Abstract
ICOS, a member of the CD28 family, represents a key molecule that regulates adaptive responses to foreign Ags. ICOS is prominently expressed on T follicular helper (TFH) cells, a specialized CD4+ T cell subset that orchestrates B cell differentiation within the germinal centers and humoral response. However, the contribution of ICOS and TFH cells to autoantibody profiles under pathological conditions has not been thoroughly investigated. We used the Sle1 lupus-prone mouse model to examine the role of ICOS in the expansion and function of pathogenic TFH cells. Genetic deletion of ICOS impacted the expansion of TFH cells in B6.Sle1 mice and inhibited the differentiation of B lymphocytes into plasma cells. The phenotypic changes observed in B6.Sle1-ICOS–knockout mice were also associated with a significant reduction in class-switched IgG, and anti-nucleosomal IgG-secreting B cells compared with B6.Sle1 animals. The level of vascular cell adhesion protein 1, a molecule that was shown to be elevated in patients with SLE and in lupus models, was also increased in an ICOS-dependent manner in Sle1 mice and correlated with autoantibody levels. The elimination of ICOS-expressing CD4+ T cells in B6.Sle1 mice, using a glyco-engineered anti-ICOS–depleting Ab, resulted in a significant reduction in anti-nucleosomal autoantibodies. Our results indicate that ICOS regulates the ontogeny and homeostasis of B6.Sle1 TFH cells and influences the function of TFH cells during aberrant germinal center B cell responses. Therapies targeting the ICOS signaling pathway may offer new opportunities for the treatment of lupus and other autoimmune diseases.
Introduction
The pathological hallmark of systemic lupus erythematosus (SLE) is the production of Abs against nuclear self-antigens. Increased expression of ICOS on T follicular helper (TFH) cells was observed in several autoimmune conditions, including rheumatoid arthritis (1, 2) and SLE (3–6). This expression correlates with increased cytokine and IgG autoantibody production (7, 8). ICOS is a costimulatory molecule expressed on activated CD4+ T cells that binds to its ligand partner ICOS ligand (ICOSL; also called B7RP-1) expressed on B cells and dendritic cells (9–14). Expression of ICOS is induced following the activation of naive Th cells by APCs (e.g., dendritic cells) and is essential for T cell activation that supports germinal center (GC) reactions and the differentiation of Ab-secreting plasma cells (PCs) (3, 15–21). ICOS expression is found on activated Th1 and Th2 cells, with the greatest levels observed on TFH cells located within the GCs of secondary lymphoid tissues. TFH cells can be distinguished from Th1 and Th2 cells by the concomitant expression of the chemokine receptor CXCR5, programmed death-1 (PD-1), and the master repressor transcription factor B cell lymphoma 6 (Bcl6), which is regulated by ICOS signaling (22–24). Following activation, Bcl6 induces the production of several cytokines, including IL-6 and IL-21. These cytokines are required for the differentiation of B cells into Ab-producing cells (25–27). TFH cells are critical for the formation and maintenance of GCs in secondary lymphoid tissues in which they provide help for the generation of high-affinity memory B cells and long-lived PCs. Altering the regulation of ICOS expression on CD4+ T cells, as exemplified by Sanroque mice, results in the aberrant formation and accumulation of ICOS-bearing TFH cells that are responsible for a profound increase in the number of GC B cells and Ab-producing PCs in secondary lymphoid tissues (28). Sanroque mice develop an autoimmune lupus-like phenotype that resembles the clinical condition of patients with lupus. The phenotype in Sanroque mice is dependent on a mutation in the RNA-binding protein Roquin. It was later shown that Roquin, through interaction with miR-146a, regulates the expression of ICOS and the expansion of TFH cells (29, 30). The increase in the number of TFH cells correlates with high levels of autoantibody and disease activity in Sanroque animals.
Mice containing the Sle1 locus also were reported to express increased levels of ICOS, which may contribute to abnormalities in TFH cell homeostasis and the selective breakdown in tolerance against endogenous nucleosomes (31).
In contrast, lack of ICOS affects the formation of memory T cells and dampens humoral immunity in mice (32, 33). ICOS deficiency correlates with a severe reduction in circulating CXCR5+ Th cells and is associated with common variable immunodeficiency in humans (17, 34).
Recent research suggested that the induction of ICOS on CD4+ T cells does not simply represent a general marker of activation but is rather a critical biological response to dangerous Ag(s) (35). ICOS is predicted to be a key regulator of immune responses to foreign Ags and plays a pivotal role in establishing tolerance or triggering autoimmunity.
In this study, we evaluated the contribution of ICOS to the ontogeny and accumulation of TFH cells and the role of ICOS in the loss of tolerance to self-antigens that characterize Sle1 (B6.Sle1) mice. Our data indicate that the elevated expression of ICOS in B6.Sle1 mice contributed to the expansion of TFH cells and the break of peripheral tolerance. This mechanism drives the expansion of GC B cells and the formation of several pathogenic autoantibody specificities. We demonstrate that Ab-mediated depletion of CD4+ ICOS+ T cells inhibits the anti-nucleosomal response, suggesting that ICOS is a critical regulator of autoimmunity in B6.Sle1 mice.
Materials and Methods
Mice
C57BL/6 (B6) and C57BL/6J-Tcrαtm1 MOM mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.ICOS-knockout (KO) mice were obtained from Japan Tobacco (Osaka, Japan) and maintained at The Jackson Laboratory. Sle1-ICOS–KO mice were generated at The Jackson Laboratory and were backcrossed for more than six generations. All animal experiments were approved by the MedImmune Institutional Animal Use and Care Committee.
Genotype of B6.Sle1-ICOS–KO mice
B6.Sle1-ICOS–KO mice were generated at The Jackson Laboratory after multiple crosses. B6.Sle1 mice were crossed with B6.ICOS−/− mice to generate B6.Sle1+/− × ICOS+/− mice. B6.Sle1+/− × ICOS+/− mice were then crossed with B6.Sle1 mice to produce B6.Sle1 × ICOS+/− mice. Finally, B6.Sle1 × ICOS+/− mice were crossed to generate the B6.Sle1-ICOS–KO mice. To ensure the correct phenotype, genotype analysis was performed on tail snips using the REDExtract-N-Amp Tissue PCR Kit (Sigma). Primers were purchased from Eurofins Operon (Huntsville, AL). The primer sequences were wild-type (wt) forward primer: 5′-AACACACTTGCTTTGTTCTCTTTT-3′, mutant forward primer: 5′-CGTTGGCTACCCGTGATATT-3′, and common reverse primer: 5′-CTTGAAAAGGAGGTGGGTCA-3′. The wt forward primer and the common reverse primer pair amplify a 336-bp region. The mutant forward primer and the common reverse primer pair amplify a 236-bp region. Further verification of the Sle1 genotype was performed on selected mice containing the ICOS−/− genotype and one band at 236 bp. Three primer sets corresponding to different loci in chromosome 1 were used to differentiate B6 from B6.Sle1. The primer pairs were D1MIT15a: 5′-TCCACAGAACTGTCCCTCAA-3′ and D1MIT15b: 5′-ATACACTCACACCACCCCGT-3′; D1MIT47a: 5′-CTGACCTCCACACGAACCC-3′ and D1MIT47b: 5′-GCTTGGGAAACTGGATGAAA-3′; and D1MIT17a: 5′-GTGTCTGCCTTTGCACCTTT-3′ and D1MIT17b: 5′-CTGCTGTCTTTCCATCCACA-3′. PCR products were electrophoresed on 2% E-gels (Life Technologies).
T cell adoptive transfers
Splenocytes were pooled from 7-mo-old B6 mice, B6.Sle1 mice, and B6.Sle1-ICOS–KO mice. T cells from each strain were enriched from splenocytes using Miltenyi Biotec CD19 bead purification, according to the manufacturer’s instructions. Phenotypic analysis of the T cells to evaluate purity indicated that ∼95% of the cells were CD3+. Less than 0.5% of cells were CD19+. Ten million donor T cells from each strain in a volume of 200 μl were administered by tail vein injection to 3-mo-old sex-matched TCRαβ−/− mice. Blood was collected from mice once per month (2–5 mo posttransfer) to measure histone-DNA autoantibody levels in the serum. Serum and spleens were harvested 6 mo posttransfer. Flow cytometric analysis and ELISPOT assays were performed on splenocytes, and histone-DNA autoantibody levels were measured from the serum.
Abs
Anti-mouse ICOS-depleting Ab (mAb) was generated by linking the V-region sequences of rat IgG2a (JmAb-51) with a sequence of human IgG1. A vector containing the complete sequence of J51-hG1 was transfected into CHO cells deficient in the gene encoding for fucosyl transferase 8 (FUT8) (Potelligent Cell Line technology). FUT8 is the enzyme responsible for adding fucose to sugar chains. The resulting fucose-free J51-hG1 (ICOS-depleting) Ab was purified from supernatants.
A-fucosylated R347 IgG1 served as a negative isotype-matched control (36, 37). All Abs used for the in vivo study were tested at <0.05 EU/mg.
In vivo depletion
Four-month-old B6.Sle1 mice were injected i.p. with 10 mg/kg ICOS-depleting Ab, control Ab (A-fucosylated R347 IgG1), or PBS twice a week for 12 consecutive weeks. Serum was collected monthly for 3 mo. Serum levels of histone-DNA autoantibodies were measured by ELISA. A cohort of ICOS and isotype-control mAb-treated B6.Sle1 mice were sacrificed at day 8, and FACS analysis of B and T cells was performed on the splenocytes.
Flow cytometry and intracellular staining
Single-cell suspensions from spleens were isolated by gentle disruption using Miltenyi Biotec’s GentleMACS Dissociator and RBC lysis with an ammonium chloride solution (STEMCELL Technologies). Single-cell suspensions were prepared from thymus by mashing through a filter and from bone marrow by flushing the femur with a syringe attached to a 21-gauge needle. The cell suspensions were used for flow cytometric analysis. Splenocytes were plated and stained with Fcγ III/II block, followed by incubation with anti-CXCR5–biotin Ab. They were then treated with fluorescently labeled Abs targeting CD3, CD4, CD8, CD44, CD62L, PD-1, ICOS (eBio C398.4A), and streptavidin. The B cell preparation included incubation with Fcγ III/II block, followed by Abs against CD3, CD19, GL-7, CD95, CD138, peanut agglutinin (PNA), FAS, and IgD. Cells were incubated for 20 min on ice, washed in FACS buffer, and acquired using an LSR II (BD Biosciences). Cells were stained for surface markers using the T cell–staining preparation and then fixed and permeabilized using Cytofix/Cytoperm buffer (BD Biosciences) for intracellular detection of Bcl6 and Foxp3. Abs against Bcl6 and Foxp3 were added, and the cells were stained on ice for 30 min. Cells were washed in FACS buffer and acquired using an LSR II (BD Biosciences). To stain for expression of ICOSL, splenocytes were incubated with an Ab preparation containing CD3, SiglecH, CD11c, ICOSL, and B220 after a preincubation step with Fcγ III/II block. The T cell–staining panel was used, including Tcrβ to verify the transfer of T cells into Tcrα−/− mice. Data were analyzed using FlowJo software (TreeStar).
Immunohistochemistry
The immunohistochemistry staining procedures for PNA/CD3 dual stain were performed using a modified avidin–biotin complex (ABC) method and a DISCOVERY ULTRA (Ventana Medical Systems, Tucson, AZ). Formalin-fixed, paraffin-embedded sections of spleen were sliced at 5 μm on a rotary microtome. Sections were deparaffinized by immersing for 4 min in solutions of xylene (three times), 100% ethanol (ETOH) (three times), 95% ETOH, and 75% ETOH. Samples were then rinsed in distilled water. Heat-mediated Ag retrieval was performed with Dako citrate buffer (pH 6) in a decloaker at 120°C for 4 min. Slides were stained on a Dako Autostainer Plus with a Vector Avidin/Biotin Blocking Kit (catalog no. SP-2002; Vector Laboratories), followed by Vector Carbo free blocking solution. The primary Ab, PNA (catalog no. B-1075; Vector Laboratories), was applied at 2 μg/ml at room temperature for 30 min, followed by ABC solution (VECTASTAIN Elite ABC Kit, catalog no. PK-6100; Vector Laboratories) and 3,3′-diaminobenzidine (3,3′-diaminobenzidine kit, catalog no. D-6815; Sigma), 30 μl chromogen/1 ml buffer. Slides were transferred to a DISCOVERY ULTRA (Ventana Medical Systems). Anti-CD3 (2GV6; catalog no. 790-4341; Ready to Use) was applied at 37°C for 12 min, followed by UltraMap anti-Rb Alk Phos multimer (catalog no. 760-4314; both from Ventana Medical Systems) for 16 min. Dako Ab diluent (catalog no. S0809) was used as the negative primary Ab control. Slides were counterstained with Hematoxylin II (catalog no. 790-2208; Roche), rinsed, and dehydrated, and a coverslip was applied.
In vitro Ig and autoantibody assays
ELISPOT.
Polyvinylidene difluoride plates (catalog no. MAIP SWU; Millipore) were pretreated with 50 μl 70% ETOH to prewet the membrane. For Ig assays, the filter plates were precoated with 15 μg/ml capture Ab against mouse anti-mouse IgG, IgG1, IgG2b, IgG2c, or IgG3 (Southern Biotech) overnight at 4°C. For the dsDNA IgG autoantibody assay, calf thymus DNA was diluted to 50 μg/ml and coated on plates at 4°C overnight. Splenocytes from 5- and 7-mo-old B6, B6.Sle1, and B6.Sle1-ICOS–KO mice were serially diluted in culture media (RPMI 1640 containing 10% FBS and 5% penicillin-streptomycin) and transferred to coated plates that were blocked with cell culture media. After incubation at 37°C for 48 h, the level of Ab-secreting cells (ASCs) was detected with the sequential addition of biotinylated anti-mouse IgG (Southern Biotech) and Streptavidin-ALP (Mabtech). A colorimetric detection with BCIP/NBT substrate (Mabtech) was then performed. The number of ASCs was counted using a Biosys plate reader.
ELISA.
Autoantibody, immune complexes, and β-2 microglobulin (B2M) ELISAs were performed as follows. For the autoantibody ELISA, Nunc Maxisorp plates were coated with 50 μg/ml dsDNA for 3 h at 37°C and postcoated with 10 μg/ml total histone, H1, H3 (Roche), or human recombinant histone, H2a/H2b (Millipore), overnight at 4°C. For the immune complexes and B2M ELISAs, plates were coated with 10 μg/ml recombinant mouse C1q-related factor (Cusabio) or mouse B2M (Sino Bio) overnight at 4°C. After blocking with PBS containing 3% BSA, sera from 7-mo-old B6, B6.Sle1, and B6.Sle1-ICOS–KO mice were serially diluted 100- and 1000-fold and added to coated plates. Serially diluted sera, pooled from 10-mo-old B6.NZB/NZW mice, were used as standards. After a 2-h incubation at room temperature, goat anti-mouse IgG HRP (Invitrogen) was added to the plates, followed by detection with a colorimetric substrate (TMB Stop solution; KPL). The absorbance was read on a plate reader at 450 nm, and the data were analyzed using Softmax Pro software.
Analyte detection
Autoantigen microarray was performed by UT Southwestern Medical Center on serum samples from B6, B6.Sle1, and B6.Sle1-ICOS–KO mice. Serum samples were also sent to Myriad RBM for a multianalyte analysis using Rodent MAP.
Statistical analysis
ANOVA/Tukey tests were used to analyze grouped data. In the event that assumptions were violated, the Kruskal–Wallis/Dunn tests were used to analyze the data. A multiplicity adjusted p value is reported for each pairwise comparison. Where indicated, a one-tailed t test was used to analyze the data. Prism 6.03 for Windows was used for data analysis. A p value <0.05 was considered significant.
Results
ICOS affects the T cell phenotype in B6.Sle1 mice
To examine whether the B6.Sle1 locus affected intrathymic T cell development, thymocytes collected from 6–8-wk-old B6.Sle1 mice, B6.Sle1 crossed with B6.ICOS–KO (B6.Sle1-ICOS–KO) mice, and B6 mice were subjected to flow cytometric analysis. Single-positive CD4+ and double-negative CD4− CD8− T cell profiles in the thymus of B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice were similar (data not shown). However, the percentage and number of CD8+ thymic T cells in B6.Sle1 mice were increased compared with B6 mice (Supplemental Table I). These results suggest that the mechanisms that govern CD8+ T cell central tolerance in B6.Sle1 could be impaired.
As previously described (31), splenic B6.Sle1 CD4+ T cells had significantly higher percentages of ICOS compared with T cells from B6 animals (Fig. 1A, Supplemental Fig. 1). We confirmed the lack of ICOS expression on CD4+ T cells from the spleen of B6.Sle1-ICOS–KO animals (Fig. 1A). The increased percentages of ICOS in B6.Sle1 mice had no effect on the percentages of ICOSL on APCs, including B cells and plasmacytoid dendritic cells, compared with B6 mice (Fig. 1E, 1F). This result suggests that expression of ICOSL may not represent a suitable marker for T cell hyperactivation (6). B6.Sle1-ICOS–KO mice exhibited high levels of ICOSL on APCs relative to B6 mice (Fig. 1E, 1F).
FIGURE 1.
The lack of ICOS expression in B6.Sle1 mice significantly alters the number of T cell subsets. Spleens were harvested from 7-mo-old B6.Sle1 (Sle1), B6.Sle1-ICOS–KO (Sle1ICOS), and C57BL/6 (B6) mice and analyzed by flow cytometry. Immunophenotyping was performed on B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice at 7 mo of age to determine the effect of ICOS expression on CD4 T cells. Total percentages (A) and numbers (B) of CD4+ICOS+ T cells from each strain of mice. Each point represents one mouse. (C) Profiles from CD4 naive and memory splenic T cells from representative mice. (D) Total numbers of CD4 memory and naive T cells from B6 (n = 11), B6.Sle1 (n = 13), and B6.Sle1-ICOS–KO (n = 12) mice were enumerated by flow cytometry analysis and plotted. Error bars are the SD of the mean. Percentages of ICOSL-expressing B220+ cells (E) and plasmacytoid dendritic cells (B220+ CD11c+ SiglecH+) (F). Total numbers of CD8+ICOS+ T cells (G) and memory CD8+ICOS+ T cells (H) from each strain of mice. Each point represents one mouse. *p < 0.05, ***p < 0.001, ****p ≤ 0.0001.
Augmented ICOS expression on CD4+ T cells in B6.Sle1 mice resulted in a >3-fold expansion in the total number of CD4+ICOS+ T cells compared with B6 animals (Fig. 1B) and correlated with a significant expansion of CD4+ CD44+ CD62L+/− total memory T cells (Fig. 1C, 1D). The loss of ICOS expression in B6.Sle1 mice was sufficient to re-establish physiological numbers of naive and memory CD4+ T cells comparable to B6 mice (Fig. 1C, 1D).
We evaluated the expression of cytokines from total splenocytes cultured in the absence or presence of mitogenic anti-CD3 Ab to determine the effect of Sle1 loci on T cells. ELISPOT analysis demonstrated that levels of IFN-γ, IL-4, and IL-6 were comparable among B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice (data not shown). Similar results were also obtained using purified CD4+ T cells (data not shown).
In addition to CD4+ T cells, we determined that expression levels of ICOS were increased significantly in total CD8+ T cells isolated from the spleens of B6.Sle1 mice compared with B6 mice (Fig. 1G). Within the CD8 T cell pool, ICOS was predominantly upregulated on memory CD8+ T cells from B6.Sle1 mice relative to B6 animals (Fig. 1H). The percentages of total CD8+ T cells in B6.Sle1-ICOS–KO mice returned to wt baseline levels (data not shown), suggesting that a potential ICOS-dependent mechanism is involved in the expansion of CD8+ T cells in the B6.Sle1 lupus model.
ICOS is required for the expansion of TFH cells in B6.Sle1 mice
Because CD4+ memory T cells are decreased in B6.Sle1 mice devoid of ICOS expression compared with B6.Sle1 mice, the frequency of TFH cells was examined. Flow cytometry analysis demonstrated that the percentage of splenic TFH cells, as assessed by the cell surface expression of CXCR5 and PD-1 (gated on CD3+, CD4+, CD44+, and CD62L−), was reduced significantly by 28 wk of age in B6.Sle1 mice lacking ICOS relative to B6.Sle1 mice (Fig. 2A). Longitudinal analysis indicated that there were minimal differences in the number of TFH cells at 5 wk of age among the strains. At 12 wk, the frequency and number of TFH cells were increased in B6.Sle1 mice compared with B6.Sle1-ICOS–KO mice (Fig. 2A, 2B). The loss of ICOS in B6.Sle1 mice results in TFH cell numbers similar to those found in nonautoimmune B6 mice at all ages (Fig. 2A, 2B). Assessment of the frequency and total number of TFH cells, as defined by concomitant expression of PD-1 and Bcl6 (gated on TCRαβ+, CD4+, CD44+, CD62L−, CXCR5+, and Foxp3−), confirmed that TFH cells from B6.Sle1-ICOS–KO mice were decreased relative to those from B6.Sle1 mice (Fig. 2C, 2D). However, the levels were higher relative to age-matched B6.ICOS-KO mice (data not shown). These data suggest that, in the context of the Sle1 locus, ICOS contributes to the ontogeny and expansion of TFH cells.
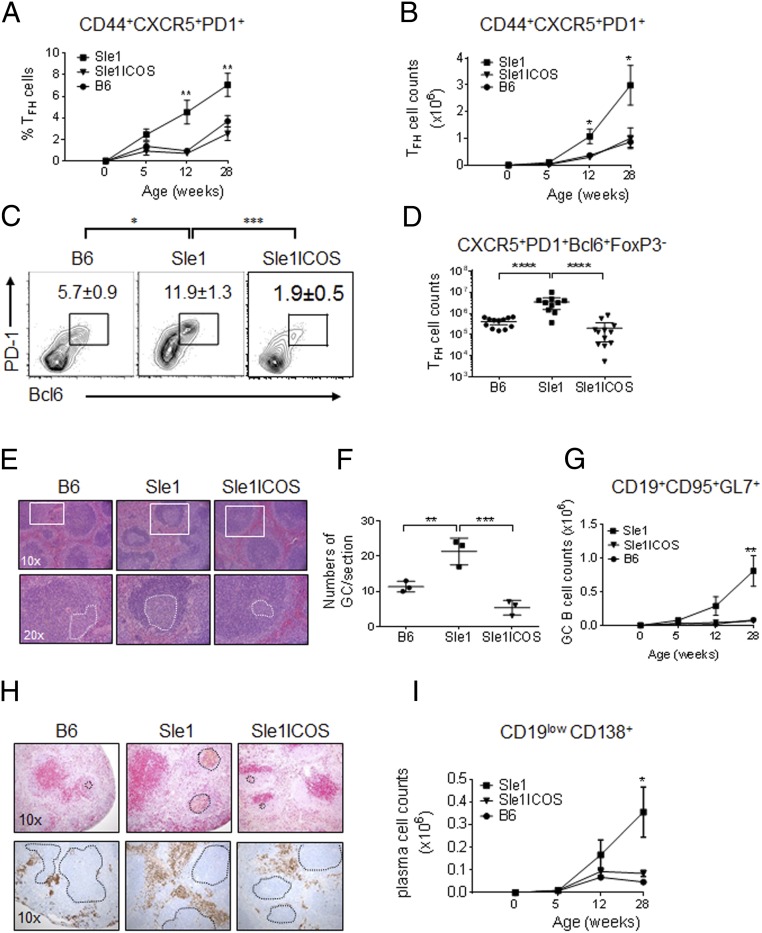
FIGURE 2.
Decreased number of TFH cells in B6.Sle1-ICOS–KO mice affects the numbers of GC B cells and PCs. Flow cytometry analysis was performed on total splenocytes collected from age-matched B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice. The kinetics of splenic CXCR5+ PD-1+ TFH cells (gated on CD4+ CD44high CD62L+/−) are depicted for 5-wk-old through 7-mo-old mice as percentages (A) and total cell numbers (B). (C) Average percentage of splenic CXCR5+ PD-1+ Bcl6+ TFH cells (gated on CD4+ CD44high CD62L+/− and Foxp3−) from the indicated mice at 7 mo of age. (D) Total splenic TFH cells. (E) The size of splenic GCs in 7-mo-old mice was determined by staining formalin-fixed spleens with H&E. Insets define the follicles (top panels) and white dotted circles represent the GCs (bottom panels). (F) The number of GCs was enumerated on a splenic cross-section. (G) The kinetics of GC B cells (CD19+CD95+GL-7+) for B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice are depicted from 5 wk to 7 mo of age. (H) Spleen sections from 7-mo-old mice were stained with anti-CD3 Ab (red) and biotinylated PNA (brown) to highlight the GCs (black dotted circles, upper panels) and with anti-CD138 Ab (brown) to highlight the PCs on the periphery of the follicle (black dotted circles, lower panels). (I) Total PC (CD19lowCD138+) numbers from 5 wk to 7 mo. The symbols on the kinetic graphs represent the combined data from three staggered necropsy dates with n = 3 mice/group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p ≤ 0.0001.
TFH cells are critical for the formation and maintenance of GCs during an immune response and are required to provide cognate help for the differentiation of B lymphocytes into memory B cells and PCs (38). H&E staining of splenic sections from 7-mo-old mice demonstrated smaller GCs in B6.Sle1-ICOS–KO mice compared with B6.Sle1 and B6 mice (Fig. 2E). Enumeration validated that GCs are also less abundant in B6.Sle1-ICOS–KO mice (Fig. 2F). Quantitative flow cytometry analysis confirmed that B6.Sle1-ICOS–KO mice contain significantly fewer GC B cells (gated on CD19+, CD95+, and GL-7+) compared with B6.Sle1 animals (Fig. 2G). Decreased numbers of TFH cells and GC B cells in B6.Sle1-ICOS–KO mice were correlated with decreased splenic CD19low, CD138+ PCs compared with the parental strain. No differences in the number and percentage of PCs were observed in the bone marrow (data not shown). The kinetics of GC B cells and PCs between 5-wk-old mice and 7-mo-old mice demonstrated similar trends as observed for TFH cells (Fig. 2G, 2I). Immunohistochemical analysis confirmed that the GCs, dense brown-stained clusters (black dashed circles) with CD138+ PCs surrounding their borders (white dashed outline), were larger and more abundant in B6.Sle1 mice compared with B6.Sle1 mice lacking ICOS (Fig. 2H). These data suggest that ICOS regulates the homeostasis and function of TFH cells on autoimmune and nonautoimmune backgrounds and is required for TFH cell and B cell interactions within the GCs that drive the expansion of PCs.
B6.Sle1 mice have increased numbers of Foxp3+ regulatory T cells
Previous research indicated that the number of CD4+ Foxp3+ regulatory T cells (Tregs) in mice expressing different B6.Sle1 subloci are either decreased or unaltered compared with the parental B6 animals (31, 39–41). We re-examined the Treg compartment in B6.Sle1 mice to determine the contribution of ICOS to Treg development in B6.Sle1 mice. Assessment of thymic single-positive CD4+ Foxp3+ T cells (Supplemental Table I) and double-positive CD4+CD8+ Foxp3+ T cells (data not shown) did not reveal any abnormality between young B6.Sle1 and B6 mice. Although young splenic B6.Sle1 Tregs were also comparable to B6 wt Tregs, B6.Sle1-ICOS–KO mice had significantly lower frequency and numbers of Tregs (Fig. 3A, 3B). However, thymic CD4+ single-positive (Supplemental Table I) and CD4+CD8+ double-positive (data not shown) Foxp3+ T cells were unaltered among B6.Sle1-ICOS–KO, B6.Sle1, and B6 animals, indicating that an impaired thymic T cell development was not the cause for the reduction of splenic Tregs in young B6.Sle1-ICOS–KO animals.
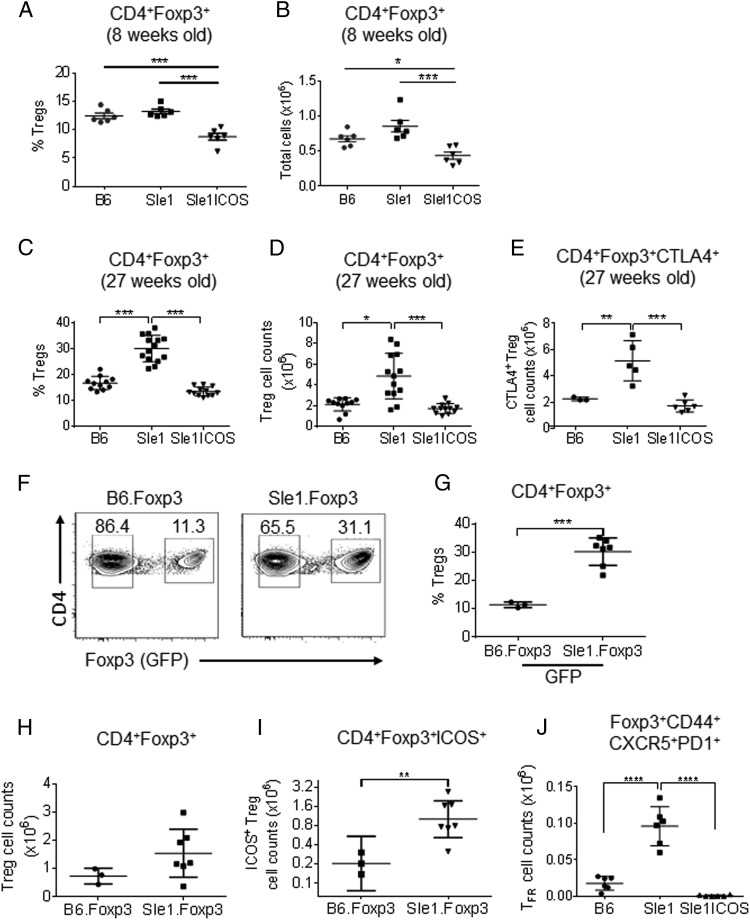
FIGURE 3.
B6.Sle1 mice have increased numbers of splenic Tregs. Total splenocytes were stained with preparations of mAbs, followed by fixation, permeabilization, and Foxp3 and CTLA-4 mAb staining. Immunophenotyping was performed on B6.Sle1, B6.Sle1-ICOS–KO, B6, B6.Foxp3-GFP, and B6.Sle1.Foxp3-GFP mice. Percentages (A and C) and numbers (B and D) of CD4+ Foxp3+ Tregs from 8-wk-old (A and B) and 7-mo-old (C and D) B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice. (E) Numbers of CD4+ Foxp3+ CTLA-4+ cells from 7-mo-old B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice. (F) Representative plots comparing CD4+-gated Foxp3− and Foxp3+ cells from B6.Foxp3-GFP and B6.Sle1.Foxp3-GFP mice (boxed areas) and the percentages of GFP+ Tregs from both strains (G) with the number of Tregs (H) and ICOS+ Tregs (I) from 7-mo-old mice. (J) Number of TFR cells in B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice. *p < 0.05, **p < 0.01, ***p < 0.001, ****p ≤ 0.0001 versus B6.Sle1 mice.
However, the frequency and total number of splenic CD4+ Foxp3+ Tregs were increased significantly in 7-mo-old B6.Sle1 mice compared with wt B6 mice (Fig. 3C, 3D). These results were confirmed using B6.Sle1-Foxp3-GFP mice (Fig. 3F–H). ICOS expression was increased significantly on Tregs from B6.Sle1-Foxp3-GFP mice, which correlated with an expansion of the ICOS+ Treg compartment compared with B6-Foxp3-GFP animals (Fig. 3I). Although reduced in young animals, 7-mo-old B6.Sle1-ICOS–KO mice had Tregs comparable to B6 wt animals.
One of the molecules that plays a role in the development and function of Tregs is CTL-associated Ag 4 (CTLA-4). The expression level and total number of CTLA-4+ Tregs were increased significantly in our B6.Sle1 mice (Fig. 3E, data not shown) compared with B6.Sle1-ICOS–KO and B6 mice. This result suggests that the re-established homeostasis of Th cells observed in B6.Sle1-ICOS–KO mice may also affect the Treg compartment. Finally, we evaluated the levels of T follicular regulatory (TFR) cells, a recently discovered Th cell population that was shown to inhibit B cell responses within the GCs (42–44). We found that the TFR cell compartment was significantly increased in B6.Sle1 mice relative to B6 and B6.Sle1-ICOS–KO mice (Fig. 3J). These results suggest that the number of Tregs and their activation status are increased in Sle1 mice relative to B6 mice and that the Treg compartment in B6.Sle1-ICOS–KO mice was comparable to B6 animals.
Ig and anti-nucleosome Ab levels are severely diminished in B6.Sle1 mice lacking ICOS
We assessed whether the lack of ICOS in B6.Sle1 mice affected the number of IgG-producing ASCs by subjecting total splenocytes to ELISPOT analysis. The IgG ASCs in B6.Sle1 mice were significantly higher compared with B6 control mice, whereas the lack of ICOS in B6.Sle1 mice diminished the IgG ASCs (Fig. 4A). The kinetics demonstrated that, although the number of IgG-producing ASCs increased dramatically in B6.Sle1 mice from 20 to 28 wk of age, little or no increase was observed in B6.Sle1 mice devoid of ICOS (Fig. 4A). B6.Sle1 mice had greater numbers of IgG-secreting cells compared with B6.Sle1-ICOS–KO or B6 mice, with significance observed for IgG1, IgG2c, and IgG2b subtypes (Fig. 4B). The numbers of ASCs for IgM, as well as IgA serum levels, were also reduced significantly in B6.Sle1 mice deficient for ICOS compared with B6.Sle1 mice (Fig. 4E, 4F).
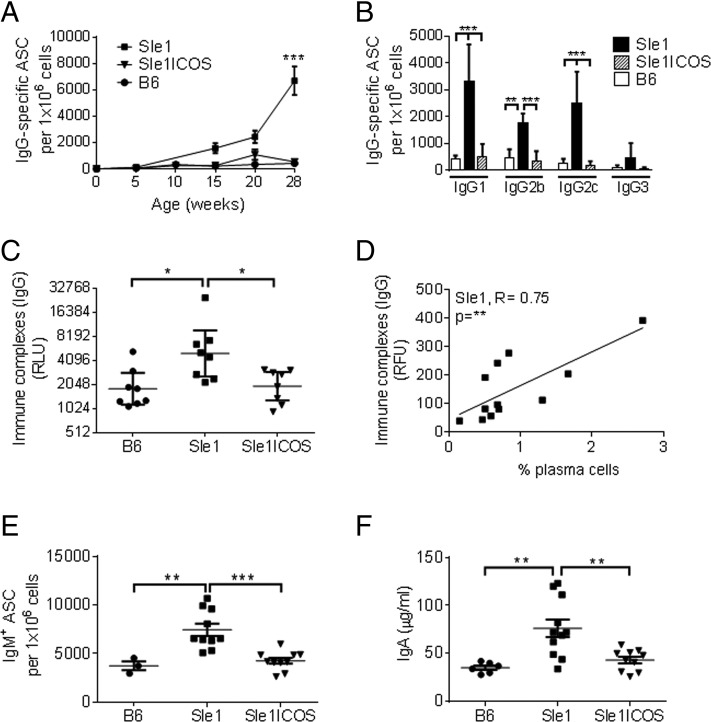
FIGURE 4.
B6.Sle1-ICOS–KO mice exhibit a severely decreased production of Igs. Total splenocytes from 7-mo-old B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice were collected and serially diluted, and the number of IgG-producing cells was determined by ELISPOT. (A) The kinetics of the number of ASCs in mice at 5 wk to 7 mo of age. Symbols at 5 mo of age represent the averaged data from nine mice/group. Symbols at 7 mo of age represent the averaged data from 16 mice/group. (B) The number of ASCs from the splenocytes of 7-mo-old B6.Sle1 mice (black bars), B6.Sle1-ICOS–KO (stippled bars), and B6 mice (white bars) was determined for the IgG subtypes. (C) IgG-specific immune complex levels from the serum of 7-mo-old Sle1, Sle1-ICOS–KO, and B6 mice were analyzed by ELISA. (D) The correlation between sera immune complexes levels, as analyzed by protein Ag arrays, and the percentage of PCs in B6.Sle1 mice was determined (R = +0.75, **p < 0.01). (E) ELISPOT analysis of IgM-secreting splenocytes at 7 mo of age for each group. (F) ELISA-based (Myriad RBM) IgA levels in serum collected from 7-mo-old B6, B6.Sle1, and B6.Sle1-ICOS–KO mice. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA Tukey test versus B6.Sle1 mice.
Levels of immune complexes were elevated significantly in sera collected from B6.Sle1 mice (Fig. 4C) compared with B6.Sle1-ICOS–KO and B6 mice and correlated strongly with increased levels of PCs (Fig. 4D).
We then examined whether the reduction in Ig and immune complex levels in B6.Sle1-ICOS–KO mice corresponded to a reduction in serum levels of anti-nuclear autoantibodies (ANAs). Autoantigen microarray profiling of sera indicated that 7-mo-old B6.Sle1 mice have high titers of IgG autoantibodies against dsDNA, chromatin, and histones (Fig. 5A). B6.Sle1-ICOS–KO mice were protected from ANA-associated signature and also exhibited a significant reduction in the serum level of dsRNA autoantibodies (Fig. 5A).
FIGURE 5.
The lack of ICOS expression in B6.Sle1 mice is associated with decreased levels of autoantibodies. (A) Sera were obtained from 7-mo-old B6.Sle, B6.Sle1-ICOS–KO, and B6 mice and assayed for the production of IgG autoantibodies of different specificities using protein Ag arrays. Values are expressed as normalized signal intensities using standard control Ags. Red represents values > row mean, blue represents values < row mean. (B) Sera from 7-mo-old B6.Sle1 (black bars, n = 24), B6.Sle1-ICOS–KO (stippled bars, n = 24), and B6 (white bars, n = 12) mice were assayed by ELISA for levels of ANAs directed against histone-DNA, H2a/H2b-DNA, H1-DNA, H3-DNA, dsDNA, and histone. Data are mean ANA levels. IgG-specific histone-DNA autoantibody levels from the serum of 7-mo-old mice were compared with the percentage of PCs (C) and CD4+ memory T cells (D) in B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice, with R values displayed for B6.Sle1 animals. *p < 0.05, ***p < 0.001, one-way ANOVA Tukey test.
As reported previously, 7-mo-old B6.Sle1 mice have increased serum levels of anti-nuclear autoantibodies that specially bind to histone-DNA complexes (45–49). The most abundant are anti-H2a/H2b-DNA Abs, with lower levels of anti-dsDNA Abs, anti-histone Abs, and anti-H1 and anti-H3 Abs (Fig. 5B). B6.Sle1 mice lacking ICOS were significantly protected with sera ANAs comparable to the levels measured in B6 mice. Levels of autoantibodies and the percentage of PCs and CD4+ memory T cells were compared. We observed that increased levels of anti–histone-DNA autoantibodies correlated with augmented percentages of PCs (R = +0.78) and CD4+ memory T cells (R = +0.69) in B6.Sle1 mice (Fig. 5C, 5D).
ICOS regulates the production of cellular autoantibodies in B6.Sle1 mice
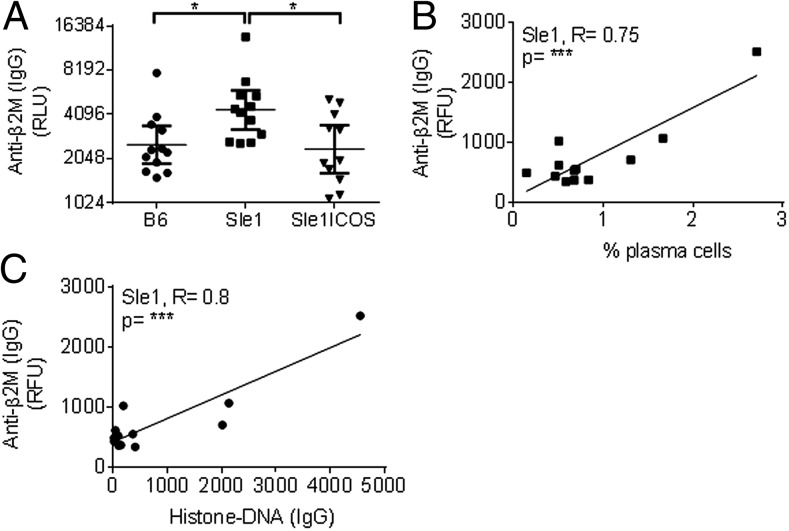
SLE is characterized by the presence of autoantibodies against nuclear proteins. However, there is evidence that pathogenic Abs recognizing nonnuclear Ags may also contribute to the pathogenesis of this disease. Multiplex array data analysis revealed that IgG-specific autoantibodies against B2M were significantly elevated in the sera collected from B6.Sle1 mice relative to B6 mice (Fig. 6A). This elevation correlated strongly with increased levels of splenic PCs and DNA-histone autoantibodies (Fig. 6B, 6C, respectively). B2M is required for the assembly and function of cell surface molecules, such as classical MHC class I protein family and neonatal FcR, as well as for the development of CD8+ T cells (50, 51). B6.Sle1-ICOS–KO mice exhibited significantly lower levels of IgG autoantibodies against B2M compared with B6.Sle1 mice, suggesting that lack of ICOS in B6.Sle1 animals was sufficient to re-establish tolerance against this protein. Our data suggest that ICOS contributes to the production of autoantibodies against B2M, which may play a role in the development of lupus in B6.Sle1 animals.
FIGURE 6.
ICOS contributes to increased levels of nonnuclear B2M autoantibodies in B6.Sle1 mice. (A) DNase-treated sera from 7-mo-old B6.Sle1, B6.Sle1-ICOS–KO, and B6 mice were assayed by ELISA for IgG-specific Abs against B2M. The relative fluorescent units for these graphs were plotted in log2. IgG-specific B2M Ab levels from the serum of 7-mo-old mice measured using protein Ag arrays correlated with (B) the frequency of B6.Sle1 PCs (***R = 0.75) and (C) with IgG-specific histone-DNA autoantibodies (***R = 0.8). *p < 0.05, ***p < 0.001.
B6.Sle1-ICOS–KO mice do not generate DNA-histone–specific T cells that induce anti-nuclear IgG Ab production
We evaluated the contribution of CD4+ T cells isolated from ICOS-sufficient or -deficient B6.Sle1 mice to the induction of total Igs and anti–histone-DNA autoantibodies to examine the contribution of ICOS to the pathogenesis of the B6.Sle1 lupus-prone model. Pools of splenocytes depleted of CD19+ B cells (<0.5%) were purified from B6.Sle1 or B6.Sle1-ICOS–KO mice (CD3 purity > 95%) and transferred into B6.TCRαβ−/− mice. The successful transfer of T cells from B6.Sle1 and B6.Sle1-ICOS–KO mice into B6.TCRαβ−/− mice was confirmed by staining the total splenocytes 6 mo posttransfer for CD4+ TCRβ+ T cells. Significantly higher percentages of CD4+TCRβ+ T cells were observed in B6.Sle1 and B6 animals (data not shown) compared with B6.Sle1-ICOS–KO mice (Fig. 7A, 7B). However, the percentage of CD4+ TCRβ+ T cells was negligible in the no-transfer control group. The percentage of TFH cells was significantly higher at 6 mo posttransfer of B6.Sle1 T cells into B6.TCRαβ−/− mice relative to B6.Sle1-ICOS–KO mice (Fig. 7C). The results correlated with an elevated frequency of bone marrow and splenic PCs (Fig. 7D, 7E, respectively). Quantitative analysis of B6.Sle1-ICOS–KO mice demonstrated a significant reduction in the number of ASCs specific for total IgG and anti–histone-DNA (Fig. 7F, 7G, respectively) in the spleen and bone marrow relative to ICOS-sufficient Sle1 animals. We believe that the small amount of B cells transferred along with T cells (<0.5%) did not account for the difference in IgG-specific histone-DNA ASCs observed between the transfer of B6.Sle1 and B6.Sle1-ICOS–KO T cells into B6.TCRαβ−/− recipient mice. A similar conclusion was also made in a recent study in which the investigators showed that comparable B cell contamination (4–6 × 104 cells) did not contribute to autoantibody production (52). Our results demonstrated that ICOS drives the production of anti-nucleosomal autoantibodies during the cognate interaction between donor B6.Sle1 T cells and B cells in B6.TCRαβ−/− animals. Additional studies are warranted to determine whether B6.Sle1 T cells, in addition to recipient (B6.TCRαβ−/−) B cells, may also contribute to autoantibody production via interaction with the small fraction of contaminant B6.Sle1 B cells (<0.5%).
FIGURE 7.
Transfer of B6.Sle1-ICOS–KO T cells into B6.TCRαβ−/− mice did not mediate production of autoantibodies. Total splenic lymphocytes collected from B6.TCRαβ−/− mice 6 mo after adoptive transfer with T cells from B6.Sle1 and B6.Sle1-ICOS–KO mice were stained with preparations including CD3, CD4, and Tcrβ to verify successful transfer of T cells in TCRαβ−/− mice. (A) Representative FACS plot for each strain. (B) Percentages of Tcrβ+ cells from adoptive transfers of B6.Sle1 and B6.Sle1-ICOS–KO T cells in B6.TCRαβ−/− mice are shown with B6.Sle1 (black bars, n = 6), B6.Sle1-ICOS–KO (gray bars, n = 6), and no transfer (NT) control (white bars, n = 4). Percentages of splenic CD4+ CD44+ TFH cells (C), bone marrow PCs (D), and splenic PCs (E) among the groups with B6.Sle1 (black bars, n = 6), B6.Sle1-ICOS–KO (gray bars, n = 6), and NT control (white bars, n = 4). The number of ASCs from total splenocytes and bone marrow collected from B6.Sle1 and B6.Sle1-ICOS–KO mice at 6 mo posttransfer were enumerated for total IgG (F) and IgG-specific histone-DNA autoantibodies (G). *p < 0.05 **p < 0.01, ***p < 0.001, ****p ≤ 0.0001.
ICOS regulates the level of IFN-dependent chemokines CXCL10 and CCL19 in B6.Sle1 mice
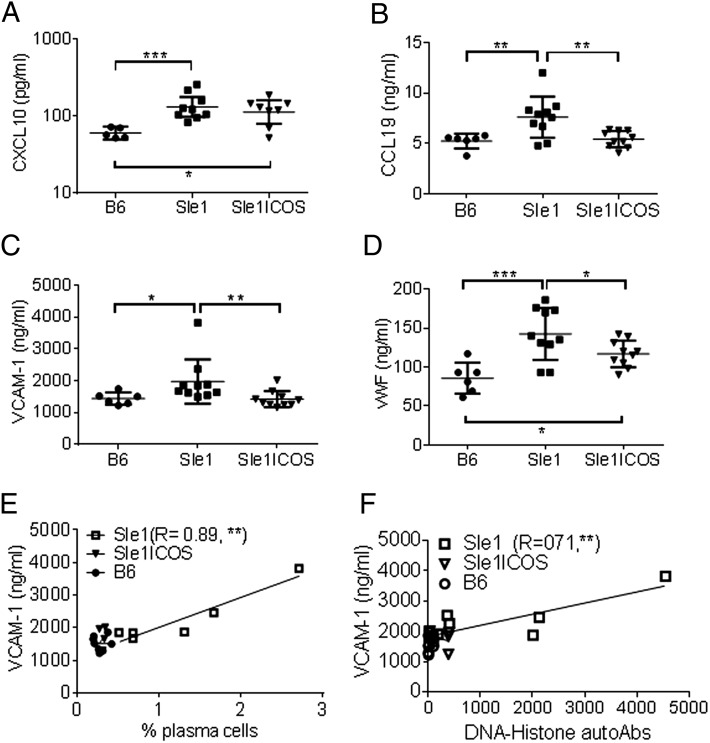
In addition to increased serum levels of ANAs and nonnuclear Abs, we determined which cytokine and chemokine levels correlated with the immunophenotypic features of B6.Sle1 mice. Analysis of >65 biomarkers using multi-analyte profile technology revealed that levels of type I IFN–inducible chemokines CXCL10 (IP-10) and CCL19 (MIP-3β) were increased in the sera of B6.Sle1 mice compared with B6.Sle1-ICOS–KO and B6 mice (Fig. 8A, 8B, respectively).
FIGURE 8.
B6.Sle1-ICOS–KO mice show reduced levels of lupus-associated soluble factors and inflammatory molecules. Sera collected from 7-mo-old mice were assayed using Myriad RBM’s MAP rodent technology platform. Mean analyte levels of CXCL10 (A), CCL19 (B), VCAM-1 (C), and vWF (D). Correlative graphs of VCAM levels versus percentage of PCs (E) and histone-DNA autoantibodies (F) for each strain, with R values for B6.Sle1. Each point represents an individual animal. CXCL10 levels were plotted in log10, and VCAM-1 levels were plotted in log2. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA Tukey test.
Because levels of IFN-γ were similar between B6 and B6.Sle1 mice (data not shown), we examined the expression of type I IFN and its contribution to the phenotype of B6.Sle1 mice. The expression levels of type I IFN–inducible genes (CXCL10, ISG15, IFIT1, RTP4, and BAFF) were investigated using RNA isolated from the peripheral blood of B6.Sle1 mice relative to B6 mice (Supplemental Fig. 2). In general, type I IFN–inducible gene expression in young B6.Sle1 mice (5 wk old) was increased, with IFIT1 and RTP4 showing a statistically significant increase relative to B6 animals. The increased IFN-inducible gene signature in 5-wk-old B6.Sle1 mice preceded any of the autoimmune phenotypic features that characterize this model. By 27 wk, type I IFN–dependent genes CXCL10, ISG15, IFIT1, and RTP4 were increased significantly in B6.Sle1 mice compared with B6 animals and correlated with increased levels of autoantibodies. BAFF (Supplemental Fig. 2), MX1, and IRF7 gene expression (data not shown) increased in B6.Sle1 mice, although the changes were not statistically significant relative to B6 mice. Although lack of ICOS had a significant impact on the expression of ISG15, IFIT1, and RTP4 in young mice (5 wk old), only the level of the ISG15 gene remained significantly inhibited in aged B6.Sle1-ICOS–KO mice (27 wk old) compared with B6.Sle1 or B6 wt mice (Supplemental Fig. 2).
Analysis of the serum collected from 7-mo-old B6.Sle1 mice revealed significantly increased levels of vascular cell adhesion protein 1 (VCAM-1) and von Willebrand factor (vWF) (Fig. 8C, 8D). VCAM-1 is associated with the migration and recruitment of inflammatory cells, and vWF is associated with endothelial cell dysfunction (53–55). ICOS-deficient B6.Sle1 mice had reduced levels of vWF and VCAM-1, similar to wt B6 animals (Fig. 8C, 8D, respectively). Although VCAM-1 is elevated in the endothelium and the kidneys of MRL/lpr mice (56), the total protein urine levels in B6.Sle1 mice were comparable to control B6 mice (data not shown). Histopathology analysis did not reveal any significant inflammation or damage to the glomeruli or in the tubulointerstitium of kidneys (4–7-mo-old B6.Sle1 animals; data not shown). Serum levels of VCAM-1, but not vWF, correlated with percentages of PCs (Fig. 8E) and with anti–DNA-histone autoantibody titers (Fig. 8F) in Sle1 mice.
We conclude that the serum levels of VCAM-1 are a useful activity marker in the B6.Sle1 autoimmune animal model.
Treatment of B6.Sle1 mice with ICOS mAb results in decreased autoantibody levels
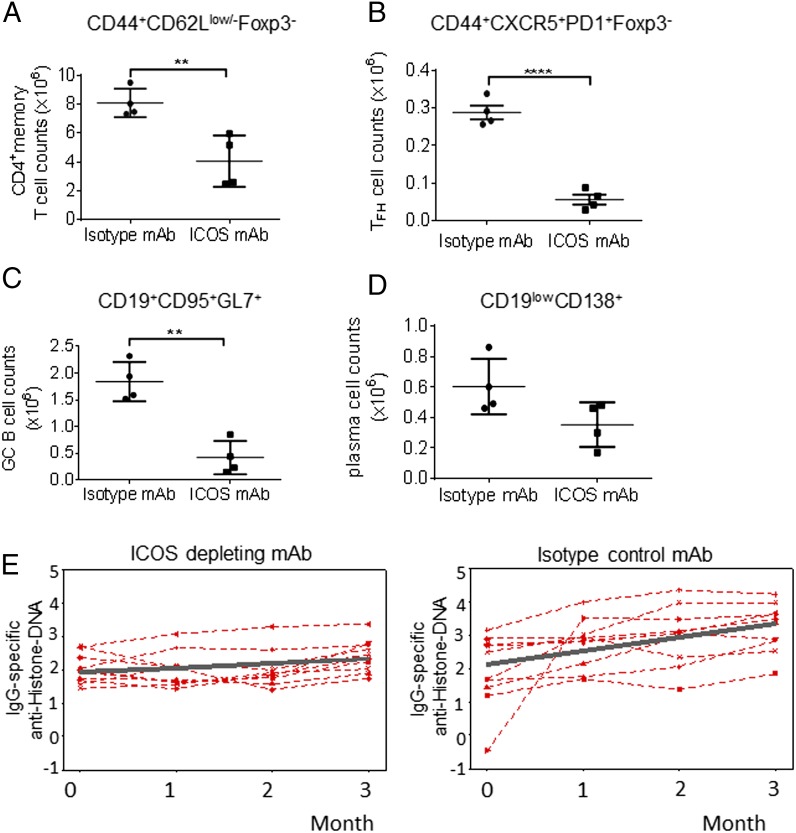
To confirm the contribution of ICOS to the increased levels of anti–histone-DNA autoantibodies, 12-wk-old B6.Sle1 mice were injected with an Ab to deplete ICOS-expressing T cells. Immunophenotype analysis after two doses of Ab (day 8) indicated that the ICOS-depleting mAb treatment significantly decreased the levels of splenic CD4 memory T cells and TFH cells compared with the isotype-control treatment (Fig. 9A, 9B). Splenic naive CD4+ T cells and total CD8+ T cells were not affected by the ICOS-depleting mAb treatment (data not shown). The number of GC B cells was significantly decreased by day 8 in the spleens of B6.Sle1 mice treated with the ICOS-depleting mAb. The PCs were reduced by the ICOS-depleting Ab, but the difference was not statistically significant (Fig. 9C, 9D). The reduction in CD4+ICOS+ T cells following treatment with the ICOS-depleting mAb correlated with significantly decreased serum levels of VCAM-1 and vWF (Supplemental Fig. 3). This result suggests that these molecules are potential predictive and pharmacodynamic biomarkers for therapies targeting the ICOS-ICOSL pathway in the Sle1 lupus model.
FIGURE 9.
Autoantibody levels are reduced by ICOS depletion in B6.Sle1 mice. Four to five–mo-old B6.Sle1 mice were treated with an ICOS-depleting mAb (250 μg/mouse) or an isotype-control Ab (250 μg/mouse) twice a week for 13 wk. At day 8 (after two doses of Ab), mice were sacrificed, and the total splenocytes were analyzed by FACS. Total memory CD4+ Foxp3− T cell (A), TFH cell (CD4+ CD44+ CD62L−/+ CXCR5+ PD-1+ and Foxp3−) (B), GC B cell (CD19+ CD95+ GL-7+) (C), and PC (CD19lowCD138+) (D) numbers/spleen. Each point represents an individual animal. (E) Sera from the isotype control and ICOS-depleting mAb treatment groups were analyzed by ELISA for levels of IgG-specific ANAs directed against histone-DNA at the 3-mo study end point. Each symbol represents the level of ANAs from one mouse/group at different time points. The levels of anti–histone-DNA are plotted in log10. The dark gray line represents the average slope of the lines within the group in which p < 0.05. The symbols on the kinetic graphs represent the combined data from two independent studies with n = 4 mice/group. **p < 0.01, ****p ≤ 0.0001, one-way ANOVA Tukey test.
B6.Sle1 mice treated with the ICOS-depleting mAb exhibited a statistically significant inhibition in the serum anti–histone-DNA autoantibody levels over the course of the 12-wk treatment compared with the isotype-control treatment group (Fig. 9E). These data confirm the critical function of ICOS in the immunopathogenesis of Sle1 mice.
Discussion
The costimulatory receptor ICOS plays an important role in the development and regulation of TFH cells. TFH cells are a specialized T cell population that is critical for humoral immune responses, and it has been implicated in a number of autoantibody-driven autoimmune diseases. We used the Sle1 lupus-prone mouse model to further dissect the functional role of ICOS in TFH cell development and function and its contribution to loss of tolerance and autoimmunity.
We first generated B6.Sle1-ICOS–KO mice to provide a comprehensive analysis of the functions of ICOS in the development of CD4+ T cells, specifically TFH cells. The results demonstrated that ICOS is critical for the B6.Sle1 autoimmune phenotype that is characterized by significant expansion of TFH cells and the generation and differentiation of PC-secreting Abs against several self-antigens.
We hypothesized that this is a primary mechanism for the phenotypic characteristics associated with B6.Sle1 mice because ICOS regulates the survival and function of effector memory CD4+ T cells (32, 57). However, analyses conducted in heterozygous B6.Sle1 mice (B6.Sle1-ICOS+/−), in which ICOS expression is halved, did not reveal any impact on autoantibody profiles (data not shown). This result suggested that a quantitative difference in ICOS expression does not unequivocally correlate with the immunophenotypic abnormalities of B6.Sle1 mice. The complete deletion of ICOS in B6.Sle1 mice normalizes the numbers of naive and memory CD4+ and CD8+ T cells comparable to wt animals. We found no difference in the levels of Th-associated cytokines (IL-4, IFN-γ, and IL-6) between B6.Sle1 and B6.Sle1-ICOS–KO or B6 animals upon ex vivo stimulation. Our data diverged from a recent report in which B6.Sle1 CD4+ T cells produced increased levels of IL-4 and IFN-γ after in vitro stimulation compared with B6 mice (52). Different assay conditions may explain the inability to measure any difference in the levels of these cytokines. It is possible that alternative unidentified molecules also play a key role in the pathogenesis of the B6.Sle1 model.
Our observations for B6.Sle1-ICOS+/− mice differed from a recent report (58) in which the investigators showed that modulation of ICOS expression in Sanroque mice (San/San × ICOS+/−) balanced the ratio of naive/memory T cell populations and affected autoimmunity compared with San/San mice. A defect in the regulation of the transcript levels of ICOS, as a result of a mutation in the RNA-binding protein Roquin, is the main mechanism responsible for the autoimmune features of San/San mice in which T cells are responsible for driving the formation of high levels of GC B cells and ANA-producing cells (28, 29). Pratama et al. (30) shed new light on the role of miR-146a in the downstream regulation of ICOS expression on T cell subsets, including TFH and GC B cells. By treating miR-146a–deficient mice with a low dose of ICOSL Ab or by lowering the expression levels of ICOS on miR146a−/− mice (miR146a−/− × ICOS+/−), the investigators inhibited the expansion of TFH cells.
Our study further expands on these findings, showing that normalization of the frequency and total number of TFH cells, either through genetic deletion of ICOS in B6.Sle1 mice or treatment of B6.Sle1 animals with ICOS-depleting Ab, restores B and T cell tolerance and correlates with a significant decrease in the levels of autoantibodies against dsDNA-histone and class I cellular Ag B2M.
In addition to the increased levels of TFH cells, lupus-prone B6.Sle1 mice displayed more Tregs at the peak of disease (week 27). Based on increased CTLA-4 expression, the status of Treg activation in B6.Sle1 mice was elevated relative to B6 animals. The Treg phenotype found in aged B6.Sle1 mice was presumably a consequence of chronic T cell activation because the frequency and numbers of Tregs in young B6.Sle1 and B6 wt control animals were comparable. Our analysis also indicates that, although the Treg compartment was comparable between aged (27-wk-old) B6.Sle1-ICOS–KO and B6 mice, young B6.Sle1-ICOS–KO mice had significantly lower frequency and total number of splenic Tregs compared with B6 and B6.Sle1 mice. However, the numbers of thymic CD4+ single-positive (Supplemental Table I) and CD4+CD8+ double-positive Foxp3+ T cells (data not shown) were unaltered in young B6.Sle1.ICOS–KO, B6.Sle1, and B6 animals. These results suggest that impaired generation of Tregs in the thymus of B6.Sle1.ICOS–KO mice is not the cause of their reduced frequency in the secondary lymphoid tissue. Although it was suggested that ICOS has a differentiation and regulatory function (59–61), no study has examined the role of ICOS on Tregs in lupus. Further research is necessary to understand the properties of Tregs in the context of the ICOS-sufficient and ICOS-deficient B6.Sle1 lupus-prone models.
We observed a significant correlation between the increased levels of memory CD4+ T cells in B6.Sle1 mice and the elevated levels of anti–histone-DNA ASCs that constitute the hallmark phenotype of this model. The profile of autoantibodies detected in B6.Sle1 animals was not limited to reactivity against nuclear proteins, such as H2a-H2b-DNA histone; it also included B2M. B2M is a small m.w. cellular protein involved in class I Ag presentation. A peptide derived from B2M only activated T cells isolated from young MRL/lpr mice (62), suggesting that this protein may represent a target for autoantibodies. Autoantibodies against B2M were increased in patients with SLE, although their occurrences are not substantially linked to clinical manifestations (63, 64). Our data demonstrated that increased levels of IgG-specific B2M autoantibodies correlated with the elevated frequency of PCs and with increased levels of DNA-histone autoantibodies in B6.Sle1 mice. The result supports the notion that MHC class I+ cells possess a suppressive regulatory function in autoimmunity (65). The lack of ICOS completely restored tolerance against ANAs, as well as to a cellular Ag, B2M. These data reinforce the critical role of ICOS in the autoimmune phenotype of B6.Sle1 animals.
Our study is in line with other studies that dissected the role of ICOS in autoimmunity. For instance, work by Tada et al. (66) was one of the first to demonstrate the contribution of ICOS to the pathogenesis of the MRL/lpr lupus-prone animal model. The lack of ICOS in MRL/lpr animals reduced the frequency of splenic CD4+ T cells and impacted dsDNA autoantibodies. Using B6.Sle1 mice, we now show that the expression of ICOS is critical for the expansion of CD4+ TFH cells that assist in the generation and maintenance of GCs and humoral immunity. TFH cells, which are characterized by the concomitant expression of CXCR5, PD-1, ICOS, and the transcription repressor Bcl6, are critical for the migration of B cells in follicles. TFH cells also provide signals required for the formation of B cell GC responses. However, in contrast to the study by Tada et al. (66), the levels of Th1- and Th2-associated cytokines among B6.Sle1, B6.Sle1-ICOS–KO, and B6 wt control mice were comparable, suggesting that alternative unidentified molecules contribute to the pathogenesis of B6.Sle1 animals. These differences may alternatively rely on the fact that the MRL/lpr cells are primarily involved in extrafollicular autoantibodies responses (67–69), but the B6.Sle1 model reflects abnormal T lymphocyte activation that occurs within the GCs.
Increased numbers of TFH cells were described in different autoimmune disorders, including patients with SLE, and are associated with Ab-mediated end-organ inflammation, including glomerulonephritis (4, 70, 71). Our longitudinal analysis demonstrated that the number of TFH cells expanded during the onset of B6.Sle1 disease in an ICOS-dependent manner. The genetic ablation of ICOS significantly affected TFH cell frequency, similar to that observed in B6 mice, and reversed the autoimmune phenotype of B6.Sle1 animals.
Increased levels of TFH cells correlated with the significant expansion of the size and numbers of GCs, resulting in a significant increase in PCs in B6.Sle1 mice. The expansion occurred primarily in the spleen and was not associated with the accumulation of PCs in the bone marrow of B6.Sle1 mice. We hypothesized that this feature might be regulated by type I IFN. IFN acts subsequent to high-affinity somatic mutations that occur within the GC reactions via the downregulation of chemotactic molecules involved in the migration of newly formed PCs to the bone marrow (72–75). Our study demonstrated that B6.Sle1 lupus-prone animals have an upregulated IFN signature early in life, before expansion of ASCs can be detected. This upregulation modestly correlated with increased levels of autoantibodies, suggesting that an immune complex–mediated mechanism for type I IFN does not play a major role in B6.Sle1 lupus-prone mice. The deletion of ICOS in B6.Sle1 mice did not completely normalize the expression of type I IFN–responsive genes. This result suggests the existence of an alternative ICOS-independent pathway(s) that drives the upregulation of the IFN gene signature. Additional analysis is necessary to understand the contribution of other leukocyte populations to the elevated IFN-dependent genes in B6.Sle1 mice.
We transferred T cells isolated from B6.Sle1 mice into Tcrαβ−/− mice, which are expected to have type I IFN levels comparable to B6 wt animals. These experiments address the roles of TFH cells and type I IFN in the generation and migration of autoreactive ASCs. We confirmed that the intrinsic pathogenic properties of B6.Sle1 TFH cells driving the production of histone-DNA autoantibodies required the expression of ICOS. The mechanism may be related to survival and/or proliferation advantages conferred by ICOS to B6.Sle1 T cells (57). The transfer of autoreactive B6.Sle1 TFH cells mediated the formation of IgG-specific histone-DNA ASCs that accumulated primarily in the bone marrow of TCRαβ−/− animals.
Together, these results indicate that upregulation of type I IFN limits the circulation of autoreactive ASCs in the bone marrow of B6.Sle1 animals. Although homeostatic proliferation contributes to the expansion of T cells in this model, TCRαβ−/− recipient mice only developed IgG-specific anti–histone-DNA autoantibodies following the transfer of ICOS-sufficient B6.Sle1 T cells, further validating the requirement of ICOS to drive autoimmunity. The requirement of B6.Sle1 T cells for the production of anti–histone-DNA autoantibodies by TCRαβ−/− host cells is also in agreement with a previous study by Chen and Morel (40).
To further validate our results using the genetic model (Sle1-ICOS–KO mice), we used a pharmacological approach to decipher the contribution of the ICOS-ICOSL pathway to the autoimmune phenotype of B6.Sle1 animals. We glyco-engineered an ICOS mAb that possesses enhanced Ab-dependent cellular cytotoxicity to deplete ICOS-bearing CD4+ T cells. The Ab-dependent cellular cytotoxicity function is mediated via the increased affinity of the Fc portion of the ICOS mAb for mouse FcγRIV, which is expressed on effector cells. We demonstrated that, following the administration of two doses of the ICOS-depleting mAb, B6.Sle1 mice exhibited a significant reduction in ICOS-expressing cells, predominantly TFH cells. The effect was selective because ICOS-depleting mAb treatment did not alter the number of total CD4+ and CD8+ T cells.
The depletion of TFH cells resulted in a significant reduction in the number of GC B cells and a reduction in PCs (not statistically significant).
At 12 wk after treatment, the serum levels for histone-DNA autoantibodies were reduced significantly in B6.Sle1 mice treated with the ICOS-depleting mAb compared with animals receiving the isotype-control mAb.
These results demonstrate that ICOS governs the activation threshold of CD4+ T cells in B6.Sle1 mice and drives the early loss of tolerance that results in the autoimmune phenotype in the animals.
Our study also provides novel information with regard to other aspects of the disease, particularly in the context of clinical pathologies that characterize lupus, including tissue damage and cardiovascular complications. We showed that depletion of TFH cells with the anti-ICOS mAb also resulted in a significant reduction in serum levels of VCAM-1 and vWF. These two markers correlate with cardiovascular events that represent a major cause of premature mortality among patients with SLE (76). Because of the strong association of ICOS and TFH cells with elevated levels of autoantibody-producing PCs in B6.Sle1 mice, the abundant circulating immune complexes and autoantibodies in B6.Sle mice may contribute to the vascular endothelial insult that causes the release of vWF and the upregulation of adhesion molecules. Over time, this process may facilitate the recruitment of leukocytes. The reduction in autoreactive humoral activity, by depleting TFH cells or blocking the ICOS-ICOSL pathway, may result in an improvement in SLE-associated comorbidities.
This work supports the rationale for targeting the ICOS-ICOSL pathway for the treatment of lupus by inhibiting the expansion of TFH cell–dependent pathogenic GC B cell responses.
Supplementary Material
Acknowledgments
We thank Terrence O’Day, Li Yu, and Stephanie Oldham for technical support; Rachel Ettinger and Gary Sims for critically reading the manuscript; and Chandra Mohan for providing the B6.Sle1 mice.
The online version of this article contains supplemental material.
- ABC
- avidin–biotin complex
- ANA
- anti-nuclear autoantibody
- ASC
- Ab-secreting cell
- Bcl6
- B cell lymphoma 6
- B2M
- β-2 microglobulin
- B6
- C57BL/6
- CTLA-4
- CTL-associated Ag 4
- ETOH
- ethanol
- GC
- germinal center
- ICOSL
- ICOS ligand
- KO
- knockout
- PC
- plasma cell
- PD-1
- programmed death-1
- PNA
- peanut agglutinin
- SLE
- systemic lupus erythematosus
- TFH
- T follicular helper
- TFR
- T follicular regulatory
- Treg
- regulatory T cell
- VCAM-1
- vascular cell adhesion protein-1
- vWF
- von Willebrand factor
- wt
- wild-type.
Disclosures
All authors are employees of MedImmune LLC and are shareholders in MedImmune/AstraZeneca.
References
- 1.Okamoto T., Saito S., Yamanaka H., Tomatsu T., Kamatani N., Ogiuchi H., Uchiyama T., Yagi J. 2003. Expression and function of the co-stimulator H4/ICOS on activated T cells of patients with rheumatoid arthritis. J. Rheumatol. 30: 1157–1163. [PubMed] [Google Scholar]
- 2.Ma J., Zhu C., Ma B., Tian J., Baidoo S. E., Mao C., Wu W., Chen J., Tong J., Yang M., et al. 2012. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2012: 827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King C., Tangye S. G., Mackay C. R. 2008. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 26: 741–766. [DOI] [PubMed] [Google Scholar]
- 4.Le Coz C., Joublin A., Pasquali J. L., Korganow A. S., Dumortier H., Monneaux F. 2013. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One 8: e75319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morel L., Blenman K. R., Croker B. P., Wakeland E. K. 2001. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc. Natl. Acad. Sci. USA 98: 1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutloff A., Büchner K., Reiter K., Baelde H. J., Odendahl M., Jacobi A., Dörner T., Kroczek R. A. 2004. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum. 50: 3211–3220. [DOI] [PubMed] [Google Scholar]
- 7.Kawamoto M., Harigai M., Hara M., Kawaguchi Y., Tezuka K., Tanaka M., Sugiura T., Katsumata Y., Fukasawa C., Ichida H., et al. 2006. Expression and function of inducible co-stimulator in patients with systemic lupus erythematosus: possible involvement in excessive interferon-gamma and anti-double-stranded DNA antibody production. Arthritis Res. Ther. 8: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X., Lindwall E., Gauthier C., Lyman J., Spencer N., Alarakhia A., Fraser A., Ing S., Chen M., Webb-Detiege T., et al. 2015. Circulating CXCR5+CD4+ helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 24: 909–917. [DOI] [PubMed] [Google Scholar]
- 9.Hutloff A., Dittrich A. M., Beier K. C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R. A. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397: 263–266. [DOI] [PubMed] [Google Scholar]
- 10.Coyle A. J., Lehar S., Lloyd C., Tian J., Delaney T., Manning S., Nguyen T., Burwell T., Schneider H., Gonzalo J. A., et al. 2000. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 13: 95–105. [DOI] [PubMed] [Google Scholar]
- 11.Khayyamian S., Hutloff A., Büchner K., Gräfe M., Henn V., Kroczek R. A., Mages H. W. 2002. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc. Natl. Acad. Sci. USA 99: 6198–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swallow M. M., Wallin J. J., Sha W. C. 1999. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity 11: 423–432. [DOI] [PubMed] [Google Scholar]
- 13.Dong H., Zhu G., Tamada K., Chen L. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5: 1365–1369. [DOI] [PubMed] [Google Scholar]
- 14.Yoshinaga S. K., Whoriskey J. S., Khare S. D., Sarmiento U., Guo J., Horan T., Shih G., Zhang M., Coccia M. A., Kohno T., et al. 1999. T-cell co-stimulation through B7RP-1 and ICOS. Nature 402: 827–832. [DOI] [PubMed] [Google Scholar]
- 15.McAdam A. J., Greenwald R. J., Levin M. A., Chernova T., Malenkovich N., Ling V., Freeman G. J., Sharpe A. H. 2001. ICOS is critical for CD40-mediated antibody class switching. Nature 409: 102–105. [DOI] [PubMed] [Google Scholar]
- 16.Tafuri A., Shahinian A., Bladt F., Yoshinaga S. K., Jordana M., Wakeham A., Boucher L. M., Bouchard D., Chan V. S., Duncan G., et al. 2001. ICOS is essential for effective T-helper-cell responses. Nature 409: 105–109. [DOI] [PubMed] [Google Scholar]
- 17.Bossaller L., Burger J., Draeger R., Grimbacher B., Knoth R., Plebani A., Durandy A., Baumann U., Schlesier M., Welcher A. A., et al. 2006. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 177: 4927–4932. [DOI] [PubMed] [Google Scholar]
- 18.Akiba H., Takeda K., Kojima Y., Usui Y., Harada N., Yamazaki T., Ma J., Tezuka K., Yagita H., Okumura K. 2005. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175: 2340–2348. [DOI] [PubMed] [Google Scholar]
- 19.Stone E. L., Pepper M., Katayama C. D., Kerdiles Y. M., Lai C. Y., Emslie E., Lin Y. C., Yang E., Goldrath A. W., Li M. O., et al. 2015. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity 42: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong C., Temann U. A., Flavell R. A. 2001. Cutting edge: critical role of inducible costimulator in germinal center reactions. J. Immunol. 166: 3659–3662. [DOI] [PubMed] [Google Scholar]
- 21.Dong C., Juedes A. E., Temann U. A., Shresta S., Allison J. P., Ruddle N. H., Flavell R. A. 2001. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409: 97–101. [DOI] [PubMed] [Google Scholar]
- 22.Johnston R. J., Poholek A. C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A. L., Craft J., Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325: 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevalier N., Jarrossay D., Ho E., Avery D. T., Ma C. S., Yu D., Sallusto F., Tangye S. G., Mackay C. R. 2011. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol. 186: 5556–5568. [DOI] [PubMed] [Google Scholar]
- 24.Haynes N. M., Allen C. D., Lesley R., Ansel K. M., Killeen N., Cyster J. G. 2007. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179: 5099–5108. [DOI] [PubMed] [Google Scholar]
- 25.Diehl S. A., Schmidlin H., Nagasawa M., Blom B., Spits H. 2012. IL-6 triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol. Cell Biol. 90: 802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant V. L., Ma C. S., Avery D. T., Li Y., Good K. L., Corcoran L. M., de Waal Malefyt R., Tangye S. G. 2007. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 179: 8180–8190. [DOI] [PubMed] [Google Scholar]
- 27.Kuchen S., Robbins R., Sims G. P., Sheng C., Phillips T. M., Lipsky P. E., Ettinger R. 2007. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 179: 5886–5896. [DOI] [PubMed] [Google Scholar]
- 28.Vinuesa C. G., Cook M. C., Angelucci C., Athanasopoulos V., Rui L., Hill K. M., Yu D., Domaschenz H., Whittle B., Lambe T., et al. 2005. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435: 452–458. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava M., Duan G., Kershaw N. J., Athanasopoulos V., Yeo J. H., Ose T., Hu D., Brown S. H., Jergic S., Patel H. R., et al. 2015. Roquin binds microRNA-146a and Argonaute2 to regulate microRNA homeostasis. Nat. Commun. 6: 6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratama A., Srivastava M., Williams N. J., Papa I., Lee S. K., Dinh X. T., Hutloff A., Jordan M. A., Zhao J. L., Casellas R., et al. 2015. MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nat. Commun. 6: 6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuda C. M., Wan S., Sobel E. S., Croker B. P., Morel L. 2007. Murine lupus susceptibility locus Sle1a controls regulatory T cell number and function through multiple mechanisms. J. Immunol. 179: 7439–7447. [DOI] [PubMed] [Google Scholar]
- 32.Burmeister Y., Lischke T., Dahler A. C., Mages H. W., Lam K. P., Coyle A. J., Kroczek R. A., Hutloff A. 2008. ICOS controls the pool size of effector-memory and regulatory T cells. J. Immunol. 180: 774–782. [DOI] [PubMed] [Google Scholar]
- 33.Marriott C. L., Carlesso G., Herbst R., Withers D. R. 2015. ICOS is required for the generation of both central and effector CD4(+) memory T-cell populations following acute bacterial infection. Eur. J. Immunol. 45: 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yong P. F., Salzer U., Grimbacher B. 2009. The role of costimulation in antibody deficiencies: ICOS and common variable immunodeficiency. Immunol. Rev. 229: 101–113. [DOI] [PubMed] [Google Scholar]
- 35.Lischke T., Hegemann A., Gurka S., Vu Van D., Burmeister Y., Lam K. P., Kershaw O., Mollenkopf H. J., Mages H. W., Hutloff A., Kroczek R. A. 2012. Comprehensive analysis of CD4+ T cells in the decision between tolerance and immunity in vivo reveals a pivotal role for ICOS. J. Immunol. 189: 234–244. [DOI] [PubMed] [Google Scholar]
- 36.Matlawska-Wasowska K., Ward E., Stevens S., Wang Y., Herbst R., Winter S. S., Wilson B. S. 2013. Macrophage and NK-mediated killing of precursor-B acute lymphoblastic leukemia cells targeted with a-fucosylated anti-CD19 humanized antibodies. Leukemia 27: 1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbst R., Wang Y., Gallagher S., Mittereder N., Kuta E., Damschroder M., Woods R., Rowe D. C., Cheng L., Cook K., et al. 2010. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J. Pharmacol. Exp. Ther. 335: 213–222. [DOI] [PubMed] [Google Scholar]
- 38.Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29: 621–663. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y., Perry D., Boackle S. A., Sobel E. S., Molina H., Croker B. P., Morel L. 2005. Several genes contribute to the production of autoreactive B and T cells in the murine lupus susceptibility locus Sle1c. J. Immunol. 175: 1080–1089. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y. F., Morel L. 2005. Genetics of T cell defects in lupus. Cell. Mol. Immunol. 2: 403–409. [PubMed] [Google Scholar]
- 41.Perry D. J., Yin Y., Telarico T., Baker H. V., Dozmorov I., Perl A., Morel L. 2012. Murine lupus susceptibility locus Sle1c2 mediates CD4+ T cell activation and maps to estrogen-related receptor γ. J. Immunol. 189: 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung Y., Tanaka S., Chu F., Nurieva R. I., Martinez G. J., Rawal S., Wang Y. H., Lim H., Reynolds J. M., Zhou X. H., et al. 2011. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linterman M. A., Pierson W., Lee S. K., Kallies A., Kawamoto S., Rayner T. F., Srivastava M., Divekar D. P., Beaton L., Hogan J. J., et al. 2011. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wollenberg I., Agua-Doce A., Hernández A., Almeida C., Oliveira V. G., Faro J., Graca L. 2011. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 187: 4553–4560. [DOI] [PubMed] [Google Scholar]
- 45.Mohan C., Alas E., Morel L., Yang P., Wakeland E. K. 1998. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J. Clin. Invest. 101: 1362–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morel L., Mohan C., Yu Y., Croker B. P., Tian N., Deng A., Wakeland E. K. 1997. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J. Immunol. 158: 6019–6028. [PubMed] [Google Scholar]
- 47.Liang Z., Chang S., Youn M. S., Mohan C. 2009. Molecular hallmarks of anti-chromatin antibodies associated with the lupus susceptibility locus, Sle1. Mol. Immunol. 46: 2671–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang S., Yang L., Moon Y. M., Cho Y. G., Min S. Y., Kim T. J., Kim Y. J., Patrick W., Kim H. Y., Mohan C. 2009. Anti-nuclear antibody reactivity in lupus may be partly hard-wired into the primary B-cell repertoire. Mol. Immunol. 46: 3420–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fritsch R. D., Shen X., Sims G. P., Hathcock K. S., Hodes R. J., Lipsky P. E. 2005. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J. Immunol. 175: 6489–6497. [DOI] [PubMed] [Google Scholar]
- 50.Simister N. E., Mostov K. E. 1989. An Fc receptor structurally related to MHC class I antigens. Nature 337: 184–187. [DOI] [PubMed] [Google Scholar]
- 51.Koller B. H., Marrack P., Kappler J. W., Smithies O. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248: 1227–1230. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y., Cuda C., Morel L. 2005. Genetic determination of T cell help in loss of tolerance to nuclear antigens. J. Immunol. 174: 7692–7702. [DOI] [PubMed] [Google Scholar]
- 53.Singh S., Wu T., Xie C., Vanarsa K., Han J., Mahajan T., Oei H. B., Ahn C., Zhou X. J., Putterman C., et al. 2012. Urine VCAM-1 as a marker of renal pathology activity index in lupus nephritis. Arthritis Res. Ther. 14: R164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curiel R. V., Bhagati R., Basavaraju L., Norton D., Katz J., Haile E., Weinstein A. 2008. Von Willebrand factor, red cell fragmentation, and disease activity in systemic lupus erythematosus. HSS J. 4: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin-Rodriguez S., Reverter J. C., Tàssies D., Espinosa G., Heras M., Pino M., Escolar G., Diaz-Ricart M. 2015. Reduced ADAMTS13 activity is associated with thrombotic risk in systemic lupus erythematosus. Lupus 24: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 56.Wuthrich R. P. 1992. Vascular cell adhesion molecule-1 (VCAM-1) expression in murine lupus nephritis. Kidney Int. 42: 903–914. [DOI] [PubMed] [Google Scholar]
- 57.Moore T. V., Clay B. S., Ferreira C. M., Williams J. W., Rogozinska M., Cannon J. L., Shilling R. A., Marzo A. L., Sperling A. I. 2011. Protective effector memory CD4 T cells depend on ICOS for survival. PLoS One 6: e16529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu D., Tan A. H., Hu X., Athanasopoulos V., Simpson N., Silva D. G., Hutloff A., Giles K. M., Leedman P. J., Lam K. P., et al. 2007. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450: 299–303. [DOI] [PubMed] [Google Scholar]
- 59.Bauquet A. T., Jin H., Paterson A. M., Mitsdoerffer M., Ho I. C., Sharpe A. H., Kuchroo V. K. 2009. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 10: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herman A. E., Freeman G. J., Mathis D., Benoist C. 2004. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 199: 1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prevot N., Briet C., Lassmann H., Tardivel I., Roy E., Morin J., Mak T. W., Tafuri A., Boitard C. 2010. Abrogation of ICOS/ICOS ligand costimulation in NOD mice results in autoimmune deviation toward the neuromuscular system. Eur. J. Immunol. 40: 2267–2276. [DOI] [PubMed] [Google Scholar]
- 62.Suh C. H., Freed J. H., Cohen P. L. 2003. T cell reactivity to MHC class II-bound self peptides in systemic lupus erythematosus-prone MRL/lpr mice. J. Immunol. 170: 2229–2235. [DOI] [PubMed] [Google Scholar]
- 63.Revillard J. P., Vincent C., Rivera S. 1979. Anti-beta2-microglobulin lymphocytotoxic autoantibodies in systemic lupus erythematosus. J. Immunol. 122: 614–618. [PubMed] [Google Scholar]
- 64.Ooi B. S., Ooi Y. M., Pesce A. J., Pollak V. E. 1977. Antibodies to beta 2 microglobulin in the sera of patients with systemic lupus erythematosus. Immunology 33: 535–541. [PMC free article] [PubMed] [Google Scholar]
- 65.Konya C., Goronzy J. J., Weyand C. M. 2009. Treating autoimmune disease by targeting CD8(+) T suppressor cells. Expert Opin. Biol. Ther. 9: 951–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tada Y., Koarada S., Tomiyoshi Y., Morito F., Mitamura M., Haruta Y., Ohta A., Nagasawa K. 2006. Role of inducible costimulator in the development of lupus in MRL/lpr mice. Clin. Immunol. 120: 179–188. [DOI] [PubMed] [Google Scholar]
- 67.Mandik-Nayak L., Seo S. J., Sokol C., Potts K. M., Bui A., Erikson J. 1999. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J. Exp. Med. 189: 1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.William J., Euler C., Christensen S., Shlomchik M. J. 2002. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 297: 2066–2070. [DOI] [PubMed] [Google Scholar]
- 69.Vinuesa C. G., Sanz I., Cook M. C. 2009. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 9: 845–857. [DOI] [PubMed] [Google Scholar]
- 70.Simpson N., Gatenby P. A., Wilson A., Malik S., Fulcher D. A., Tangye S. G., Manku H., Vyse T. J., Roncador G., Huttley G. A., et al. 2010. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 62: 234–244. [DOI] [PubMed] [Google Scholar]
- 71.Liarski V. M., Kaverina N., Chang A., Brandt D., Yanez D., Talasnik L., Carlesso G., Herbst R., Utset T. O., Labno C., et al. 2014. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci. Transl. Med. 6: 230ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathian A., Gallegos M., Pascual V., Banchereau J., Koutouzov S. 2011. Interferon-α induces unabated production of short-lived plasma cells in pre-autoimmune lupus-prone (NZB×NZW)F1 mice but not in BALB/c mice. Eur. J. Immunol. 41: 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z., Zou Y., Davidson A. 2011. Plasma cells in systemic lupus erythematosus: the long and short of it all. Eur. J. Immunol. 41: 588–591. [DOI] [PubMed] [Google Scholar]
- 74.Liu Z., Bethunaickan R., Huang W., Lodhi U., Solano I., Madaio M. P., Davidson A. 2011. Interferon-α accelerates murine systemic lupus erythematosus in a T cell-dependent manner. Arthritis Rheum. 63: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barbalat R., Ewald S. E., Mouchess M. L., Barton G. M. 2011. Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 29: 185–214. [DOI] [PubMed] [Google Scholar]
- 76.Gustafsson J., Gunnarsson I., Börjesson O., Pettersson S., Möller S., Fei G. Z., Elvin K., Simard J. F., Hansson L. O., Lundberg I. E., et al. 2009. Predictors of the first cardiovascular event in patients with systemic lupus erythematosus - a prospective cohort study. Arthritis Res. Ther. 11: R186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.