Abstract
Lattice oxygen can play an intriguing role in electrochemical processes, not only maintaining structural stability, but also influencing electron and ion transport properties in high-capacity oxide cathode materials for Li-ion batteries. Here, we report the design of a gas–solid interface reaction to achieve delicate control of oxygen activity through uniformly creating oxygen vacancies without affecting structural integrity of Li-rich layered oxides. Theoretical calculations and experimental characterizations demonstrate that oxygen vacancies provide a favourable ionic diffusion environment in the bulk and significantly suppress gas release from the surface. The target material is achievable in delivering a discharge capacity as high as 301 mAh g−1 with initial Coulombic efficiency of 93.2%. After 100 cycles, a reversible capacity of 300 mAh g−1 still remains without any obvious decay in voltage. This study sheds light on the comprehensive design and control of oxygen activity in transition-metal-oxide systems for next-generation Li-ion batteries.
 Oxygen activity can play a vital role in determining charge transport properties of materials. Here, the authors demonstrate a method to create oxygen vacancies on layered oxides via a gas-solid interface reaction, leading to materials with enhanced energy and power densities for Li-ion batteries.
Oxygen activity can play a vital role in determining charge transport properties of materials. Here, the authors demonstrate a method to create oxygen vacancies on layered oxides via a gas-solid interface reaction, leading to materials with enhanced energy and power densities for Li-ion batteries.
The functionality of many transition metal oxides can be significantly altered by oxygen vacancies on the surface. Oxygen vacancies can behave as charge carriers for solid-oxide fuel cells1, as well as important adsorption sites and as active sites for electro-photocatalysts2. In Li-ion cathode materials, these vacancies play a vital role in determining the material's electron and ion transport properties3,4,5. The influence of oxygen vacancies at the surface on electrochemical performance can be completely different depending on the type of Li-ion cathode material6,7,8. Li-rich layered oxides, either as a solid solution or as a nano-composite of layered Li2MnO3 and Li(TM)O2 (TM=Ni, Co, Mn), are drawing attention as next-generation cathode materials for high-energy-density Li-ion batteries in electric vehicles9,10,11. Over the past 20 years, the discharge capacity at room temperature of these cathode materials9,12,13,14 has been improved, from 200 mAh g−1, given in the first report12, to over 320 mAh g−1 (ref. 14) today as summarized by Hy et al.15, and even higher at elevated temperatures16. While research has continued to push the limit of the available capacity of the materials over the years, debates on the origins of the ultrahigh capacity beyond the redox of transition metal have intensified recently.
Numerous studies10,17,18,19,20,21,22,23 exemplify the contributions of bulk and surface oxygen on high charge–discharge capacity in Li-rich layered oxides, although this hypothesis has not been fully verified. Tarascon and co-workers21,22 concluded that the extra-high capacity is mainly attributable to reversible anionic redox processes (O2−/O22− or O2−/O2n−, where 3>n>1) within the bulk of Li-rich layered oxides. Delmas and co-workers19,20 proposed that surface oxygen is oxidized to O2 gas and irreversibly lost from the structure, leaving some oxygen vacancies on the surface or in the sub-surface layers. Our previous work24,25,26 illustrated that oxygen vacancies generated during the high-potential electrochemical process can facilitate the transition metal ion migration and surface structural transformation, finally leading to their potential degradation during extended cycles.
To reduce oxygen gas generation and utilize reversible oxygen redox activity during charging and discharging, oxygen vacancies have been proposed to form on the surface of the as-synthesized Li-rich layered oxides before electrochemical processing. The influence of the surface oxygen vacancies on the oxygen gas generation is discussed in detail in Supplementary Note 1. The creation of surface oxygen vacancies was previously attempted utilizing a reducing atmosphere27,28 and leaching with acid accompanied by heat treatment29,30. However, the bulk structure reported in previous work easily transforms from a pure layered phase to spinel- and/or rock-salt phases28,29,30,31,32,33, which diminishes the rate capability and cycling stability.
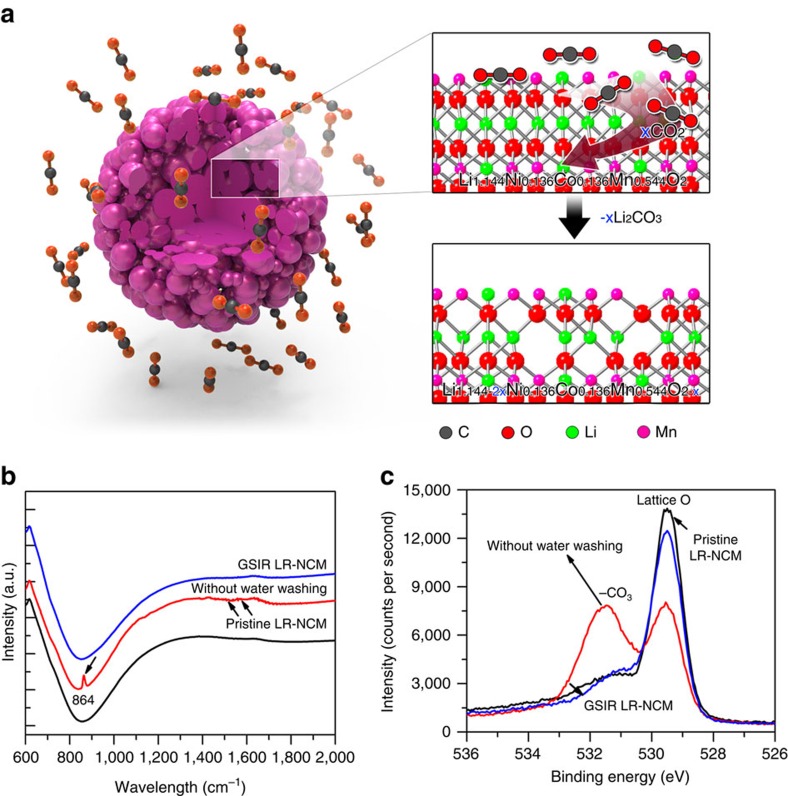
Motivated by the considerations above, we propose a strategy based on a gas–solid interface reaction (GSIR) between Li-rich layered oxides and carbon dioxide gas to create oxygen vacancies on the particles' surface. The schematic of GSIR process between Li-rich layered oxides and carbon dioxide is displayed in Fig. 1a, and the procedure is detailed in the ‘Methods' section. Oxygen vacancies in the surface regions up to 20-nm thick are created on the particles without affecting structural integrity. After the surface modification, the target material Li[Li0.144Ni0.136Co0.136Mn0.544]O2 exhibits discharge capacity as high as 300 mAh g−1 with no obvious voltage degradation after 100 cycles at a current density of 25 mA g−1. As confirmed by both theoretical calculations and experimental characterization, the improved electrochemical performance is ascribed to the full utilization of oxygen activity through the creation of oxygen vacancies on the surface of the as-synthesized Li-rich layered oxides.
Figure 1. GSIR.
(a) Schematic of GSIR between Li-rich layered oxides and carbon dioxide. (b) FTIR spectrum. The GSIR LR-NCM without water washing (red line), compared with the pristine LR-NCM, has three additional peaks, corresponding to the IR-active modes of –CO3. The sharp peak (864 cm−1) is the CO3 group bending mode, while the other two peaks (1,438 and 1,500 cm−1) are from the C–O asymmetric and symmetric stretching modes; (c) XPS spectra of the O 1s. It is clear that the GSIR LR-NCM has lower intensity than that of the pristine LR-NCM at lower-banding energy, which indicates some lattice O has been extracted by CO2.
Results
Characterizations of oxygen vacancies after GSIR process
Li-rich layered oxide Li[Li0.144Ni0.136Co0.136Mn0.544]O2 (denoted as LR-NCM) was prepared by a co-precipitation method (see the ‘Methods' section). After the GSIR process is completed, the general morphology remains the same as in the pristine LR-NCM (Supplementary Fig. 1). Both synchrotron X-ray diffraction (SXRD) refinements and transition metal K-edge X-ray absorption near-edge structure show no major difference for the pristine and GSIR LR-NCM samples (Supplementary Figs 2 and 3). These results preliminarily imply that the GSIR process does not heavily influence the average crystal structures and bulk electronic environments of the LR-NCM sample.
To investigate the GSIR reaction mechanism of the formation of surface oxygen vacancies, we applied Fourier-transformed infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) to three samples (Fig. 1b,c). The FTIR results (Fig. 1b) indicate that carbonate formed on their surfaces after the GSIR. The lithium concentration of the GSIR LR-NCM is slightly lower (6.4%) than that of the pristine LR-NCM, as was determined by inductive coupled plasma-atomic emission spectrometry. On the other hand, in the oxygen 1s XPS spectra (Fig. 1c), the intensity of the binding energy at 529.5 eV representing TM-O covalency for the GSIR LR-NCM after washing is much lower than that of the pristine LR-NCM. In contrast, the intensity of the binding energy at 531.5 eV representing the carbonate group (–CO3) for the GSIR LR-NCM without washing is much larger34,35. Both changes indicate some lattice oxygen was extracted by CO2, forming oxygen vacancies on the sub-surfaces.
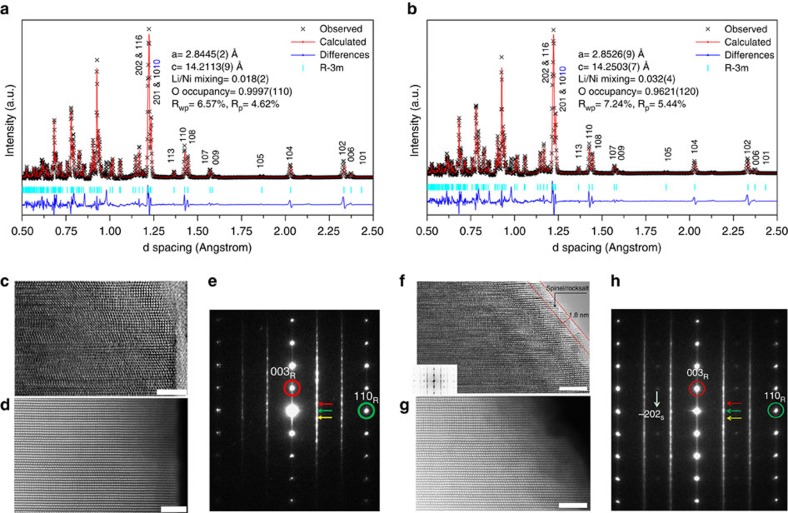
The amount of oxygen vacancies in the GSIR LR-NCM sample was determined by neutron diffraction (ND), a technique with high sensitivity for detecting light elements36, such as Li and O. Figure 2a,b demonstrates the as-collected time-of-flight (TOF) ND patterns with ‘Rietveld' refinement for both samples (patterns refined with a solid solution of ‘R-3m' symmetry; the normalized TOF ND patterns are shown in Supplementary Fig. 4). The lattice parameters of the pristine layer are a=2.8445(2) Å, and c=14.2113(9) Å. The oxygen occupancy of the pristine LR-NCM is 99.97±1.10%. In comparison, the lattice parameters of the GSIR LR-NCM are a=2.8526(8) Å and c=14.2503(7) Å, which are slightly larger than those of the pristine LR-NCM. The oxygen occupancy is reduced to 96.21±1.20% after surface modification. The amount of Ni2+ in the Li layer, and the occupancy of Li in the Li layer are also relatively larger than that of in the pristine LR-NCM. In addition, the data are also analysed using the two-phase model (the as-collected TOF ND patterns are shown in Supplementary Fig. 5), that is, a combination of LiTMO2 (R-3m) phase, and Li2MnO3 (C/2m) phase. The results show that no oxygen vacancies (VO) are observed in the pristine LR-NCM sample, while the GSIR LR-NCM sample exhibits 7.20±3.3% VO on the 4i site and 3.8±3.0% VO on the 8j site in Li2MnO3 phase and 2.96±1.86% VO in the overall composition. Both models are consistent with the observed oxygen vacancies in the GSIR LR-NCM. The lower oxygen occupancy in the GSIR LR-NCM proves that the oxygen vacancies are successfully created after applying the GSIR process.
Figure 2. Structural characterizations of the pristine and GSIR LR-NCM.
(a,b) TOF ND patterns for the pristine and GSIR LR-NCM. On the basis of these results, the oxygen vacancy for the GSIR LR-NCM is around three per cent higher than that of the pristine LR-NCM sample; (c) high-resolution transmission electron microscopy (HRTEM) image and, (d) high-angle annular dark-field—STEM (HAADF-STEM) image for the pristine LR-NCM; (e) electron-diffraction (ED) pattern for the pristine LR-NCM. The elongation of these spots are along [001]R* as well as [001]M* direction. The spots indicated with red, green and yellow arrows represent (110), (020) and (−110) of the monoclinic Li2MnO3 (donated as M) variants [1–10]M, [100]M and [110]M, respectively. The spot marked by a red circle is shared by (003)R of the rhombohedral (donated as R), as well as the structure (001)M of all monoclinic variants. The spot marked by a green circle is shared by (110)R of the rhombohedral, as well as the structure (33-1)M, (060)M and (–331)M of the monoclinic. (f) HRTEM image with fast Fourier transform (FFT) about the surface and (g). HAADF-STEM image for the GSIR LR-NCM; (h) ED for the GSIR LR-NCM. The weak spot indicated by the white arrow can only be indexed as the structure (−202)S of the spinel (donated as S). Scale bars, 5 nm.
High-resolution transmission electron microscopy (TEM; Fig. 2c,f) was used to further illustrate the changes between the pristine and GSIR LR-NCM. A clean surface with Li and TM layers extending to the very edge of atomic planes is observed in the pristine LR-NCM (Fig. 2c). Meanwhile, a non-uniform surface (varying in thickness from 10 to 20 nm) is detected on the GSIR LR-NCM sample. In addition, high-angle annular-dark-field scanning-TEM images (Fig. 2d,g) for the pristine and GSIR LR-NCM are compared to demonstrate the lack of any obvious structural change. Although electron diffraction from some particles shows spinel/rock-salt-like phase in the GSIR LR-NCM compared with the pristine LR-NCM sample (Fig. 2e,h), it should be noted this phase transformation only occurs on the surface of GSIR LR-NCM within several atomic layers, as indicated in Fig. 2f, while the oxygen vacancy presence are 10–20-nm deep. These results are consistent with our SXRD and ND refinements, which verify that the GSIR process does not significantly alter the bulk structure.
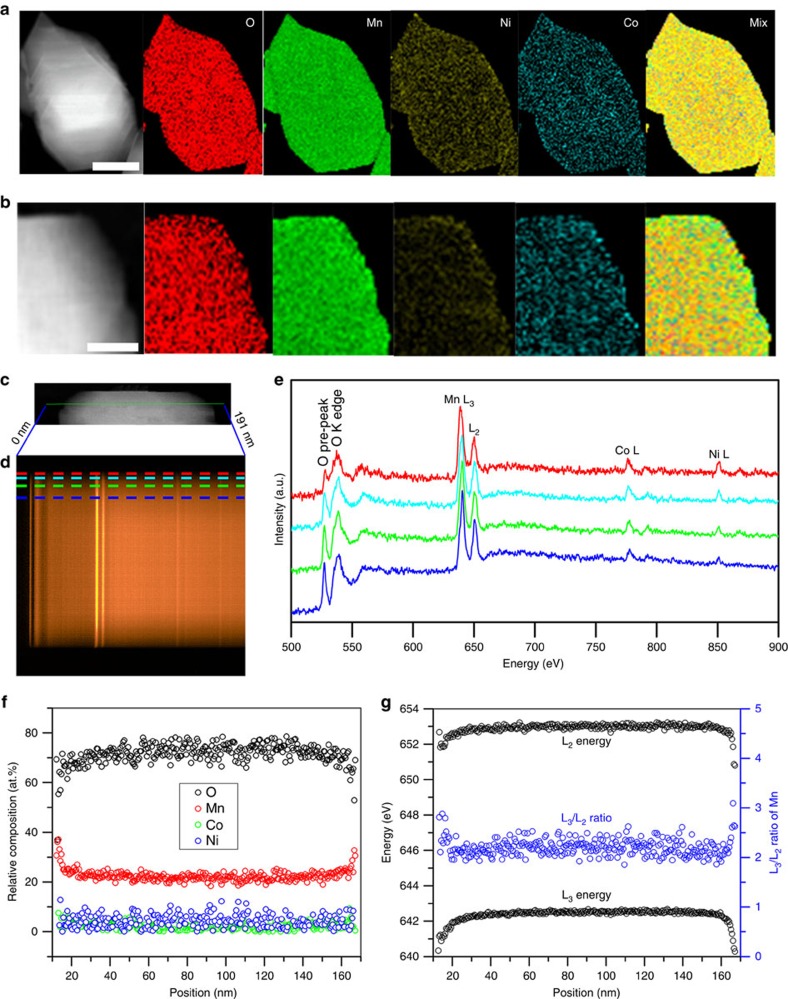
Electron energy-loss spectroscopy (EELS) mapping was applied to compare relative surface composition changes for the pristine and GSIR LR-NCM sample (Fig. 3a,b). Oxygen reduction at mixed colour images is well-demonstrated on the edge of GSIR LR-NCM in comparison with the pristine LR-NCM. To study the electronic states of oxygen and transition metal from the particle, EELS was collected at different positions from bulk to surface of the GSIR LR-NCM (represented by colours in Fig. 3c,d). The spectra of the O-K-edge main peaks in Fig. 3e are normalized with the Mn L3 peak. Interestingly, it is observed that reduced O pre-peak intensities are from the surface in the GSIR LR-NCM sample. The weak pre-peak intensities can be even seen at 20 nm for the GSIR LR-NCM sample although the difference is small. The reduced ratio of the O-K-edge pre-peak intensities to the main peak can result from a change in the local environment of oxygen, especially from the oxygen vacancies formed on the surface26,37. The relative distribution of chemical composition is plotted in Fig. 3f. The content of O on average is 55% in the first 10 nm surface layer compared with the bulk value of 65%. The Mn L3,2 white lines provide information on Mn valence (oxidation); The Mn L3,2 white lines for the GSIR LR-NCM sample display a significant energy shift, and their L3/L2 intensity ratio remains low until 20 nm away from the surface of the GSIR LR-NCM sample (Fig. 3g), indicating a lower Mn valence on the particles' surface. When oxygen vacancies are created on the particles' surface, the valence of Mn is reduced simultaneously with the extraction of Li to compensate for the changes in charge. Therefore, as confirmed by both the ND and STEM/EELS results, oxygen vacancies have been successfully introduced on the 10–20 nm of the surface without a noticeable interruption in the bulk structure through the GSIR approach.
Figure 3. Relative surface composition changes for the pristine and GSIR LR-NCM.
(a) STEM-EELS spectrum images for the pristine LR-NCM. Scale bar, 50 nm. (b) The GSIR LR-NCM. Scale bar, 25 nm. Oxygen reduction at mixed colour images is well-demonstrated on the edge in comparison with the pristine LR-NCM (a). (c) STEM image of a particle of the GSIR LR-NCM. (d) EELS spectrum image from the vertical green line (0–191 nm) in the left STEM image (c), showing O K, Mn L2, 3, Co L2, 3, Ni L2, 3 edges. The dispersion is 0.25 eV/channel. The scan step is 0.127 nm. (e) EELS spectrum profiles from the surface to the interior as marked by the horizontal dashed lines with the same colour as in d. The spectra are normalized with Mn L3 peak, and each spectrum was averaged vertically with 10 individual spectra to improve the signal noise ratio. Weak O pre-peak on the surface indicates the oxygen vacancies are formed. (f) Relative atomic composition of O (black), Mn (red), Co (blue) and Ni (green) as a function of position calculated based on the integrated EELS peak intensity in e. (g) Mn L3, and L2 energy (top and bottom of panel) from refinement, and L3/L2 intensity ratio.
Electrochemical properties of the pristine and GSIR LR-NCM
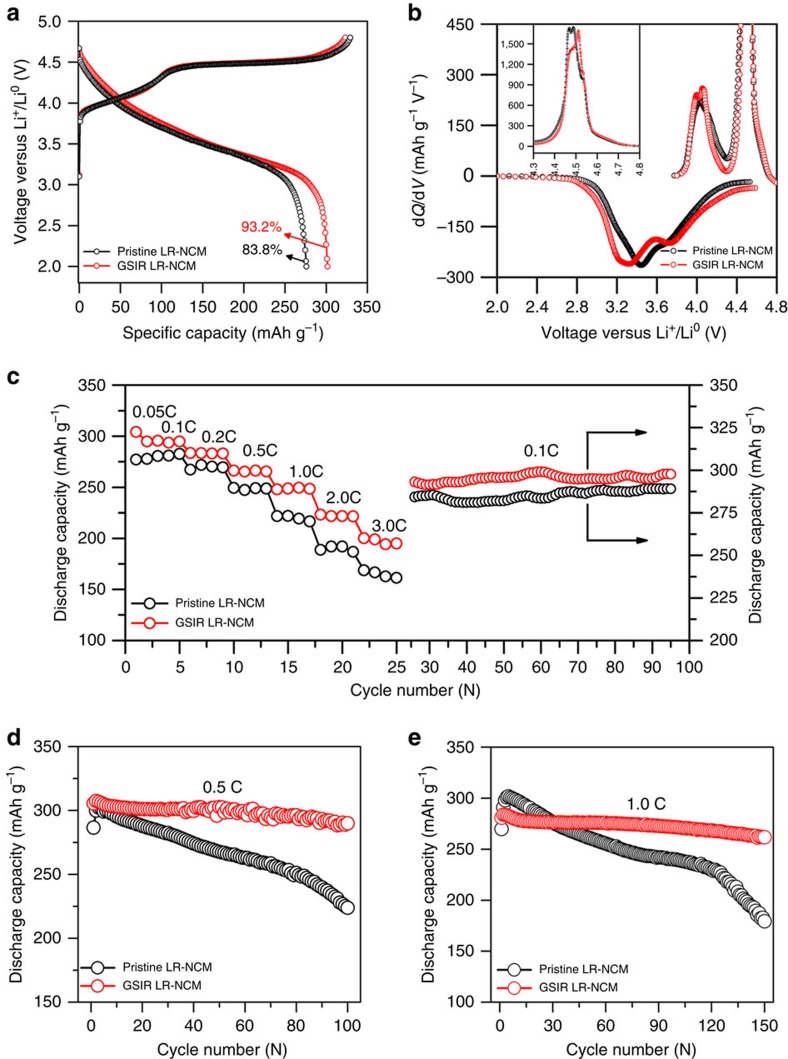
CR2032 coin cells with metallic Li as the counter electrode were assembled to investigate the electrochemical performance of the pristine and GSIR LR-NCM samples. Similar to other Li-rich layered oxides, both of them (Fig. 4a) exhibit a long plateau region at ∼ 4.5 V versus Li+/Li0, which usually is ascribed to the electrochemical activation of the Li2MnO3 component during the initial charge process38,39,40, although an electrolyte/electrode side reaction is expected in this region. Interestingly, the value of the initial discharge capacity for the GSIR LR-NCM can reach as high as 301 mAh g−1 compared with that of 276 mAh g−1 for the pristine LR-NCM. The initial coulombic efficiency also increases from 83.8 to 93.2%. The corresponding differential capacity versus voltage (dQ/dV) curves are plotted in Fig. 4b. A lower shift in the oxidation peak after the GSIR process is due to the decrease in electrochemical impedance (Supplementary Fig. 6 and Supplementary Table 1). In addition, the decline of the oxidation peak around 4.5 V versus Li+/Li0 observed in the enlarged dQ/dV curves (inset in Fig. 4b) indicates that the GSIR process has pre-activated the Li2MnO3 component responsible for the 4.5 V plateau. The rate capability and cycling stability further highlight the advantages of our GSIR LR-NCM sample (Fig. 4c). At all tested rates, the GSIR LR-NCM exhibits a higher capacity than that of the pristine LR-NCM. The unique characteristic at different rates for the GSIR LR-NCM is that the additional discharge capacity (Supplementary Fig. 7a–f) results only from the lower-potential region (<3.5 V versus Li+/Li0). It is remarkable that the GSIR LR-NCM delivers a higher discharge capacity of 298 mAh g−1 when it returns to the 0.1 C-rate, compared with that of 288 mAh g−1 for the pristine LR-NCM. More importantly, the charge–discharge plots at subsequent cycles (Supplementary Fig. 7g,h) for the GSIR LR-NCM demonstrate a slight degradation in potential after 100 cycles, even for a discharge capacity as high as 300 mAh g−1. To track the origin of the additional capacity after the GSIR, the energy density and discharge capacity below and above 3.5 V versus Li+/Li0 are plotted as a function of the cycle number, as depicted in Supplementary Fig. 8i,j. It proves that the additional capacity in discharge capacity also comes from the lower-potential region (<3.5 V versus Li+/Li0).
Figure 4. Charge–discharge characteristics of the pristine and GSIR LR-NCM.
(a) First charge–discharge profiles of the pristine and GSIR LR-NCM obtained from a 2032-type coin cells at 0.05 C-rate, where 1.0 C-rate corresponds to the current density of 250 mA g−1. (b) Differential capacity (dQ/dV) plots of the initial cycle curves of the pristine and GSIR LR-NCM. The inset shows the enlarged dQ/dV curves about 4.5 V. (c) Discharge-rate capacity after charging galvanostatically at 0.1 C-rate before each discharge. The capacity retention when performing charge–discharge cycles at constant 0.1 C-rate for 70 cycles after all rates tested. (d,e) Cycling performance of the pristine and GSIR LR-NCM at 55 °C by applying a constant current density of 0.5 C-rate and 1.0 C-rate (250 mA g−1), respectively. The loading density of the active material on the electrode was around 5.5 mg cm−2. It is noticeable that the electrochemical data are derived from the average value of at least five coin cells, and the errors on the specific capacity are about 3 mAh g−1.
To evaluate the stability of the GSIR LR-NCM, a more challenging measurement was selected (Fig. 4d,e). Cells based on the GSIR LR-NCM show a higher initial capacity of 306 mAh g−1 (0.5 C-rate) and 280.9 mAh g−1 (1.0 C-rate), compared with that of 287 mAh g−1 (0.5 C-rate) and 269 mAh g−1 (1.0 C-rate) for the pristine LR-NCM at the elevated temperature of 55 °C. The initial charge–discharge curves (Supplementary Fig. 8a,b) are similar to the results at room temperature (Fig. 4a). In addition, the cells based on the pristine LR-NCM display only 223 mAh g−1 (at 0.5 C-rate) and 179 mAh g−1 (at 1.0 C-rate) after 100 cycles and 150 cycles, respectively, whereas the GSIR LR-NCM shows an excellent capacity of about 290 mAh g−1 (at 0.5 C-rate) and 262 mAh g−1 (at 1.0 C-rate) during the same cycling period. Moreover, the charge–discharge plots of subsequent cycles (Supplementary Fig. 8d,f) for the GSIR LR-NCM exhibit much slower potential degradation profiles at different rates than those of the pristine LR-NCM (Supplementary Fig. 8c,e). The difference clearly signifies that surface oxygen vacancies introduction in Li-rich layered oxides without severe structural destruction has a considerable effect on improving electrochemical performance.
Discussion
The influence of surface oxygen vacancies introduced by the GSIR process on the electrochemical performance can be discussed in two aspects. (1) The generation of highly reactive oxygen radicals on the particles' surface during the electrochemical process, which determines the electrochemical resistance at the electrode/electrolyte interface; (2) the participation of the oxygen reaction in the form of O2−/O− or O22− in the bulk of the material, which are the dominant factors in compensating for extra electrons on the superior capacity of Li-rich layered oxides.
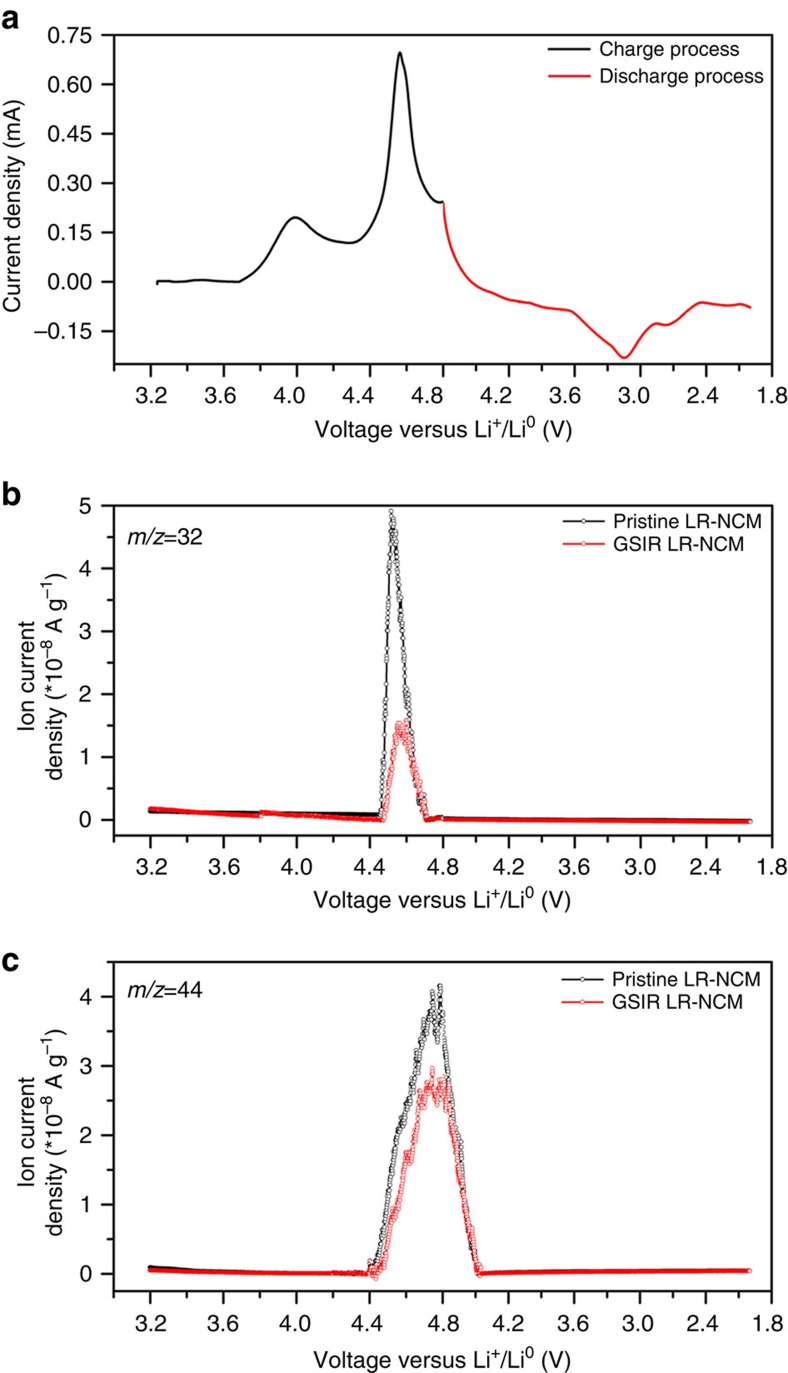
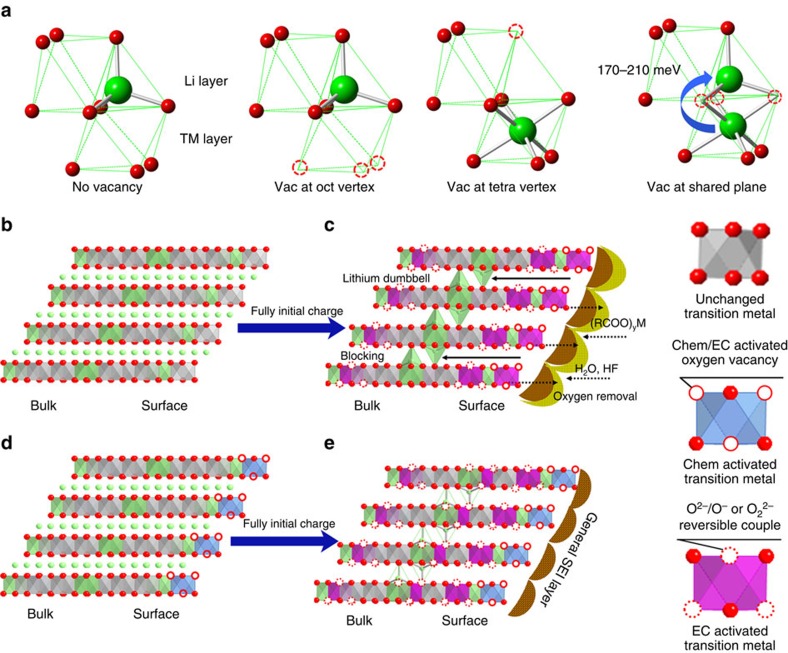
First, operando differential electrochemical mass spectrometry (DEMS) experiments were performed to evaluate the gas evolution during the initial charge–discharge process (Fig. 5a), which is largely related to the activity of oxygen on the particles' surface18,41,42. Irrespective of the cycling profile, as shown in Fig. 5b, O2 is detected near 4.5–4.6 V versus Li+/Li0 for both the pristine and GSIR LR-NCM; however, the O2 gas released from the GSIR LR-NCM electrode is much less than that of the pristine electrode. It is suggested that the formation of oxygen vacancies through GSIR could greatly suppress the evolution of O2 gas. According to the equation 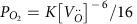 in the Supplementary note 1, less O2 gas will be released due to the reduced partial pressure of oxygen on the surface of the GSIR LR-NCM electrode after oxygen vacancies are created. At the same time, the released CO2 gas for the GSIR LR-NCM (Fig. 5c), which is usually considered as the source of decomposition of the electrolyte at high potentials42, is smaller than that of the pristine LR-NCM. Therefore, the oxygen vacancies on the surface can hinder the highly reactive oxygen radicals from generating during the electrochemical process.
in the Supplementary note 1, less O2 gas will be released due to the reduced partial pressure of oxygen on the surface of the GSIR LR-NCM electrode after oxygen vacancies are created. At the same time, the released CO2 gas for the GSIR LR-NCM (Fig. 5c), which is usually considered as the source of decomposition of the electrolyte at high potentials42, is smaller than that of the pristine LR-NCM. Therefore, the oxygen vacancies on the surface can hinder the highly reactive oxygen radicals from generating during the electrochemical process.
Figure 5. Operando DEMS.
(a) Initial cyclic voltammetry (CV) curve of the pristine and GSIR LR-NCM electrode. (b) Oxygen gas profile of the initial charge–discharge process for the pristine and GSIR LR-NCM electrode. (c) Carbon dioxide gas profile of the initial charge–discharge process as the above electrodes. The loading density mass for these two electrodes are the same about 7.76 mg. The scan rate was ∼0.1 mV s−1, and the potential range was between 2.0 and 4.8 V versus Li+/Li0 at an elevated temperature of 55 °C.
Second, the GSIR LR-NCM sample exhibits almost the same capacity in the plateau region as in the pristine sample with much less oxygen redox participation on the surface. To compensate for ionic changes during the charge process, more oxygen reactions in the form of O2−/O− or O2− (instead of the O2−/O2 redox couple) are triggered in the bulk of the GSIR LR-NCM. The influence of the bulk oxygen activity in the diffusivity of lithium ions was investigated by first-principles calculations. For simplicity, the supercell was defined as Li28Ni6Mn14O48, which was adopted as a computational model in the previous work24. A specific Li20/28 concentration was chosen to simulate the last stage of discharge (below ∼3.2 V), in which the GSIR LR-NCM demonstrates the biggest difference in electrochemical performance compared with the pristine LR-NCM. This stage is also generally acknowledged as a rate-determining step of the whole discharge process in Li-rich layered oxides43. In this stage, the Li-ion migrates from the octahedral site of the Li layer into the octahedral site of the adjacent transition metal layer through an empty tetrahedral site in the former (as shown in Fig. 6a). When no oxygen vacancy is introduced, the Li tetrahedron site, which is face-sharing with the TM layer Li, is most stable. There is a high chance of trapping the Li-ion in the tetrahedral site (so-called ‘Li–Li dumbbell') without diffusing into the adjacent octahedral site in the transition metal layer24. These dumbbells finally block the pathways of lithium diffusion, thereby decreasing ionic conductivity. In contrast, when oxygen vacancies are introduced the under-coordinated site is no longer stable, and the migration barrier is either zero or only around 170–210 meV (ref. 44). The trapped Li-ion has a higher chance of escaping from the tetrahedral sites and continuing its diffusion process.
Figure 6. Reaction mechanisms during charging and discharging.
(a) Calculated site stability and activation barrier under conditions of no vacancy, vacancy at octahedron (Oct) vertex, vacancy (Vac) at tetrahedron (Tetra) vertex and vacancy (Vac) at shared plane. (b,c). The pristine LR-NCM before charging and after full initial charge. (d,e) The GSIR LR-NCM before charging and after full initial charge. Green, Li; red, O. The fundamental contribution of the novel surface modification is the uniform creation of lithium and oxygen vacancies on the surface of particles before electrochemical cycling. Consequently, more Mn4+/Mn3+ redox couples in Li2MnO3 component are pre-activated; less oxygen gas is released from the material and less oxygen vacancies are created in the bulk during the charging process; limited electrode/electrolyte corrosion and bulk material structure transformation are formed in the battery system.
Overall, Fig. 6b,e summarizes the proposed reaction mechanism for the pristine and GSIR LR-NCM during charging and discharging. Before charging, uniform oxygen and lithium vacancies are created on the particle surface of the GSIR LR-NCM, accompanied by the pre-activation of certain amount of the Li2MnO3 component. The sloped region (<4.4 V) of the charging curve is attributed to the extraction of Li+ ions from the lithium layer. No difference is expected between the pristine and GSIR LR-NCM material during this process. During the charging plateau region, more lithium ions can be extracted from the Li2MnO3 component, together with the oxidation of lattice oxygen19,20,22,23. The pre-activated surface layer of the GSIR sample with oxygen vacancies reduces the partial pressure of oxygen on the surface, and then prevents gaseous oxygen from evolving during plateau charging. A thinner SEI layer is thus expected to form on the particles' surface of the GSIR LR-NCM due to less side reactions between released oxygen and the electrolyte species, thereby decreasing the electrochemical resistance at the electrode/electrolyte interface, as supported by our electrochemical impedance-spectroscopy (EIS) results (Supplementary Fig. 6 and Supplementary Table 1). At the same time, the more reversible oxygen redox-reaction is triggered in the bulk of the GSIR LR-NCM during the high-potential-charging process. These oxygen vacancies can activate the Li trapped in the tetrahedral sites to provide a favourable environment for Li-ion diffusion during the rate-determining step (the last stage of discharging). Higher levels of Li-ion diffusivity in the bulk, together with smaller resistance to charge-transfer and larger numbers of activated Mn4+/Mn3+ couples lead to higher capacity and better rate capability of the GSIR LR-NCM sample. Furthermore, minimal structure changes enable the superior cycling stability after GSIR modification.
In summary, a facile but effective approach to surface modification based on GSIR has been applied to create oxygen vacancies homogeneously without causing structural destruction. Oxygen vacancies in the surface regions up to 20-nm thick are found to facilitate Li diffusion by activating the Li in the tetrahedral sites and suppress the release of gas from the surface, which finally lead to higher discharge capacity and a better rate capability in the Li-rich layered oxides. To the best of our knowledge, this study also clarifies for the first time the critical influence of the activity of surface oxygen in enhancing the electrochemical performance. We believe this facile, scalable and inexpensive approach opens a door for comprehensive designing and controlling oxygen activity in transition-metal-oxide systems to achieve excellent energy and power density in their commercial applications.
Methods
Synthesis of Li[LO0.144Ni0.136Co0.136Mn0.544]O2
The synthesis procedures of Li[LO0.144Ni0.136Co0.136Mn0.544]O2 were detailed as follows45: we pumped an aqueous solution containing NiSO4·6H2O, CoSO4·7H2O and MnSO4·4H2O with a concentration of 2.0 mol l−1 into a continuously stirred tank reactor (CSTR, capacity of 50 l); at the same time, a 2.0 mol l−1 Na2CO3 solution and a 0.2 mol l−1 NH4OH solution were added separately into the reactor. The co-precipitation temperature was held at 60 °C, and the pH value was fixed to 7.8. The resulting (Ni1/6Co1/6Mn4/6)CO3 powders were washed several times with distilled water to remove residual Na+, and dried in a vacuum oven at 80 °C for over 20 h. The resulting precipitates were mixed with Li2CO3 and the exact molar ratio between them was 0.7. The mixed powders were first pre-treated at 500 °C for 5 h in the air and then calcinated at 850 °C for 15 h in the air. Then, they were cooled to room temperature in the furnace. The as-obtained sample was labelled as pristine LR-NCM.
GSIR process
The GSIR process of the pristine LR-NCM sample with CO2 was carried out as follows: a set amount of LR-NCM cathode materials and a given amount of NH4HCO3, which is as the source of CO2 after decomposition at certain temperatures, were placed in an enclosed reactor in an argon-filled glove box (O2<0.1 p.p.m.) with a total volume of 100 ml, and heated at 200 °C for 10 h. The optimal molar ratio between carbon dioxide and Li-rich layered oxides was about 1:5. It is important that the pristine LR-NCM cathode materials and the NH4HCO3 must be separated at the beginning for ensuring a homogeneous GSIR. To remove the reaction products on the surface of the LR-NCM, the sample obtained was washed with water several times and dried at 120 °C for 12 h. The as-obtained product was termed the GSIR LR-NCM. We would like to stress that the same procedures have been adopted in both NIMTE and UCSD labs, and all the electrochemical data have been completely reproduced.
Materials characterization
A field-emission scanning electron microscopy image was acquired on a Hitachi S-4800. A FTIR analysis was performed on a pellet made of active materials and KBr powder, using an FTIR spectrometer (Nicolet, 6700 series, USA). XPS measurements were collected using an AXIS Ultra DLD spectrometer with Al Kα (1,253.6 eV) radiation, to investigate the changes in the compositions on the cathode surface. The compositions of the pristine, and GSIR LR-NCM sample were confirmed quantitatively by inductive coupled plasma-atomic emission spectrometry with an emission spectrometer (Optima 2100 DV, Perkin-Elmer). The tap density of the pristine LR-NCM sample was measured and calculated by tap density instrument (FSZ-4, Ruike).
TOF powder ND
The TOF powder ND data were collected on the VULCAN beamline at the Spallation Neutron Sources (SNS) in the Oak Ridge National Laboratory46. Around 0.6 g of powder was packed into a vanadium sample can. An incident beam (5 mm × 12 mm) of 0.7- to 3.5-Å bandwidth allowing a 0.5–2.5 Å d-spacing in the diffracted patterns in the±90° 2θ detector banks was selected, using the double-disk choppers at a speed of 30 Hz. A high-resolution mode was used with Δd/d ∼0.25%. The SNS was at a nominal 1,100 kW. Powder-neutron-diffraction data were collected at a high-resolution mode for 3 h and reduced by the VDRIVE software. Full-pattern Rietveld refinement was performed using the GSAS programs with the EXPGUI interface47.
TEM characterization
TEM images and EELS spectra were carried out using the JEM-ARM200CF scanning/transmission electron microscope at Brookhaven National Laboratory. The instrument is equipped with a cold field-emission source, two aberration-correctors and a high-resolution dual electron energy-loss spectrometer (Quantum GIF). The optimal energy-resolution was ∼0.35 eV, as judged by the full-width at half-maximum of the zero-loss peak. Spectra were recorded either using a convergent-beam in STEM mode, or parallel-beam in TEM mode, for diffraction, imaging and spectroscopy. The refinement Mn L3, and L2 energy, and L3/L2 intensity ratio were based on Pearson's method48.
X-ray diffraction and X-ray absorption spectroscopy
SXRD data were collected using beamline BL14B1 at the Shanghai Synchrotron Radiation Facility (SSRF). These data were recorded in the 2θ range between 10o and 85o, using a fixed wavelength of 1.2398 Å. The structural composite study was carried out by Rietveld refinement analysis, using the GSAS program. For X-ray absorption fine-structure spectroscopy studies, we performed measurements at beamline BL14W1 of the SSRF. The incident beam was mono-chromatized using a Si (111) fixed-exit, double-crystal monochromator. Foils of nickel, manganese and cobalt were used for energy calibration with zero energy (E0), defined according to Kraft et al.49. Spectra were acquired in transmission mode, utilizing gas-ionization chambers as detectors. Data on X-ray absorption near-edge structure was extracted with established methods using the ATHENA software package.
Electrochemical measurements
The negative electrodes were prepared by casting the slurry with a mixture of 80 wt.% the pristine or GSIR LR-NCM, 10 wt.% acetylene black and 10 wt.% polyvinylidene fluoride binder on an aluminium foil. Electrode discs of 13-mm diameter were punched from the negative electrode, pressed at 8 Mpa per disc and dried at 80 °C for 12 h in vacuum to remove any residual NMP and traces of water. The prepared electrodes then were assembled in an Ar-filled glove box (H2O<0.1 p.p.m. and O2<0.1 p.p.m.). Metallic Li was used as the counter electrode, and Celgard 2502 as a separator. The electrolyte solution was 1 M LiPF6 in a 3:7 (volume ratio) mixture of ethylene carbonate–dimethyl carbonate (Zhangjiagang Guotai-Huarong New Chemical Materials Co., Ltd.). The cells were galvanostatically charged and discharged on a LAND-CT2001A battery-test system. Unless otherwise specified, the cells typically cycled between 2.0 and 4.8 V versus Li+/Li0, and the temperature for testing was about 28 °C. EIS measurements were performed on an Autolab83710 impedance analyser with a.c. voltage amplitude of 5 mV, and frequency range of 0.01–100,000 Hz. The metallic Li served as both the counter and reference electrodes during the EIS measurements.
Operando DEMS
Operando DEMS analysis was carried out to detect the gases generated during the initial charge–discharge process. The DEMS cell was assembled in an Ar-filled glove box. In all, 1 M LiPF6 in 1:1 ethylene carbonate:dimethyl carbonate was used as the electrolyte, and a glass-fiber filter (Whatman GF/D) was used as a separator. Electrodes were prepared by casting a slurry with a composition of 80 wt. % active materials, 10 wt. % Super C65 (Timcal) and 10 wt.% PVDF (Kynar FLEX 761A, Arkema Group) on to an aluminium current-collector foil. Electrode tapes then were punched into 22-mm diameter discs, and dried at 120 °C in an under vacuum oven for 24 h. The electrodes were assembled into Swagelok-type cells, using metallic Li as both the counter and the reference. Thereafter, the cell was connected to the mass spectrometer. The cell was purged continuously with gaseous Ar, which flowed from the cell into the mass spectrometer carrying the evolved gases for MS analysis. Operando DEMS cells were conducted on a VSP electrochemical workstation (Biologic, France). The mass signals were recorded as a function of time and the cell voltage. The time resolution of ion-current intensity is optimized by selectively scanning the m/z 32 and 44 signals. In line with previous studies41,42, only O2 and CO2 were evolved during the initial cycling of Li-rich layered-oxide electrodes.
Computation methodology
First-principles calculations were performed in the spin-polarized GGA+U approximations to the density functional theory. The core electron states were represented by the projector-augmented-wave method50 as implemented in the Vienna ab initio simulation package51,52,53. We used the Perdew–Burke–Ernzerhof54 exchange correlation, and a plane-wave representation for the wave function with a cutoff energy of 450 eV. The Brillouin zone was sampled with a dense k-points mesh by Gamma packing. Our supercell, composed of 24 formula units of Li[Ni1/4Li1/6Mn7/12]O2, used in our previous work was used again to obtain the site's stability and activation barrier. The Hubbard U correction was introduced to describe the effect of localized d electrons of the transition-metal ions. The applied effective U value given to Mn ions is 5 eV, and that to Ni ions is 5.96 eV (ref. 55). In this calculation, a concentration of Li20/28 was applied, in which the GSIR LR-NCM exhibits a difference in electrochemical performance compared with pristine LR-NCM. The nudged elastic band method was used to find the minimum-energy path and the energy barrier for Li diffusion inside the materials.
Data availability
The data that support the findings of this study are available from the corresponding authors on request.
Additional information
How to cite this article: Qiu, B. et al. Gas–solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat. Commun. 7:12108 doi: 10.1038/ncomms12108 (2016).
Supplementary Material
Supplementary Figures 1-8, Supplementary Table 1, Supplementary Note 1 and Supplementary References
Acknowledgments
NIMTE is grateful for financial support from Strategic Priority Research Program of Chinese Academy of Sciences (CAS, Grant No. XDA09010101), Key Projects in Cooperation between CAS and Department of Energy, USA (CAS-DOE, Grant No. 174433KYSB20150047), and Ningbo Science and Technology Innovation Team (Grant No. 2012B82001). UC San Diego's efforts are supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the U.S. Department of Energy (DOE) under Contract No. DE-AC02-05CH11231, Subcontract No. 7073923, under the Advanced Battery Materials Research (BMR) Program. Work at Brookhaven National Laboratory is supported by the U.S. DOE, Office of Basic Energy Science, Division of Materials Science and Engineering, under Contract No. DE-AC02-98CH10886. The synchrotron XRD and XAS measurements were carried out at SSRF of the beamline BL14B1 and BL14W1, respectively. The neutron experiments benefit from the SNS user facilities (VULCAN beamline) sponsored by the office of Basic Energy Sciences (BES), the Office of Science of the U.S. DOE. We acknowledge Dr Ding-Yi Tong for the design of the schematic.
Footnotes
Author contributions B.Q. prepared the samples. M.Z., H.L. and Y.S.M. performed the mechanism analysis. B.Q. and M.Z. tested the electrochemical performances and performed analysis. L.W. and Y.Z performed the electron microscopy imaging, diffraction and spectroscopy experiments and analyses. J.W. performed the operando mass spectrometry analysis. D.Q. and Y.S.M. performed the first-principles calculations. H.L., Y.C., K.A. and S.H. recorded ND experiment data and analyses. Z.L., Y.S.M., and Y.X. supervised the research. B.Q. and M.Z. co-wrote the manuscript. All authors contributed to the discussion and provided feedback on the manuscript.
References
- Adler S. B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 104, 4791–4843 (2004). [DOI] [PubMed] [Google Scholar]
- Liao P. & Carter E. A. New concepts and modeling strategies to design and evaluate photo-electro-catalysts based on transition metal oxide. Chem. Soc. Rev. 42, 2401–2422 (2013). [DOI] [PubMed] [Google Scholar]
- Huang R. C. et al. Oxygen-vacancy ordering at surfaces of lithium manganese (III, IV) oxide spinel nanoparticles. Angew. Chem. Int. Ed. 50, 3053–3057 (2011). [DOI] [PubMed] [Google Scholar]
- Okamoto Y. Ambivalent effect of oxygen vacancies on Li2MnO3: a First-Principles study. J. Electrochem. Soc. 159, A152–A156 (2012). [Google Scholar]
- Sushko P. V., Rosso K. M., Zhang J.-G., Liu J. & Sushko M. L. Oxygen vacancies and ordering of d-levels control voltage suppression in oxide cathodes: the case of spinel LiNi0.5Mn1.5O4-δ. Adv. Funct. Mater. 23, 5530–5535 (2013). [Google Scholar]
- Kim J.-H., Myung S.-T., Yoon C. S., Kang S. G. & Sun Y.-K. Comparative study of LiNi0.5Mn1.5O4-δ and LiNi0.5Mn1.5O4 cathodes having two crystallographic structures: Fd-3m and P4332. Chem. Mater. 16, 904–916 (2007). [Google Scholar]
- Jung S.-K. et al. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 4, 201300787 (2014). [Google Scholar]
- Lin F. et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5, 3529 (2014). [DOI] [PubMed] [Google Scholar]
- Yu H. et al. Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2 cathode material for lithium-ion batteries. Angew. Chem. Int. Ed. 52, 5969–5973 (2013). [DOI] [PubMed] [Google Scholar]
- Johnson C. S. et al. The significance of the Li2MnO3 component in ‘composite' xLi2MnO3·(1-x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Commun. 6, 1085–1091 (2004). [Google Scholar]
- Thackeray M. M. et al. Li2MnO3-stabilized LiMO2 (M=Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 17, 3112–3125 (2007). [Google Scholar]
- Rossouw M. H. & Thackeray M. M. Lithium manganese oxides from Li2MnO3 for rechargeable lithium battery applications. Mater. Res. Bull. 26, 463–473 (1991). [Google Scholar]
- Lu Z., MacNeil D. D. & Dahn J. R. Layered cathode materials Li[NixLi1/3-2x/3Mn2/3−x/3]O2 for lithium-ion batteries. Electrochem. Solid-State Lett. 4, A191–A194 (2001). [Google Scholar]
- Xia Q. et al. A Li-rich layered@spinel@carbon heterostructured cathode material for high capacity and high rate lithium-ion batteries fabricated via an in situ synchronous carbonization-reduction method. J. Mater. Chem. A 3, 3995–4003 (2015). [Google Scholar]
- Hy S. et al. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ. Sci. 9, 1931–1954 (2016). [Google Scholar]
- Ohzuku T., Nagayama M., Tsuji K. & Ariyoshi K. High-capacity lithium insertion materials of lithium nickel manganese oxides for advanced lithium-ion batteries: toward rechargeable capacity more than 300 mAh g−1. J. Mater. Chem. 21, 10179–10188 (2011). [Google Scholar]
- Lu Z. & Dahn J. R. Understanding the anomalous capacity of Li/ Li[NixLi1/3-2x/3Mn2/3−x/3]O2 cells using in situ X-ray diffraction and electrochemical studies. J. Electrochem. Soc. 149, A815–A822 (2002). [Google Scholar]
- Armstrong A. R. et al. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 128, 8694–8698 (2006). [DOI] [PubMed] [Google Scholar]
- Koga H. et al. Reversible oxygen participation to the redox processes revealed for Li1.2Mn0.54Co0.13Ni0.13O2. J. Electrochem. Soc. 160, A786–A792 (2013). [Google Scholar]
- Koga H. et al. Different oxygen redox participation for bulk and surface: a possible global explanation for the cycling mechanism of Li1.20Mn0.54Co0.13Ni0.13O2. J. Power Sources 236, 250–258 (2013). [Google Scholar]
- Sathiya M. et al. High performance Li2Ru1-yMnyO3 (0.2 ≤ y ≤ 0.8) cathode materials for rechargeable lithium-ion batteries: their understanding. Chem. Mater. 25, 1121–1131 (2013). [Google Scholar]
- Sathiya M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013). [DOI] [PubMed] [Google Scholar]
- Oishi M. et al. Direct observation of reversible charge compensation by oxygen ion in Li-rich manganese layered oxide positive electrode material, Li1.16Ni0.15Co0.19Mn0.50O2. J. Power Sources 276, 89–94 (2015). [Google Scholar]
- Xu B., Fell C. R., Chi M. & Meng Y. S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: a joint experimental and theoretical study. Energy Environ. Sci. 4, 2223–2233 (2011). [Google Scholar]
- Fell C. R. et al. Correlation between oxygen vacancy, microstrain, and cation distribution in lithium-excess layered oxides during the first electrochemical cycle. Chem. Mater. 25, 1621–1629 (2013). [Google Scholar]
- Qian D., Xu B., Chi M. & Meng Y. S. Uncovering the roles of oxygen vacancies in cation migration in lithium excess layered oxides. Phys. Chem. Chem. Phys. 16, 14665–14668 (2014). [DOI] [PubMed] [Google Scholar]
- Kubota K. et al. Direct synthesis of oxygen-deficient Li2MnO3-x for high capacity lithium battery electrodes. J. Power Sources 216, 249–255 (2012). [Google Scholar]
- Song B. et al. High rate capability caused by surface cubic spinels in Li-rich layer-structured cathodes for Li-ion batteries. Sci. Rep. 3, 3094–3105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. H., Johnson C. S., Vaughey J. T., Amine K. & Thackeray M. M. The effects of acid treatment on the electrochemical properties of 0.5Li2MnO3-0.5LiNi0.44Co0.25Mn0.31O2 electrodes in lithium cells. J. Electrochem. Soc. 153, A1186–A1192 (2006). [Google Scholar]
- Zhao J. et al. An ion-exchange promoted phase transition in a Li-excess layered cathode material for high-performance lithium ion batteries. Adv. Energy Mater 5, 201401937 (2015). [Google Scholar]
- Yu D. Y. W., Yanagida K. & Nakamura H. Surface modification of Li-excess Mn-based cathode materials. J. Electrochem. Soc. 157, A1177–A1182 (2010). [Google Scholar]
- Sun Y. K. et al. The role of AlF3 coatings in improving electrochemical cycling of Li-enriched nickel-manganese oxide electrodes for Li-ion batteries. Adv. Mater. 24, 1192–1196 (2012). [DOI] [PubMed] [Google Scholar]
- Villevieille C., Lanz P., Bünzli C. & Novák P. Bulk and surface analyses of ageing of a 5V-NCM positive electrode material for lithium-ion batteries. J. Mater. Chem. A 2, 6488–6493 (2014). [Google Scholar]
- Yabuuchi N., Yoshii K., Myung S. T., Nakai I. & Komaba S. Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3-LiCo1/3Ni1/3Mn1/3O2. J. Am. Chem. Soc. 133, 4404–4419 (2011). [DOI] [PubMed] [Google Scholar]
- Qiu B. et al. Enhanced electrochemical performance with surface coating by reactive magnetron sputtering on lithium-rich layered oxide electrodes. ACS Appl. Mater. Interfaces 6, 9185–9193 (2014). [DOI] [PubMed] [Google Scholar]
- Liu H., Fell C. R., An K., Cai L. & Meng Y. S. In situ neutron diffraction study of the xLi2MnO3·(1-x)LiMO2 (x=0, 0.5; M=Ni, Mn, Co) layered oxide compounds during electrochemical cycling. J. Power Sources 240, 772–778 (2013). [Google Scholar]
- Wu L. J. et al. Structural defects of silver hollandite, AgxMn8Oy, nanorods: dramatic impact on electrochemistry. ACS Nano 9, 8430–8439 (2015). [DOI] [PubMed] [Google Scholar]
- Arunkumar T. A., Wu Y. & Manthiram A. Factors influencing the irreversible oxygen loss and reversible capacity in layered Li[Li1/3Mn2/3]O2-Li[M]O2 (M=Mn0.5-yNi0.5-yCo2y and Ni1-yCoy) solid solutions. Chem. Mater. 19, 3067–3073 (2007). [Google Scholar]
- Wu Y. & Manthiram A. High capacity, surface-modified layered Li[Li(1-x)/3Mn(2-x)/3Nix/3Cox/3]O2 cathodes with low irreversible capacity loss. Electrochem. Solid-State Lett. 9, A221–A224 (2006). [Google Scholar]
- Liu W. et al. Countering voltage decay and capacity fading of lithium-rich cathode material at 60 °C by hybrid surface protection layers. Adv. Energy Mater. 5, 201500274 (2015). [Google Scholar]
- Lanz P., Sommer H., Schulz-Dobrick M. & Novák P. Oxygen release from high-energy xLi2MnO3-(1-x)LiMO2 (M=Mn, Ni, Co): electrochemical, differential electrochemical mass spectrometric, in situ pressure, and in situ temperature characterization. Electrochim. Acta 93, 114–119 (2013). [Google Scholar]
- Castel E., Berg E. J., El Kazzi M., Novák P. & Villevieille C. Differential electrochemical mass spectrometry study of the interface of xLi2MnO3·(1-x)LiMO2 (M=Ni, Co, and Mn) material as a positive electrode in Li-ion batteries. Chem. Mater. 26, 5051–5057 (2014). [Google Scholar]
- Yu H. et al. Electrochemical kinetics of the 0.5Li2MnO3-0.5LiMn0.42Ni0.42Co0.16O2 ‘composite' layered cathode material for lithium-ion batteries. RSC Adv. 2, 8797–8807 (2012). [Google Scholar]
- Kang K., Meng Y. S., Breger J., Grey C. P. & Ceder G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 311, 977–980 (2006). [DOI] [PubMed] [Google Scholar]
- Qiu B. et al. Electrochemical investigation of Li-excess layered oxide cathode materials/mesocarbon microbead in 18650 batteries. Electrochim. Acta 123, 317–324 (2014). [Google Scholar]
- An K., Wang X. L. & Stoica A. D. Vulcan data reduction and interactive visualization software, ORNL Report, 621 (2012).
- Toby B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 34, 210–213 (2001). [Google Scholar]
- Pearson D. H. et al. White lines and d-electron occupancies for the 3d and 4d transition metals. Phys. Rev. B: Condens. Matter Mater. Phys. 47, 8471–8478 (1993). [DOI] [PubMed] [Google Scholar]
- Kraft S., Stümpel J., Becker P. & Kuetgens U. High resolution X-ray absorption spectroscopy with absolute energy calibration for the determination of absorption edge energies. Rev. Sci. Instrum. 67, 681–687 (1996). [Google Scholar]
- Kresse G. & Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999). [Google Scholar]
- Kresse G. & Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Hafner J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994). [DOI] [PubMed] [Google Scholar]
- Perdew J. P., Burke K. & Wang Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 54, 16533–16539 (1996). [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Meng Y. S., Kang K. & Ceder G. Phase transitions in the LiNi0.5Mn0.5O2 system with temperature. Chem. Mater. 19, 1790–1800 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-8, Supplementary Table 1, Supplementary Note 1 and Supplementary References
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on request.