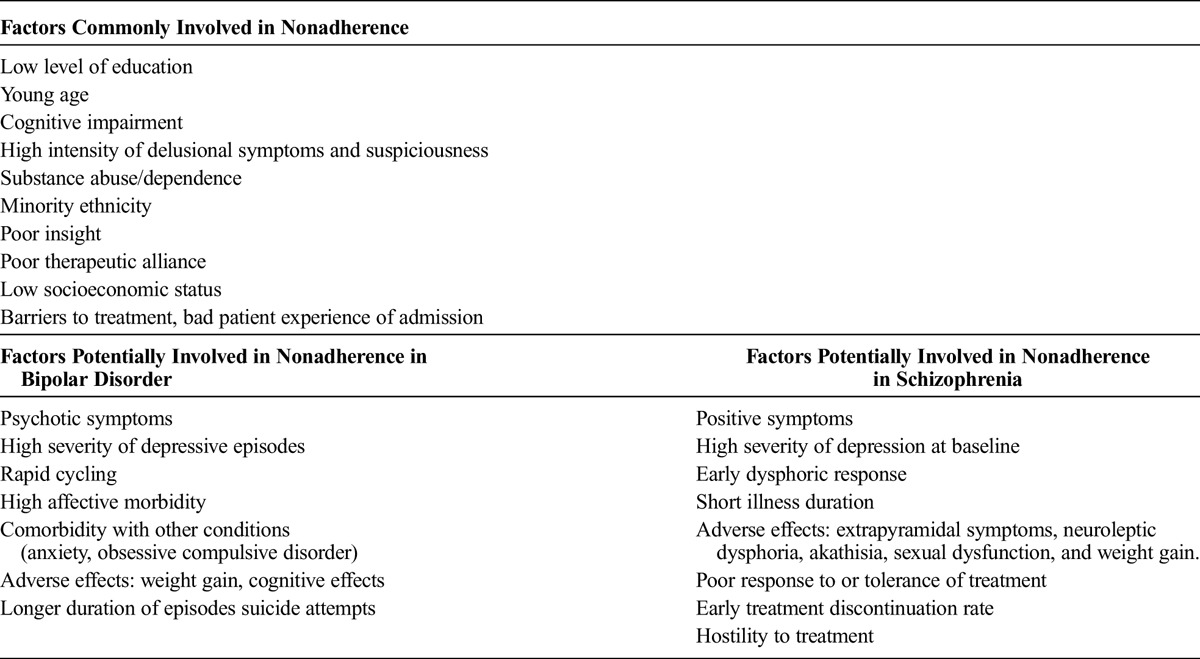
To summarize the findings in a clear way, Table 2 lists all factors associated with nonadherence rates found in literature by diagnosis. We can observe that a number of factors are common to both types of disorders, whereas other factors are more closely related to the clinical symptoms of each diagnosis.
TABLE 2.
Factors Common to Both Pathologies and Specific Factors by Diagnosis
DISCUSSION
Adherence to antipsychotics by patients diagnosed with psychosis is notably low; a review found a mean rate of 42%.14 This has negative consequences for patients, their families, and communities. For clinicians, it makes treatment nonadherence one of the most important challenges in treating these highly prevalent psychiatric conditions. Overall, it is clear that great efforts are needed to enhance adherence. From this review, we conclude that the most important factors to consider are associated with patients themselves and with the medication.
The nonmodifiable factors associated with patients themselves include young age, and although results differed between studies, as we have said previously, we found this association in most cases.31 In particular, adolescents may be less tolerant to the adverse effects of antipsychotics (sexual dysfunction, sedation), more concerned about the stigma of the illness, or more impulsive and impatient if treatment is complex or does not improve symptoms sufficiently fast, and these attitudes can lead to treatment discontinuation.55 Furthermore, ethnicity is associated with significant differences in adherence, antipsychotic adherence rates tending to be lower in black than white patients.36,37,54 On the other hand, level of education and quality of life also have an impact on adherence; patients with a low level of education or poor quality of life are more prone to nonadherence.37
Regarding modifiable factors, a psychological model has been proposed, the Health Belief Model, which aims to explain and predict health behaviors, focusing on attitudes and beliefs of individuals that may have an influence on adherence.16 This model indicates 2 behaviors that play a very important role in medication acceptance: (1) patients must be aware of their own condition (they must perceive their vulnerability and the seriousness of the illness) and (2) they must know and interiorize the benefits of treatment adherence.24,31,46 These 2 requisites are of particular importance in patients with first psychotic episodes, given that they tend to occur during adolescence,56 a critical period of development at biological, personal, and social levels.45,57 For this reason, a specialized early intervention program is needed at this stage of the illness in young patients,58,59 to attempt to minimize the consequences of the psychosis.60 A study on adolescents, all treated with antipsychotics, assessed their subjective experience with medication with the “Drug Attitude Inventory” and found that a change to more positive medication attitudes was associated with significantly greater medication adherence, decreases in psychopathology, and improvement in functioning.61 To achieve this change in patient attitude, it is essential to include psychoeducation in the treatment program, to teach patients about their illness, medication and adverse effects, and relapse prevention.19
Cognitive Behavioral Therapy is a model of psychotherapy intervention focused on understanding patient's perception of their problems and treatment. Cognitive Behavioral Therapy therapists help patients identify and modify negative automatic thoughts about medications and strengthen their belief that taking their medication is a step toward recovery and improving their well-being. This type of therapy has been found to improve adherence and symptom management and to enhance insight in patients with schizophrenia.19
In addition, psychoeducation may be extended to include the patient's family, as seen in previous studies,31,46 and then treatment becomes more effective in reducing relapse rates and the symptoms of the illness than if psychoeducation is given only to the patient.62 As long as the patient consents, involvement of a family member would help improve the management of the patient's treatment program, providing support through the course of the disease and reminding the patient take medications, attend health appointments, etc, and improving the patient environment.
Therapeutic alliance has also been identified as a relevant factor for improving adherence to antipsychotics.17,54,63 A study on patients with bipolar disorder found that patient collaboration was significantly associated with good adherence, that is, patients being involved as a comanager of their own illness, with the psychiatrist considering their opinions and comments during the intervention process, helped improve the management of the illness, and hence led to better treatment adherence.64 These 2 factors, therapeutic alliance and patient collaboration, together with social support and a positive environment31,46 are also predictive of good adherence during treatment.
Therefore, the first contact between the patient and the health system is a key factor because it influences patient perception. The following factors help patients develop a more positive perception of their illness and drug treatment: approachable clinicians, who discuss the beliefs, fears, and needs of patients regarding their illness and treatment; continuity of care provided by a single health care team; more frequent and/or longer visits19; and easy access to their health center.65 Regarding the last of these factors, physical or economic barriers, such as a lack of public transport to reach the health center or difficulties meeting the costs of new antipsychotics, clearly hinder patients' capacity to adhere to medication.19,46 Health centers should explore ways to facilitate access, and clinicians should provide support and advice as part of the treatment, being proactive in the breaking down of barriers, for example, offering free samples of drugs to start the treatment,66 or informing patients and families about the drugs, and helping them obtain grants to cover the cost of drugs, especially in the case of people with low economic resources. These gestures could also contribute to improve patient-clinician relationships.
In line with these ideas for improvement, new strategies for therapeutic interventions include offering economic rewards to patients with a psychotic disorder to investigate whether financial incentives would affect their adherence to antipsychotic medication. A study with 73 patients with schizophrenia and bipolar disorder demonstrated the effectiveness of this type of intervention, showing benefits in adherence, contact, monitoring, and patient trust in 77% of cases.67 Further research is needed into this type of intervention; however, benefits were only found in the short-term, intrinsic demotivation being observed in the long term.63
Other important modifiable risk factors are alcohol and drug abuse, which can be said to have an almost direct relationship with nonadherence to antipsychotic drugs.23,33,43,47,52 Notably, in a study with patients with bipolar disorder, alcohol dependence was the factor most strongly associated with nonadherence, above and beyond being young, and even the potential adverse effects of treatment.29 The findings of Barbeito et al68 in the first psychotic episodes support the view that there is a link between nonadherence and cannabis use, and interestingly, they found not only that patients who had never used cannabis had better adherence but also that patients who were nonusers with a history of dependence were also good adherers to treatment. These results are in line with those of the 2006 study of Sajatovic et al,69 in which past substance use disorder did not differ between adherent and nonadherent patients. Hence, we conclude that cannabis abuse does not cause irreversible damage in patients and that the aim of interventions should be to create a targeted and personalized treatment, not only to increase medication adherence but also to encourage the cessation of substance abuse.
Among the adverse effects of antipsychotics, weight gain is probably the health problem that is most likely to result in nonadherence.19 In fact, there is an association between adherence and patient BMI, adherence being lower among those with higher BMIs, and more subjective distress was related to weight gain.53 Extrapyramidal adverse effects such as pseudoparkinsonism, akathisia, dyskinesia, and sexual dysfunction were also found to be of great importance in nonadherence.46 One way to address this type of factor would be to create strategies for offering specific treatments depending on patients' characteristics, carefully considering the risk-benefit ratio of each drug and selecting those least likely to have relevant adverse effects in given patients.70–74 Type of antipsychotic may be a factor underlying loss of adherence in some patients, related to low efficacy or severe adverse effects, but results were mixed across the articles reviewed. Specifically, not all studies found significant differences in adherence between FGA and SGA drugs that would be able to guide our choice, and more importantly, loss of adherence was observed with both types of antipsychotic.
Regarding the route of administration, depot formulations are the type most widely chosen for patients with severe lack of adherence, although again data are mixed, results differing by trial design.75 Despite the use of depot medication, patient lack of insight or poor therapeutic alliance over time and among others factors mean that patients tend to become nonadherent again.
We conclude that neither lack of medication effectiveness nor the choice of route of administration is the real factor that prevents patients from continuing treatment. If possible, it is important to accompany treatment with an informative and explanatory discussion about the benefits thereof and to reduce polypharmacy (which increases the risk of adverse events and pharmacokinetic interactions, thereby increasing the likelihood of nonadherence). In addition, reducing the number of pills, when possible, is a good way to increase adherence, making the treatment easier for patients to remember and follow.66
Regarding adverse events, there are innovations in personalized medicine, with growth in the area of pharmacogenetics. Numerous studies have found polymorphism in genes that are involved in the metabolism of antipsychotics. Moreover, in relation to adherence, there is a direct relationship between some polymorphisms and the development of adverse events. For example, it has been found that genetic polymorphisms in the genes encoding cytochrome P450 enzymes CYP2D6 and CYP2C19 provide an explanation as to why some patients do not respond to drugs as expected, whereas others show an exaggerated response or serious adverse effects after receiving a standard dose that should have been safe for them. These differential responses to treatment are related to 2 phenotypes in the population, the extensive metabolizer and the poor metabolizer. The gene coding for CYP2D6 is highly polymorphic, and several mutations have been identified in poor metabolizers, all leading to the absence of functional CYP2D6. It is relatively common that poor metabolizers of CYP2D6 and CYP2C19 show an exaggerated drug response and adverse effects when they receive standard doses, whereas at the other extreme, so-called ultrarapid metabolizers do not respond to standard doses. Recently, the molecular basis of ultrarapid metabolism has been identified as the CYP2D6 gene amplification.76,77 Given this, new personalized medicine has the potential to reduce adverse events and indirectly increase adherence.78
Another new area of knowledge has emerged, namely, pharmacogenovigilance.79 The most common adverse effects of drug therapy are observed before approval for clinical use. The less common adverse effects may not be observed, however, until after regulatory approval in clinical practice; in some cases, serious effects may be discovered many decades after a drug receives regulatory approval.80 The aim of pharmacovigilance is to monitor drug safety and effectiveness after approval and understand the epidemiology and mechanisms of vast heterogeneity in drug-related outcomes, at individual and population levels. This area together with pharmacogenomics, seeking to explain the genomic basis of interindividual differences in efficacy and safety of drugs, creates the new term “pharmacogenovigilance.” This union enables a more mechanical approach, allowing extrapolation of early signs of drug-related events from 1 population to another, when the worldwide distribution of pharmacogenomics biomarkers linked to a given drug safety or efficacy event is known.79 It also helps us understand the pharmacokinetic and pharmacodynamic performance of drugs in population extremes, such as poor and ultrarapid metabolizers, mentioned previously and thus prompts a population-scale overview during postmarketing surveillance.81
A third new area of knowledge is pharmamicrobiomics. In relation to the Human Microbiome Project, it has been observed that drug-microbiome interactions may shed light on the influence that individual microbiota can have on the effects and adverse events of therapies in individuals. Gut microbiota can vary from 1 person to other because of differences in diet, health, use of medicines, place of residence, or age. Some drugs are particularly affected by gut microbes, and this is a little explored area that may help us understand patterns of adherence.78 For instance, it has been demonstrated that the gut microbiota has a role in the metabolic dysfunction associated with olanzapine in an animal model.82 In the future, the microbiome will be taken into account along with other factors, in personalized medicine. It is likely that considering the microbiome in the development of personalized medicine will initially be too expensive. Nevertheless, the use of this new tool may be justified and provide benefits in some patients with serious adverse events.
On the other hand, it has been observed that long hospital stays favor medication adherence. In particular, they allow pharmacotherapy to be optimized and to be more effective, given that patients' beliefs and attitudes regarding their illness and medication can slowly change during admission, enabling a therapeutic alliance to develop, and this subsequently helps maintain treatment adherence.83 In relation to this, psychoeducation therapies mentioned previously play a very important role in the preparation of patients for the type of response they should expect, how their symptoms will improve, the management of adverse effects, and how to adjust their medication dosage.
Another very important area in which there is margin for improving practice relates to cognitive impairment in patients with psychiatric illnesses. In recent years, several studies have been conducted in an attempt to clarify the relationship between cognitive dysfunction and nonadherence. Jeste et al5 indicated that memory and conceptualization dysfunction were very good predictors of poor medication management. However, the results regarding predictive factors are mixed. On the one hand, Martinez-Aran et al18 analyzed cognitive dysfunction in a sample of patients with bipolar disorder and found that patients with the lowest levels of adherence had greater cognitive impairments. In this type of patients, adherence can be improved with the use of electronic pill boxes or alerts, to remind them to take their medication and hence adhere to their treatment.84 Furthermore, Perlis et al48 observed that memory impairment was the only significant predictor of nonadherence in 3460 patients with bipolar disorder, which might suggest that nonadherence is likely to result, at least in part, from the cognitive deficits that are increasingly recognized in these patients.85
In line with this, a study by Torrent et al86 in patients with bipolar disorder and moderate to severe cognitive disability showed functional improvement after a functional remediation program compared with usual care and psychoeducation. In this new type of intervention, patients perform exercises to improve memory, attention, problem solving and reasoning, multitasking, and organization, to strengthen their cognitive and general functioning. With the same objective, Velligan et al87 developed a program called cognitive adaptation training, which seems to be a promising strategy to improve adherence. Cognitive adaptation training focused on medication adherence uses individually tailored environmental supports (eg, signs, checklists, electronic cuing devices, organization of belongings) to cue adaptive behavior in the patient's home environment and help compensate for cognitive deficits. It also addresses logistic issues related to obtaining medication (eg, picking up prescriptions) and getting to appointments. In a study published in 2008 involving patients with schizophrenia, Velligan et al87 found that a full cognitive adaptation training program, focused on many aspects of community adaptation, and a cognitive adaptation training program, focused only on adherence to medication and appointments, were both better than treatment as usual in improving adherence, reducing relapse rates, and increasing time to relapse or exacerbation of symptoms. The full program produced greater improvements in functional outcome than the other 2 interventions.
On the other hand, unlike the aforementioned studies, Perkins et al47 and Maeda et al41 found that patients with the poorest adherence had better cognitive performance. Such contradictions between studies make it necessary to conduct further research in this area and, in turn, identify techniques that are useful for patients.
In several studies, symptom at baseline was found to be a relevant factor. In particular, the duration of episodes was observed to be a key factor in patients with bipolar disorder, adherence being better in patients with short episodes than those with longer ones.24 In patients with schizophrenia, Lindenmayer et al1 found that the time to medication discontinuation was significantly longer in patients with high levels of hostility toward the study and those with poor insight.33,88 Taking into account these results, characterizing the symptom, monitoring symptom response on an ongoing basis (eg, using a daily checklist or mood chart),19 and reducing patient hostility may contribute to preventing future nonadherence.
Table 3 summarizes factors that it might be feasible to modify through interventions to improve patient adherence. A psychoeducational intervention in which patients are provided with a global overview of all these factors, with emphasis on aspects that are most relevant to their own profiles, may help encourage them to take a proactive role in the management of their illness.
TABLE 3.
Potential Areas for Intervention to Improve Adherence

Limitations
The findings of the review are limited by the wide range of rates of adherence found in the scientific literature (from 10 to 76% in schizophrenia14 and 20 to 66% in bipolar disorder),15 this being attributable to the different measures and definitions of adherence used. The percentages of studies included in our review that used subjective (66%), objective (2%), or both (16%) kinds of measures are consistent with figures in other studies described in the clinical guidelines developed by Velligan et al.19 These authors19 evaluated 161 studies on adherence, and 77% used only subjective measures. Nosé et al2 also found that only 1% of studies used objective measures (urine tests).
As we explained previously, the rate of adherence differs markedly between studies that use subjective measures (34%52–80%39). Errors associated with this approach can be seen in the study of Baldessarini et al29: adherence measured by self-report resulted in more than half (56%) of patients being classified as nonadherent, whereas in assessments carried by psychiatrists, only 6% of patients were classified as nonadherent.
There are also sources of error when using objective measures. Plasma or urine measures only determine whether the patient is taking the medication at the time but cannot be considered proof of their usual behavior.43 If the patient only took the medication before the test, adherence would be overestimated.13 In particular, it is essential to use objective measures for testing adherence, when nonadherence is denied by the patient and ignored by the family.18
Pill counting can also overestimate adherence, because patients can throw away pills without ingesting them.1 In brief, by describing these results, we want to underline the wide range of measurements in the literature and the need for agreeing on an appropriate methodology, to enable more accurate research in this field and comparisons between studies.
In terms of our methodology, another limitation is that the assessment of the quality of each article using critical reading sheets is open to a degree of subjective interpretation, although we have attempted to compensate for this to some extent by 2 different researchers reviewing each article independently.
Despite these limitations, in this systematic review, we have been able to classify the multiple factors associated with adherence to antipsychotics, in patients from the 38 selected studies, into 4 groups related to patients themselves, the drug treatment, their environment (social issues), and the health system provider. Finally, all factors were grouped by diagnosis to clarify the results, allowing us to produce a summary of all the key factors that may affect patients in the management of their medication.
We can conclude that great efforts must be made to enhance adherence in patients with schizophrenia and bipolar disorder. Among the most important factors influencing this behavior, there are nonmodifiable factors, such as young age (adolescents having lower levels of adherence) and ethnicity but also many potentially modifiable factors, and these include the following: symptom at baseline, alcohol and drug abuse, illness awareness, therapeutic alliance and family support, adverse effects (weight gain and extrapyramidal adverse effects being the most important for patients), quality of life, level of education, previous experience with health services, and level of cognitive impairment.
Improvements in patient symptoms and quality of life are dependent on good adherence to drug treatment. In the era of precision psychiatry, the choice of the right treatment for the right patient may be an affordable unmet need,89 and this may be particularly relevant when trying to predict poor treatment adherence. Hence, early interventions focused on adherence enhancement may be particularly relevant.90 Accordingly, this systematic review seeks to facilitate efforts to improve patient behavior, by identifying factors associated with adherence in specific diagnoses and proposing potential strategies to address modifiable factors.
AUTHOR DISCLOSURE INFORMATION
This study was supported by health research funds from the Spanish Government, cofinancing FEDER (PI12/02077, PI11/01977, PI14/01900); the Basque Foundation for Health Innovation and Research (BIOEF); Networking Center for Biomedical Research in Mental Health (CIBERSAM); and the University of the Basque Country (GIC12/84). The psychiatric research department in University Hospital Araba is supported by the Stanley Research Foundation (03-RC-003). Dr Gonzalez-Pinto has received grants and served as consultant, advisor, or CME speaker for the following entities: Almirall, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Otsuka, Pfizer, Sanofi-Aventis, Servier, Shering-Plough, Solvay, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute), the Basque Government, the Stanley Medical Research Institute, and Wyeth. Dr Vieta has received grants and served as consultant, advisor, or CME speaker for the following entities: AB-Biotics, Actavis, Allergen, AstraZeneca, Bristol-Myers Squibb, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, Telefónica, the Brain and Behaviour Foundation, the Spanish Ministry of Economy and Competitiveness (CIBERSAM and PI 12/00910), the Seventh European Framework Programme (ENBREC), the Stanley Medical Research Institute, and the Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya (2014 SGR 398), Aequus, Adamed, Alexza, Bial, Dainippon Sumitomo Pharma, Elan, Eli Lily, Gedeon Ritcher, Janssen-Cilag, Jazz, Johnson & Johnson, Merck, Novartis, Organon, Pierre-Fabre, Qualigen, Shering-Plough, Solvay, Sumitomo Dainippon, Telenófica, Teva, the Spanish Ministry of Science and Innovation (CIBERSAM), United Biosource Corporation, and Wyeth . The other authors declare no conflicts of interest.
REFERENCES
- 1.Lindenmayer JP, Liu-Seifert H, Kulkarni PM, et al. Medication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J Clin Psychiatry. 2009;70:990–996. [DOI] [PubMed] [Google Scholar]
- 2.Nosé M, Barbui C, Tansella M. How often do patients with psychosis fail to adhere to treatment programmes? A systematic review. Psychol Med. 2003;33:1149–1160. [DOI] [PubMed] [Google Scholar]
- 3.Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1:1265–1268. [DOI] [PubMed] [Google Scholar]
- 4.Kampman O, Lehtinen K. Compliance in psychoses. Acta Psychiatr Scand. 1999;100:167–175. [DOI] [PubMed] [Google Scholar]
- 5.Jeste SD, Patterson TL, Palmer BW, et al. Cognitive predictors of medication adherence among middle-aged and older outpatients with schizophrenia. Schizophr Res. 2003;63:49–58. [DOI] [PubMed] [Google Scholar]
- 6.Colom F, Vieta E, Martínez-Arán A, et al. Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiatry. 2000;61:549–555. [DOI] [PubMed] [Google Scholar]
- 7.Novick D, Haro JM, Suarez D, et al. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176:109–113. [DOI] [PubMed] [Google Scholar]
- 8.Schumann C, Lenz G, Berghöfer A, et al. Non-adherence with long-term prophylaxis: a 6-year naturalistic follow-up study of affectively ill patients. Psychiatry Res. 1999;89:247–257. [DOI] [PubMed] [Google Scholar]
- 9.Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159:1927–1929. [DOI] [PubMed] [Google Scholar]
- 10.Vieta E. Improving treatment adherence in bipolar disorder through psychoeducation. J Clin Psychiatry. 2005;66(Suppl 1):24–29. [PubMed] [Google Scholar]
- 11.Kemp R, David A. Psychological predictors of insight and compliance in psychotic patients. Br J Psychiatry. 1996;169:444–450. [DOI] [PubMed] [Google Scholar]
- 12.Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23:637–651. [DOI] [PubMed] [Google Scholar]
- 13.Jónsdóttir H, Opjordsmoen S, Birkenaes AB, et al. Predictors of medication adherence in patients with schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2013;127:23–33. [DOI] [PubMed] [Google Scholar]
- 14.Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49:196–201. [DOI] [PubMed] [Google Scholar]
- 15.Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105:164–172. [DOI] [PubMed] [Google Scholar]
- 16.Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892–909. [DOI] [PubMed] [Google Scholar]
- 17.Day JC, Bentall RP, Roberts C, et al. Attitudes toward antipsychotic medication: the impact of clinical variables and relationships with health professionals. Arch Gen Psychiatry. 2005;62:717–724. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Aran A, Scott J, Colom F, et al. Treatment nonadherence and neurocognitive impairment in bipolar disorder. J Clin Psychiatry. 2009;70:1017–1023. [DOI] [PubMed] [Google Scholar]
- 19.Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(Suppl 4):1–46 quiz 47–8. [PubMed] [Google Scholar]
- 20.Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33:261–263. [DOI] [PubMed] [Google Scholar]
- 21.Häfner H, Hambrecht M, Löffler W, et al. Is schizophrenia a disorder of all ages? A comparison of first episodes and early course across the life-cycle. Psychol Med. 1998;28:351–365. [DOI] [PubMed] [Google Scholar]
- 22.Mutsatsa SH, Joyce EM, Hutton SB, et al. Clinical correlates of early medication adherence: West London first episode schizophrenia study. Acta Psychiatr Scand. 2003;108:439–446. [DOI] [PubMed] [Google Scholar]
- 23.Verdoux H, Lengronne J, Liraud F, et al. Medication adherence in psychosis: predictors and impact on outcome. A 2-year follow-up of first-admitted subjects. Acta Psychiatr Scand. 2000;102:203–210. [DOI] [PubMed] [Google Scholar]
- 24.González-Pinto A, Reed C, Novick D, et al. Assessment of medication adherence in a cohort of patients with bipolar disorder. Pharmacopsychiatry. 2010;43:263–270. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Pinto A, Mosquera F, Alonso M, et al. Suicidal risk in bipolar I disorder patients and adherence to long-term lithium treatment. Bipolar Disord. 2006;8:618–624. [DOI] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López de Argumedo M, Reviriego E, Andrío E, et al. Revisión externa y validación de instrumentos metodológicos para la Lectura Crítica y la síntesis de la evidencia científica. Madrid: Madrid: Plan Nacional para el SNS del MSC; Servicio de Evaluación de Tecnologías Sanitarias del País Vasco (Osteba); Report No.: OSTEBA No 2006/02. [Google Scholar]
- 28.Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101:119–124. [DOI] [PubMed] [Google Scholar]
- 29.Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol. 2008;23:95–105. [DOI] [PubMed] [Google Scholar]
- 30.Bond DJ, Pratoomsri W, Yatham LN. Depot antipsychotic medications in bipolar disorder: a review of the literature. Acta Psychiatr Scand Suppl. 2007:3–16. [DOI] [PubMed] [Google Scholar]
- 31.Coldham EL, Addington J, Addington D. Medication adherence of individuals with a first episode of psychosis. Acta Psychiatr Scand. 2002;106:286–290. [DOI] [PubMed] [Google Scholar]
- 32.Hayward P, Chan N, Kemp R, et al. Medication self-management: a preliminary report on an intervention to improve medication compliance. J Ment Health. 1995;4:511–517. [Google Scholar]
- 33.Czobor P, Van Dorn RA, Citrome L, et al. Treatment adherence in schizophrenia: a patient-level meta-analysis of combined CATIE and EUFEST studies. Eur Neuropsychopharmacol. 2015;25:1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis JM, Matalon L, Watanabe MD, et al. Depot antipsychotic drugs. Place in therapy. Drugs. 1994;47:741–773. [DOI] [PubMed] [Google Scholar]
- 35.Faries D, Ascher-Svanum H, Phillips G, et al. Construct validity of 2 measures to assess reasons for antipsychotic discontinuation and continuation from patients' and clinicians' perspectives in a clinical trial. BMC Med Res Methodol. 2012;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleck DE, Keck PE, Corey KB, et al. Factors associated with medication adherence in African American and white patients with bipolar disorder. J Clin Psychiatry. 2005;66:646–652. [DOI] [PubMed] [Google Scholar]
- 37.Johnson FR, Ozdemir S, Manjunath R, et al. Factors that affect adherence to bipolar disorder treatments: a stated-preference approach. Med Care. 2007;45:545–552. [DOI] [PubMed] [Google Scholar]
- 38.Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371:1085–1097. [DOI] [PubMed] [Google Scholar]
- 39.Karow A, Czekalla J, Dittmann RW, et al. Association of subjective well-being, symptoms, and side effects with compliance after 12 months of treatment in schizophrenia. J Clin Psychiatry. 2007;68:75–80. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman JA. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: efficacy, safety and cost outcomes of CATIE and other trials. J Clin Psychiatry. 2007;68:e04. [DOI] [PubMed] [Google Scholar]
- 41.Maeda K, Kasai K, Watanabe A, et al. Effect of subjective reasoning and neurocognition on medication adherence for persons with schizophrenia. Psychiatr Serv. 2006;57:1203–1205. [DOI] [PubMed] [Google Scholar]
- 42.Menzin J, Boulanger L, Friedman M, et al. Treatment adherence associated with conventional and atypical antipsychotics in a large state Medicaid program. Psychiatr Serv. 2003;54:719–723. [DOI] [PubMed] [Google Scholar]
- 43.Miller R, Ream G, McCormack J, et al. A prospective study of cannabis use as a risk factor for non-adherence and treatment dropout in first-episode schizophrenia. Schizophr Res. 2009;113:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olivares JM, Rodriguez-Martinez A, Burón JA, et al. Cost-effectiveness analysis of switching antipsychotic medication to long-acting injectable risperidone in patients with schizophrenia : a 12- and 24-month follow-up from the e-STAR database in Spain. Appl Health Econ Health Policy. 2008;6:41–53. [DOI] [PubMed] [Google Scholar]
- 45.Patel MX, de Zoysa N, Bernadt M, et al. A cross-sectional study of patients' perspectives on adherence to antipsychotic medication: depot versus oral. J Clin Psychiatry. 2008;69:1548–1556. [DOI] [PubMed] [Google Scholar]
- 46.Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry. 2002;63:1121–1128. [DOI] [PubMed] [Google Scholar]
- 47.Perkins DO, Gu H, Weiden PJ, et al. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry. 2008;69:106–113. [DOI] [PubMed] [Google Scholar]
- 48.Perlis RH, Ostacher MJ, Miklowitz DJ, et al. Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry. 2010;71:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sajatovic M, Valenstein M, Blow FC, et al. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8:232–241. [DOI] [PubMed] [Google Scholar]
- 50.Sajatovic M, Jenkins JH, Cassidy KA, et al. Medication treatment perceptions, concerns and expectations among depressed individuals with type I bipolar disorder. J Affect Disord. 2009;115:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sendt KV, Tracy DK, Bhattacharyya S. A systematic review of factors influencing adherence to antipsychotic medication in schizophrenia-spectrum disorders. Psychiatry Res. 2015;225:14–30. [DOI] [PubMed] [Google Scholar]
- 52.van Nimwegen-Campailla L, van Beveren N, Laan W, et al. Effect of early dysphoric response and cannabis use on discontinuation of olanzapine or risperidone in patients with early psychosis. Pharmacopsychiatry. 2010;43:281. [DOI] [PubMed] [Google Scholar]
- 53.Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. 2004;66:51–57. [DOI] [PubMed] [Google Scholar]
- 54.Zeber JE, Copeland LA, Good CB, et al. Therapeutic alliance perceptions and medication adherence in patients with bipolar disorder. J Affect Disord. 2008;107:53–62. [DOI] [PubMed] [Google Scholar]
- 55.Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56:273–282. [DOI] [PubMed] [Google Scholar]
- 56.Ballageer T, Malla A, Manchanda R, et al. Is adolescent-onset first-episode psychosis different from adult onset? J Am Acad Child Adolesc Psychiatry. 2005;44:782–789. [DOI] [PubMed] [Google Scholar]
- 57.Redmond C, Larkin M, Harrop C. The personal meaning of romantic relationships for young people with psychosis. Clin Child Psychol Psychiatry. 2010;15:151–170. [DOI] [PubMed] [Google Scholar]
- 58.Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011:CD004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl. 1998;172:53–59. [PubMed] [Google Scholar]
- 61.Molteni S, Giaroli G, Rossi G, et al. Drug attitude in adolescents: a key factor for a comprehensive assessment. J Clin Psychopharmacol. 2014;34:99–108. [DOI] [PubMed] [Google Scholar]
- 62.Lincoln TM, Wilhelm K, Nestoriuc Y. Effectiveness of psychoeducation for relapse, symptoms, knowledge, adherence and functioning in psychotic disorders: a meta-analysis. Schizophr Res. 2007;96:232–245. [DOI] [PubMed] [Google Scholar]
- 63.Montreuil TC, Cassidy CM, Rabinovitch M, et al. Case manager- and patient-rated alliance as a predictor of medication adherence in first-episode psychosis. J Clin Psychopharmacol. 2012;32:465–469. [DOI] [PubMed] [Google Scholar]
- 64.Sylvia LG, Hay A, Ostacher MJ, et al. Association between therapeutic alliance, care satisfaction, and pharmacological adherence in bipolar disorder. J Clin Psychopharmacol. 2013;33:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh SP, Merino C. Treatment of first-episode and prodromal signs. Psychiatry. 2008;7:467–471. [Google Scholar]
- 66.Abdel-Baki A, Lesage A, Nicole L, et al. Schizophrenia, an illness with bad outcome: myth or reality? Can J Psychiatry. 2011;56:92–101. [DOI] [PubMed] [Google Scholar]
- 67.Highton-Williamson E, Barnicot K, Kareem T, et al. Offering financial incentives to increase adherence to antipsychotic medication: the clinician experience. J Clin Psychopharmacol. 2015;35:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbeito S, Vega P, Ruiz de Azúa S, et al. Cannabis use and involuntary admission may mediate long-term adherence in first-episode psychosis patients: a prospective longitudinal study. BMC Psychiatry. 2013;13:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sajatovic M, Bauer MS, Kilbourne AM, et al. Self-reported medication treatment adherence among veterans with bipolar disorder. Psychiatr Serv. 2006;57:56–62. [DOI] [PubMed] [Google Scholar]
- 70.Asenjo Lobos C, Komossa K, Rummel-Kluge C, et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010:CD006633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gasquet I, Flandre P, Heurtebize N, et al. Pattern and evolution of the prescription of olanzapine during one year: Results of the cohort study ECOL [in French]. Encephale. 2009;35:25–31. [DOI] [PubMed] [Google Scholar]
- 72.Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079–1087. [DOI] [PubMed] [Google Scholar]
- 73.Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28:995–1003. [DOI] [PubMed] [Google Scholar]
- 74.Rummel-Kluge C, Komossa K, Schwarz S, et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr Bull. 2012;38:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haddad PM, Kishimoto T, Correll CU, et al. Ambiguous findings concerning potential advantages of depot antipsychotics: in search of clinical relevance. Curr Opin Psychiatry. 2015;28:216–221. [DOI] [PubMed] [Google Scholar]
- 76.Coutts RT, Urichuk LJ. Polymorphic cytochromes P450 and drugs used in psychiatry. Cell Mol Neurobiol. 1999;19:325–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eichelbaum M, Evert B. Influence of pharmacogenetics on drug disposition and response. Clin Exp Pharmacol Physiol. 1996;23:983–985. [DOI] [PubMed] [Google Scholar]
- 78.ElRakaiby M, Dutilh BE, Rizkallah MR, et al. Pharmacomicrobiomics: the impact of human microbiome variations on systems pharmacology and personalized therapeutics. OMICS. 2014;18:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Şardaş S, Endrenyi L, Gürsoy UK, et al. A call for pharmacogenovigilance and rapid falsification in the age of big data: why not first road test your biomarker? OMICS. 2014;18:663–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ladewski LA, Belknap SM, Nebeker JR, et al. Dissemination of information on potentially fatal adverse drug reactions for cancer drugs from 2000 to 2002: first results from the research on adverse drug events and reports project. J Clin Oncol. 2003;21:3859–3866. [DOI] [PubMed] [Google Scholar]
- 81.Warnich L, Drögemöller BI, Pepper MS, et al. Pharmacogenomic research in South Africa: lessons learned and future opportunities in the Rainbow Nation. Curr Pharmacogenomics Person Med. 2011;9:191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davey KJ, Cotter PD, O'Sullivan O, et al. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013;3:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reutfors J, Brandt L, Stephansson O, et al. Antipsychotic prescription filling in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2013;33:759–765. [DOI] [PubMed] [Google Scholar]
- 84.Stip E, Abdel-Baki A, Bloom D, et al. Long-acting injectable antipsychotics: an expert opinion from the Association des médecins psychiatres du Québec [in French]. Can J Psychiatry. 2011;56:367–376. [DOI] [PubMed] [Google Scholar]
- 85.Arts B, Jabben N, Krabbendam L, et al. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–785. [DOI] [PubMed] [Google Scholar]
- 86.Torrent C, Bonnin C del M, Martínez-Arán A, et al. Efficacy of functional remediation in bipolar disorder: a multicenter randomized controlled study. Am J Psychiatry. 2013;170:852–859. [DOI] [PubMed] [Google Scholar]
- 87.Velligan DI, Diamond PM, Mintz J, et al. The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophr Bull. 2008;34:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Czobor P, Volavka J, Derks EM, et al. Insight and hostility as predictors and correlates of nonadherence in the European First Episode Schizophrenia Trial. J Clin Psychopharmacol. 2013;33:258–261. [DOI] [PubMed] [Google Scholar]
- 89.Vieta E. Personalised medicine applied to mental health: precision psychiatry [in Spanish]. Rev Psiquiatr Salud Ment. 2015;8:117–118. [DOI] [PubMed] [Google Scholar]
- 90.Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet. 2016;387:1561–1572. [DOI] [PubMed] [Google Scholar]


