Abstract
Radical gastrectomy with an adequate lymphadenectomy is the main procedure which makes it possible to cure patients with resectable gastric cancer (GC). A number of randomized controlled trials and meta-analysis provide phase III evidence that laparoscopic gastrectomy is technically safe and that it yields better short-term outcomes than conventional open gastrectomy for early-stage GC. While laparoscopic gastrectomy has become standard therapy for early-stage GC, especially in Asian countries such as Japan and South Korea, the use of minimally invasive techniques is still controversial for the treatment of more advanced tumours, principally due to existing concerns about its oncological adequacy and capacity to carry out an adequately extended lymphadenectomy. Some intrinsic drawbacks of the conventional laparoscopic technique have prevented the worldwide spread of laparoscopic gastrectomy for cancer and, despite technological advances in recent year, it remains a technically challenging procedure. The introduction of robotic surgery over the last ten years has implied a notable mutation of certain minimally invasive procedures, making it possible to overcome some limitations of the traditional laparoscopic technique. Robot-assisted gastric resection with D2 lymph node dissection has been shown to be safe and feasible in prospective and retrospective studies. However, to date there are no high quality comparative studies investigating the advantages of a robotic approach to GC over traditional laparoscopic and open gastrectomy. On the basis of the literature review here presented, robot-assisted surgery seems to fulfill oncologic criteria for D2 dissection and has a comparable oncologic outcome to traditional laparoscopic and open procedure. Robot-assisted gastrectomy was associated with the trend toward a shorter hospital stay with a comparable morbidity of conventional laparoscopic and open gastrectomy, but randomized clinical trials and longer follow-ups are needed to evaluate the possible influence of robot gastrectomy on GC patient survival.
Keywords: Gastric cancer, Gastric resection, Minimally invasive surgery, Laparoscopic gastrectomy, Robot-assisted gastrectomy
Core tip: Laparoscopic gastrectomy has been demonstrated to be feasible and oncologically adequate for early gastric cancer (GC). Major criticism arose instead towards the spread of the use of laparoscopy for advanced GC, principally due to its poor suitability to complex maneuvers, such as extended lymphadenectomy. In recent years, robotic surgery techniques have been shown to make certain laparoscopic procedures easier and safer, such as during D2 lymph node dissection. Authors increasingly cite robotic-assisted gastrectomy as one of the most promising tools to extend the minimally invasive surgical indications for advanced GC patients.
INTRODUCTION
Total and distal gastrectomy with D2 lymph node dissection is the recommended surgical procedure for resectable (curable) gastric cancer (GC) patients[1]. The current medical evidence shows that standardized extended (D2) lymphadenectomy leads to better results than standardized limited (D1) lymphadenectomy in terms of lower loco-regional recurrence and gastric-cancer-related death rates, with equal postoperative mortality, morbidity and re-operation rates so far, thanks to the currently standard safer spleen-preserving D2 resection technique[1].
Laparoscopic surgery was introduced for the treatment of GC in 1991, when Kitano et al[2] performed the first laparoscopically assisted gastrectomy for GC. Since then laparoscopic assisted distal gastrectomy (LADG) for distal early-stage GC has progressively spread worldwide, especially in Eastern countries, such as Japan and South Korea[3,4]. During the years, reports have provided level III evidence that LADG is technically safe and that it yields better short-term outcomes than conventional open gastrectomy for early-stage GC[5]. While laparoscopic gastrectomy (LG) has become standard therapy for early-stage GC, especially in Asian countries, such as Japan and South Korea[5,6], a safer spleen-preserving LG with D2 resection technique for the treatment of more advanced GC did not meet the same values and is currently available only in high-volume centers. The widespread diffusion of laparoscopic surgery to manage advanced GC[7,8] was limited, mainly by the technical difficulties posed by the total gastrectomy and the complexity of D2 lymphadenectomy, which entailed the removal of node stations along the celiac trunk, left gastric artery and hepatic pedicle. This gave rise to concern regarding the oncological feasibility and long-term outcomes of laparoscopic surgery for advanced GC.
The introduction of robot-assisted techniques improved some surgical procedures, especially when precise dissection is required, and gives them an advantage over conventional laparoscopy techniques. By making it possible to overcome some intrinsic limitations of the traditional laparoscopic approach, robot-assisted LG is advocated by some authors as able to facilitate complex reconstruction after gastrectomy and the lymph node retrieval, so as to permit radical resection and adequate lymph node dissection also in advanced GC patients[9-11].
LAPAROSCOPIC GASTRECTOMY
In the past decade, laparoscopic techniques have gained wide clinical acceptance in surgical practice. The principal advantages of laparoscopic over conventional open surgery are the reduction in stress, induced by minimal manipulation of the small bowel and the use of a small incision, accounting for earlier return to normal bowel function with earlier resumption of oral intake, less postoperative nausea, vomiting, and abdominal discomfort, reduction of postoperative pain and acceleration of discharge from hospital. Mitigating surgical stress reduces the generalized inflammatory reaction; consequently, it may lead to a reduction in the overall complication rate.
Laparoscopic surgery was introduced for the treatment of GC in 1994 by Kitano et al[2], who performed the first laparoscopically assisted distal gastrectomy (LADG) for early GC. Since then LADG for distal early-stage GC has progressively spread worldwide, especially in Eastern countries, such as Japan and South Korea[3,4].
During the years a large number of outcome variables were analyzed for individual series and comparative trials between laparoscopic assisted gastrectomy (LAG) and open gastrectomy (OG)[12]. Laparoscopic surgery was reported to give similar results to those with the open method regarding the oncological principles, with generally better postoperative patient comfort[12]. However, mainly due to the heterogeneity of available data and the extreme complexity of such a structured matter, comprising different topics on GC also worthy of being treated by different approaches, any meaningful conclusions regarding the advantages or disadvantages of LAG over conventional open procedures are difficult to draw. It was decided that this review strategy of discussion would divide the topic in main areas by the separation of two essentially different laparoscopic techniques, distal and total gastrectomy, and two fundamental oncological aspects, early and advanced GCs. Finally, we discussed the potential advantage of the newer introduction of the robotic assisted laparoscopic technique.
Laparoscopic assisted distal gastrectomy
Several reports demonstrated that laparoscopic distal gastrectomy is a feasible and safe technical option in the treatment of GC, in particular for early GC[3].
However, the comparative data between this technique and the conventional open distal gastrectomy (ODG) originated mainly from retrospective and observational studies with a small number of patients, and limited essentially to early GC patients. Randomized controlled trials (RCTs) are few and have few patients. These studies largely represent the experience of Eastern countries.
In the last years, a number of meta-analysis on this topic have been published. There are 4 meta-analyses[3,13-15] based on few RCTs and a large number of retrospective and prospective studies that compare short-term outcomes of LADG and conventional ODG. The meta-analysis of Hosono et al[13] published in 2006 (12 retrospective studies, 4 RCTs) included patients with advanced GC, while Yakoub et al[3] (2009, 12 retrospective studies, 3 RCTs) and Zeng et al[15] (2012, 17 non-randomized studies, 5 RCTs) focused on early GC only. Finally, the more recent and large meta-analysis by Viñuela et al[14] (2012) included 6 RCTs and 19 high quality non-RCTs, with global 3055 patients (1658 LDG, 1397 ODG) studied, with a high proportion of stage I cancers.
All these analyses reported that LADG for gastric adenocarcinoma is associated with comparable or lower complications, reduction in the operative blood loss, less pain, faster bowel function recovery, and shorter hospital stay with respect to ODG. Moreover, all the meta-analysis demonstrated that LADG has a similar or lower overall morbidity rate, but that a higher number of lymph nodes were harvested by ODG, although this number did not become significant when less than D2 lymphadenectomy was performed.
Other 6 meta-analysis[4,16-20] focused on the comparative evaluation between LADG and ODG considering RCTs only. The meta-analysis of Memon et al[16] (2008) and Sun et al[17] (2012) focused on 4 and 8 RCTs respectively, including all GC patients independently from the tumour stage. The other 4 meta-analysis[4,18-20] focused their evaluation on respectively 4, 6, 6, and 7 RCTs comparing LADG and ODG restricted to early GC only. Currently, other 2 meta-analysis[21,22], have been published in literature which included 6 and 8 randomized studies respectively, but they are both biased due to an inclusion criteria error. These authors, in fact, claimed to have conducted a meta-analysis of RCTs on LADG vs ODG for early GC, but effectively they included two studies (Huscher et al[7] and Varela et al[23]) the former is not limited to early cancer, and the latter is not a RCT .
However, all these studies showed essentially similar results, reporting a significant reduction of intraoperative blood loss in favor of LADG, at the expense of significantly longer duration of operating time and significant reduction in lymph nodes harvesting compared to the conventional open procedure. Length of hospital stay, complications, tumour recurrence and mortality rates were found to be similar in both groups or smaller in the laparoscopy group.
Thus, globally in all these meta-analysis, whether only data from RCTs is considered or data pooled by prospective studies, or whether GC unselectively from their stages is considered, or whether the analysis is limited to early GC only, the result of an inferior number of lymph nodes harvesting by LADG compared to ODG is always constant. Just only one recent meta-analysis[24] reported a dissimilar result in regards to number of lymph nodes dissected, but it focused only on non-randomized retrospective studies. In fact, the work of Ding et al[24] included studies comparing LADG and ODG for GC only associated to D2 lymphadenectomy, while all patients submitted to lymphadenectomy inferior (D1 or D1+α or D1+β) or superior (D2+ or D3) to D2, independently from the GC stage, were excluded from the final analysis. Eight retrospective nonrandomized studies, totaling 1065 distal gastrectomies (510 LADG and 555 ODG), were considered suitable for this meta-analysis. The final results were similar to the previous ones in terms of decreased blood loss, fewer complications, faster recovery, shorter hospitalization, as well as longer operating time in the LADG group, but no evident differences regarding the number of lymph nodes dissected were found between the two groups. Probably, the main reason for this discrepancy, other than the fact that all the included studies were retrospective and thus potentially leading to some selection bias, is that the meta-analysis of Ding et al included only gastrectomies with D2 lymphdenectomy, while previous meta-analysis on LADG[3,13-20] comprised different proportions of D1, D1+ ad D2 lymph nodes dissection. Extended D2 lymphadenectomy is a more complex procedure, principally indicated for advanced GC, and thus it is a procedure only more recently performed in highly experienced centers. In fact, the series included in the study of Ding et al are essentially newer compared to those in previous meta-analysis[3,13-20]. As the operative technique has developed, the number of lymph nodes dissected by LDG has gradually increased[25] and increasingly highly skilled surgeons will report adequate D2 lymphadenectomy[24,25]. The improvements in instruments and techniques could enable ever more surgeons to carry out an extended lymphadenectomy and decrease the operating time for LADG[13,26], which is essentially related to the knowledge of and familiarity with the laparoscopic system and the skill of the operating team[27].
Therefore, as lymph node metastasis has been considered one of the most significant predictive factors for recurrence and subsequently survival in patients with GC[3,28], concern has been raised regarding the oncological adequacy of LADG, which generally yields a lower number of lymph nodes with respect to ODG. The oncologic effect of procuring less nodes after LADG is understandable only if we separate early from advanced GC, treatment of which will occur in the following sections. Instead, with regards to the technical aspects, what must be outlined here is that laparoscopic systematic D2 lymphadenectomy is technically complicated. Large vessels have to be identified and extensive lymph node dissection has to be performed. In particular, this laparoscopic procedure is complex in the dissection of the perigastric lymph nodes along the major curvature and the second tier nodes along the celiac and splenic arteries[29]. Essentially, LADG with D2 lymphadenectomy remains a challenging and time-consuming procedure, and is significantly longer than ODG[3,13]. Another important point of potential heterogeneity and bias among results of different studies is that most postoperative recovery measures, such as time to oral intake and walking, as well as administration of analgesic drugs and length of hospital stay, are rather subjective and dependent on the patient’s attitude as well as the policy of practice of the surgical team. This may explain the significant heterogeneity between studies which could not be overcome by subgroup analyses.
Moreover, clinical evidence regarding long-term outcomes of LADG for GC is still lacking. Only 1 prospective randomized controlled trial (Huscher et al[7]) reported no significant difference in the 5-year overall survival and disease-free survival rate between LADG and ODG. Several retrospective studies reported comparable results[13], of which one of the largest is the study of Gordon et al[30], that have the merit of focusing on patients with advanced GC, comparing LADG to ODG with quite a long follow-up period (mean of 49.2 mo). The study involved 201 patients, 66 of whom underwent LADG, and the authors did not find significant differences in terms of 5-year overall survival and disease specific compared to those submitted to ODG.
However, an overall shortage of statistical significance in the long-term outcomes of LADG still exists and may be attributable principally to the small sample size. Therefore, there was no sufficient data to allow a definitive conclusion on survival after LADG and long-term survival benefit remains to be proven by many high-quality RCTs with larger sample sizes.
Other limitations that warrant emphasis are the following. Most high quality data originates from Eastern experience. An intrinsic biological difference in GC tumour between Eastern and Western countries is well known. Molecular and biological evidence suggests that gastric adenocarcinoma can be divided into distinct sub-types based on the predominant histology and distinct patterns of gene expression[31,32]. Western patients typically present with more advanced lesions, have a higher proportion of proximal or gastro-oesophageal-junction tumours, and a greater proportion of diffuse-type histology[33]. Understanding geographic differences and the clinical and pathologic manifestations of various GC sub-types could in the future help to direct the surgical and medical management of this heterogeneous disease. Moreover, the advancements in diagnostic modalities and mass examination techniques employed in Eastern countries, together with nationwide screening and Helicobacter pylori eradication programs that have been in act for several years in Asia[34,35], have made the earlier detection of GC with respect to West possible. This meant more patients with early GC and an increase in the awareness of minimally invasive approaches for treatment. Conversely, the disease incidence is much lower in the West and a greater proportion of patients present with locally advanced tumor that render laparoscopic resection less feasible. Notably, in many institutions in the United States and some in Western countries an extensive prophylactic lymphadenectomy D2 in advanced GC patients is not routinely performed, firstly because complications of open gastrectomy with extended lymphadenectomy are still high enough to be considered disadvantageous in balance with oncologic benefit, and secondly because they are at low volume centers where experience with advanced laparoscopic gastric surgery is limited. A recent multi-center analysis[36] reported a median of 14 lymph nodes examined in a United States minimally invasive cohort, which is inadequate according to consensus guidelines. Conversely, higher operative case volume was associated with the greatest odds of adequate lymph node staging, confirming the association between surgical volume and surgical quality[37,38]. As long as these differences between East and West exist, an appropriate comparison of the results among studies of different geographic origin is difficult, and in particular what is not strictly appropriate is to translate Asian results to western GC patients without proper confirmation.
In conclusion, LADG is associated with a similar or lower morbidity and better short-term outcomes compared to ODG. Considering the literature evidence concerning its oncological adequacy, LADG is indicated for early GC and presently has become a routine option in South Korea and Japan for these early lesions. On the other hand, these results largely represent the Eastern experience and cannot be extrapolated to patients with advanced tumours. Significant limitations exist to draw definitive conclusions for all GC patients and for oncologic adequacy of laparoscopic D2 lymphadenectomy. The limited number of published RCTs, the small sample sizes to date and the limited duration of follow up does not make it possible to indicate the use of LADG as adequate for every advanced GC. Further large multi-center RCTs are required to increase statistical power and to delineate significantly quantifiable differences between LADG and ODG. In particular, well-designed RCTs which standardize postoperative measures and elucidate oncological clearance, including the quality of lymphadenectomy and long-term outcomes, are needed to establish whether LADG could be a standard treatment for advanced GC too.
Laparoscopic total gastrectomy
In contrast to LADG, there is not such a widespread acceptance of laparoscopic assisted total gastrectomy (LATG) as an alternative to the open approach, essentially due to its technical difficulty. In particular the procedure gives rise to concern about the complexity encountered in composite reconstruction of the alimentary tract, such as esophago-jejunal anastomosis, and potentially serious subsequent complications.
Currently, there is no standard method for restoration of continuity of the oesophagus and jejunum. In open surgery, an esophagojejunostomy is typically performed end-to-side using a circular stapler. This procedure is very difficult to reproduce in conventional laparoscopy, because placing a purse-string suture on the esophageal stump requires particular skill.
Solutions to restore the digestive transit following LATG have been reported. The most common are to perform a laparoscopic intracorporeally esophagojejunostomy using a linear stapler (side-to-side)[39] or with a circular stapler (end-to-end)[40]. Alternatively with a hybrid-open technique performing the esophagojejunostomy extra-corporeally through the same minilaparotomy used for specimen removal[41] or with a full robotic technique performing hand-sewn anastomosis[42]. The optimal method to perform anastomosis remains to be established and it is probable that there is not one single optimal method.
Opponents to LATG argue that there is a higher incidence of major intra- and post-operative complications due to the complexity of the procedure, made difficult also by the absence of tactile sensation, much longer anesthetic and operating times and insufficient surgical resection margins compared to conventional open gastrectomy[16]. The incidence of post-operative complications is reported to be higher when compared with distal gastrectomy too. The rates of infra-operative and post-operative complications in LATG were 2.6% and 21.8%, respectively[43], which is still high, despite the progressive development of laparoscopic techniques and while the complication rate in LADG is decreasing year by year.
Recently a systematic review[44] was conducted to research studies comparing LATG with open total gastrectomy (OTG) in GC. Only 8 non randomized comparative or case-control studies fulfilled quality criteria and were selected for this meta-analysis. LATG demonstrated compared with OTG a significant reduction of intra-operative blood loss, a reduced risk of post-operative complications, a shorter hospital stay, at the cost of longer operative time. Fewer lymph nodes were dissected in LATG than in OTG, even though the difference was not significant. Data on long-term survival were not sufficiently addressed in the included studies. In conclusion, thus far, no randomized clinical trials evaluating LATG have been conducted and studies available to date can be seen as pioneer work.
Recent meta-analyses[45,46] of LATG have shown that this procedure is feasible in terms of safety and survival, leading to a reduced risk of post-operative complications compared with OTG similar to the risk after LADG, despite the expense of longer operative time. But overall, data available in literature are poor. The sample sizes in these studies were small, there have been no high-quality RCTs, and the existing studies have limitations of potential bias and heterogeneity. The majority of the available studies focuses on early GC and originates from Asian countries[5,23,47-49]. Moreover, among the few reports which focused on the procedure of LATG for advanced GC the majority included less advanced disease, such as those at stage II or stage IIIa[50,51], while the effects of LATG for more advanced GC, such as stages IIIb and IIIc disease, have been rarely reported. Just one recent retrospective single institution study[52] focused on laparoscopic total gastrectomy (LTG) for advanced GC, including quite a large proportion of stage IIIb and IIIc tumours also. The authors demonstrated that in these patients LTG (n = 976) yield comparable oncological and surgical outcomes compared to LADG (n = 646). Finally, most of these studies documented the success of laparoscopic-assisted gastric resection, while only a few studies have examined the totally laparoscopic approach[53], and prospective studies comparing totally laparoscopic total gastrectomy to the open methods are lacking. Thus the results were not conclusive.
Early GC
Since it was introduced, the laparoscopic technique has been progressively used by several specialized centers for the treatment of early GC. Phase III trials (Table 1)[54-60], all originated from Eastern series, and a number of meta-analysis[3,4,14,15,18-20] demonstrated benefits on short-term postoperative outcomes (including less blood loss, less pain, lower incidence of postoperative complications, shorter hospital stay and rapid recovery) of LADG compared to ODG in the treatment of GC at early stage. As discussed in the “Laparocopic distal gastrectomy” section to date, several investigations have reported no differences in recurrence or survival following LADG and ODG for early GC long term[5,7,25,61], although all the available meta-analysis on more relevant pooled data showed that the overall number of lymph nodes retrieved was less in LADG compared to ODG[4,16,18]. An Asian meta-analysis involved studies comparing LAG vs OG, independently from the type of gastrectomy (total or subtotal) for early GC[62] included 5 RCTs and 11 case controls and reported essentially comparable results to those of previous meta-analysis.
Table 1.
Randomized controlled trials comparing laparoscopy-assisted distal gastrectomy with open distal gastrectomy in the treatment early gastric cancer
| Ref. | Year | Country |
Patients (n) |
Operative time1 (mean ± SD, min) |
Blood loss1 (mean ± SD, mL) |
Harvested nodes1 (mean ± SD, n) |
Complications rate (%) |
Hospital stay1 (mean ± SD, d) |
|||||||||||
| LDG | ODG | LDG | ODG | P value | LDG | ODG | P value | LDG | ODG | P value | LDG | ODG | P value | LDG | ODG | P value | |||
| Kitano et al[54] | 2002 | Japan | 14 | 14 | 227 ± 7 | 171 ± 13 | < 0.05 | 177 ± 30 | 258 ± 53 | < 0.05 | 20.2 ± 3.6 | 24.9 ± 3.5 | < 0.05 | 14.3 | 28.6 | < 0.05 | 17.6 ± 2.6 | 16 ± 0.4 | < 0.05 |
| Fujii et al[55] | 2003 | Japan | 10 | 10 | 225 ± 35 | 179 ± 37 | < 0.05 | 134 ± 110 | 205 ± 75 | < 0.05 | NR | NR | - | 2.0 | 2.0 | NS | NR | NR | - |
| Hayashi et al[56] | 2005 | Japan | 14 | 14 | 378 ± 97 | 235 ± 71 | < 0.05 | 327 ± 245 | 489 ± 281 | < 0.05 | 28 ± 14 | 27 ± 10 | NS | 28.6 | 57.1 | < 0.05 | 12 ± 2 | 18 ± 6 | < 0.05 |
| Lee et al[57] | 2005 | South Korea | 24 | 23 | 319 ± 16 | 235 ± 71 | < 0.05 | 336 ± 180 | 294 ± 156 | NS | 31.8 ± 13.5 | 38.1 ± 15.9 | < 0.05 | 12.5 | 43.5 | < 0.05 | 11 ± 4 | 17 ± 15 | < 0.05 |
| Kim et al[58] | 2008 | South Korea | 82 | 82 | 252 ± 48 | 170 ± 27 | < 0.05 | 111 ± 85 | 267 ± 155 | < 0.05 | 39 ± 11.9 | 45.1 ± 13.8 | < 0.05 | 0.0 | 4.9 | < 0.05 | 7.2 ± 1.4 | 8.6 ± 2 | NS |
| Sakuramoto et al[59] | 2013 | Japan | 31 | 32 | 182 ± 37 | 113 ± 21 | < 0.05 | 64 ± 48 | 167 ± 135 | < 0.05 | 31.6 ± 12.2 | 33.8 ± 13.4 | NS | 3.2 | 15.6 | < 0.05 | 9.1 ± 1.1 | 10 ± 3.1 | NS |
| Takiguchi et al[60] | 2013 | Japan | 20 | 20 | 185 ± 23 | 119 ± 17 | < 0.05 | 65 ± 51 | 180 ± 111 | < 0.05 | 33.0 ± 13.7 | 32 ± 5.2 | NS | 0.0 | 10.0 | < 0.05 | 10 ± 0.2 | 11 ± 0.7 | NS |
Mean value. LDG: Laparoscopic distal gastrectomy; ODG: Open distal gastrectomy; NS: Not statistically significant; NR: Not reported.
Why lower number of harvested lymph nodes did not affect long-term survival in early GC patients can be explained by the fact that survival outcome is likely to be more tolerant to a less extensive lymphadenectomy compared to advanced GC stages. In fact, the pattern of lymph node metastasis to the 2nd tier (N2) of nodes is only 3.5% even in submucosal cancer[63] and the prevalence of distant metastasis in recurrence patients indicates that treatment failure may be due to systemic tumour spread rather than local spread[3]. Accordingly, the Japanease GC Association (JGCA) recommended a conservative sub-D2 lymphadenectomy, designated modified gastrectomy A and B, to Stages IA and IB GCs[64].
Thus, for early GCs, particularly for those with infrequent lymph node metastasis, LADG has become a widely accepted alternative treatment option and in Asian countries, such as Japan and South Korea, it has become a standard therapy[5,6].
In conclusion, despite the lower number of retrieved lymph nodes in LADG compared to ODG, a less extensive lymphadenectomy in LADG is oncologically adequate for early GC and thus LADG will not compromise survival in these patients even when performed with a sub-D2 lymphadenectomy. On the contrary, this conclusion cannot be extrapolated for cases with more advanced stage, in which the survival benefit for radical lymphadenectomy is well established[1]. However, one important aspect should be outlined: it is very difficult to diagnose early GC accurately before operation and the underestimation of the preoperative stage is a well known problem[65]. A risk of under staging GC has been reported to occur in up to 25% of patients diagnosed preoperatively as early GC[66-68]. For this reason, care should be taken to increase the accuracy of the preoperative diagnosis before performing minimally invasive gastrectomy by selecting properly indicated patients; conversely, on the basis of this risk, other investigators emphasized the routine need for D2 dissection, even in cases preoperatively suspected as early GC[66,67].
Advanced GC
The use of laparoscopic surgery in the management of advanced GC, contrary to early GC, has not yet met analogous widespread acceptance, mainly due to the controversial issue on the technical difficulty of carrying out D2 lymphadenectomy and insufficient data related to the procedure’s oncological adequacy[69-71]. As treatment options differ for these different stages of GC, the results of laparoscopic surgery for early stage cannot be directly applied to advanced GC.
Even experienced minimally-invasive surgeons reported the technical difficulty of laparoscopic extraperigastric lymphadenectomy[72]. While a number RCTs and meta-analysis have been published for early GC patients (as discussed in “Laparoscopic distal gastrectomy” and “Early GC” sections), such studies have not been conducted for the majority of cases with advanced gastric tumour.
Since Uyama et al[8] first reported laparoscopy assisted total gastrectomy with D2 lymphadenectomy and distal pancreaticosplenectomy for advanced upper-third GCs in 1999, several studies have been reported to determine the technical feasibility of D2 lymphadenectomy in patients with advanced GC[66,73-78]. Although most of these series are retrospective and small, the first results have shown no differences in terms of oncological adequacy, recurrence, morbidity and survival rates of LAG compared with the open approach, confirming at the same time the well known advantages of minimally invasive surgery in improvement of postoperative outcomes[7,71,73,76,79-82].
In 2013, Qiu et al[83] conducted a systematic review and meta-analysis on LADG vs ODG for advanced GC. No RCTs were found by the authors’ search. Conclusively, 7 case-control studies involving 1271 patients (626 LADG and 645 ODG) were considered eligible for the final pooled analysis. The meta-analysis revealed that LADG patients had longer operative time, less estimated blood loss, fewer analgesic requirements, and a shorter hospital stay compared to ODG. There were no significant differences between the 2 groups in number of lymph node dissections, post-operative mortality and complication rates, and 3-year overall survival rate.
Recently, 2 meta-analysis have been conducted[84,85] comparing the clinical outcome of both partial and total LAG and OG for the treatment of advanced GC. In the first[84] overall 7 studies were selected for the analysis (1 prospective RCT, 1 comparative prospective study and 5 comparative retrospective studies), including a total of 452 patients (174 in the LAG and 278 in the OG group). In the second one[85], overall 26 studies were included (1 prospective RCT, 1 comparative prospective study and 24 comparative retrospective studies), totaling 5061 patients, of which 2193 (43.3%) treated by LAG and 2868 (56.7%) underwent OG. The results of both these meta-analysis are overlapping. Compared to OG, laparoscopic total and partial gastrectomy demonstrated a longer operative time but lower blood loss and shorter postoperative hospital stay. Moreover, there were similar outcomes between both approaches in terms of number of dissected lymph nodes, and overall survival and disease-free survival.
The Korean Laparoscopic Gastrointestinal Surgery Study Group (KLASS) recently published their experience on long-term outcomes in patients undergoing laparoscopic resection for advanced lesions[86]. In this multi-center retrospective trial, the long-term outcomes of LAG for advanced GC was analyzed in a relatively large number of cases (n = 239). The median follow-up period was 55.4 mo with an overall 5-year survival rate of 78.8% and disease-specific 5-year survival rate of 85.6%, which were considered comparable to those previously reported for open gastrectomy.
These results make it possible to conclude that laparoscopic resection for advanced GC is feasible with oncologic equivalence to open resection. Although more time was needed to perform LAG, it had some advantages over OG in achieving faster postoperative recovery. However, most studies to date were retrospective, their case volumes varied greatly, and a high heterogeneity between them can be recognized, which could significantly affect the final results of pooled data meta-analysis. Thus, these results are promising, but need to be confirmed in further prospective controlled randomized trials.
SPECIFIC CONSIDERATIONS ON LAPAROSCOPIC D2 LYMPHADENECTOMY
Although only gradually accepted by Western investigators, gastric resection with extended (D2) lymphadenectomy is the standard procedure for advanced GC. The long-term results of RCTs have reported superiority in the survival rate of patients who underwent D2 dissection relative to that of limited (D1) lymphadenectomy[1,87].
Thus, extended D2 lymph node dissection has its proper indication for advanced GC and the ability to perform an adequate D2 lymphadenectomy, with low morbidity and mortality, is crucial for the treatment of local advanced GC.
Gradually, with the passing of time, together with the improvement of laparoscopic technology, an increasing number of surgeons have demonstrated their ability to perform an adequate laparoscopic D2 lymphadenectomy. For example, Huang et al[88] respectively compared 66 vs 69 advanced GC patients who underwent LADG and ODG with D2 lymphadenectomy, and found that similar numbers of lymph nodes were collected in the two groups. Furthermore, the LADG group showed less infra-operative blood loss, earlier bowel recovery, and shorter hospital stays, without increasing the risk of postoperative morbidity and mortality. Zhao et al[89] objectively compare the surgical outcomes of 133 LADG and 133 ODG in a well-matched design trial, showing that LADG with D2 lymphadenectomy is comparable to open surgery in terms of its technical feasibility and safety, and with total numbers of collected lymph nodes similar in the two groups.
Recently two prospective RCTs[65,90] have been conducted to better elucidate the proficiency in performing D2 lymphadenectomy by laparoscopic tool. Cai et al[90] focused their analysis on only advanced GC, comparing open vs laparoscopy-assisted D2 radical gastrectomy: 96 patients were randomly assigned to the LAG group (n = 49) and to the OG group (n = 47) cases. A similar number of harvested lymph nodes was obtained in both groups (22.98 ± 2.704 vs 22.87 ± 2.428, P = 0.839), at the price of significantly longer mean operating time for the LAG group. The postoperative morbidity rate was similar in the two groups, however pulmonary infection was observed more frequently in the OG group. Moreover, after a mean follow-up of 22 mo, the authors did not find a statistically significant difference in the overall estimated survival rate for patients in both groups. The second RCT[65] was conducted again with the specific aim of evaluating the radicalness and safety of laparoscopic D2 dissection, but in this case with unselective criteria for GC stage. The authors included 270 patients (128 in LAG and 142 in OG) with either early and advanced GC, submitted to either subtotal or total gastrectomy. Similir to Cai et al[90], the authors did not find significant differences in the number of harvested lymph nodes and morbidity rates between the two groups.
In adjunct to these above mentioned studies, 2 meta-analysis[91,92] have been published which included pooled data on this issue and which reproduced essentially similar results. The more recent meta-analysis published in 2014 by Zou et al[91] focused on studies comparing open and laparoscopic D2 gastrectomy for the treatment of advanced GC. The analysis included only one RCT, that of Cai et al, and 13 non-RCTs, with a total of 2596 eligible for the meta-analysis. Instead, the analysis of Wei et al[92] involved trials comparing laparoscopic and open gastrectomy with D2 lymphadenectomy for GC unselectively from the tumor stage, thus including also early GC. The authors found 10 trials eligible for inclusion in the meta-analysis, of which one (i.e., a work of Lee et al[25] that is restricted to early GC) partially conducted in a randomized way and the rest case-control retrospective studies. In both this 2 meta-analysis, the authors concluded that LAG associated to laparoscopic D2 gastrectomy in comparison to the open procedure showed no significant differences regarding number of harvested lymph nodes, and tumour recurrence, disease-free and overall survival rates. However, laparoscopic gastrectomy had a longer operative time.
On the basis of these results, all the authors concluded that LAG with D2 lymph node dissection, despite being a technically demanding and time-consuming procedure, is a safe and feasible procedure with adequate lymphadenectomy, good curability and survival rate for the treatment of GC. With regard to lymph nodes harvested, this conclusion is slightly discordant with respect to the result of meta-analysis restricted to RCTs on LADG: as discussed in the “Laparoscopic distal gastrectomy” section, ODG demonstrated essentially to be associated with a major number of harvested lymph nodes than LADG. In order to explain this a main reason could be hypothesized. Laparoscopic procedures, in particular those as complex as extended lymphadenectomy and total gastrectomy, are characterized by a typical steep learning-curve. The discrepancy on results of previous meta-analysis regarding the number of harvested lymph nodes may be due to the combination of data from studies with different lymphadenectomy levels and from different periods of publication, other than the heterogeneity and low quality of studies involved which could lead to bias. In fact, a similar discrepancy in the number of lymph nodes retrieved was revealed also for the results of LADG when the procedure is meta-analyzed associated with D2 lymphadenectomy only, while excluding LADG with lymphadenectomies inferior to D2[24]. As confirmed by the meta-analysis of Ding et al[24], if this type of restriction is applied, the number of lymph node harvested by laparoscopy tended to become similar to those retrieved by open procedure. On the other hand, the RCTs of Cai et al[90] and Cui et al[65] introduced in their analysis a significant proportion of total gastrectomies. Both these procedures, i.e. laparoscopic extended D2 lymphadenectomy and laparoscopic total gastrectomy, are more complex, and only recently more frequently performed in highly experienced centres, than LADG with D1+ lymphadenectomy, which conversely are typically performed for early GC. Thus, these techniques are described in essentially newer series on advanced GC with respect to the meta-analysis limited to LADG and early GC[3,4,14,15,18-20]. So the heterogeneity is founded on the different skills among different surgical teams achieved during the years. The increasingly reported adequacy of D2 dissection in literature is indeed probably due to the progressively higher number of surgeons reaching the plateau of the traditionally steep learning curve of LAG.
In other words, as the operative technique has developed, the number of lymph nodes dissected during either distal and total laparoscopic gastrectomies has gradually increased and ever more highly skilled surgeons will report an adequate D2 lymphadenectomy[24,25,57]. Thus, while laparoscopic D2 dissection is probably not adequate at the beginning of the experience of every surgeon at the initial phase of the learning curve, it progressively increases and the number of retrieved lymph nodes in LADG tends to be close to or even greater than that in ODG[15].
The importance of the learning curve for LAG has been confirmed by other indications. Kim et al[27] found that LADG with systemic lymph node dissection for early GC requires a long learning curve, at least 50 cases, a surgeon’s familiarity with the endoscopic instruments, and the cooperation of the whole operation team. Ikeda et al[93], with the aim of assessing the oncological quality of laparoscopic D2 lympadenectomy, reported no significant differences in the viewpoint of lymph node dissection between 102 patients treated by LADG and 90 treated by ODG if the LADG was performed by an experienced laparoscopic surgeon. Kunisaki et al[94] demonstrated that the number of harvested lymph nodes during LADG did not differ significantly from that under open surgery, or better still was greater after experience of over 80 cases for an institution or over 40 cases for a surgeon. Moreover, it is reported that optimum proficiency can be achieved with experience in 40 to 60 cases and that a well executed educational system minimizes the steep learning curve[27,94,95].
Thus, in conclusion, the quality of laparoscopic lymph node dissection differs between institutes and depends essentially on the surgeon’s technical proficiency. In future years, increasing numbers of medical institutions will be capable of performing adequate laparoscopic D2 dissections, also for cases of advanced GC. Thanks to the development of laparoscopic technique, the use of LAG for treating GC has expanded also in the historically poor proficient West, such as in the United States, Europe, and other countries[7,43]. Globally, these reasons could explain why the passing of time and more trials demonstrate the oncological adequacy of laparoscopic D2 lymphadenectomy compared with traditional open procedure and the reason for the slight variation from results of earlier meta-analysis with respect to the newer ones.
ROBOT-ASSISTED LAPAROSCOPIC GASTRECTOMY
To achieve wider application, new minimally invasive techniques will necessarily demonstrate that they do not represent a disadvantage with respect to oncologic outcome. In order for GC treatment to be considered oncologically sound, the minimally invasive laparoscopic procedures need to include an appropriate lymphadenectomy. The concern is mainly due to the technical difficulties posed by laparoscopic D2 lymphadenectomy, which requires a highly skilled laparoscopic surgeon, effort and time.
The anatomic complexity of the vascular structures and the technical limits of the conventional laparoscopic instrumentation can make this procedure quite complex even for minimally-invasive well-trained surgeons, and can be associated with significant bleeding during dissection around the hepatic, celiac, and splenic arteries. Relatively difficult areas to access during laparoscopic lymphadenectomy include lymph node stations 4, 6, 9, and 11p[29]. For advanced GC, the Japanese GC Association[64] indicated as the standard therapy complete D2 lymphadenectomy including lymph nodes along the hepatic artery (No. 12a), along the proximal splenic artery (No. 11p), and when carcinoma is located in the lower third of the stomach along the superior mesenteric vein (No. 14v). These are traditionally difficult points of the laparoscopic dissections. Miura et al[29] reported a lower compliance (i.e., no nodal tissue documented at a node station that should have been resected) rate for nodes along the hepatic, celiac, and splenic arteries and a significantly lower number of harvested lymph nodes for the perigastric lymph nodes along the major curvature (Nos. 4 and 6) and second tier nodes along the celiac and splenic arteries (Nos. 9 and 11) when laparoscopic D2 dissection was performed, as compared to open surgery. In a similar station-specific lymph node yield analysis, Bouras et al[96] revealed a statistically significant lower number of lymph nodes retrieved for LDG than for ODG in the common hepatic artery station, and Son et al[97] reported a statistically significant higher mean number of lymph nodes harvested around splenic vessels through a robotic spleen-preserving total gastrectomy with D2 dissection compared to those obtained by a laparoscopic approach. These areas contain the suprapancreatic or splenic hilar lymph nodes and are crucial for D2 lymph node dissection.
It is in this context that robotics is worth looking at, being a potentially valid tool which, within the laparoscopic procedure itself, could allow significant improvement. Robotic technology has been employed in areas of surgery in which precise movements are required and in 1994 it gained the approval of the United States Food and Drug Administration (FDA)[98]. The robotic surgical system can overcome some of the intrinsic drawbacks of conventional laparoscopy surgery, improving maneuverability and vision. The main specific technical disadvantages of conventional laparoscopy are, in fact, the unstable positioning of the two-dimensional (2D) camera; instruments with restricted degrees of motion, increasing the physiologic tremor of surgeon’s hand, with limited manipulation and ergonomic discomfort; and the “fulcrum effect” (i.e., the need for the surgeon to move his/her hand in the opposite direction to that in which the tip of the instrument is intended to go in the abdominal cavity). In particular, the not ideal and often shallow angulation, together with the traditional non ergonomic nature of laparoscopic instrumentation, make the laparoscopic D2 dissection difficult, requiring particular ability. Moreover, the difficulty is associated with the need for all the members of the operating team to be skilled in laparoscopic procedures, including accurate maneuvering of the camera to view the site of dissection, as well as very careful handling of forceps to prevent accidental bleeding from adipose tissue and lymph nodes.
While robotic surgery is poorly suited for dissections involving multiple quadrants and heavy structures, such as in gastric surgery for omentectomy, conversely, when precise dissection is needed, especially in a relatively small field of the abdomen, its superiority compared to a traditional laparoscopic technique is crucial. The hand movements of the surgeon, who sits at the master console, are transmitted to the robotic arms through a computerized interface software that removes the natural tremor of the hand. At the same time, the system provides improved dexterity with an internal articulated endoscopic wrist (EndoWristTM System) that allows 7 degrees of freedom, via 180º articulation and 540º rotation and enabling the hand movements of the surgeon into the abdomen to a scale motions filtered at a ratio of 3:1 or 5:1. Finally, the system provides magnified three-dimensional (3D) high-resolution images and stereoscopic vision supported by a dual light supply and dual three-chipped camera[98]. The view system is characterized by a particular stability of the camera platform, which is held by a robotic arm controller by the first surgeon, that overcomes the physiologic human handling tremor of the traditional laparoscopic camera.
Another advantages of robotic surgery is to facilitate the technical limitation of traditional laparoscopy to perform the digestive restoration after total gastrectomy. To place a hand-sewn purse-string suture on the esophagus is simpler using robotic assistance and the esophageal anastomosis can then be performed using a circular stapler, just as with open surgery[9,41]. An alternative is to perform a full robotic hand-sewn esophagojejunal anastomosis, thanks to the ability of the robotic system to provide surgeons to perform precise sutures, even in deep and narrow spaces, which would otherwise be impossible with traditional laparoscopic tools. Finally, although the experience of laparoscopic surgery could affect the learning process of robotic gastrectomy, robotic surgery seems to require globally earlier adaptation with respect to a laparoscopic procedure that traditionally has a steep learning curve. Operation time analysis showed that an experienced laparoscopic surgeon requires fewer cases of robotic gastrectomy to reach a steady state[99-102].
Robotic D2 lymphadenectomy
It is widely accepted that D2 lymph node (along the hepatic, celiac, and splenic vessels) dissection is the more critical part of the minimally invasive gastrectomy procedure for cancer patients. Thanks to significant technical advantages in performing the dissection of the lymphatic tissue around the portal vein, common hepatic artery, celiac trunk, and splenic artery, the D2 lymph node dissection can be a primary indication for the robot assisted procedure.
The robotic extended lymphadenectomy begins at the hepatic pedicle, along the common hepatic artery above the pancreas, and continues into the portal hepatis distally. The first assistant provides a gentle pressure on the pancreatic head to obtain an optimal tension of the hepato-duodenal ligament and to allow a complete dissection of the lymphatic tissue of the proper hepatic artery (station 12a). The dissection is prolonged above the pancreas along the common hepatic artery (station 8a) (Figure 1), which is exposed from the bifurcation of the gastroduodenal artery toward the root of the left gastric artery (Figure 2). The No. 8a lymph nodes and the right side of the No. 9 lymph nodes are dissected by exposing the right border of the celiac artery (Figure 2). Lymph nodes are removed ‘‘en bloc’’ until the left gastric artery is reached (station 7). Particularly in these sites the EndoWrist® function enables the surgeon to reach these deep areas that would otherwise be unreachable with the conventional straight forceps used in conventional laparoscopic surgery. Moreover, the convex body of the pancreas often interferes with the laparoscopic instruments and hinders surgeons from performing delicate dissection. Conversely, the robotic scope can provide a much more stable view of this narrow surgical field with better depth perception, and the articulating instruments of the robotic system could allow radical dissection over the pancreas with relative ease.
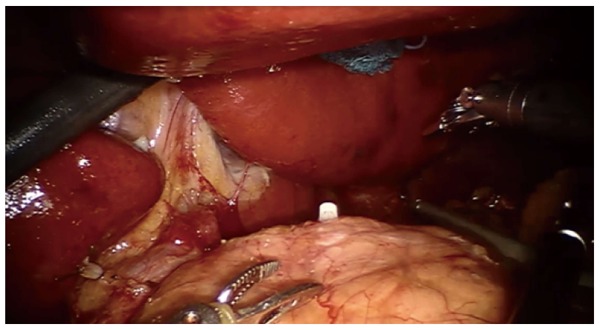
Figure 1.
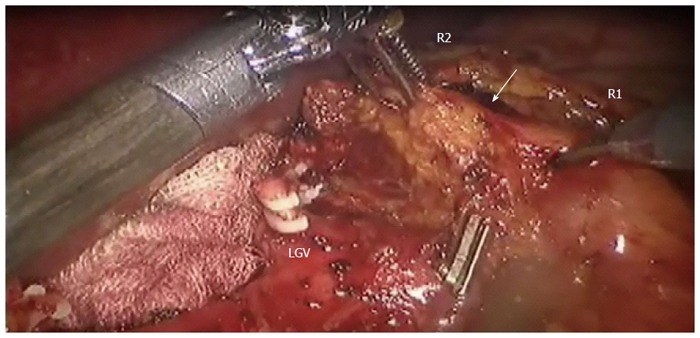
Adipose tissue including the station No. 8a lymph nodes (white arrow) is pulled up by the 2nd robotic (R2) arm dissected by the 1st robotic (R1) arm. Clipped on Hem-o-lock is Left gastric vein (LGV). Provided by Roviello F, University of Siena.
Figure 2.
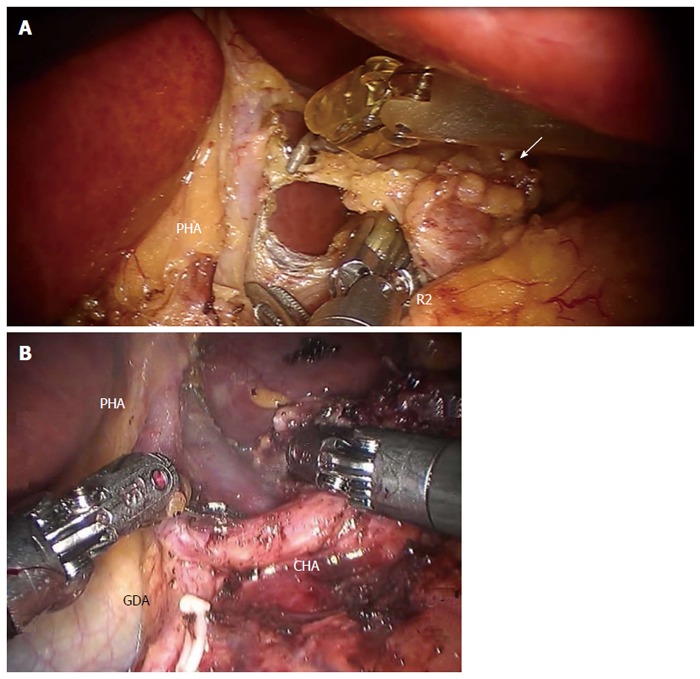
Dissection of No. 8 lymph nodes (white arrow) continues medially, through the traction of the 2nd robotic arm (R2), exposing (A) the proper hepatic artery and (B) the common hepatic artery. Provided by A: Coratti A, University of Florence; B: Patriti A. USL2 Spoleto. PHA: Proper hepatic artery; CHA: Common hepatic artery; GDA: Gastroduodenal artery.
Proximally, the lymphatic dissection is continued along the celiac truck, the left gastric vessels are identified and, taking great care, the avascular space of the left gastric artery is dissected bilaterally. The left gastric artery is exposed (Figure 3) and ligated at the origin using endoclips (Hem-o-lock) or ties. Robotics make this maneuver easier to execute than with a typically straight laparoscopy tool, because during a laparoscopy the combination of surgeon tremors and flat two-dimensional imaging make it technically demanding to maintain appropriate tension between lymphatic tissue and the main artery. Over stretching of the suprapancreatic adipose tissue by the surgeon often leads to tissue laceration, hemorrhage, and inadequate nodal dissection.
Figure 3.
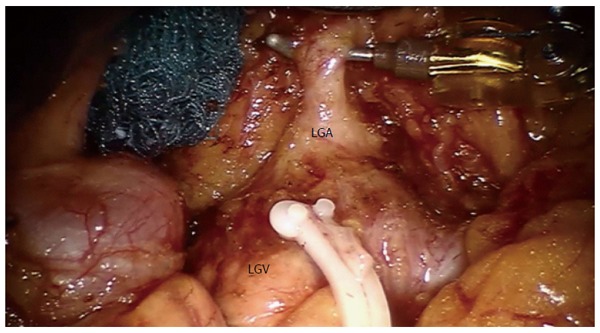
Exposition of left gastric artery after No. 7 lymph nodes dissection. Clipped on Hem-o-lock is the left gastric vein (LGV). Provided by Coratti A, University of Florence. LGA: Left gastric artery.
Next, the left side of the celiac artery and the origin of the splenic artery are also exposed. The splenic artery is identified along its route and skeletonized by the surrounding lymphatic tissue (station 11p) (Figure 4) up to the splenic helium (station 10). During the dissection of the splenic vessels the small branches can be more easily visualized by the larger robotic 3D stereoscopic imaging compared to the traditional laparoscopic technique, allowing vascular dissection under the tunica adventitia, completely clearing the lymphatic tissue and identifying and preserving the vascular supply of the pancreas and spleen. That makes it safer and easier to complete the spleen-pancreatic-preserving D2 lymph node dissection (Figure 5). Moreover, similar to the proper hepatic (station 12a), the splenic arteries lymph nodes (station 11p) are located in the dorsal plane and the approach to these target nodes by laparoscopic forceps lacking articulation is troublesome. The robot assisted technique makes it easier to accomplish this phase laparoscopically, as robotic instruments can easily overcome this typical laparoscopic drawback when the dissection is driven circumferentially around the major vessels. The EndoWrist property of robotic arms is particularly useful to enable these movements and makes it easier to perform the dissection, during which conversely the conventional straight laparoscopic instrument does not provide the surgeon with enough freedom. In conventional laparoscopy it is very difficult to effectively reach the posterior side of the suprapancreatic node-bearing area, even with excessive downward compression of the pancreas. Further, this may cause pancreatic injuries and pancreatitis.
Figure 4.
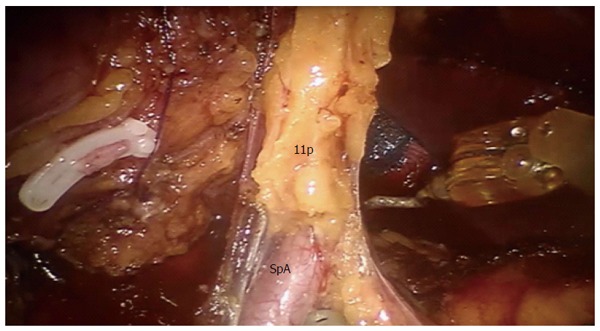
Dissection of 11p lymph nodes. Provided by Coratti A, University of Florence. SpA: Splenic artery.
Figure 5.
Exposure of the supra pancreatic area after supra pancreatic lymph nodes dissection. Provided by Coratti A, University of Florence.
Moreover, the significant technical advantage of robotic capacity permits a safer control of eventual bleeding vessel injury. In fact, the infra pyloric area and the inferior mesenteric vein, including stations 6 and 14, and the supra pancreatic area including stations 7, 8, and 9 are reported in literature as the most frequent sources of intra operative bleeding[72]. Moreover, in cases of vascular injury, the recovery from bleeding is easier than with conventional laparoscopy. The surgeon has direct control of vision and can use three surgical tools for clamping and suturing. In the meantime, the assistant surgeon can maintain a clean operating field using a sponge, suction, and irrigation. It is impossible to reproduce these same working conditions during a conventional laparoscopic procedure.
Literature evidence
After the earliest experiences of robot-assisted gastrectomy (RAG) published in 2003 by Hashizume et al[103] and Giulianotti et al[104], in recent years several reports have demonstrated the safety and feasibility of robotic gastrectomy in the treatment of GC[105,106]. Most of the experience thus far comes from small, non randomized, retrospective studies. Table 2 summarizes some of the published data[9-11,42,66,105-122].
Table 2.
Robot-assisted laparoscopic gastrectomy series for treatment of gastric cancer
| Ref. | Year | Country | Patients (n) |
Resection type |
Operative time1 (mean ± SD, min) | Blood loss1 (mean ± SD, mL) | Open conversion (%) | Harvested nodes1 (mean ± SD, n) | Morbidity (%) | Mortality (%) | Hospital staya (mean ± SD, d) | |
| Total | Subtotal | |||||||||||
| Anderson et al[10] | 2007 | United States | 7 | - | 7 | 420 ± NR | 300 ± NR | 0 | 24 ± NR | 11.1 | 0 | 4 ± NR |
| Patriti et al[9] | 2008 | Italy | 13 | 4 | 9 | 286 ± 32.6 | 103 ± 87.5 | 0 | 28.1 ± 8.3 | 7.7 | 0 | 11.2 ± 4.3 |
| Song et al[11] | 2009 | South Korea | 100 | 33 | 67 | 231.3 ± 43.2 | 128.2 ± 217.5 | 0 | 36.7 ± NR | 13.0 | 1 | 7.8 ± 17.1 |
| Pugliese et al[66] | 2010 | Italy | 16 | - | 16 | 344 ± 62 | 90 ± 48 | 12 | 25 ± 4.5 | 6.0 | 6 | 10 ± 3 |
| Kim et al[107] | 2010 | South Korea | 16 | - | 16 | 259.2 ± 38.9 | 30.3 ± 15.1 | 0 | 41.1 ± 10.9 | 0.0 | 0 | 5.1 ± 0.3 |
| Eom et al[108] | 2011 | South Korea | 30 | - | 30 | 229.1 ± NR | 152.8 ± NR | 0 | 30.2 ± NR | 13.3 | 0 | 7.9 ± NR |
| Lee et al[109] | 2011 | South Korea | 12 | - | 12 | 253.7 ± 53.0 | 135.8 ± 133.9 | 0 | 46.0 ± 25.5 | 8.3 | 0 | 6.6 ± 1.6 |
| D’Annibale et al[110] | 2011 | Italy | 24 | 11 | 13 | 267.5 ± NR | 30 ± NR | 0 | 28 ± NR | 8.3 | 0 | 6 ± NR |
| Woo et al[106] | 2011 | South Korea | 236 | 64 | 172 | 219.5 ± 46.8 | 91.6 ± 152.6 | 0 | 39.0 ± 15.2 | 11.0 | 0.4 | 7.7 ± 17.2 |
| Caruso et al[111] | 2011 | Italy | 29 | 12 | 17 | 290 ± 67 | 197.6 ± 202.1 | 0 | 28.0 ± 11.2 | 41.4 (13.8)2 | 0 | 9.6 ± 2.8 |
| Isogaki et al[112] | 2011 | Japan | 61 | 14 | 47 | 520 ± 177 TG | 150 ± 234 TG | 0 | 43 ± 14 TG | 4.9 | 1.6 | 13.3 ± NR |
| 388 ± 85 SDG | 61.8 ± 46.5 SDG | 42 ± 18 SDG | ||||||||||
| Huang et al[113] | 2012 | Taiwan | 39 | 7 | 32 | 430 ± NR | 50 ± NR | 0 | 32.0 ± 13.7 | 15.4 | 2.6 | 7 ± NR |
| Uyama et al[114] | 2012 | Japan | 25 | - | 25 | 361 ± 58.1 | 51.8 ± 38.2 | 0 | 44.3 ± 18.4 | 8.0 | 0 | 12.1 ± NR |
| Kang et al[115] | 2012 | South Korea | 100 | 16 | 84 | 202.05 ± 52.31 | 93.25 ± 84.59 | 0 | NR | 14.0 | 0 | 9.81 ± 12.16 |
| Kim et al[116] | 2012 | South Korea | 436 | 109 | 327 | 226 ± 54 | 85 ± 60 | NR | 40.2 ± 5.5 | 10.1 | 0.5 | 7.5 ± 13.7 |
| Yoon et al[117] | 2012 | South Korea | 36 | 36 | - | 305.8 ± 115.8 | NR | 0 | 42.8 ± 12.7 | 16.7 | 0 | 8.8 ± 3.3 |
| Liu et al[42] | 2013 | China | 104 | 54 | 50 | 272.52 ± 53.91 | 80.78 ± 32.37 | 2 | 23.1 ± 5.3 | 11.5 | 0 | 6.2 ± 2.5 |
| Hyun et al[118] | 2013 | South Korea | 38 | 9 | 29 | 234.4 ± 48.0 | 131.3 ± 10.1 | 0 | 32.8 ± 13.8 | 47.3 (13.1)2 | 0 | 10.5 ± 5.9 |
| Park et al[119] | 2013 | South Korea | 200 | 46 | 154 | 248.8 ± 55.6 | 146.1 ± 130.3 | 7 | 37.9 ± NR | 10.0 | 0.5 | 8.0 ± 3.7 |
| Son et al[120] | 2014 | South Korea | 51 | 51 | - | 264.1 ± 46.7 | 163.4 ± 255.1 | 0 | 47.2 ± NR | 15.7 | 1.9 | 8.6 ± NR |
| Junfeng et al[105] | 2014 | China | 120 | 26 | 94 | 234.8 ± 42.4 | 118.3 ± 55.8 | 0 | 34.6 ± 10.9 | 5.8 | 0 | 7.8 ± 3.0 |
| Shen et al[121] | 2015 | China | 93 | 23 | 70 | 257.1 ± 74.5 | 176.6 ± 217.2 | 0 | 33 ± 8.5 | 9.8 | NR | 9.4 ± 7.5 |
| Kim et al[122] | 2016 | South Korea | 223 | 43 | 180 | 226 ± NR | 50 ± NR | 2 | 33 ± NR | 13.5 (1.3)2 | 0 | 4 ± NR |
Mean value;
Total morbidity rate, including minor and major complications; between parenthesis major complications rate base on Clavien-Dindo classification ≥ 3, such as anastomotic and duodenal lekeage. NR: Not reported; TG: Total gastrectomy; SDG: Subtotal distal gastrectom.
The studies prevalently originate from Asian countries. The Western experience with robotic gastrectomy is limited to smaller series that assess feasibility and safety. In the United States, Anderson et al[10] were the first, in 2007, to report on outcomes after robot-assisted subtotal gastrectomy, in a pilot series of 7 patients. While no direct comparison was made with laparoscopy, the authors showed that robotic gastrectomy was feasible, with acceptable length of stay and low morbidity[10].
Among the largest single institution robotic series on short-term oncological and clinical outcomes to date are (Table 2): Woo et al[106] in 2011, Kim et al[116] in 2012, Kim et al[122] in 2016, Park et al[119] in 2013, and Junfeng et al[105] in 2014. They included respectively 236, 436, 223, 200 and 120 patients who underwent robot-assisted LG for cancer. All these studies confirmed the safety and feasibility of RAG with lymphadenectomy for the treatment of GC, but they did not provide data on long-term survival.
While there are so many reports about the feasibility of RAG, only few robotic studies reported a high quality comparative analysis of RAG vs laparoscopic and/or open gastrectomy. Preliminary results showed better short-term surgical outcomes of RAG than those of open and laparoscopic methods. Kim et al[107] were the first to compare post-operative outcomes between robotic, laparoscopic and open gastrectomy in a small pilot series of (16 robotic, 11 laparoscopic, 12 open) patients with early stage disease. The robotic group demonstrated longer operative times, but lower blood loss and shorter hospital stay. There was no difference in terms of number of harvested lymph nodes or post-operative morbidity or mortality between groups.
The largest comparative study to date was conducted by Kim et al[116]. They retrospectively reviewed data on surgical complications of a prospectively collected GC patients data-base. In a total of 5839 patients (4542 open, 861 laparoscopic and 436 robotic gastrectomies), overall complication, re-operation and mortality rates significant differences between the three groups were not found. The results of this study should be interpreted with some caution, as any retrospective comparison has its limitations and selection bias. In particular, the work included different surgical modalities of gastrectomy in a wide heterogeneous groups of patients. Patients in the OG group had more proximally located, more poorly differentiated and more locally advanced GCs, and so the proportion of total gastrectomies and adjacent organ resections was significantly higher in this group. Thus, consequently, it is obvious that a more positive lymph nodes harvested and intra operative blood loss were significantly greater in open operations, most likely attributable to higher rates of total gastrectomy and more extensive surgery for more advanced tumours. On the other hand, it is quite probable that patients who were expected to need a more radical operation based on preoperative evaluation did not undergo laparoscopic or robotic surgery, and even within the same pathological stage patients in the OG group may have had more advanced disease and more extensive surgery. The intrinsic bias which necessarily originates from such a heterogeneity study cohort, and specifically the different level of learning curve at which every group was treated, could determine why in this comparative analysis anastomotic leaks were significantly more common with the minimally invasive approach (twice as high after laparoscopic and robotic procedures than after an open approach), despite the more advanced stage of cancer in this last group. For these reasons, the work of Kim et al should be seen more as a study of feasibility rather than a comparative analysis of RAG vs LAG and OG.
Another among the largest single institute comparative series, prospectively collecting patients who underwent curative resection of GC, compared robotic surgery with open and laparoscopic surgery respectively in 39, 586 and 64 patients[113] (Table 2). Robotic gastrectomy was associated with less blood loss and shorter hospital stay, at the price of longer operative time than both open and laparoscopic gastrectomy. Postoperative morbidity rates were similar among the three groups. The number of retrieved lymph nodes was similar between the open and robotic groups, while the laparoscopic group had fewer retrieved lymph nodes than the open and robotic. In particular, the authors noted how much easier it was with the aid of robotic instruments to perform lymphadenectomy than the traditional laparoscopic gastrectomy, especially in the infra-pyloric and supra-pancreatic area. Similar results were reported by Junfeng et al[105] in an another of the largest comparative studies, which retrospectively compared 120 vs 394 GC patients who underwent to RAG and LAG respectively. In addition to showing once more less intraoperative blood loss and longer operative time of RAG with respect to LAG, interestingly the authors revealed that the numbers of collected lymph nodes were significantly higher in the RAG group at tier 2. Similarly, Kim et al[123] revealed in a series of 87 patients who underwent robot-assisted distal gastrectomy (RADG) compared to 288 patients who underwent to LADG that RADG could provide an advantage over LADG in the dissection of the D2 area lymph nodes, especially around the splenic artery area. Son’s study[120] found that robotic gastric surgery yielded significantly greater number of retrieved lymph nodes around splenic vessels and splenic hilum compared with those obtained by a laparoscopic approach.
These results would confirm the advantage of robotic surgery over LAG in the D2 dissection, in particular providing a better exposure and wider operating field visualization of the second tier lymph node stations (No. 7, No. 8a, No. 9, and No. 11) which are traditionally the more difficult sites to be laparoscopically harvested.
To date, 9 meta-analysis[124-132] have been published in literature to elucidate the issue of RAG in the treatment of GC patients. Of these meta-analysis, 1 included selected reports comparing RAG with OG[124]; 5 recruited high quality studies comparing RAG and LG[125-129]; the other 3 consists of a systematic review and meta-analysis of trials evaluating the safety and short-term efficacy of RAG compared with laparoscopic and open gastrectomy[130-133]. Only non-randomized comparative controlled trials were found eligible for inclusion in these meta-analysis. These meta-analysis demonstrated that the short-term clinical outcomes of RAG were essentially comparable to those of LG and OG. Specifically, RAG was superior to LG and OG in terms of blood loss, despite an increased operative time; there were no differences between RAG and LG groups in the number of retrieved lymph nodes and conversion to open; hospital stay for RAG was slightly inferior or similar to that for LAG, but significantly shorter than OG; postoperative complications were similar for all three operative approaches.
The advantage of RAG with respective blood loss may be mainly attributable to the typical features of the robotic device which, compared to conventional laparoscopy, enable better detection of vessels, due to the greater field of vision and stereoscopic vision, and facilitate control of intra abdominal bleeding with tremor filtration and stable hemostatic pressure supplied by the robotic arms. On the other hand, the longer duration of robotic surgical procedure is mainly because of the additional setup- and docking-time required for the robotic system. However, the operating time significantly decreased with the accumulation of surgical experience in robotic gastrectomy[11,100,119].
However, significant limitations exist in the interpretation of the comparative data among RAG vs LG and OG available so far, due to the lack of RCTs, the limited number of published high quality observational and retrospective studies, the small sample sizes to date, and the limited duration of follow up. Large multi-centre prospective RCTs are required to delineate significantly quantifiable advantages of RAG over LAG and OG, thus to draw conclusive considerations. Due to this shortfall of studies, at the present time, the real, long-term benefits of RAG for the treatment of GC remain unreported. Pugliese et al[66] in Italy are one of the few groups to study long term outcomes in patients with early and locally advanced lesions undergoing minimally invasive subtotal gastrectomy. Of the 70 patients included (37 early and 33 advanced lesions), all patients had a D2 lymphadenectomy and 18 underwent robotic surgery. Short-term results were similar between laparoscopic and robotic surgery groups. The 5-year overall survival for the entire cohort was 81% (97% for early and 67% for advanced lesions). Recently, Coratti et al[133] reported the long-term results of 98 consecutive patients submitted to RAG for early and advanced GC, with a mean follow-up of 46.9 mo and 5-year overall survival of 73.3%. Son et al[120] reported the longest follow-up study after RAG for GC, with a median follow-up of 70 mo, and found no difference in overall survival or disease-free survival. The 5-year overall survival rate was 89.5% in the RAG group and 91.1% in the LGS group while the 5-year disease-free survival rate was 90.2% in the RAG group and 91.2% in the LAG group. These results are promising, but these studies included limited numbers of cases and selection bias is a concern as the study design was non-randomized. To demonstrate oncologic outcomes follow-up periods longer than 5 years are needed, thus definitive conclusions need to be validated by further RCTs.
A recent prospective multi-centre comparative study[122] comparing short-term surgical outcomes of robotic (n = 223) and laparoscopic (n = 211) gastrectomy introduced also financial cost analysis. Both groups showed similar overall complication rates, estimated blood loss, and length of hospital-stay. The Robotic group showed significantly longer operative time and significantly higher total cost (robotic US$ 13.432 vs laparoscopic US$ 8090; P < 0.001). Other studies underlined the higher costs for RAG than those for LADG owing to the substantial expense of the robotic system itself[100].
Globally, on the basis of the literature evidence available so far, the following preliminary conclusions could be drawn. Robotic gastrectomy shows short-term outcomes comparable to open and laparoscopic series, with essentially satisfactory results in terms of peri-operative outcomes and oncological adequacy. To date, RAG appears to be a valid alternative to conventional open or laparoscopic resection for the treatment of gastric carcinoma, thus essentially making some difficult traditional laparoscopy procedures easier and safer, also making it possible at the same time to maintain the typical advantages of minimally invasive surgery with respect to open. Although these initial results are promising, solid evidence of superiority of robotic gastric surgery over the conventional laparoscopic approach is not determined. Moreover, the considerable expenses remain a major drawback of robotic surgery. The role of robotic gastrectomy for GC and its long-term oncologic benefits remain poorly investigated. Only in recent years have the first studies on long-term oncological outcomes of GC patients treated with RAG been reported. Larger and randomized prospective trials are needed before robotic resection can be considered to be an acceptable alternative for all patients with resectable GC. We believe that carefully selected patients may be considered for robotic resection by experienced GC surgeons.
DISCUSSION
The introduction of a new technological modality for the treatment of cancer is acceptable if it is as oncologically sound as the traditional procedure. Minimally invasive procedures would be a valid alternative to open surgery, with best short-term outcomes, if oncologic criteria could be respected as in the open approach, and long-term survival remain uncompromised. Although overall survival represents the prime oncologic parameter, wide margin resection and number of resected lymph nodes accurately reflect the adequacy of gastric resection for adenocarcinoma. Indeed, in order for laparoscopic gastric surgery to be accepted for the surgical treatment of GC and to not represent a disadvantage with regard to oncologic outcome, the quality of lymphadenectomy is the most important factor to be considered. When a laparoscopic or robotic-assisted laparoscopic approach is used for the surgical treatment of GC, the same extent of lymph node dissection as in traditional surgery should be performed, and postoperative outcomes should also be favorable.
Laparoscopic gastrectomy with lymph node dissection has developed as a minimally invasive surgery for GC over the past 20 years. This surgery has been used mainly for early-stage GC. Sufficient data is available on the feasibility of LADG and this approach has essentially been validated for early GC, as several level III studies and meta-analysis demonstrated that laparoscopic gastrectomy with limited lymphadenectomy for patients with early GC had non-inferior oncologic outcome relative to open surgery, with instead better short-term results[3,14,18]. The potential benefits of laparoscopic gastrectomy compared to conventional open surgery include faster postoperative recovery, quicker return of gastrointestinal function, shorter hospital stay, less postoperative pain, and better cosmesis[54,79]. The incidence of operative complications is less than or the same as that with conventional open surgery.
Conversely, few reports, all containing small patient series, describe the safety of laparoscopic assisted distal and total gastrectomy with D2 lymphadenectomy for advanced GC. During the last years, some meta-analysis on this topic have been published, but conflicting results were found in particular for postoperative complications and number of collected lymph nodes[14,18-20,24,84]. The majority of the comparative trials between LG and traditional open technique are too heterogeneous to be globally evaluated. The main reasons for this heterogeneity were the different levels of laparoscopic expertise; the issue related to the learning curve; different levels of lymphadenectomy; nonblinded assessment of outcomes; lack of randomization; predominance of Asian studies. If individually taken, most of the studies on LG including advanced gastric tumour are too small to reach the necessary statistical power to draw definitive conclusions and the majority of these contain a greater proportion of patients operated upon for early distal GC, thus making it implausible to obtain results generalized to all GC stages. Several meta-analysis on LG vs OG, as discussed in the previous sections (laparoscopic assisted distal gastrectomy, laparoscopic assisted total gastrectomy, early GC, advanced GC, laparoscopic D2 lymphadenectomy sections), have been conducted to address the controversy on potential advantage of the laparoscopic procedure, yet sample size of those analysis are not large enough and homogeneous. Some authors only included the few available RCTs[18-20]; others performed meta-analyses that combined RCTs also with retrospective comparative studies, which have the advantage of potentiating the statistical power of the study but at the price of the possibility to introduce bias for potential intrinsic flaws of non-randomization[14,24,84]. Some authors focused the meta-analysis on only one type of gastrectomies, usually LADG, others included all LAG procedure; some of them combined data from RCTs of different lymphadenectomy levels, others are restricted to one type of lymphadenectomy only; some included studies restricted to early GC only, while conversely some included studies that enrolled high ratio of advanced cases; and some even synthesized duplicated publication data. All of which would introduce bias.
Recently, a meta-analysis[134] has been conducted which enclosed all the available RCTs regarding the effectiveness of LG vs OG for resectable GC, independently either of the type of gastrectomy (LADG and LATG) and gastric tumour stage; therefore, this study was not commented in the previous sections that are restricted to a specific topic. The strategy of this last meta-analysis has the advantage of increasing the study’s statistical power, expanding as much as possible the cohort patients for the analysis, but with the disadvantage of mixing different technical procedures towards different stages of cancer. Ultimately, 8 RCTs, totaling 784 patients (402 LAG and 382 open gastrectomy), were considered eligible. The study included the largest sample sizes among the meta-analyses available to date, thanks to the above mentioned non selective inclusion criteria, but again the limits of previous meta-analysis were not overcome. In fact, the results again largely represent the experience of East Asian countries (included studies from South Korea = 3, Japan = 3, Italy = 1, China = 1), mostly cases were early GC (the advanced GC patients were from 2 trials only), the laparoscopic approach was mainly focused on LADG (87.5%), and the long-term survival rate was not available because of insufficient follow-up time. Overall, even if all of the RCTs comparing LG vs OG available in literature are enclosed in this meta-analysis, they remain few and singularly small. Essentially this meta-analysis did not achieve significant superiority respect to the previous ones. Regarding the adequacy of lymph node dissection, no differences were found in the overall mean number of collected lymph nodes between the LG and OG group, but as is known for the reasons above mentioned the meta-analysis is characterized by a significant heterogeneity of type of lymphadenectomy among RCTs. In fact, subgroup analysis depending on the level of lymph node dissection revealed a not so linear concordance. Subgroup analysis showed that the number of collected lymph nodes in LG arm tended to be smaller than that in open gastrectomy arm in either the D1+ surgery or the D2 surgery subgroups, even though the differences were not statistically significant.
Thus, in conclusion, although LADG has been widely developed for early GC, many problems and controversies still exist. The therapeutic efficacy of LG in general and specifically LADG has not yet been widely investigated for the treatment of advanced GCs around the world. Although a totally LG and an extended D2 lymphadenectomy might be possible to perform laparoscopically in some patients[7,8,48,50,71], owing to the intrinsic difficulty of execution, one of the major oncologic concerns is the ability to perform a radical and suitable D2 lymph node dissection. In fact, the meta-analysis of the randomized evidence shows that when data restricted to LADG are pooled from GCs not only in the early stage but also from advanced GC the same extent of lymph node dissection as in traditional surgery could not be guaranteed[13,16].
The technical challenge of performing this meticulous procedure (D2 lymphadenectomy) is well recognized, especially in patients with abundant intrabdominal fat[135]. Precisely in order to elucidate the efficacy of LADG with D2 lymphadenectomy for patients who are clinically diagnosed with locally advanced GC, with respect to conventional open subtotal gastrectomy and D2 lymphadenectomy, currently the Korean Laparoscopic Surgical Society (KLASS) group launched the multi-centre RCT[136] (KLASS-02 RCT; registered at www.clinicaltrials.gov as NCT01456598), comparing the oncologic and surgical outcomes of these two procedures. Other ongoing studies, are being awaited, but until definitive evidence is obtained, doubts still persist on the routine use of laparoscopic procedure for advanced GC. Moreover, the operating time for LG in general remains significantly longer compared to its open counterpart and, as yet, there is little high-level evidence based on long-term outcome and oncologic outcome of LG as a treatment of advanced GC[7,76,80].
Thus, conclusively, for all these reasons, although LG for cancer is a safe and feasible technique, whereas laparoscopic sub-D2 lymphadenectomy can be considered adequate for almost of all early GC and to date is the standard procedure in Asian countries, the same claim could not be made for advanced GC and LG cannot be recommended as a routine approach for all GC patients.
Accordingly, Japanese GC Association (JGCA) treatment guidelines still indicate LG as an experimental procedure in the context of advanced GC[137], while on the other hand sub-D2 dissection is considered oncologically sufficient for most early GC, as lymph node metastases occur in 2%-20% of these[13]. In fact, the guidelines by the Japanese GC Association[64,137] recommend for early stage GC D1 or D1+α, β lymphadectomy in function of the tumour diameter, depth of wall infiltration and suspected nodal metastasis at preoperative investigation work-up (D1+α resection is defined as D1 plus No. 7 lymph node resection, D1+β is defined as D1 plus No. 7, 8, 9 lymph nodes resection).
However, some final considerations could be made. The correct assessment of tumour invasiveness through the gastric wall is sometimes difficult and underestimated at the preoperative investigation, thus, the role of D2 nodal clearance in early GC from some authors is emphasized[8,138]. Moreover, the applicability of Eastern evidence to Western countries remains uncertain and, to date, western literature has been limited to retrospective single-institution series and small nonrandomized trials. GC in the West is characterized by more advanced disease at initial diagnosis and a larger proportion of proximal tumors[33]. Intrinsic differences in gastric tumor biological aggressiveness, genetic arrangement and geographic spread between eastern and western countries are well known. These factors, in conjunction with the lower overall incidence of GC and increased prevalence of obesity in the West, and significant length of the learning curve associated with LG, may account for the widespread low acceptance of minimally invasive approaches for gastrectomy.
In the future, a well-designed RCT with a large sample size would be required to aptly compare the controversial outcome measures of LG over traditional open procedure, particularly for the quality of lymphadenectomy.
Robotic surgery probably may overcome some intrinsic limitations of traditional laparoscopy, expanding the application of minimally invasive procedures, in particular for the D2 lymphadenectomy. As long as drawbacks of laparoscopic gastrectomy technique exist, such as in extended lymphadenetomy, the introduction of new technologies and medical devices, such as robotic gastrectomy, that would be able to improve health care and patients' outcome, are desirable. When precise dissection is needed, such as during the lymphadenectomy along major abdominal vessels, robotic technology takes advantage of the excellent stereoscopic visualization, and the improved dexterity, stability (tremor filter) and superior movements of the robotic arm whose internal articulated endoscopic wrist makes it possible to perform the dissection with greater ease. For these reasons the use of the robotic techniques represents a technical advantage for a minimally invasive approach by making it possible to carry out a safe and effective lymphadenectomy. The median number of nodes retrieved reported by many investigators who used robotic techniques for D2 lymph node dissection is similar to that of open and in some cases superior to that laparoscopic[10,111,124-132].
Possible disadvantages of robot assisted laparoscopy could be decreased sense of touch, and a lack of sense of the tissue tension forces. Therefore, particular attention should be made during movements of the robotic instruments and traction with the robotic arms to avoid tissue damage.
The use of the robot is indeed a valuable adjunct facilitating some traditional difficult laparoscopic procedures. However, although it probably may overcame some intrinsic limitations of traditional laparoscopy, expanding the application of minimally invasive procedure, recent years have seen steady improvements in dissection techniques with the spread of those purely laparoscopic (without the aid of robot) and some medical institutions are now capable of performing laparoscopic D2 dissection with safe outcomes.
Moreover, the superiority of robot assisted laparoscopic gastric surgery regarding laparoscopic oncological outcomes has not yet shown clear benefit for early GC patients, for whom an increasing number of harvested lymph nodes does not necessarily improve overall survival, but does at least influence accurate staging, for which highly experienced surgeons’ present levels of proficiency with conventional LG procedure seem to be sufficient.
Thus, there is expected to be a major impact of the robotic system on procedures that are technically demanding by laparoscopy rather than those that are relatively simple, such as overcoming the technical difficulties of laparoscopic total gastrectomy and extended D2 lymphadenectomy, with particular reference to reconstruction of the alimentary tract and the supra-pancreatic area lymph nodes dissection respectively. Therefore, it is expected that the major area of research in robotic gastric surgery in the near future will be on advanced GC, and in Western countries where GC is mainly detected in advanced stages, for which the importance of D2 lymph node dissection has been advocated in order to improve long-term survival. This fact may justify the application of a robotic system.
It must be considered that robotic surgery requires an experienced operative team, additional surgical space and high costs of the procedure are still superior to conventional open or laparoscopic gastrectomy. From the point of view of technological development, and diffusion of laparoscopic and robotic instruments that could in the near future reduce costs, it is necessary to clarify the important controversy on the cost-effectiveness of robotic surgery. In fact, the main criticism to the robotic approach is the low ratio between the advantage over classic laparoscopic minimally invasive technique in spite of the clear cost-effectiveness gap. Other still persistent disadvantages of using the robot include the relatively restricted field of vision when compared with laparoscopy, increased operative times, and lack of data on long term oncologic equivalency. Due to inadequate long-term follow-up results and a limited number of studies, it is still too early to draw definite conclusions. Randomized controlled studies are required for long-term survival outcomes.
Thus, although robot assisted laparoscopic surgery has evident benefits, it is difficult to assess and compare some advantages at the moment with respect to traditional surgery. Larger randomized prospective trials are needed before robotic resection can be considered an acceptable alternative for patients with local advanced resectable GC. Probably, the main indication for robotic gastrectomy is when it serves as an adjunct to laparoscopic resection in selected patients with locally advanced tumors requiring a D2 lymphadenectomy. However, what should be borne in mind is that robotic procedures are not independent from traditional laparoscopic ones, but are technically an adjunctive tool which can improve the effectiveness of laparoscopic technique and overcome some of its limits. Thus, well designed cost-effectiveness analysis, and high-quality comparative-effectiveness research are required to assess the strengths and disadvantages of robotic surgery compared with laparoscopic more than open surgery, in order to demonstrate if the addition of robotics to laparoscopy is truly beneficial.
CONCLUSION
The available clinical evidence implies that LG with less than D2 or D2 lymphadenectomy may be a valid option to open surgery for the treatment of early GC. Level III evidence of safety and oncological adequacy of this procedure has been reported. Principally the evidence originated from Eastern studies and at present LADG is routinely used for early GC in countries as Japan and South Korea. Conversely, data available on the advantages of LG for advanced GC are not so consistent. LAG with D2 lymphadenectomy is a time-consuming procedure even in the experienced surgeon’s hands. The reports are once more prevalently from Eastern studies, where the patient population and disease biology may differ with respect to the West. Due to clinicopathological dissimilarities between Eastern and Western GC population, not all high quality and large amount of results from Asian studies can be applied to Europe and Unite States.
Oncological outcome such as lymph node yield and margin status appear similar between open and laparoscopic approaches, but a slight superiority of open surgery still exists regarding capacity to obtain a major, complete and extended (D2) lymphadenectomy. The first RCT recruiting a large number of patients to compare laparoscopic D2 lymphadenectomy with open conventional lymphadenectomy in patients preoperatively diagnosed with locally advanced GC is ongoing and its results are being awaited[136]. Emerging long-term data on survival after LG suggest that outcomes are similar to open surgery, but high quality RCTs are needed to claim definitive conclusions.
At present, minimally invasive approaches to GC are currently indicated for patients with T1 and T2N0 adenocarcinomas, while careful selection of patients with locally advanced tumours (T3/4 or N+ disease) should be made only in high volume centers with advanced laparoscopic skills, and in a clinical research setting. Thanks to the improvements on minimally invasive techniques, the diffusion of neoadjuvant chemotherapy protocols and the introduction of robotic surgery, the indications for laparoscopic gastrectomy are expected to expand to all stages of gastric adenocarcinoma.
In general, RAG demonstrates it has overcome some intrinsic limitations of conventional laparoscopic techniques for GC, however the full potential of robotic surgery still remains to be balanced against the lack of a clear oncological superiority to its counterpart. The major technical advantages of the robot-assisted approach are appreciated in routine reproduction of D2-lymphadenectomy and complex reconstructions, such as intracorporeal reconstruction following total gastrectomy. These demanding procedures are not easily overcome by the surgeon’s experience or current laparoscopic instruments, thus representing the best indication for the use of the robot, particularly in radical gastrectomy for advanced GC for which the D2 dissection is crucial.
The learning curve and reproducibility of RAG seems to be shorter and more feasible than conventional laparoscopy, so that probably robotic surgery could permit major diffusion of minimally invasive surgery for GC in the near future. With acceptable complications and radical resection, RAG is a promising approach that improves LAG. Larger comparative series comparing robotic surgery with conventional laparoscopic procedure are needed to definitely elucidate eventual advantages in terms of long-term oncological results, in spite of more cost-effectiveness of the robotic procedure to date. An impartial assessment should be made in order to determine whether the progress so far identified in favor of robotic gastrectomy is really worth the higher expense.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 26, 2016
First decision: May 12, 2016
Article in press: June 15, 2016
P- Reviewer: El Nakeeb A, Hou X, Noshiro H S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 2.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 3.Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009;18:322–333. doi: 10.1016/j.suronc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Zhang Y, Guo TK. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: A meta-analysis based on seven randomized controlled trials. Surg Oncol. 2015;24:71–77. doi: 10.1016/j.suronc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N; Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MC, Kim HH, Jung GJ. Surgical outcome of laparoscopy-assisted gastrectomy with extraperigastric lymph node dissection for gastric cancer. Eur J Surg Oncol. 2005;31:401–405. doi: 10.1016/j.ejso.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230–234. doi: 10.1007/s101200050069. [DOI] [PubMed] [Google Scholar]
- 9.Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, Casciola L. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc. 2008;22:2753–2760. doi: 10.1007/s00464-008-0129-0. [DOI] [PubMed] [Google Scholar]
- 10.Anderson C, Ellenhorn J, Hellan M, Pigazzi A. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc. 2007;21:1662–1666. doi: 10.1007/s00464-007-9266-0. [DOI] [PubMed] [Google Scholar]
- 11.Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009;249:927–932. doi: 10.1097/01.sla.0000351688.64999.73. [DOI] [PubMed] [Google Scholar]
- 12.Sato H, Shimada M, Kurita N, Iwata T, Nishioka M, Morimoto S, Yoshikawa K, Miyatani T, Goto M, Kashihara H, et al. Comparison of long-term prognosis of laparoscopy-assisted gastrectomy and conventional open gastrectomy with special reference to D2 lymph node dissection. Surg Endosc. 2012;26:2240–2246. doi: 10.1007/s00464-012-2167-x. [DOI] [PubMed] [Google Scholar]
- 13.Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006;12:7676–7683. doi: 10.3748/wjg.v12.i47.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 15.Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39–52. doi: 10.1097/SLA.0b013e3182583e2e. [DOI] [PubMed] [Google Scholar]
- 16.Memon MA, Khan S, Yunus RM, Barr R, Memon B. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22:1781–1789. doi: 10.1007/s00464-008-9925-9. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Li J, Wang J, Pan T, Zhou J, Fu X, Zhang S. Meta-analysis of randomized controlled trials on laparoscopic gastrectomy vs. open gastrectomy for distal gastric cancer. Hepatogastroenterology. 2012;59:1699–1705. doi: 10.5754/hge12259. [DOI] [PubMed] [Google Scholar]
- 18.Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, Oba H, Aoki T, Minami M, Hirakawa K. A meta-analysis of randomized controlled trials that compared laparoscopy-assisted and open distal gastrectomy for early gastric cancer. J Gastrointest Surg. 2010;14:958–964. doi: 10.1007/s11605-010-1195-x. [DOI] [PubMed] [Google Scholar]
- 19.Chen XZ, Hu JK, Yang K, Wang L, Lu QC. Short-term evaluation of laparoscopy-assisted distal gastrectomy for predictive early gastric cancer: a meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech. 2009;19:277–284. doi: 10.1097/SLE.0b013e3181b080d3. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Li G, Chen P, Yu J, Zhang C. Laparoscopic versus open gastrectomy for early distal gastric cancer: a meta-analysis. ANZ J Surg. 2011;81:673–680. doi: 10.1111/j.1445-2197.2010.05599.x. [DOI] [PubMed] [Google Scholar]
- 21.Peng JS, Song H, Yang ZL, Xiang J, Diao DC, Liu ZH. Meta-analysis of laparoscopy-assisted distal gastrectomy and conventional open distal gastrectomy for early gastric cancer. Chin J Cancer. 2010;29:349–354. doi: 10.5732/cjc.009.10548. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Wang S, Huang ZQ, Chou WP. Meta-analysis of laparoscopy assisted distal gastrectomy and conventional open distal gastrectomy for EGC. Surgeon. 2014;12:53–58. doi: 10.1016/j.surge.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Varela JE, Hiyashi M, Nguyen T, Sabio A, Wilson SE, Nguyen NT. Comparison of laparoscopic and open gastrectomy for gastric cancer. Am J Surg. 2006;192:837–842. doi: 10.1016/j.amjsurg.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 24.Ding J, Liao GQ, Liu HL, Liu S, Tang J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol. 2012;105:297–303. doi: 10.1002/jso.22098. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759–1763. doi: 10.1007/s00464-008-0198-0. [DOI] [PubMed] [Google Scholar]
- 26.Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg. 2006;202:874–880. doi: 10.1016/j.jamcollsurg.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 27.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isozaki H, Tanaka N, Okajima K. General and specific prognostic factors of early gastric carcinoma treated with curative surgery. Hepatogastroenterology. 1999;46:1800–1808. [PubMed] [Google Scholar]
- 29.Miura S, Kodera Y, Fujiwara M, Ito S, Mochizuki Y, Yamamura Y, Hibi K, Ito K, Akiyama S, Nakao A. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection: a critical reappraisal from the viewpoint of lymph node retrieval. J Am Coll Surg. 2004;198:933–938. doi: 10.1016/j.jamcollsurg.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Gordon AC, Kojima K, Inokuchi M, Kato K, Sugihara K. Long-term comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy in advanced gastric cancer. Surg Endosc. 2013;27:462–470. doi: 10.1007/s00464-012-2459-1. [DOI] [PubMed] [Google Scholar]
- 31.Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, Zhang S, Lee M, Wu J, Lim KH, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–684. doi: 10.1136/gutjnl-2011-301839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, Coit DG, Brennan MF. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–646. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 34.Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419–424. doi: 10.1136/pgmj.2004.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong J, Jackson P. Gastric cancer surgery: an American perspective on the current options and standards. Curr Treat Options Oncol. 2011;12:72–84. doi: 10.1007/s11864-010-0136-y. [DOI] [PubMed] [Google Scholar]
- 36.Spolverato G, Kim Y, Ejaz A, Valero V, Squires MH, Poultsides G, Fields RC, Bloomston M, Weber SM, Acher AW, et al. A multi-institutional analysis of open versus minimally-invasive surgery for gastric adenocarcinoma: results of the US gastric cancer collaborative. J Gastrointest Surg. 2014;18:1563–1574. doi: 10.1007/s11605-014-2562-9. [DOI] [PubMed] [Google Scholar]
- 37.Bilimoria KY, Talamonti MS, Wayne JD, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Effect of hospital type and volume on lymph node evaluation for gastric and pancreatic cancer. Arch Surg. 2008;143:671–68; discussion 678. doi: 10.1001/archsurg.143.7.671. [DOI] [PubMed] [Google Scholar]
- 38.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okabe H, Obama K, Tsunoda S, Tanaka E, Sakai Y. Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a long-term follow-up study. Ann Surg. 2014;259:109–116. doi: 10.1097/SLA.0b013e31828dfa5d. [DOI] [PubMed] [Google Scholar]
- 40.Bracale U, Marzano E, Nastro P, Barone M, Cuccurullo D, Cutini G, Corcione F, Pignata G. Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc. 2010;24:2475–2479. doi: 10.1007/s00464-010-0988-z. [DOI] [PubMed] [Google Scholar]
- 41.Coratti A, Annecchiarico M, Di Marino M, Gentile E, Coratti F, Giulianotti PC. Robot-assisted gastrectomy for gastric cancer: current status and technical considerations. World J Surg. 2013;37:2771–2781. doi: 10.1007/s00268-013-2100-z. [DOI] [PubMed] [Google Scholar]
- 42.Liu XX, Jiang ZW, Chen P, Zhao Y, Pan HF, Li JS. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol. 2013;19:6427–6437. doi: 10.3748/wjg.v19.i38.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etoh T, Inomata M, Shiraishi N, Kitano S. Minimally invasive approaches for gastric cancer-Japanese experiences. J Surg Oncol. 2013;107:282–288. doi: 10.1002/jso.23128. [DOI] [PubMed] [Google Scholar]
- 44.Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:1509–1520. doi: 10.1007/s00464-012-2661-1. [DOI] [PubMed] [Google Scholar]
- 45.Chen K, Xu XW, Zhang RC, Pan Y, Wu D, Mou YP. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:5365–5376. doi: 10.3748/wjg.v19.i32.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen H, Shan C, Liu S, Qiu M. Laparoscopy-assisted versus open total gastrectomy for gastric cancer: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2013;23:832–840. doi: 10.1089/lap.2013.0152. [DOI] [PubMed] [Google Scholar]
- 47.Dulucq JL, Wintringer P, Stabilini C, Solinas L, Perissat J, Mahajna A. Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc. 2005;19:933–938. doi: 10.1007/s00464-004-2172-9. [DOI] [PubMed] [Google Scholar]
- 48.Pugliese R, Maggioni D, Sansonna F, Scandroglio I, Ferrari GC, Di Lernia S, Costanzi A, Pauna J, de Martini P. Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc. 2007;21:21–27. doi: 10.1007/s00464-005-0409-x. [DOI] [PubMed] [Google Scholar]
- 49.Usui S, Yoshida T, Ito K, Hiranuma S, Kudo SE, Iwai T. Laparoscopy-assisted total gastrectomy for early gastric cancer: comparison with conventional open total gastrectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:309–314. doi: 10.1097/01.sle.0000191589.84485.4a. [DOI] [PubMed] [Google Scholar]
- 50.Tanimura S, Higashino M, Fukunaga Y, Takemura M, Tanaka Y, Fujiwara Y, Osugi H. Laparoscopic gastrectomy for gastric cancer: experience with more than 600 cases. Surg Endosc. 2008;22:1161–1164. doi: 10.1007/s00464-008-9786-2. [DOI] [PubMed] [Google Scholar]
- 51.Kim KH, Kim MC, Jung GJ, Kim HH. Long-term outcomes and feasibility with laparoscopy-assisted gastrectomy for gastric cancer. J Gastric Cancer. 2012;12:18–25. doi: 10.5230/jgc.2012.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Jun L, Chen QY, Lin M, Tu R. Evaluation of laparoscopic total gastrectomy for advanced gastric cancer: results of a comparison with laparoscopic distal gastrectomy. Surg Endosc. 2016;30:1988–1998. doi: 10.1007/s00464-015-4429-x. [DOI] [PubMed] [Google Scholar]
- 53.Moisan F, Norero E, Slako M, Varas J, Palominos G, Crovari F, Ibañez L, Pérez G, Pimentel F, Guzmán S, et al. Completely laparoscopic versus open gastrectomy for early and advanced gastric cancer: a matched cohort study. Surg Endosc. 2012;26:661–672. doi: 10.1007/s00464-011-1933-5. [DOI] [PubMed] [Google Scholar]
- 54.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 55.Fujii K, Sonoda K, Izumi K, Shiraishi N, Adachi Y, Kitano S. T lymphocyte subsets and Th1/Th2 balance after laparoscopy-assisted distal gastrectomy. Surg Endosc. 2003;17:1440–1444. doi: 10.1007/s00464-002-9149-3. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 57.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 58.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 59.Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Watanabe M, Okutomi T, Wang G, Bax L. Laparoscopy versus open distal gastrectomy by expert surgeons for early gastric cancer in Japanese patients: short-term clinical outcomes of a randomized clinical trial. Surg Endosc. 2013;27:1695–1705. doi: 10.1007/s00464-012-2658-9. [DOI] [PubMed] [Google Scholar]
- 60.Takiguchi S, Fujiwara Y, Yamasaki M, Miyata H, Nakajima K, Sekimoto M, Mori M, Doki Y. Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg. 2013;37:2379–2386. doi: 10.1007/s00268-013-2121-7. [DOI] [PubMed] [Google Scholar]
- 61.Fujiwara M, Kodera Y, Misawa K, Kinoshita M, Kinoshita T, Miura S, Ohashi N, Nakayama G, Koike M, Nakao A. Longterm outcomes of early-stage gastric carcinoma patients treated with laparoscopy-assisted surgery. J Am Coll Surg. 2008;206:138–143. doi: 10.1016/j.jamcollsurg.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Zhang CD, Chen SC, Feng ZF, Zhao ZM, Wang JN, Dai DQ. Laparoscopic versus open gastrectomy for early gastric cancer in Asia: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2013;23:365–377. doi: 10.1097/SLE.0b013e31828e3e6e. [DOI] [PubMed] [Google Scholar]
- 63.Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, Saragoni L, Di Leo A, Gaudio M, Nanni O, Carli A, Cordiano C, Dell’Amore D, Vio A; Italian Research Group for Gastric Cancer (IRGGC) Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC) Jpn J Clin Oncol. 2001;31:495–499. doi: 10.1093/jjco/hye107. [DOI] [PubMed] [Google Scholar]
- 64.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 65.Cui M, Li Z, Xing J, Yao Z, Liu M, Chen L, Zhang C, Yang H, Zhang N, Tan F, et al. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med Oncol. 2015;32:241. doi: 10.1007/s12032-015-0680-1. [DOI] [PubMed] [Google Scholar]
- 66.Pugliese R, Maggioni D, Sansonna F, Costanzi A, Ferrari GC, Di Lernia S, Magistro C, De Martini P, Pugliese F. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc. 2010;24:2594–2602. doi: 10.1007/s00464-010-1014-1. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu S, Uchiyama A, Mizumoto K, Morisaki T, Nakamura K, Shimura H, Tanaka M. Laparoscopically assisted distal gastrectomy for early gastric cancer: is it superior to open surgery? Surg Endosc. 2000;14:27–31. doi: 10.1007/s004649900005. [DOI] [PubMed] [Google Scholar]
- 68.Kitano S, Shiraishi N. Minimally invasive surgery for gastric tumors. Surg Clin North Am. 2005;85:151–64, xi. doi: 10.1016/j.suc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Memon MA, Butler N, Memon B. The issue of lymphadenectomy during laparoscopic gastrectomy for gastric carcinoma. World J Gastrointest Oncol. 2010;2:65–67. doi: 10.4251/wjgo.v2.i2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huscher C, Mingoli A, Sgarzini G, Sansonetti A, Piro F, Ponzano C, Brachini G. Value of extended lymphadenectomy in laparoscopic subtotal gastrectomy for advanced gastric cancer. J Am Coll Surg. 2005;200:314. doi: 10.1016/j.jamcollsurg.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 71.Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Soga R, Wakayama A, Okamoto K, Ohyama A, Hasumi A. Completely laparoscopic extraperigastric lymph node dissection for gastric malignancies located in the middle or lower third of the stomach. Gastric Cancer. 1999;2:186–190. doi: 10.1007/s101200050044. [DOI] [PubMed] [Google Scholar]
- 72.Kim MC, Choi HJ, Jung GJ, Kim HH. Techniques and complications of laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer. Eur J Surg Oncol. 2007;33:700–705. doi: 10.1016/j.ejso.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Noshiro H, Nagai E, Shimizu S, Uchiyama A, Tanaka M. Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc. 2005;19:1592–1596. doi: 10.1007/s00464-005-0175-9. [DOI] [PubMed] [Google Scholar]
- 74.Song KY, Kim SN, Park CH. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc. 2008;22:655–659. doi: 10.1007/s00464-007-9431-5. [DOI] [PubMed] [Google Scholar]
- 75.Ziqiang W, Feng Q, Zhimin C, Miao W, Lian Q, Huaxing L, Peiwu Y. Comparison of laparoscopically assisted and open radical distal gastrectomy with extended lymphadenectomy for gastric cancer management. Surg Endosc. 2006;20:1738–1743. doi: 10.1007/s00464-006-0031-6. [DOI] [PubMed] [Google Scholar]
- 76.Hur H, Jeon HM, Kim W. Laparoscopic pancreas- and spleen-preserving D2 lymph node dissection in advanced (cT2) upper-third gastric cancer. J Surg Oncol. 2008;97:169–172. doi: 10.1002/jso.20927. [DOI] [PubMed] [Google Scholar]
- 77.Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138–1142. doi: 10.1001/archsurg.2009.223. [DOI] [PubMed] [Google Scholar]
- 78.Haverkamp L, Ruurda JP, Offerhaus GJ, Weijs TJ, van der Sluis PC, van Hillegersberg R. Laparoscopic gastrectomy in Western European patients with advanced gastric cancer. Eur J Surg Oncol. 2016;42:110–115. doi: 10.1016/j.ejso.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 79.Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211:33–40. doi: 10.1016/j.jamcollsurg.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 80.Hwang SI, Kim HO, Yoo CH, Shin JH, Son BH. Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc. 2009;23:1252–1258. doi: 10.1007/s00464-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 81.Li HT, Han XP, Su L, Zhu WK, Xu W, Li K, Zhao QC, Yang H, Liu HB. Short-term efficacy of laparoscopy-assisted vs open radical gastrectomy in gastric cancer. World J Gastrointest Surg. 2014;6:59–64. doi: 10.4240/wjgs.v6.i4.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 83.Qiu J, Pankaj P, Jiang H, Zeng Y, Wu H. Laparoscopy versus open distal gastrectomy for advanced gastric cancer: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2013;23:1–7. doi: 10.1097/SLE.0b013e3182747af7. [DOI] [PubMed] [Google Scholar]
- 84.Martínez-Ramos D, Miralles-Tena JM, Cuesta MA, Escrig-Sos J, Van der Peet D, Hoashi JS, Salvador-Sanchís JL. Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig. 2011;103:133–141. doi: 10.4321/s1130-01082011000300005. [DOI] [PubMed] [Google Scholar]
- 85.Quan Y, Huang A, Ye M, Xu M, Zhuang B, Zhang P, Yu B, Min Z. Comparison of laparoscopic versus open gastrectomy for advanced gastric cancer: an updated meta-analysis. Gastric Cancer. 2016;19:939–950. doi: 10.1007/s10120-015-0516-x. [DOI] [PubMed] [Google Scholar]
- 86.Park do J, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, Ryu SW, Song KY, Lee HJ, Cho GS, Kim HH; Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548–1553. doi: 10.1007/s00464-011-2065-7. [DOI] [PubMed] [Google Scholar]
- 87.Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, Borasi A, Capussotti L, Fronda G, Morino M; Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 88.Huang JL, Wei HB, Zheng ZH, Wei B, Chen TF, Huang Y, Guo WP, Hu B. Laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer. Dig Surg. 2010;27:291–296. doi: 10.1159/000281818. [DOI] [PubMed] [Google Scholar]
- 89.Zhao XF, Jeong O, Jung MR, Ryu SY, Park YK. A propensity score-matched case-control comparative study of laparoscopic and open extended (D2) lymph node dissection for distal gastric carcinoma. Surg Endosc. 2013;27:2792–2800. doi: 10.1007/s00464-013-2809-7. [DOI] [PubMed] [Google Scholar]
- 90.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 91.Zou ZH, Zhao LY, Mou TY, Hu YF, Yu J, Liu H, Chen H, Wu JM, An SL, Li GX. Laparoscopic vs open D2 gastrectomy for locally advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2014;20:16750–16764. doi: 10.3748/wjg.v20.i44.16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei HB, Wei B, Qi CL, Chen TF, Huang Y, Zheng ZH, Huang JL, Fang JF. Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2011;21:383–390. doi: 10.1097/SLE.0b013e31822d02dc. [DOI] [PubMed] [Google Scholar]
- 93.Ikeda O, Sakaguchi Y, Toh Y, Oogaki K, Oki E, Minami K, Okamura T, Baba H. Evaluation of oncological adequacy of laparoscopic distal gastrectomy with special attention to lymph node dissection: a comparison with conventional open gastrectomy. Hepatogastroenterology. 2012;59:627–632. doi: 10.5754/hge10089. [DOI] [PubMed] [Google Scholar]
- 94.Kunisaki C, Makino H, Yamamoto N, Sato T, Oshima T, Nagano Y, Fujii S, Akiyama H, Otsuka Y, Ono HA, et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2008;18:236–241. doi: 10.1097/SLE.0b013e31816aa13f. [DOI] [PubMed] [Google Scholar]
- 95.Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, Han SU. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc. 2007;21:28–33. doi: 10.1007/s00464-005-0634-3. [DOI] [PubMed] [Google Scholar]
- 96.Bouras G, Lee SW, Nomura E, Tokuhara T, Tsunemi S, Tanigawa N. Comparative analysis of station-specific lymph node yield in laparoscopic and open distal gastrectomy for early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2011;21:424–428. doi: 10.1097/SLE.0b013e3182367dee. [DOI] [PubMed] [Google Scholar]
- 97.Son T, Kwon IG, Hyung WJ. Minimally invasive surgery for gastric cancer treatment: current status and future perspectives. Gut Liver. 2014;8:229–236. doi: 10.5009/gnl.2014.8.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alimoglu O, Atak I, Eren T. Robot-assisted laparoscopic (RAL) surgery for gastric cancer. Int J Med Robot. 2014;10:257–262. doi: 10.1002/rcs.1566. [DOI] [PubMed] [Google Scholar]
- 99.Kim HI, Park MS, Song KJ, Woo Y, Hyung WJ. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol. 2014;40:1346–1354. doi: 10.1016/j.ejso.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 100.Park JY, Jo MJ, Nam BH, Kim Y, Eom BW, Yoon HM, Ryu KW, Kim YW, Lee JH. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg. 2012;99:1554–1561. doi: 10.1002/bjs.8887. [DOI] [PubMed] [Google Scholar]
- 101.Obama K, Sakai Y. Current status of robotic gastrectomy for gastric cancer. Surg Today. 2016;46:528–534. doi: 10.1007/s00595-015-1190-7. [DOI] [PubMed] [Google Scholar]
- 102.Terashima M, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Miki Y, Makuuchi R, Honda S, Tatsubayashi T, Takagi W, et al. Robotic surgery for gastric cancer. Gastric Cancer. 2015;18:449–457. doi: 10.1007/s10120-015-0501-4. [DOI] [PubMed] [Google Scholar]
- 103.Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am. 2003;83:1429–1444. doi: 10.1016/S0039-6109(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 104.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 105.Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, Feng Q, Peiwu Y. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc. 2014;28:1779–1787. doi: 10.1007/s00464-013-3385-6. [DOI] [PubMed] [Google Scholar]
- 106.Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, Choi SH, Noh SH. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086–1092. doi: 10.1001/archsurg.2011.114. [DOI] [PubMed] [Google Scholar]
- 107.Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610–615. doi: 10.1007/s00464-009-0618-9. [DOI] [PubMed] [Google Scholar]
- 108.Eom BW, Yoon HM, Ryu KW, Lee JH, Cho SJ, Lee JY, Kim CG, Choi IJ, Lee JS, Kook MC, et al. Comparison of surgical performance and short-term clinical outcomes between laparoscopic and robotic surgery in distal gastric cancer. Eur J Surg Oncol. 2012;38:57–63. doi: 10.1016/j.ejso.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 109.Lee HH, Hur H, Jung H, Jeon HM, Park CH, Song KY. Robot-assisted distal gastrectomy for gastric cancer: initial experience. Am J Surg. 2011;201:841–845. doi: 10.1016/j.amjsurg.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 110.D’Annibale A, Pende V, Pernazza G, Monsellato I, Mazzocchi P, Lucandri G, Morpurgo E, Contardo T, Sovernigo G. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res. 2011;166:e113–e120. doi: 10.1016/j.jss.2010.11.881. [DOI] [PubMed] [Google Scholar]
- 111.Caruso S, Patriti A, Marrelli D, Ceccarelli G, Ceribelli C, Roviello F, Casciola L. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot. 2011;7:452–458. doi: 10.1002/rcs.416. [DOI] [PubMed] [Google Scholar]
- 112.Isogaki J, Haruta S, Man-I M, Suda K, Kawamura Y, Yoshimura F, Kawabata T, Inaba K, Ishikawa K, Ishida Y, et al. Robot-assisted surgery for gastric cancer: experience at our institute. Pathobiology. 2011;78:328–333. doi: 10.1159/000330172. [DOI] [PubMed] [Google Scholar]
- 113.Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, Li AF, Chiou SH, Wu CW. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1303–1310. doi: 10.1007/s11605-012-1874-x. [DOI] [PubMed] [Google Scholar]
- 114.Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg. 2012;36:331–337. doi: 10.1007/s00268-011-1352-8. [DOI] [PubMed] [Google Scholar]
- 115.Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer. 2012;12:156–163. doi: 10.5230/jgc.2012.12.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681–1687. doi: 10.1002/bjs.8924. [DOI] [PubMed] [Google Scholar]
- 117.Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, Choi IJ, Kim CG, Lee JY, Cho SJ, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26:1377–1381. doi: 10.1007/s00464-011-2043-0. [DOI] [PubMed] [Google Scholar]
- 118.Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, Kim JH, Park SH, Mok YJ, Park SS. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. 2013;20:1258–1265. doi: 10.1245/s10434-012-2679-6. [DOI] [PubMed] [Google Scholar]
- 119.Park JY, Kim YW, Ryu KW, Eom BW, Yoon HM, Reim D. Emerging Role of Robot-assisted Gastrectomy: Analysis of Consecutive 200 Cases. J Gastric Cancer. 2013;13:255–262. doi: 10.5230/jgc.2013.13.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014;28:2606–2615. doi: 10.1007/s00464-014-3511-0. [DOI] [PubMed] [Google Scholar]
- 121.Shen W, Xi H, Wei B, Cui J, Bian S, Zhang K, Wang N, Huang X, Chen L. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc. 2016;30:574–580. doi: 10.1007/s00464-015-4241-7. [DOI] [PubMed] [Google Scholar]
- 122.Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016;263:103–109. doi: 10.1097/SLA.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 123.Kim YW, Reim D, Park JY, Eom BW, Kook MC, Ryu KW, Yoon HM. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc. 2016;30:1547–1552. doi: 10.1007/s00464-015-4372-x. [DOI] [PubMed] [Google Scholar]
- 124.Liao G, Chen J, Ren C, Li R, Du S, Xie G, Deng H, Yang K, Yuan Y. Robotic versus open gastrectomy for gastric cancer: a meta-analysis. PLoS One. 2013;8:e81946. doi: 10.1371/journal.pone.0081946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol. 2012;21:274–280. doi: 10.1016/j.suronc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 126.Xiong J, Nunes QM, Tan C, Ke N, Chen Y, Hu W, Liu X, Mai G. Comparison of short-term clinical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: a meta-analysis of 2495 patients. J Laparoendosc Adv Surg Tech A. 2013;23:965–976. doi: 10.1089/lap.2013.0279. [DOI] [PubMed] [Google Scholar]
- 127.Liao GX, Xie GZ, Li R, Zhao ZH, Sun QQ, Du SS, Ren C, Li GX, Deng HJ, Yuan YW. Meta-analysis of outcomes compared between robotic and laparoscopic gastrectomy for gastric cancer. Asian Pac J Cancer Prev. 2013;14:4871–4875. doi: 10.7314/apjcp.2013.14.8.4871. [DOI] [PubMed] [Google Scholar]
- 128.Shen WS, Xi HQ, Chen L, Wei B. A meta-analysis of robotic versus laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2014;28:2795–2802. doi: 10.1007/s00464-014-3547-1. [DOI] [PubMed] [Google Scholar]
- 129.Chuan L, Yan S, Pei-Wu Y. Meta-analysis of the short-term outcomes of robotic-assisted compared to laparoscopic gastrectomy. Minim Invasive Ther Allied Technol. 2015;24:127–134. doi: 10.3109/13645706.2014.985685. [DOI] [PubMed] [Google Scholar]
- 130.Hyun MH, Lee CH, Kim HJ, Tong Y, Park SS. Systematic review and meta-analysis of robotic surgery compared with conventional laparoscopic and open resections for gastric carcinoma. Br J Surg. 2013;100:1566–1578. doi: 10.1002/bjs.9242. [DOI] [PubMed] [Google Scholar]
- 131.Marano A, Choi YY, Hyung WJ, Kim YM, Kim J, Noh SH. Robotic versus Laparoscopic versus Open Gastrectomy: A Meta-Analysis. J Gastric Cancer. 2013;13:136–148. doi: 10.5230/jgc.2013.13.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zong L, Seto Y, Aikou S, Takahashi T. Efficacy evaluation of subtotal and total gastrectomies in robotic surgery for gastric cancer compared with that in open and laparoscopic resections: a meta-analysis. PLoS One. 2014;9:e103312. doi: 10.1371/journal.pone.0103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coratti A, Fernandes E, Lombardi A, Di Marino M, Annecchiarico M, Felicioni L, Giulianotti PC. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol. 2015;41:1106–1113. doi: 10.1016/j.ejso.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 134.Jiang L, Yang KH, Guan QL, Cao N, Chen Y, Zhao P, Chen YL, Yao L. Laparoscopy-assisted gastrectomy versus open gastrectomy for resectable gastric cancer: an update meta-analysis based on randomized controlled trials. Surg Endosc. 2013;27:2466–2480. doi: 10.1007/s00464-012-2758-6. [DOI] [PubMed] [Google Scholar]
- 135.Lee J, Kim YM, Woo Y, Obama K, Noh SH, Hyung WJ. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc. 2015;29:3251–3260. doi: 10.1007/s00464-015-4069-1. [DOI] [PubMed] [Google Scholar]
- 136.Hur H, Lee HY, Lee HJ, Kim MC, Hyung WJ, Park YK, Kim W, Han SU. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015;15:355. doi: 10.1186/s12885-015-1365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 138.Mochiki E, Nakabayashi T, Kamimura H, Haga N, Asao T, Kuwano H. Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg. 2002;26:1145–1149. doi: 10.1007/s00268-002-6286-8. [DOI] [PubMed] [Google Scholar]


