Abstract
Insecticides are still largely applied in public health to control disease vectors. In Brazil, organophosphates (OP) and pyrethroids (PY) are used against Aedes aegypti for years. Since 2009 Insect Growth Regulators (IGR) are also employed in the control of larvae. We quantified resistance to temephos (OP), deltamethrin (PY), and diflubenzuron (IGR) of A. aegypti samples from 12 municipalities distributed throughout the country, collected between 2010 and 2012. High levels of resistance to neurotoxic insecticides were detected in almost all populations: RR95 to temephos varied between 4.0 and 27.1; the lowest RR95 to deltamethrin was 13.1, and values higher than 70.0 were found. In contrast, all samples were susceptible to diflubenzuron (RR95 < 2.3). Biochemical tests performed with larvae and adults discarded the participation of acetylcholinesterase, the OP target, and confirmed involvement of the detoxifying enzymes esterases, mixed function oxidases, and glutathione-S-transferases. The results obtained were discussed taking into account the public chemical control component and the increase in the domestic use of insecticides during dengue epidemic seasons in the evaluated municipalities.
1. Introduction
Currently dengue is spreading worldwide, placing at risk around 40% of the global population [1]. To date, no specific drugs are available and dengue treatment is restricted to supportive care. Although several candidate vaccines, directed against the four dengue serotypes, are presently submitted to human clinical trials, or even licensed for commercialization, none of them attains high protection levels [2]. The major dengue vector is Aedes aegypti (Diptera: Linnaeus, 1762), a highly anthropophilic and synanthropic mosquito, distributed throughout tropical and subtropical areas of the world [3–5], mainly between latitudes 35°N and 35°S [6, 7]. In addition, the recent chikungunya and Zika virus dispersion throughout the globe is also primarily attributed to A. aegypti [8].
Actions against dengue are mostly focused on the reduction of mosquito densities, and vector control can be accomplished through mechanical, biological, and chemical approaches. Mechanical control is based on the elimination or on the adequate protection of potential breeding sites; biological control makes use of larvae predators, such as small fishes, or formulations with entomopathogenic bacteria, like Bacillus thuringiensis var. israelensis (Bti); chemical control consists in the use of insecticides against larvae or adults of the vector mosquito [6, 7, 9].
Insecticides, still largely utilized by a number of vector control programs, belong to four main classes; all of them are neurotoxic compounds: carbamates (CA), organochlorates (OC), organophosphates (OP), and pyrethroids (PY) [10]. Nowadays, PY and OP are the most used. Recently two additional classes became available, the spinosyns, modulators of acetylcholine receptors [11], and the Insect Growth Regulators (IGR), a group that includes the chitin synthesis inhibitors (CSI) [12]. It should be noted that the Brazilian Dengue Control Program (PNCD) only employs insecticides that are recommended by the World Health Organization Pesticide Evaluation Scheme (WHOPES) for use in potable water or properly approved for space spraying applications [13, 14].
The intensive and prolonged use of insecticides can select resistant specimens in the natural vector populations, decreasing the frequency of susceptible individuals and reducing variability of field populations [5]. Insecticide resistance can derive from different mechanisms, the main ones being modifications in the target sites and higher ability to detoxify xenobiotic compounds; the former mechanism is known as target site resistance and the other as metabolic resistance [5, 6].
The voltage gated sodium channel (NaV) is the target site of pyrethroids; these insecticides keep NaV in its opened conformation, resulting in repetitive pulses. NaV substitutions that affect its susceptibility to PY are known as knockdown resistant ones (kdr) [15]. Such mutations have been reported in A. aegypti populations from several countries worldwide [16–18]. In Brazil two major kdr NaV alleles related to PY resistance are spreading and increasing in frequency. A clear regional distribution pattern is observed with NaV R1 (mutant at position 1534 of the channel protein) present throughout the country while NaV R2 (mutant at both 1534 and 1016 positions) is more frequent in central and southeastern municipalities [19, 20].
The target site of OP insecticides is acetylcholinesterase (AChE), an enzyme that hydrolyzes acetylcholine molecules; as a consequence, this neurotransmitter persists in the synaptic cleft, resulting in the exacerbation of nerve impulse transmission [21, 22]. To our knowledge there are no confirmed evidences of AChE alterations related to OP resistance in field A. aegypti populations.
The main detoxifying enzyme classes participating in the xenobiotic metabolizing processes are the Phase I mixed function oxidases (MFO) and esterases (EST) that trigger chemical modifications in the substrates and the Phase II conjugating enzymes, glutathione-S-transferases (GST) [21]. Each of these enzyme families is composed of several molecular entities, bearing distinct levels of specificity [5]. In general, evaluations of A. aegypti detoxifying mechanisms worldwide associate ESTs and OP resistance as well as GST and MFO alterations with PY resistance [23–26]. However such relations are not always straightforward due to the variability of enzymes participating in the insecticides detoxification and to the resistance multifactorial character [5, 10].
In Brazil, during more than three decades, only temephos was employed in the control of A. aegypti larvae. Resistance to this OP was originally detected at the end of the years 1990, and registers of the dissemination of this phenomenon persist up to the present [23, 24, 27–30]. Since 2009 temephos is being substituted by IGR in the country and a strategy of larvicide rotation, each 3-4 years, is attempted. Development of resistance was also verified for PY shortly after its use has been implemented for the control of adults, since 2000 [23, 31, 32].
The insecticide susceptibility profiles of several Brazilian field A. aegypti populations are presented (Figure 1). Resistance to compounds employed by the PNCD was investigated, in order to collaborate with the elucidation of both the resistance dynamics and the potential related mechanisms. Chemical insecticides are still a relevant control tool employed by public managers against dengue vectors. In addition, in general, dengue epidemic periods are related to a significant increase in the domestic use of insecticides, mainly adulticides. This collective behavior has the potential to contribute to a rapid increase in resistance levels, and it has already been detected in Brazil against pyrethroid compounds [33]. Taking these aspects into account, the results obtained were evaluated in the scope of several parameters, such as the Ministry of Health (MoH) supply of insecticides to the Brazilian States, the local historic of dengue outbreaks, and the frequency of kdr mutations, majorly responsible for PY resistance in the country.
Figure 1.
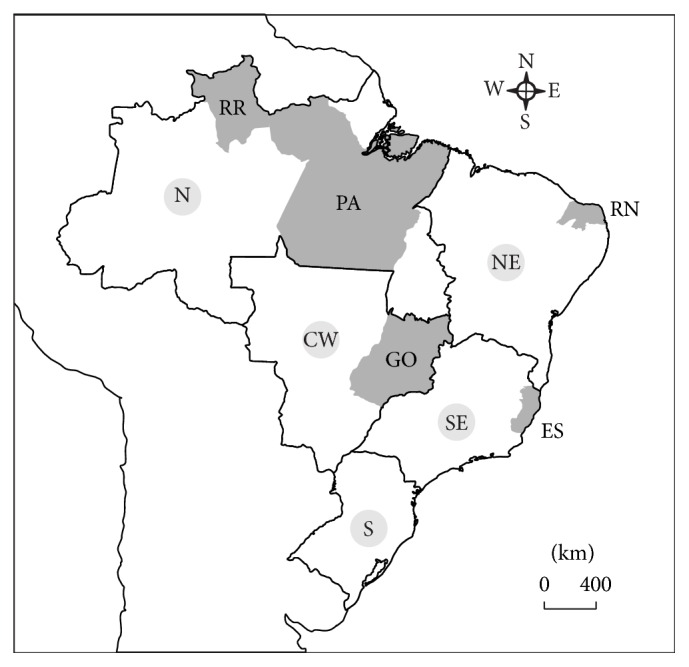
Brazilian map with the states used in the study in grey. RR: Roraima; PA: Pará; RN: Rio Grande do Norte; ES: Espírito Santo; and GO: Goiás. The continuous lines indicate the different regions of the country. N: north; NE: northeast; SE: southeast; S: south; and CW: central-west.
2. Materials and Methods
2.1. Data on Insecticide Distribution and Dengue Cases
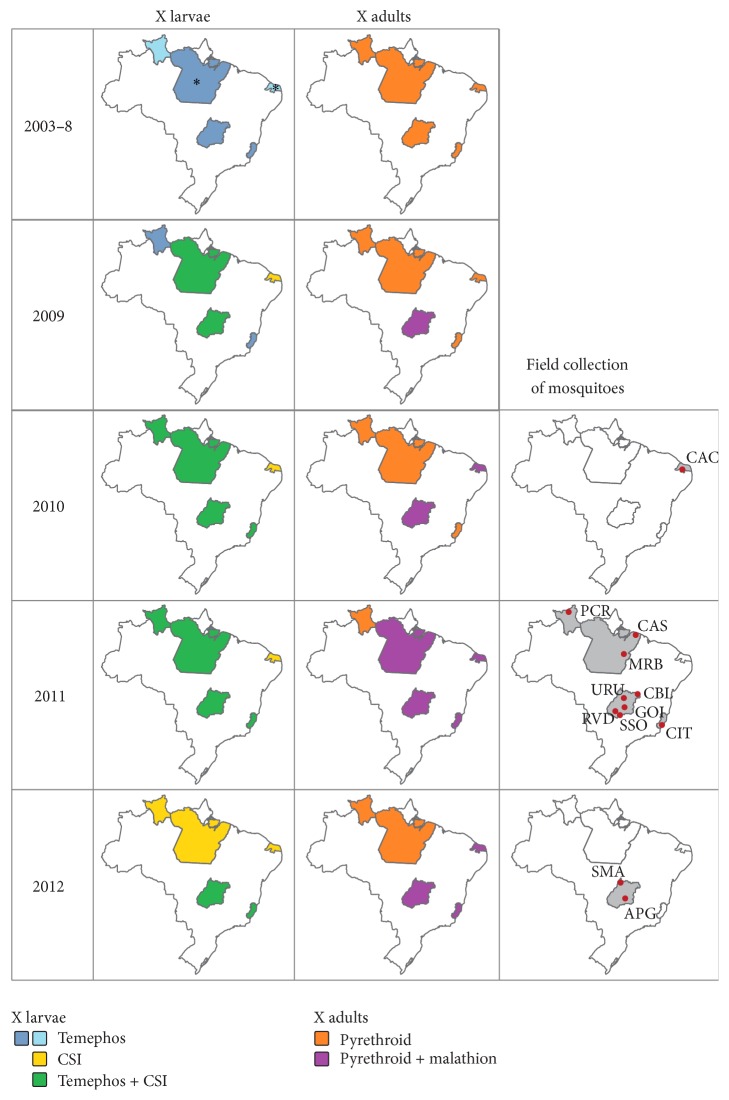
The Brazilian MoH coordinates the distribution of insecticides used in public health to all states and to all disease vector control programs. Insecticides are stored in a warehouse of Rio de Janeiro State Health Secretariat, in charge of stock control and supply of the products to the different States. We got MoH authorization to access these data, sorted by year, since 2003. Figure 2 illustrates the insecticides employed by PNCD from 2003 until 2012, the latter corresponding to the year of collection of the last samples in the field.
Figure 2.
Historic of insecticide distribution, between 2003 and 2012, by the Brazilian MoH to the States where mosquitoes samples were collected, in the indicated years. The municipalities evaluated are highlighted with red dots in the panels at right. During 2003–2008, two states, shown in lighter blue in the upper map at left, did not receive temephos continuously: at Roraima (RR) temephos supply started at 2005; at Rio Grande do Norte (RN) it ended at 2004. (∗) States that also received CSI (diflubenzuron or novaluron) during 2003–2008 (RN at 2004 and PA at 2008). Compounds against adults: all pyrethroids distributed by the MoH were considered, and not only deltamethrin (see text for details). Municipalities—APG: Aparecida de Goiânia; CAC: Caicó; CAS: Castanhal; CBL: Campos Belos; CIT: Cachoeiro de Itapemirim; GOI: Goiânia; MRB: Marabá; PCR: Pacaraima; RVD: Rio Verde; SMA: São Miguel do Araguaia; SSO: São Simão; and URU: Uruaçu.
Dengue incidence rates were based on the historical series of cases available at the MoH website for each municipality [34] and on the 2010 population census data conducted by the Brazilian Institute of Geography and Statistics [35].
2.2. Mosquitoes
Natural A. aegypti populations were collected between 2010 and 2012, in 12 municipalities belonging to a total of five States (Figures 1 and 2): Roraima (RR) and Pará (PA) at the north, Rio Grande do Norte (RN) at the northeast, Espírito Santo (ES), at the southeast, and Goiás (GO) at the central-west region. In all cases, sampling of vector eggs was performed with ovitraps according to MoReNAa (the Brazilian A. aegypti insecticide resistance monitoring network) guidelines, as described elsewhere [28, 36]. Depending on the number of buildings in each municipality, 150 to 300 ovitraps were installed during 5–7 days, representing the whole area.
Rockefeller mosquitoes (Rock), a reference strain of insecticide susceptibility [37], were employed as control in all bioassays and also in the biochemical and molecular analysis.
2.3. Mosquitoes Rearing
Eggs derived from field populations were allowed to hatch for two days in plastic cups containing 2.5 L of dechlorinated water and a small amount of cat food (Friskies®, Purina, São Paulo, SP). Pools of 1,000 larvae were then transferred to transparent plastic trays (33 × 24 × 8 cm) filled with 1.0 L of water and fed with 0.5 g of cat food every three days. The resulting pupae were transferred to cartoon cages (18 × 17 cm) and the A. aegypti emerging female and male adults were separated from other mosquito species, scored and reared in cages in order to proceed to blood feeding and egg laying. Adult females were fed on xylazine and ketamine-anaesthetized guinea pigs [38] for 30 minutes; oviposition cups were placed inside the cages three days later. Achievement of F1 and F2 generations in the laboratory was performed essentially as described elsewhere [28]. The whole procedure took place at 26 ± 1°C and 80 ± 10% relative humidity.
2.4. Larval Bioassays
In order to maximize synchronous development, egg hatching was induced during one hour in dechlorinated water. Afterwards, groups of 1,000 larvae were reared in plastic trays, as described above, until the third instar (L3).
Dose response bioassays with temephos (Pestanal®, Sigma-Aldrich) were performed with 10 different concentrations of the OP, designed to kill between 10 and 95% of each population. Four 100 mL replicas were employed per concentration and 20 L3 larvae per replica. Mortality was registered after 24 hours of exposure [29, 39, 40].
For the CSI diflubenzuron (Sigma-Aldrich), each dose response bioassay employed eight insecticide concentrations, also designed to be effective between 10 and 95%. Four 150 mL replicas per concentration and 10 L3 larvae per replica were employed. Both the bioassay methodology and the evaluation criteria were adapted from previous work [41, 42]. In this case, records were made each other day. Replicas were covered with a nylon mesh in order to avoid escaping of adults. The assay was considered terminated when all the specimens from the control group, nonexposed to the CSI, emerged as adults.
Two internal controls were placed at every bioassay: (a) Rockefeller larvae exposed to two different insecticide concentrations, the ED99 (effective dose) and half of it, and (b) field specimens kept with the solvent, in the same amount used for the experimental samples.
2.5. Adult Bioassays
Female adults were submitted to dose response bioassays to quantify resistance to the pyrethroid deltamethrin (Sigma-Aldrich) following the World Health Organization [43] methodology of impregnated papers, with some modifications [40, 44]. Assays employed 10 deltamethrin concentrations, ideally killing between 10 and 95% of each mosquito population. In all cases three replicas with 15–20 non-blood-fed females, 1–5 day-old, were used. After exposure to the pyrethroid during one hour, mosquitoes were recovered for 24 hours in insecticide-free compartments, when mortality was recorded. Adult bioassay controls followed the same rational employed for larvae ones: Rockefeller specimens exposed to two different deltamethrin concentrations and field derived adults exposed to the solvent.
2.6. Biochemical Assays
The potential mechanisms involved with resistance were evaluated through biochemical assays that quantified the activity of several classes of enzymes according to WHO and CDC procedures [23, 45, 46]. Two Phase I enzyme classes were evaluated, MFO and EST. While MFO was indirectly measured, three substrates were employed for EST: α- and β-naphtyl and ρ-nitrophenyl acetates, accounting, respectively, for activities named α-EST, β-EST, and ρNPA-EST. The Phase II GST and the OP target site AChE were also evaluated. For AChE, both total activity and activity inhibited by the carbamate propoxur were assayed. According to WHO criterion [47], AChE inhibition higher than 70% points to an activity compatible with insecticide susceptibility. Dosage of total proteins was done with the Bio-Rad protein assay/dye reagent concentrate (500-0006), and the results were used to calculate enzymatic specific activities.
Tests with each population employed approximately 90 individual non-blood-fed young females (up to 24 hours after adult emergence) and 90 late L3-early L4 larvae. All specimens were stored at −80°C until use.
Enzyme activities were classified essentially according to what was established previously [23]: after calculating the Rockefeller 99 percentile, the rate of specimens above this value was estimated for each enzyme and population. Activities were classified as unaltered, altered, or highly altered if this rate was, respectively, below 15, between 15 and 50, or above 50%.
2.7. Molecular Assays
The results of kdr genotyping were previously published [20] and herein explored in parallel with the bioassays. Briefly, the genotyping was conducted with a customized real-time PCR TaqMan SNP Genotyping Assay (Thermo Fisher), for Val1016Ile (AHS1DL6) and Phe1534Cys (AHUADFA). In general 30 individual adult males preferentially from the parental generation were used in two independent reactions, one for each NaV kdr SNP (1016 and 1534). The allelic and genotypic frequencies of each population were calculated based on variations at both positions, assuming that they are under linkage disequilibrium, which resulted in the alleles NaV S (1016 Val+ + 1534 Phe+), NaV R1 (1016 Val+ + 1534 Cyskdr), and NaV R2 (1016 Ilekdr + 1534 Cyskdr) [20].
2.8. Interpretation of Results
Results of bioassays for each population and every active compound derived from three or four tests performed in different days. The lethal concentrations (LC) in the case of neurotoxic insecticides or the concentrations inhibiting adult emergence (EI), when the IGR was considered, were calculated using probit analyses [48] (Polo-PC, LeOra Software, Berkeley, CA). Resistance ratios (RR50, RR95) were acquired dividing the results obtained for each population by the equivalent Rockefeller's values. For all insecticides, the resistance status of mosquito populations was classified according to the criterion utilized in the country to temephos evaluation. This criterion, recommended by PNCD, considers that populations with RR95 above 3.0 are resistant [23, 49] (see Section 4).
3. Results
3.1. Insecticides Employed against Aedes aegypti in the Field
Figure 2 exhibits the recent history of insecticides distributed by the Brazilian MoH to the States where field collection of A. aegypti populations took place. All larvicides evaluated in the present work are depicted. Beyond these products, Bti was also employed in the field (not shown), during most of the period between 2003 and 2009, except for the central-west State of Goiás. For adulticides, besides the organophosphate malathion, deltamethrin was the pyrethroid elected against A. aegypti. However, several PY compounds were also used in the scope of the control of other vectors. Therefore, Figure 2 depicts all pyrethroids distributed for this purpose by the MoH, since these products can interfere with A. aegypti populations' susceptibility status. It should also be taken into account that the uncontrolled domestic use of pyrethroids plays an important role in the dissemination of insecticide resistance [32]. However, information regarding domestic use is very difficult to obtain.
Up to 2011, the larvicide temephos was continuously distributed to the states evaluated, with two exceptions: temephos supply to RR started only in 2005 and to RN it lasted until 2004 (Figure 2, light blue states in the 2003–8 line). In this latter state, control of larvae employed Bti between 2005 and 2008. Since 2009-2010 the CSI diflubenzuron was introduced in the A. aegypti larvae control, in addition to the organophosphate temephos in all states. The exception was RN where, as mentioned above, temephos had been previously discontinued; in this case, the CSI remained the sole larvicide adopted in A. aegypti control from 2009 on.
Control of adult mosquitoes was performed exclusively with pyrethroids between 2003 and 2008. Since 2009, the organophosphate malathion was gradually introduced. At 2011, all the states received both compounds, except RR that employed exclusively PY. At 2012, malathion was not distributed to the state of Pará.
3.2. Dengue Incidence in the Evaluated Municipalities
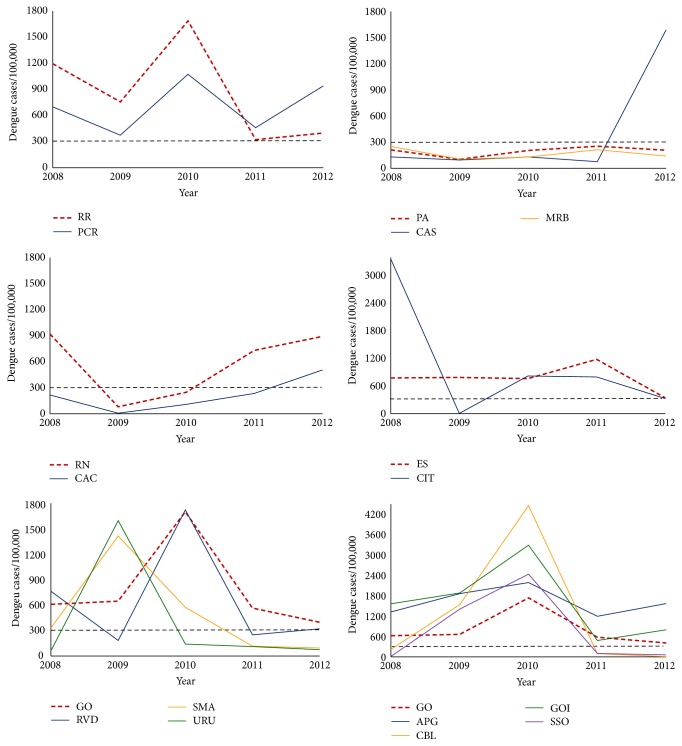
Figure 3 shows the incidence of dengue reported cases for all municipalities evaluated here. The aim in this case was to investigate if there were local outbreaks that could be related to a domestic intensification of insecticide use and, potentially, to an increase in A. aegypti resistance to these compounds. The period chosen ranged from 2008, two years before the collection of the first A. aegypti samples here evaluated, up to 2012. Incidence values for municipalities and the corresponding states are also presented in Table S1 of the Supplementary Material available online at http://dx.doi.org/10.1155/2016/8603263.
Figure 3.
Incidence of reported dengue cases in the municipalities evaluated from 2008 to 2012 (values refer to the rate of notified cases per 100,000 inhabitants). In each panel, the thick red line refers to the dengue incidence in the corresponding State. The dashed line indicates the point above which dengue incidence is considered high. Note that in some panels it was necessary to change the y-scale in order to include all values.
According to the Brazilian MoH, dengue incidence rates higher than 300 cases/100,000 inhabitants are already indicative of an epidemic situation [50]. In almost all municipalities, at least once, the dengue incidence of notified cases was compatible with this scenario. When the whole 2008–2012 period was considered, only Marabá, PA, was the exception. However, the localities of Castanhal, PA, and Caicó, RN, only presented high dengue incidence at 2012, after collection of vector samples was made (compare Figures 2 and 3). In some situations this “epidemic status” persisted throughout the whole evaluated period; this was the case of Pacaraima, RR, and of the GO adjacent municipalities Goiânia and Aparecida de Goiânia. The number of registers well above the threshold value of 300 cases per 100,000 inhabitants also attracted attention. For instance, reported incidence equivalent to at least 1% (1,000 cases/100,000 inhabitants) was found during 2009 and 2010 in half of the evaluated municipalities. Notably, the dengue incidence of Aparecida de Goiânia remained above 1% during the whole evaluated period. In the adjacent locality, Goiânia, those high dengue rates were registered during three years between 2008 and 2012. One should be aware that in general dengue epidemic periods are related to a significant increase in the domestic use of insecticides against adult mosquitoes (see Section 4).
3.3. Bioassays with Larvae
Table 1 summarizes the results of quantitative bioassays performed with the two main larvicides recently employed against A. aegypti in Brazil, the OP temephos and the CSI diflubenzuron. Table S2 shows details of these assays, such as effective concentrations and confidence intervals. Data are organized by year and then by geographic region.
Table 1.
Resistance status of several Brazilian municipalities to the larvicides temephos (OP) and diflubenzuron (CSI).
| Year | Region | State | Municipality/strain | Generation | Temephos | Generation | Diflubenzuron | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR50 | RR95 | Slope | RR50 | RR95 | Slope | ||||||
| 2010 | NE | RN | Rockefeller | 1.0 | 1.0 | 6.20 | 1.0 | 1.0∗ | 4.85 | ||
| Caicó | F2 | 8.4 | 9.6 | 5.07 | F2 | 2.2 | 1.7 ∗ | 6.56 | |||
|
| |||||||||||
| 2011 | N | RR | Rockefeller | 1.0 | 1.0 | 5.03 | 1.0 | 1.0 | 4.16 | ||
| Pacaraima | F2 | 4.3 | 4.0 | 5.70 | F2 | 1.7 | 1.5 | 5.05 | |||
| PA | Castanhal | F2 | 8.2 | 11.2 | 3.53 | F2 | 1.4 | 1.2 | 4.75 | ||
| Marabá | F2 | 8.0 | 10.3 | 3.76 | F3 | 1.8 | 1.6 | 4.91 | |||
| SE | ES | Cachoeiro de Itapemirim | F1 | 18.4 | 17.1 | 5.57 | F2 | 1.9 | 1.6 | 5.03 | |
| CW | GO | Campos Belos | F2 | 9.1 | 12.0 | 3.68 | F2 | 1.7 | 1.6 | 4.38 | |
| Goiânia | F2 | 7.9 | 8.6 | 4.56 | F2 | 1.6 | 1.8 | 3.63 | |||
| Rio Verde | F2 | 11.5 | 14.8 | 3.77 | F2 | 2.0 | 1.6 | 5.32 | |||
| São Simão | F2 | 12.1 | 14.8 | 3.98 | F2 | 2.0 | 2.3 | 3.57 | |||
| Uruaçu | F2 | 10.5 | 12.5 | 4.08 | F2 | 1.9 | 1.5 | 5.78 | |||
|
| |||||||||||
| 2012 | CW | GO | Aparecida de Goiânia | F1 | 17.9 | 16.6 | 5.59 | F2 | 1.1 | 2.1 | 2.43 |
| São Miguel do Araguaia | F1 | 21.1 | 27.1 | 3.77 | F1 | 1.6 | 1.7 | 3.82 | |||
RR50 and RR95: resistance ratios; profiles corresponding to RR95 below or above 3.0 (italic font or bold font numbers) were classified as susceptible or resistant, respectively.
∗RR80 is informed. See Table S2 for additional details.
All the populations evaluated were considered resistant to temephos. The higher resistance values were obtained in 2012. Nevertheless, since there were no municipalities examined in consecutive years, it is not possible to claim that temephos resistance is increasing in the country, based on the data presented here. In general, temephos resistance was higher at the central-west region: six out of seven municipalities presented RR95 above 10. São Miguel do Araguaia, GO, exhibited the higher temephos RR95 value, above 27. In contrast, Pacaraima, the municipality with the lower resistance level to the OP, is located at RR, a state where temephos supply started later than in the other states (Figure 2). In comparison with Rockefeller, a general higher heterogeneity of field populations was detected, as judged by their low slope values.
In contrast to the results obtained for temephos, all populations analyzed were susceptible to diflubenzuron (RR95 < 3.0). This was true even for mosquito populations bearing high temephos resistance rates, suggesting absence of cross resistance between these compounds in the localities examined. In contrast to the results obtained with temephos, field populations seemed more homogeneous than Rockefeller strain regarding diflubenzuron resistance profiles.
3.4. Bioassays and Molecular Assays with Adults
Resistance rates resulting from bioassays with the adulticide PY deltamethrin are depicted in Table 2, together with the NaV allelic frequencies, where R1 and R2 are the kdr alleles related to PY target site resistance. Additional details of the bioassays are presented in Table S3 that also includes the kdr allelic frequencies for each position (1016 and 1534) separately [20].
Table 2.
Resistance status of several Brazilian municipalities to the pyrethroid deltamethrin and allelic frequencies of the major kdr mutations found in the country.
| Year | Region | State | Municipality/strain | Generation | RR50 | RR95 | Slope | NaV allelic frequencies | ||
|---|---|---|---|---|---|---|---|---|---|---|
| S | R1 | R2 | ||||||||
| 2010 | NE | RN | Rockefeller | 1.0 | 1.0 | 2.96 | ||||
| Caicó | F2 | 6.0 | 13.1 | 2.51 | 0.917 | 0.067 | 0.017 | |||
|
| ||||||||||
| 2011 | N | RR | Rockefeller | 1.0 | 1.0 | 4.55 | ||||
| Pacaraima | F2 | 33.2 | 60.3 | 2.65 | 0.000 | 0.600 | 0.400 | |||
| PA | Castanhal | F2 | 9.9 | 14.9 | 3.05 | 0.667 | 0.300 | 0.033 | ||
| Marabá | F2 | 47.4 | 70.7 | 3.07 | 0.690 | 0.310 | 0.000 | |||
| SE | ES | Cachoeiro de Itapemirim | F1 | 49.0 | 78.6∗ | 2.16 | 0.103 | 0.224 | 0.672 | |
| CW | GO | Campos Belos | F2 | 25.3 | 52.3 | 2.43 | 0.310 | 0.086 | 0.603 | |
| Goiânia | F2 | 47.6 | 46.5 | 4.68 | ||||||
| Rio Verde | F2 | 32.5 | 56.2 | 2.74 | 0.241 | 0.207 | 0.552 | |||
| São Simão | F2 | 30.4 | 51.6 | 2.78 | 0.083 | 0.383 | 0.533 | |||
| Uruaçu | F2 | 38.6 | 51.6 | 3.37 | 0.450 | 0.117 | 0.433 | |||
|
| ||||||||||
| 2012 | CW | GO | Aparecida de Goiânia | F1 | 33.0 | 57.2 | 2.74 | 0.207 | 0.362 | 0.463 |
| São Miguel do Araguaia | F1 | 39.6 | 49.4 | 3.59 | 0.414 | 0.293 | 0.293 | |||
RR50 and RR95: resistance ratios; profiles corresponding to RR95 above 3.0 (bold font numbers) were classified as resistant.
∗RR80 is informed. See Table S3 for additional details.
Kdr allelic frequencies S, R1, and R2 refer to the positions 1016 and 1534 of the gene coding for the voltage gated sodium channel (NaV) as follows: S (susceptible) = 1016 Val+/1534 Phe+; R1 (single mutant) = 1016 Val+/1534 Cyskdr; and R2 (double mutant) = 1016 Ilekdr/1534 Cyskdr. Kdr data have been originally published by Linss et al. 2014 [20].
Very high deltamethrin resistance levels were found for all populations; RR95 was always above 10.0. Caicó, RN, at the northeast region, and Castanhal, PA, in the north region, exhibited the lowest RR95, respectively, 13.1 and 14.9. Accordingly, these municipalities presented the lower frequencies of R1 and R2 kdr alleles. In all other municipalities values remained above 45.0. In two localities, Marabá, PA, and Cachoeiro de Itapemirim, ES, RR95 was higher than 70.0. In this latter locality, due to the high resistance level detected, there was lack of enough specimens to reach LC95 (note that in Tables 2 and S3 the higher value shown for Cachoeiro de Itapemirim corresponds to LC80). The supply of pyrethroids for the bulk of the states evaluated here was continuous since at least 2003 (Figure 2). Comparison of the slope values obtained for deltamethrin assays shows that, in general, field populations evaluated demonstrate higher heterogeneity than the Rockefeller strain.
3.5. Biochemical Assays
Tables 3 and 4 present the results of biochemical assays for, respectively, larval and adult stages. In both tables, data are organized by decreasing RR95 order for the neurotoxic insecticides temephos (larvae) and deltamethrin (adults). Additional details of these assays are shown in Tables S4 and S5.
Table 3.
Quantification of the enzymatic activity of A. aegypti larvae from different Brazilian municipalities. Numbers refer to the rate of specimens with activity higher than the 99 percentile of Rockefeller (%>p99). Municipalities are arranged in descending order of temephos resistance (RR95 OP).
| Year | Region | State | Municipality/strain | RR95 OP | ACE | MFO | GST | A-EST | B-EST | PNPA-EST |
|---|---|---|---|---|---|---|---|---|---|---|
| 2012 | CO | GO | São Miguel do Araguaia | 27.1 | 0 | 0 | 59 | |||
| 2012 | CO | GO | Aparecida de Goiânia | 16.6 | 5 | 85 | 8 | 54 | 63 | |
| 2011 | CO | GO | Rio Verde | 14.8 | 1 | 0 | 8 | |||
| 2011 | CO | GO | São Simão | 14.8 | 1 | 4 | ||||
| 2011 | CO | GO | Campos Belos | 12.0 | 1 | 15 | 9 | 1 | 5 | 0 |
| 2011 | N | PA | Castanhal | 11.2 | 0 | 3 | 75 | |||
| 2010 | NE | RN | Caicó | 9.6 | 0 | 4 | 0 | 0 | 0 | |
| 2011 | CO | GO | Goiânia | 8.6 | 3 | 6 | 5 | |||
| 2011 | N | RR | Pacaraima | 4.0 | 6 | 1 | 0 | 14 | 3 |
Activities were classified as normal (regular font), altered (italic and underlined font) or highly altered (italic and bold) if these values ranged respectively below 15%, between 15 and 50% or above 50%.
Table 4.
Quantification of the enzymatic activity of A. aegypti adults from different Brazilian municipalities. Numbers refer to the rate of specimens with activity higher than the 99 percentile of Rockefeller (%>p99). Municipalities are arranged in descending order of deltamethrin resistance (RR95 PI).
| Year | Region | State | Municipality/strain | RR95 PI | ACE | MFO | GST | A-EST | B-EST | PNPA-EST |
|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | SE | ES | Cachoeiro de Itapemirim | 78.6∗ | 0 | 80 | 98 | 67 | 3 | 70 |
| 2011 | N | RR | Pacaraima | 60.3 | 0 | 13 | 2 | |||
| 2012 | CO | GO | Aparecida de Goiânia | 57.2 | 1 | 57 | 94 | 70 | 10 | 73 |
| 2011 | CO | GO | Rio Verde | 56.2 | 13 | 74 | 4 | 81 | 8 | 13 |
| 2011 | CO | GO | Campos Belos | 52.3 | 0 | 8 | 65 | 14 | 1 | 6 |
| 2011 | CO | GO | São Simão | 51.6 | 9 | 59 | 58 | 14 | 15 | |
| 2012 | CO | GO | São Miguel do Araguaia | 49.4 | 4 | 97 | 8 | 0 | 8 | |
| 2011 | CO | GO | Goiânia | 46.5 | 0 | 99 | 78 | 55 | 5 | |
| 2010 | NE | RN | Caicó | 13.1 | 3 | 0 | 11 | 63 | 6 |
Activities were classified as normal (regular font), altered (italic and underlined font) or highly altered (italic and bold) if these values ranged respectively below 15%, between 15 and 50% or above 50%. ∗RR80 is informed.
According to the WHO criterion, measurements of inhibition of AChE activity, the OP target site, point to unaltered activity for all populations and development stages (data not shown). This was verified because the carbamate propoxur induced more than 70% of AChE activity inhibition in all cases [47]. In addition, quantification of total AChE activity [45, 46] revealed values compatible with susceptibility, in all cases, with exception of adults from one population (São Simão, GO, Table 4).
For all the remaining enzymes, changes were detected for both stages. In the larval stage, major changes were noted in the activities of GST, β-EST, and ρNPA-EST, while moderate increases were noted for MFO and α-EST. The intensity of enzymatic alterations appeared to be higher at the adult stage, mainly when MFO, GST, and α-EST were considered. In general, populations with a higher RR also tended to exhibit a higher increase in detoxifying enzymes activity, taking into account both the number of altered classes and the intensity of activity increment.
4. Discussion
The use of insecticides in the control of the dengue vector in Brazil has been broad and continuous, a procedure that favored the selection of resistant specimens over the years. In order to assist in the rational use of pesticides, in 2006, the Brazilian Dengue Control Program adopted a functional criterion for the evaluation of the temephos status of A. aegypti populations. This criterion, also employed here to classify both deltamethrin and diflubenzuron resistance status, considers that populations with RR95 above 3.0 are resistant. This is the cutoff to conduct insecticide substitution in the field, and the adoption of this parameter took into account Brazilian operational aspects of insecticide management, like the period of time necessary for the effective implementation of the insecticide substitution in all affected localities. This strategy aimed to preserve the insecticides in the field [23].
Functional validation of this criterion has been previously obtained through simulated field assays with temephos and, more recently, with pyrethroids [23, 51]. Resistance to diflubenzuron was not established in the country and therefore a functional criterion has not yet been defined for this IGR by PNCD. However, Fontoura et al. [42], using simulated assays, did not find impairment of the efficacy of another CSI, novaluron, in A. aegypti populations bearing RR90 < 2.0.
The use of OP pesticides in Brazil for A. aegypti control dates back to the 1960s, and it was intensified since 1986, when the DENV-1 virus was introduced in the country [52, 53]. As a result, resistance to temephos has been reported in Brazilian populations of A. aegypti collected from 1998 on [23, 27, 28]. Resistance to temephos spread around the country so intensely that, since 2009, PNCD does not recommend the use of this OP as the larvicide of choice [54]. Accordingly, all populations here evaluated between 2010 and 2012 were resistant to temephos.
Investigation of putative resistance mechanisms present in A. aegypti larvae excluded the participation of acetylcholinesterase, the OP target site. Regarding metabolic resistance, MFO enzymes are strongly associated with insecticide resistance in several A. aegypti populations around the world [25, 26, 55]. We found only discrete alterations in this class of enzymes in larvae from Brazilian A. aegypti populations, while adult specimens exhibited levels of MFO alteration equivalent to EST and GST enzymes. In 2007, A. aegypti adult EST activities were associated with resistance to both OP and PY in Brazil [23]. Connections between OP resistance and significant alterations of EST as well as association between PY resistance and both MFO and GST elevated activity rates were also reported in other countries [25, 26, 55].
In general, higher RR levels against OP and PY neurotoxic insecticides correlated to increased metabolic alterations in terms of both number of enzymes affected and intensity of activity enhancement [30, 55, 56]. Usually detoxifying enzymes that trigger metabolic resistance participate in the general insect metabolism. These are somewhat generic molecules, with a variable affinity for a number of insecticides or other xenobiotics. Although resistance to the IGR diflubenzuron has not yet been detected in the country, the development of metabolic resistance against these compounds is a potential mechanism that should be monitored.
For some localities here depicted, previous evaluations of the temephos susceptibility status are available. In these cases, the resistance dynamics profiles were compared to the insecticide distribution performed by the MoH to each state (Figure 2). Increase in the temephos resistance status was noted whenever the application of this OP persisted. Examples are the central-west municipalities of Goiânia (GOI) and Aparecida de Goiânia (APG), at GO state: between 2003 and 2011, GOI RR95 increased more than twice, from 3.3 to 8.6 (Table 1, [23]). In APG the temephos RR95 also increased significantly between 2006 (11.2) and 2012 (16.6) (Table 1, [57]). In contrast, only a low decrease in the temephos resistance status was noted following its interruption. This was the case of Caicó (CAC), at RN state, where no temephos was provided since 2004 (Figure 2). At that year, CAC temephos RR95 was 12.5; six years later this value dropped only slightly, to 9.6 (Table 1, [23]).
One major consequence of the high and disseminated Brazilian A. aegypti temephos resistance status was the inclusion of the CSI diflubenzuron in the chemical control of larvae since 2009. Bioassays of mosquito samples obtained between 2010 and 2012 confirmed the susceptible status of all evaluated populations to this product. Diflubenzuron resistance ratios below 3.0 were also found for field A. aegypti populations from Cabo Verde, Malaysia, and Martinique [58–60]. Together, these data point that the use of this class of insecticides in the control of larvae of the dengue vector is still viable.
The bulk of results obtained by the A. aegypti insecticide resistance monitoring Brazilian network guided the option to rotate products against larvae in the country. Insect Growth Regulators were adopted. Due to operational issues, the maximum period of four years was fixed for alternation of products [61]. The aim of this resistance management strategy was to preserve the few available larvicides. In this regard, it is ought to mention that Brazil only employs larvicides recommended by WHOPES for use in drinking water [14]. Currently, the IGR used against A. aegypti larvae is the juvenile hormone analogue (JHA) pyriproxyfen [62].
Some decades were necessary until the spread of resistance to the OP temephos in Brazil could compromise its use in the control of A. aegypti. In contrast, in the case of pyrethroids, introduced in the whole country in 2000 by PNCD, only a few years were enough to the achievement of extremely high resistance levels [23, 31, 32]. If, on the one hand, chemical control of A. aegypti adults is recommended by the MoH only to block outbreaks or on the imminence of a dengue epidemic [9], on the other hand, unlike temephos, PY insecticides are available in the retail market. The domestic use of pyrethroids is intensified at every dengue epidemic period and certainly contributes significantly to the rapid dissemination of resistance [33]. Accordingly, A. aegypti deltamethrin resistance levels doubled between 2009 and 2011 at Cachoeiro de Itapemirim and Goiânia; in the same period, an eightfold increase was observed for this parameter at Marabá (Table 2; Bellinato D, personal communication). Cachoeiro de Itapemirim and Goiânia faced dengue outbreaks in this interval, corroborating the hypothesis that the intensification of the domestic use of PY collaborated in the resistance increase. However no dengue outbreak was noted at Marabá in this period. Marabá is located in the Amazon region, where almost all Brazilian malaria cases are reported. Control of Anopheles malaria vectors also employs PY and this could explain the increased resistance observed. In contrast, the lowest PY resistance levels were found for Castanhal at 2010 and Caicó at 2011, two municipalities that had not experienced dengue epidemics since 2008.
To date, the PY resistance ratios found here are among the highest ones reported in the country. In spite of that, heterogeneity levels exhibited by those vector populations suggest that the insecticide resistance character is still not irreversibly fixed. In relation to the PY target site resistance, the voltage gated sodium channel, this heterogeneity confirms previous observations, reporting the presence of the NaV susceptible allele (S) with allelic frequencies between 0.0 and 0.92 (Table 2). Regarding metabolic resistance of adult mosquitoes, as for temephos resistance and larvae biochemical profile (mentioned above), a general positive correlation between the resistance level and the magnitude of altered enzymes was also found. It should be noted that, in this case, except for Caicó, all populations exhibited PY resistance levels above 45.0. Hence, while Caicó detoxifying enzymes effects were restricted to esterases, in the remaining populations, GST or MFO activities (and both enzymes in some cases) were significantly enhanced. In addition, GST, MFO, and EST have already been correlated with PY resistance [5, 23, 44, 63].
Due to the spread of high pyrethroid resistance levels throughout the country, since 2009 PY is being gradually replaced by the OP malathion for the control of A. aegypti adults, employed in residual applications and UBV [16, 54, 64].
Currently, chemical control is still largely applied in public health, favoring the insecticide resistance dissemination of vector populations. Accordingly, we detected high resistance levels against the OP temephos and the PY deltamethrin, insecticides long used in the country. In some municipalities, comparison with the incidence of dengue outbreaks suggested significant participation of the domestic use of PY insecticides in the rapid resistance increase. The introduction of IGRs is recent in Brazil, and all A. aegypti populations here evaluated were susceptible to the CSI diflubenzuron. Together our results indicate the potential of this IGR against the vector but also point to the need for rational use of chemical tools. In this sense, the adoption of rotation of compounds with different mechanisms of action is a positive step. It still remains, however, to invest in awareness campaigns, directed to both managers and the general society, regarding the importance of the mechanical control of vectors as a priority. Spreading the concept that chemical control is a complementary antivector strategy is the best way to preserve insecticides.
Supplementary Material
Table S1 corresponds to data used to construct Figure 3. It presents the incidence of dengue cases, between 2008 and 2012, in the evaluated municipalities. Tables S2 and S3 contain additional data obtained for respectively larvae and adults (with bioassays and molecular assays): Table S2 complements Table 1 (temephos and diflubenzuron results) and Table S3 complements Table 2 (deltamethrin bioassays and pyrethroid target site data). Regarding bioassays, effective doses and confidentiality intervals are shown. For molecular assays, the frequencies of individual substitutions, at 1016 and 1534 positions, are depicted. Tables S4 and S5 give details of the biochemical assays, such as number of individual specimens tested in each case, the median values of all enzyme activities for the evaluated populations and the Rockefeller 99 percentile used to classify activities. Tables S4 and S5 also repeat the rate of specimens with activity higher than the Rockefeller 99 percentile, the parameter used to classify the populations (that is shown here in colors).
Acknowledgments
This study is funded by Fundação Oswaldo Cruz (Fiocruz), Programa Nacional de Controle da Dengue/Secretaria de Vigilância em Saúde/Ministério da Saúde (PNCD/SVS/MS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular (INCT-EM). The authors acknowledge Heloisa Maria Nogueira Diniz from the Serviço de Produção e Tratamento de Imagem/IOC for help with the figures, Marcos Batata from the Secretaria Estadual de Saúde do Rio de Janeiro for sharing information regarding insecticide supply, and the Laboratório de Fisiologia e Controle de Artrópodes Vetores personnel for help with bioassays.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Ademir J. Martins, Diogo Fernandes Bellinato, Denise Valle, José Bento Pereira Lima, and Simone Costa Araújo conceived and designed the experiments. Diogo Fernandes Bellinato and Priscila Fernandes Viana-Medeiros performed the experiments. Diogo Fernandes Bellinato, Denise Valle, Priscila Fernandes Viana-Medeiros, and Simone Costa Araújo analyzed the data. Denise Valle and José Bento Pereira Lima contributed reagents/materials/analysis tools. Diogo Fernandes Bellinato and Denise Valle wrote the paper. All authors read and approved the final version of the paper.
References
- 1.WHO. A Global Brief on Vector-Borne Diseases. 2014. (WHO/DCO/WHD/2014.1). [Google Scholar]
- 2.Schwartz L. M., Halloran M. E., Durbin A. P., Longini I. M., Jr. The dengue vaccine pipeline: implications for the future of dengue control. Vaccine. 2015;33(29):3293–3298. doi: 10.1016/j.vaccine.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consoli R., Lourenço-de-Oliveira R. Principais Mosquitos de Importância Sanitária no Brasil. Rio de Janeiro, Brazil: Editora Fiocruz; 1994. [Google Scholar]
- 4.Jansen C. C., Beebe N. W. The dengue vector Aedes aegypti: what comes next. Microbes and Infection. 2010;12(4):272–279. doi: 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Valle D., Belinato T. A., Martins A. J. Controle químico de Aedes aegypti. Resistência a inseticidas e alternativas. In: Valle D., Pimenta D. N., Cunha R. V., editors. Dengue: Teorias e Práticas. Rio de Janeiro, Brazil: Editora Fiocruz; 2015. pp. 93–126. [Google Scholar]
- 6.Braga I. A., Valle D. Aedes aegypti: inseticidas, mecanismos de ação e resistência. Epidemiologia e Serviços de Saúde. 2007;16(4):279–293. [Google Scholar]
- 7.WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: WHO Press; 2009. [PubMed] [Google Scholar]
- 8.Cardoso C. W., Paploski I. A. D., Kikuti M., et al. Outbreak of exanthematous illness associated with Zika, Chikungunya, and dengue viruses, Salvador, Brazil. Emerging Infectious Diseases. 2015;21(12):2274–2276. doi: 10.3201/eid2112.151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil. Diretrizes Nacionais Para a Prevenção e Controle de Epidemias de Dengue. 1st. Brasília, Brazil: Ministério da Saúde, Secretaria de Vigilância em Saúde; 2009. (Normas e Manuais Técnicos, Série A). [Google Scholar]
- 10.Ranson H., Burhani J., Lumjuan N., Black W. C. Insecticide resistance in dengue vectors. TropIKA.net Journal. 2010;1(1) [Google Scholar]
- 11.Darriet F., Duchon S., Hougard J. M. Spinosad: a new larvicide against insecticide-resistant mosquito larvae. Journal of the American Mosquito Control Association. 2005;21(4):495–496. doi: 10.2987/8756-971x(2006)21[495:sanlai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Mulla M. S. The future of insect growth regulators in vector control. Journal of the American Mosquito Control Association. 1995;11(2):269–273. [PubMed] [Google Scholar]
- 13.WHO. World Health Organization Recommended Insecticides for Space Spraying. Geneva, Switzerland: World Health Organization; 2012. http://www.who.int/whopes/Insecticides_for_space_spraying_Jul_2012.pdf. [Google Scholar]
- 14.WHO. WHOPES-Recommended Compounds and Formulations for Control of Mosquito Larvae. Geneva, Switzerland: World Health Organization; 2013. http://www.who.int/whopes/Mosquito_Larvicides_25_Oct_2013.pdf. [Google Scholar]
- 15.Martins A. J., Valle D. The pyrethroid knockdown resistance. In: Soloneski S., Larramendy M. S., editors. Insecticides: Basic and Other Applications. Rijeka, Croatia: InTech; 2012. [Google Scholar]
- 16.García G. P., Flores A. E., Fernández-Salas I., et al. Recent rapid rise of a permethrin knock down resistance allele in Aedes aegypti in México. PLoS Neglected Tropical Diseases. 2009;3(10, article e531) doi: 10.1371/journal.pntd.0000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcombe S., Mathieu R. B., Pocquet N., et al. Insecticide resistance in the dengue vector Aedes aegypti from Martinique: distribution, mechanisms and relations with environmental factors. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0030989.e30989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C.-X., Kaufman P. E., Xue R.-D., et al. Relationship between insecticide resistance and kdr mutations in the dengue vector Aedes aegypti in Southern China. Parasites & Vectors. 2015;8, article 325 doi: 10.1186/s13071-015-0933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martins A. J., Lima J. B. P., Peixoto A. A., Valle D. Frequency of Val1016Ile mutation in the voltage-gated sodium channel gene of Aedes aegypti Brazilian populations. Tropical Medicine and International Health. 2009;14(11):1351–1355. doi: 10.1111/j.1365-3156.2009.02378.x. [DOI] [PubMed] [Google Scholar]
- 20.Linss J. G. B., Brito L. P., Garcia G. A., et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasites and Vectors. 2014;7(1, article 25) doi: 10.1186/1756-3305-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemingway J., Ranson H. Insecticide resistance in insect vectors of human disease. Annual Review of Entomology. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 22.Ranson H., Paton M. G., Jensen B., et al. Genetic mapping of genes conferring permethrin resistance in the malaria vector, Anopheles gambiae . Insect Molecular Biology. 2004;13(4):379–386. doi: 10.1111/j.0962-1075.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 23.Montella I. R., Martins A. J., Jr., Viana-Medeiros P. F., Lima J. B. P., Braga I. A., Valle D. Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. American Journal of Tropical Medicine and Hygiene. 2007;77(3):467–477. [PubMed] [Google Scholar]
- 24.Araújo A. P., Diniz D. F. A., Helvecio E., et al. The susceptibility of Aedes aegypti populations displaying temephos resistance to Bacillus thuringiensis israelensis: a basis for management. Parasites and Vectors. 2013;6(1, article 297) doi: 10.1186/1756-3305-6-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strode C., Wondji C. S., David J.-P., et al. Genomic analysis of detoxification genes in the mosquito Aedes aegypti . Insect Biochemistry and Molecular Biology. 2008;38(1):113–123. doi: 10.1016/j.ibmb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Poupardin R., Srisukontarat W., Yunta C., Ranson H. Identification of carboxylesterase genes implicated in temephos resistance in the dengue vector Aedes aegypti . PLoS Neglected Tropical Diseases. 2014;8(3, article e2743) doi: 10.1371/journal.pntd.0002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macoris M. L. G., Andrighetti M. T. M., Takaku L., Glasser C. M., Garbeloto V. C., Cirino V. C. B. Alteration in susceptibility response of Aedes aegypti to organophosphates in cities in the state of S. Paulo, Brazil. Revista de Saúde Pública. 1999;33(5):521–522. doi: 10.1590/s0034-89101999000500013. [DOI] [PubMed] [Google Scholar]
- 28.Lima J. B. P., Pereira da-Cunha M., da Silva Júnior R. C., et al. Resistance of Aedes aegypti to organophosphates in several municipalities in the State of Rio de Janeiro and Espírito Santo, Brazil. American Journal of Tropical Medicine and Hygiene. 2003;68(3):329–333. [PubMed] [Google Scholar]
- 29.Braga I. A., Lima J. B. P., Da Silva Soares S., Valle D. Aedes aegypti resistance to temephos during 2001 in several municipalities in the states of Rio de Janeiro, Sergipe, and Alagoas, Brazil. Memórias do Instituto Oswaldo Cruz. 2004;99(2):199–203. doi: 10.1590/s0074-02762004000200015. [DOI] [PubMed] [Google Scholar]
- 30.Lima E. P., Paiva M. H. S., de Araújo A. P., et al. Insecticide resistance in Aedes aegypti populations from Ceará, Brazil. Parasites & Vectors. 2011;4(1, article 5) doi: 10.1186/1756-3305-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da-Cunha M. P., Lima J. B. P., Brogdon W. G., Moya G. E., Valle D. Monitoring of resistance to the pyrethroid cypermethrin in Brazilian Aedes aegypti (Diptera: Culicidae) populations collected between 2001 and 2003. Memórias do Instituto Oswaldo Cruz. 2005;100(4):441–444. doi: 10.1590/s0074-02762005000400017. [DOI] [PubMed] [Google Scholar]
- 32.Maciel-de-Freitas R., Avendanho F. C., Santos R., et al. Undesirable consequences of insecticide resistance following Aedes aegypti control activities due to a dengue outbreak. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0092424.e92424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maciel-de-Freitas R., Valle D. Challenges encountered using standard vector control measures for dengue in Boa Vista, Brazil. Bulletin of the World Health Organization. 2014;92(9):685–689. doi: 10.2471/BLT.13.119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brasil 2015. Sistema de Informação de Agravos de Notificação (SINAN) Brasília, Brazil: Ministério da Saúde, Secretária de Vigilância em Saúde; http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/denguebr.def. [Google Scholar]
- 35.Brasil 2010. Sinopse do Censo Demográfico 2010. Instituto Brasileiro de Geografia e Estatística; http://www.censo2010.ibge.gov.br/sinopse/index.php?uf. [Google Scholar]
- 36.Brasil 2008. Documento da Rede Nacional de Monitoramento da Resistência de Aedes aegypti a Inseticidas (MoReNAa) Brasília, Brazil: Ministério da Saúde, Secretaria de Vigilância em Saúde; Metodologia de amostragem de Aedes aegypti por meio de armadilhas de postura (ovitrampas) [Google Scholar]
- 37.Kuno G. Early history of laboratory breeding of Aedes aegypti (Diptera: Culicidae) focusing on the origins and use of selected strains. Journal of Medical Entomology. 2010;47(6):957–971. doi: 10.1603/me10152. [DOI] [PubMed] [Google Scholar]
- 38.Hawk C. T., Leary S. L. Formulary for Laboratory Animals. Ames, Iowa, USA: Iowa State University Press; 1995. [Google Scholar]
- 39.WHO. WHO/VBC/81.807. Geneva, Switzerland: World Health Organization; 1981. Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. [Google Scholar]
- 40.Belinato T. A., Martins A. J., Valle D. Fitness evaluation of two Brazilian Aedes aegypti field populations with distinct levels of resistance to the organophosphate temephos. Memórias do Instituto Oswaldo Cruz. 2012;107(7):916–922. doi: 10.1590/s0074-02762012000700013. [DOI] [PubMed] [Google Scholar]
- 41.Martins A. J., Belinato T. A., Lima J. B. P., Valle D. Chitin synthesis inhibitor effect on Aedes aegypti populations susceptible and resistant to organophosphate temephos. Pest Management Science. 2008;64(6):676–680. doi: 10.1002/ps.1547. [DOI] [PubMed] [Google Scholar]
- 42.Fontoura N. G., Bellinato D. F., Valle D., Lima J. B. P. The efficacy of a chitin synthesis inhibitor against field populations of organophosphate-resistant Aedes aegypti in Brazil. Memórias do Instituto Oswaldo Cruz. 2012;107(3):387–395. doi: 10.1590/s0074-02762012000300014. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization (WHO) Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces. Geneva, Switzerland: World Health Organization; 1998. (WHO/CDS/CPC/MAL/98.12). [Google Scholar]
- 44.Brito L. P., Linss J. G. B., Lima-Camara T. N., et al. Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0060878.e60878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valle D., Montella I. R., Ribeiro R. A., Viana-Medeiros P. F., Martins A. J., Jr., Lima J. B. P. Quantification Methodology for Enzyme Activity Related to Insecticide Resistance in Aedes aegypti. Rio de Janeiro and Distrito Federal, Brazil: Fundação Oswaldo Cruz and Secretaria de Vigilância em Saúde, Ministério da Saúde; 2006. [Google Scholar]
- 46.Viana-Medeiros P. F. Investigações sobre os mecanismos de resistência em larvas e adultos de Aedes aegypti, Linnaeus, 1762 [Dissertação de Mestrado em Biologia Parasitária] Rio de Janeiro, Brazil: Instituto Oswaldo Cruz (IOC/FIOCRUZ); 2011. [Google Scholar]
- 47.Hemingway J. Techniques to Detect Insecticide Resistance Mechanisms (Field and Laboratory Manual) Geneva, Switzerland: World Health Organization; 1998. (WHO/CDC/CPC/MAL.98.6). [Google Scholar]
- 48.Raymond M. Presentation d'une programme d'analyse logprobit pour microordinateur. Cahiers ORSTOM: Série Entomologie Médicale et Parasitologie. 1985;22:117–121. [Google Scholar]
- 49.Brasil 2006. Reunião Técnica para Discutir Status de Resistência de Aedes aegypti. Rio de Janeiro, Brazil: Ministério da Saúde, Secretaria de Vigilância em Saúde; [Google Scholar]
- 50.Teixeira M. G., Costa M. C. N., Barreto M. L., Barreto F. R. Epidemiologia da dengue. In: Valle D., Pimenta D. N., Cunha R. V., editors. Dengue: Teorias e Práticas. Rio de Janeiro, Brazil: Editora Fiocruz; 2015. pp. 293–315. [Google Scholar]
- 51.Macoris M. L. G., Andrighetti M. T. M., Wanderley D. M. V., Ribolla P. E. M. Impact of insecticide resistance on the field control of Aedes aegypti in the State of São Paulo. Revista da Sociedade Brasileira de Medicina Tropical. 2014;47(5):573–578. doi: 10.1590/0037-8682-0141-2014. [DOI] [PubMed] [Google Scholar]
- 52.Schatzmayr H. G., Nogueira R. M., Travassos da Rosa A. P. An outbreak of dengue virus at Rio de Janeiro—1986. Memórias do Instituto Oswaldo Cruz. 1986;81(2):245–246. doi: 10.1590/s0074-02761986000200019. [DOI] [PubMed] [Google Scholar]
- 53.Braga I. A., Valle D. Aedes aegypti: histórico do controle no Brasil. Epidemiologia e Serviços de Saúde. 2007;16(2):113–118. [Google Scholar]
- 54.Brasil 2009. Nota Técnica. 146/2009 CGPNCD/DIGES/SVS/MS. Brasília, Brazil: Ministério da Saúde, Secretaria de Vigilância em Saúde; Informa mudança de uso de inseticidas larvicidas e adulticidas na rotina do Programa Nacional de Controle da Dengue. [Google Scholar]
- 55.Marcombe S., Poupardin R., Darriet F., et al. Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies) BMC Genomics. 2009;10, article 494 doi: 10.1186/1471-2164-10-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saavedra-Rodriguez K., Strode C., Flores A. E., et al. Differential transcription profiles in Aedes aegypti detoxification genes after temephos selection. Insect Molecular Biology. 2014;23(2):199–215. doi: 10.1111/imb.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martins A. J., Ribeiro C. D. M., Bellinato D. F., Peixoto A. A., Valle D., Lima J. B. P. Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0031889.e31889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marcombe S., Darriet F., Agnew P., et al. Field efficacy of new larvicide products for control of multi-resistant Aedes aegypti populations in Martinique (French West Indies) American Journal of Tropical Medicine and Hygiene. 2011;84(1):118–126. doi: 10.4269/ajtmh.2011.10-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lau K. W., Chen C. D., Lee H. L., Norma-Rashid Y., Sofian-Azirun M. Evaluation of insect growth regulators against field-collected Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Malaysia. Journal of Medical Entomology. 2015;52(2):199–206. doi: 10.1093/jme/tju019. [DOI] [PubMed] [Google Scholar]
- 60.Rocha H. D. R., Paiva M. H. S., Silva N. M., et al. Susceptibility profile of Aedes aegypti from Santiago Island, Cabo Verde, to insecticides. Acta Tropica. 2015;152:66–73. doi: 10.1016/j.actatropica.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Brasil 2012. II Seminário Internacional para Avaliações de Ações de Controle Químico de Aedes aegypti no Brasil. Rio de Janeiro, Brazil: Programa Nacional de Controle da Dengue, Secretaria de Vigilância em Saúde, Ministério da Saúde; [Google Scholar]
- 62.Brasil 2014. Larvicidas. Brasília, Brazil: Ministério da Saúde, Secretaria de Vigilância em Saúde; http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/leia-mais-o-ministerio/632-secretaria-svs/vigilancia-de-a-a-z/controle-de-vetores-inseticidas-e-larvicidas/11387-larvicidas. [Google Scholar]
- 63.Diniz F. A. D., Melo-Santos M. A. V., Santos E. M. M., et al. Fitness cost in field and laboratory Aedes aegypti populations associated with resistance to the insecticide temephos. Parasites & Vectors. 2015;8, article 662 doi: 10.1186/s13071-015-1276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiocruz. Nota Técnica. 2/2011/IOC-Fiocruz/Diretoria. Rio de Janeiro, Brazil: Instituto Oswaldo Cruz; 2011. Recomendação técnica sobre a interrupção do uso de inseticidas piretroides no controle do Aedes aegypti no Brasil. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 corresponds to data used to construct Figure 3. It presents the incidence of dengue cases, between 2008 and 2012, in the evaluated municipalities. Tables S2 and S3 contain additional data obtained for respectively larvae and adults (with bioassays and molecular assays): Table S2 complements Table 1 (temephos and diflubenzuron results) and Table S3 complements Table 2 (deltamethrin bioassays and pyrethroid target site data). Regarding bioassays, effective doses and confidentiality intervals are shown. For molecular assays, the frequencies of individual substitutions, at 1016 and 1534 positions, are depicted. Tables S4 and S5 give details of the biochemical assays, such as number of individual specimens tested in each case, the median values of all enzyme activities for the evaluated populations and the Rockefeller 99 percentile used to classify activities. Tables S4 and S5 also repeat the rate of specimens with activity higher than the Rockefeller 99 percentile, the parameter used to classify the populations (that is shown here in colors).