Abstract
AIM: To correlate gastric contractility, gastrointestinal transit, and hormone levels in non-pregnant (estrous cycle) and pregnant rats using noninvasive techniques.
METHODS: Female rats (n = 23) were randomly divided into (1) non-pregnant, (contractility, n = 6; transit, n = 6); and (2) pregnant (contractility, n = 5; transit, n = 6). In each estrous cycle phase or at 0, 7, 14, and 20 d after the confirmation of pregnancy, gastrointestinal transit was recorded by AC biosusceptometry (ACB), and gastric contractility was recorded by ACB and electromyography. After each recording, blood samples were obtained for progesterone and estradiol determination.
RESULTS: In the estrous cycle, despite fluctuations of sex hormone levels, no significant changes in gastrointestinal motility were observed. Days 7 and 14 of pregnancy were characterized by significant changes in the frequency of contractions (3.90 ± 0.42 cpm and 3.60 ± 0.36 cpm vs 4.33 ± 0.25 cpm) and gastric emptying (168 ± 17 min and 165 ± 15 min vs 113 ± 15 min) compared with day 0. On these same days, progesterone levels significantly increased compared with control (54.23 ± 15.14 ng/mL and 129.96 ± 30.52 ng/mL vs 13.25 ± 6.31 ng/mL). On day 14, we observed the highest level of progesterone and the lowest level of estradiol compared with day 0 (44.3 ± 15.18 pg/mL vs 24.96 ± 5.96 pg/mL).
CONCLUSION: Gastrointestinal motility was unaffected by the estrous cycle. In our data, high progesterone and low estradiol levels can be associated with decreased contraction frequency and slow gastric emptying.
Keywords: Reproductive physiological process, Sex hormones, Gastrointestinal motility, Magnetic fields, Gastric emptying
Core tip: In female rats, the estrous cycle and pregnancy appear to disturb gastrointestinal motility because of variations in hormone levels, although data are conflicting. In vivo gastrointestinal studies during pregnancy are limited by the lack of safe and reliable methods. AC biosusceptometry and electromyography are appropriate for recording motility while adhering to ethical standards. Sex hormone variations were not sufficient to disturb gastrointestinal motility during the estrous cycle. In our data, high progesterone and low estradiol levels can be associated with decreased contraction frequency and slow gastric emptying. These data were obtained in vivo using harmless techniques during several reproductive stages.
INTRODUCTION
Female rats typically exhibit distinct physiological changes, such as during the estrous cycle (proestrus, estrous, metestrus, and diestrus) and pregnancy. Gastrointestinal (GI) motility appears to be affected by both reproductive stages[1], but previous studies have reported conflicting results[2,3]. During the reproductive cycle, a delay in gastric emptying was observed during the luteal stage, which is characterized by high levels of estrogen and progesterone[4]. Irritable bowel syndrome, nausea, early satiety, and dysrhythmia were also observed during this phase[4-6]. In pregnancy, gastric emptying, GI transit, and contractility of the antral smooth muscle were reported to decrease[7]. Expansion of the uterus and the levels of such hormones as human chorionic gonadotropin, estradiol, progesterone, motilin, and relaxin have been implicated in the pathophysiology of GI disorders[8-10]. Furthermore, the effects of sex hormones depend on their levels and receptor sensitivity[5,11]. The inhibitory effect of progesterone on gastric smooth muscles may contribute to gastric dysmotility that is associated with such complaints as nausea during pregnancy[1,7,8]. Remarkable inconsistency has been observed regarding the role of estrogen in modulating GI physiological function[3].
Studies on GI motility during pregnancy are scarce, especially in humans, because of the lack of safe and reliable methods[2,8]. Nevertheless, specific techniques can be used for each type of GI measurement, including gastric emptying, contractility, accommodation, and sensation[12,13]. AC biosusceptometry (ACB) has been shown to be valid for recording GI motor function in several species, including gastric contractility, gastric emptying, and GI transit[14,15]. ACB is appropriate for recording motility while adhering to ethical and physiological standards. Also, ACB has been combined with serosal electromyography (EMG) and cutaneous electrogastrography to simultaneously record mechanical and electrical events in real time without invasiveness or radiation[16].
In rats, GI changes that are observed during pregnancy and the estrous cycle are comparable to those observed in humans[17,18]. Previous studies that had similar aims and used similar methodologies have reported discrepant results[2,3]. Few in vivo noninvasive studies have directly examined the relationship between sex hormones and GI motor parameters in female rats. Moreover, the effects of sex steroid hormones on transit in vivo cannot simply be determined according to their effects on contractility in vitro[19]. Our aim was to study the relationship between gastric electrical and mechanical contractility, GI transit, and hormone levels in non-pregnant rats (estrous cycle) and pregnant rats and to correlate these parameters in different reproductive stages.
MATERIALS AND METHODS
Animals
The protocol was planned to minimize pain and discomfort to the animals. Female Wistar rats, 90 d of age and 250-300 g, were obtained from the Animal Laboratory (ANILAB, Paulínia, SP, Brazil) and acclimatized to the laboratory conditions (24 °C, 50% humidity, and 12 h/12 h light/dark cycle) with ad libitum access to food (Presence Nutrição Animal, Paulínia, SP, Brazil) and tap water. Rats were housed in individual cages for 2 wk prior to experimentation.
The procedures and animal handling were executed in accordance with the guidelines provided by the NIH Guide for the Care and Use of Laboratory Animals and authorized by the Bioscience Institute/UNESP Ethics Committee on Use of Animals (CEUA Process 411). After the experimental procedures, all animals were euthanized by barbiturate overdose (via intravenous administration, 150 mg/kg pentobarbital sodium).
Experimental procedure
The animals were randomly distributed into the following groups: (I) estrous cycle or non-pregnant (n = 12); and (II) pregnant (n = 11). In both groups, two subgroups were formed: estrous cycle contractility (ECC; n = 6) and estrous cycle transit (ECT; n = 6) for group I and pregnancy contractility (PC; n = 5) and pregnancy transit (PT; n = 6) for group II. The reproductive cycle in female rats is characterized by proestrus, estrous, metestrus, and diestrus, based on the amount of three types of cells that are observed in vaginal smears: epithelial cells, cornified cells, and leukocytes[17]. For pregnancy, the female rats were mated with males overnight and vaginal smear was evaluated in the next morning. Presence of spermatozoa in the slides was indicative of gestational day 0.
Gastrointestinal recordings
On the morning of each phase (estrous cycle) or established time points after pregnancy confirmation (0, 7, 14, and 20 d), GI transit was recorded by ACB, and gastric contractility was recorded by ACB and EGG according to the assigned groups.
An ACB sensor (Br4Science®, Brazil) with excitation coils (diameter = 3.5 cm) and detection coils (diameter = 2.9 cm) was used because of its high spatial resolution and sensitivity for rodents[14]. The ACB signal intensity depends on the amount of magnetic material and its distance to the sensor. Ferrite (MgZnFe2O3, Imag, Brazil) was used as the magnetic material, unabsorbed in GI tract which is unable to absorb it[20].
Gastric contractility
A previous laparotomy was performed to implant the magnetic marker and electrode (Ethicon®, Johnson and Johnson, São Paulo, Brazil) in the gastric serosa, 3 cm from the pylorus[21]. Female rats were anesthetized with ketamine/xylazine (30/15 mg/kg, intramuscularly) for the procedure. The lead wire from the electrode was exteriorized through the abdominal wall and tunneled subcutaneously to the neck. The animals were allowed at least 7 d to recover from surgery. Afterward, they were again anesthetized (30 mg/kg pentobarbital, Abbott Laboratories, Chicago, IL, United States) and placed in the supine position during 45-min recording period. The magnetic sensor was positioned on the anterior surface of the abdomen, and continuous ACB signal recording commenced. The electrode was connected to a BIOPAC system. Simultaneous signals were acquired at a sampling rate of 20 Hz/channel, digitized using a multi-channel recorder (MP100 System; BIOPAC, Santa Barbara, CA, United States), and stored for further analysis. The bipolar configuration implemented for EMG included electrodes implanted, reference, and ground (attached to the animal’s hind leg) that were connected to an amplifier system (Biopac EGG100C amplifier; set to 1000 gain, low pass filter at 1 Hz, high pass filter at 0.005 Hz)[21].
Gastrointestinal transit
After fasting for 12 h, the rats ingested a solid magnetic pellet (0.5 g powder ferrite and 1.5 g laboratory chow) and were raised gently up by the neck to place the ACB sensor on the abdominal surface. The maximum magnetic intensity value obtained was registered and recognized as corresponding to the stomach. Sequentially, the ACB sensor was placed in the cecum projection (based on anatomical references), and the magnetic intensity value was also recorded[14]. Subsequent measurements were performed in awake rats at these two points at regular 15-min intervals for at least 6 h[22].
Hormone levels
After each GI recording, orbital sinus blood samples were obtained under ketamine/xylazine anesthesia (30/15 mg/kg, intramuscular). Blood samples from the four estrous cycle phases and 4 d of pregnancy were stored in a freezer at -80 °C for later analysis to determine progesterone and estradiol levels by chemiluminescence.
Data analysis
To quantify gastric contractility parameters, all magnetic signals were analyzed in MatLab (Mathworks, Natick, MA, United States) by visual inspection and Fast Fourier Transform (FFT) with bi-directional Butterworth band-pass filters with a cutoff frequency of 50-120 mHz. The highest frequency peak for each FFT was determined as the gastric dominant frequency, and the lowest frequency represented signal noise. The amplitude of contraction (A) was determined according to the relationship between power of gastric peak (P) and power of noise peak (P’) and expressed in decibels (dB) as the following: A = 10 log10 (P/P’)[19].
Individual gastric emptying (GE) and orocecal transit (OCT) times were designed according to statistical moments using MatLab, defined as Mean Gastric Emptying Time (MGET) for GE and Mean Cecum Arrival Time (MCAT) for OCT[14,22].
Statistical analysis
The statistical methods for the present study were reviewed by Jose Ricardo de Arruda Miranda. The normality of continuous variables was evaluated using the Kolmogorov-Smirnov test. The variables were normally distributed. Overall difference among groups was detected by ANOVA followed by Tukey’s multiple-comparison test. Pearson correlation coefficients were calculated to analyze the relationship between variables. A value of P < 0.05 was considered significant. The data are expressed as mean ± SD.
RESULTS
Figure 1 and 2 show examples of gastric contractility and gastrointestinal transit during pregnancy and the estrous cycle in female rats, respectively. The control example in Figure 2 was obtained in estrous phase, although all stages of the estrous cycle and also day 0 of pregnancy have presented the same profile. Regarding to techniques, electrical (EMG) and mechanical (ACB) activities remained coordinated in all groups.
Figure 1.
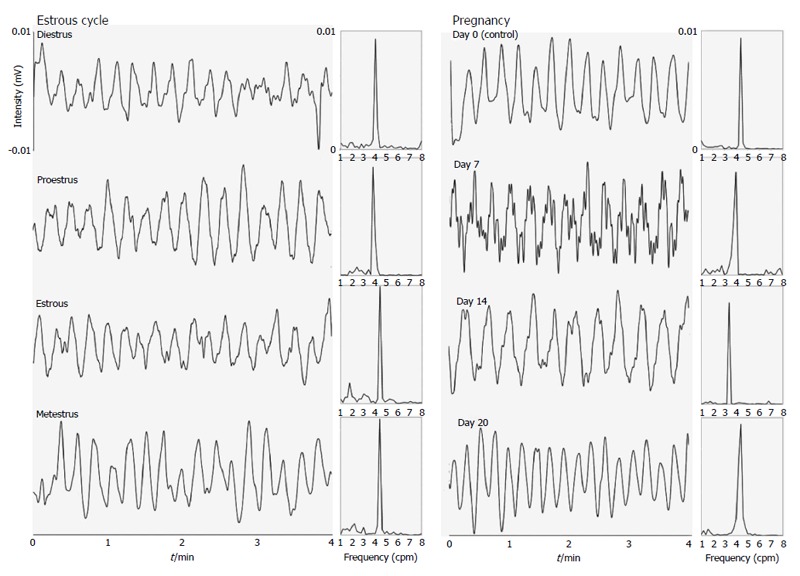
Profile of gastric contractility signals and their respective Fourier transform obtained in female rats during the estrous cycle (estrous phase) and pregnancy (day 14). The frequency values obtained were 4.3 and 3.6 cpm (71.7 and 60.0 mHz), respectively.
Figure 2.
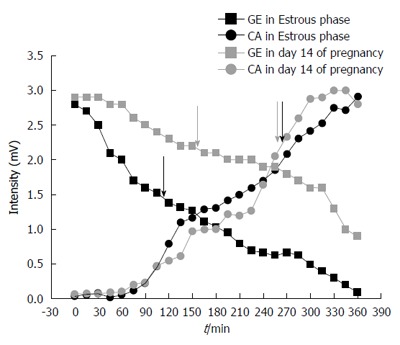
Profile of gastric emptying and cecum arrival obtained in estrous cycle (estrous phase) and pregnancy (day 14). Arrows indicate the statistical moment (minutes in X-axis), and consequently, mean gastric emptying time and mean cecum arrival time for each example.
It is possible to observe associations among gastric emptying time, frequency of gastric contractions, and progesterone levels in female rats during pregnancy and the estrous cycle (Figure 3). Considering the estrous cycle (group I), despite an expected fluctuation in sex hormone levels, no significant changes in GI motility were observed. The estradiol peak that was observed in proestrus did not modify GI motility. During estrus and metestrus, increases in progesterone levels were observed compared with proestrus. The highest levels of estradiol were observed in proestrus, and the lowest levels were observed in diestrus/metestrus. In group II, the most important days of pregnancy (days 7 and 14) were characterized by substantial changes in the frequency of contraction and gastric emptying. On these same days, progesterone levels increased compared with controls. On day 14, we observed both the highest level of progesterone and the lowest level of estradiol. This combination appeared to potentiate the effects on GI motility that were detected, including the reduction of contraction frequency that was observed by EMG. The amplitude of contractions and orocecal transit time were unaffected by pregnancy (Table 1).
Figure 3.
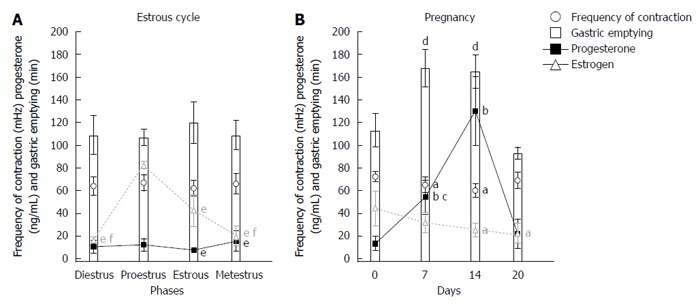
Associations among gastric emptying time (min), frequency of gastric contractions (mHz), and progesterone levels (ng/mL) in female rats during pregnancy and the estrous cycle. aP < 0.03, compared with control (day 0); bP < 0.002, compared with days 0 and 20; cP < 0.001, compared with day 14; dP < 0.001, compared with days 0 and 20; eP < 0.05, compared with proestrus; fP < 0.001 compared with estrous.
Table 1.
Gastrointestinal motility parameters recorded by AC biosusceptometry (cecum arrival and frequency of contractions) and by electromyography (frequency and amplitude of contractions) during pregnancy and the estrous cycle in female rats
| Days/phases | Frequency EMG (cpm) | Amplitude ACB (dB) | Amplitude EMG (dB) | Cecum arrival (min) | |
| Pregnancy | 0 (control) | 70.0 ± 7.0 | 66.79 ± 14.41 | 52.82 ± 7.02 | 254 ± 10.8 |
| 7 | 66.0 ± 6.0 | 72.12 ± 13.98 | 49.79 ± 9.64 | 244 ± 16.0 | |
| 14 | 59.0 ± 7.0a | 71.62 ± 18.97 | 48.41 ± 7.55 | 255 ± 14.8 | |
| 20 | 65.0 ± 9.0 | 69.42 ± 12.75 | 52.97 ± 11.12 | 259 ± 19.9 | |
| Estrous cycle | Diestrus | 65.0 ± 11.0 | 62.10 ± 7.59 | 55.54 ± 11.69 | 260 ± 26.5 |
| Proestrus | 64.0 ± 4.0 | 54.04 ± 10.16 | 54.99 ± 7.47 | 270 ± 16.7 | |
| Estrous | 65.0 ± 8.0 | 52.08 ± 13.09 | 57.20 ± 8.27 | 268 ± 17.1 | |
| Metestrus | 68.0 ± 9.0 | 54.03 ± 10.79 | 54.66 ± 8.25 | 269 ± 21.2 |
Data are expressed as mean ± SD.
P < 0.03 vs control (day 0). EGM: Electromyography; ACB: AC biosusceptometry.
An interest relationship was found among gastric emptying, the frequency of contractions, and progesterone levels (Figure 3). Low frequencies of contraction were associated with slower gastric emptying during pregnancy (Figures 1 and 2). Negative correlations were found between progesterone levels and the frequency of contractions by ACB and EMG (R = -0.93, P < 0.02, and R = -0.77, P < 0.05, respectively).
DISCUSSION
Despite several changes in body during pregnancy may contribute to impaired GI motility, our data show that high progesterone and low estradiol levels can also be associated with decreased contraction frequency and slow gastric emptying. During the estrous cycle, GI motility was unaltered, despite the occurrence of sex hormone variations. EMG and ACB were simultaneously employed, showing coordinated electric and mechanical gastric activities in female rats. Both methods can be employed in noninvasive approaches with magnetic tracer ingestion and surface electrodes[23]. Both of these techniques have enormous advantages when considering ethical issues, especially during gestation. The relatively short duration of the estrous cycle and pregnancy in rats makes these techniques ideal for investigating changes that occur during the reproductive cycle[17,24].
Previous studies have shown that progesterone delays gastric emptying in female rats and women, particularly during the third trimester of pregnancy when progesterone levels substantially increase[25,26]. This finding is consistent with relatively higher tonus in the pylorus than in gastric muscles[25]. This presumed effect of progesterone on GI transit over long periods of time may at least partially account for the disturbances in GI function frequently related by pregnant women[26]. Other studies reported that estrogen administration inhibited gastric emptying in rats[3,11]. However, assessing the influence of estrogen or progesterone alone can be difficult when considering that both of these hormones act synergistically[27], especially in the uterus[25]. In the GI tract, progesterone provokes regional gastrointestinal sensitivity differences[28] and dose-dependency[11] which leads to a divergence in findings[5].
Delayed gastric emptying was observed in pregnant guinea pigs, with no changes in gastric smooth muscles contractile[25]. In the present study, gastric smooth muscles were affected, in which both techniques showed that the frequency of contractions decreased, whereas gastric emptying slowed (Table 1 and Figure 3). Gastric dysrhythmias include abnormalities in gastric tone, myoelectrical activity and contractility, representing a pathophysiologic mechanism by which nausea is experienced in pregnant women[29].
Phases of the estrous cycle can be differentiated due to changes in the serum levels of sex hormones at different stages of the estrous cycle[27]. Estradiol reaches peak levels during proestrus and returns to baseline in estrus. Progesterone secretion rises during metestrus and diestrus and subsequently decreases thereafter. Progesterone levels increase to a second peak toward the end of proestrus[17,24]. In the present study, we collected data at the beginning of this phase. However, sex hormone variations did not appear to be sufficient to disturb GI motility, which contrasts with other in vitro and ex vivo studies[4]. Gastrointestinal transit is reportedly prolonged during the luteal phase and pregnancy[5,26], but such a finding was not observed in the present study. Constipation is a common symptom in both stages[30] and can occur through a combination of mechanical and hormonal issues that affect GI function[5,30].
The uterus will gradually enlarge during pregnancy, and this gravid uterus may have a mechanical effect that can disturb GI motility[30]. However, on day 20 of pregnancy, despite the size of the uterus, no changes in GI motility were observed in pregnant rats, supporting the theory that hormonal factors are the major influence on GI motility[30]. It is difficult to draw definitive conclusions based on the few studies that have been conducted to analyze these issues, mainly because of the different experimental designs and methods that have been used to measure GI motility[5]. Much data have been obtained using isolated muscle strips[31], the stimulation of which does not always produce the propulsion of luminal contents[32]. In vivo studies reflect a combination of factors that either stimulate or inhibit the rate of gastric emptying. Thus, contrasting effects of estrogen may be observed by increases in contractility in vitro but delayed gastric emptying[24]. Our model employed both ACB and EMG, clearly establishing the potential usefulness of such a combination as a minimally invasive monitoring system to improve clinical outcomes in obstetrics[33]. Employing such a combination of techniques allows researchers to follow the same animal over various reproductive stages.
Pregnancy in females per se is a major physiologic adjustment that affects many organ systems[9,33,34]. Understanding these physiologic adaptations is important for all clinicians because they have important implications for the diagnosis and management of various disorders[9]. When monitoring the oral intake of drugs or herbal substances, the day of pregnancy needs to be considered because changes in motility can alter the effects of such substances. In addition to the well-known elevation of sex hormones, pregnancy often alters the secretion of many hormones and peptides, including those that mediate GI motility[5,34-36]. Further investigations that utilize our model will allow us to characterize GI motility in females after different interventions, with important physiological and clinical implications.
COMMENTS
Background
In rats, gastrointestinal changes that are observed during pregnancy and the estrous cycle are comparable to those observed in humans. However, even studies that had similar aims and used similar methodologies have reported discrepant results. New studies, focusing on this traditional topic, are useful for definitely describe the physiology and for explaining certain symptoms. Few in vivo noninvasive studies have directly examined the relationship between sex hormones and gastrointestinal motor parameters in female rats. Moreover, the effects of sex steroid hormones on transit in vivo cannot simply be determined according to their effects on contractility in vitro.
Research frontiers
Studies on gastrointestinal motility during pregnancy are scarce, especially in humans, because of the lack of safe and reliable methods. AC Biosusceptometry is appropriate for recording motility while adhering to ethical and physiological standards. In the estrous cycle, despite fluctuations of sex hormone levels, no significant changes in gastrointestinal motility were observed. During pregnancy, there was a correlation between high progesterone level and slowed gastric emptying. In this context, it has been showed that gastrointestinal motor disturbance impairs drug oral treatment and intestinal nutrient absorption.
Innovations and breakthroughs
There are much controversies about which hormones provoke gastrointestinal symptoms during the estrous cycle and pregnancy. Presented data by authors data show that impaired gastrointestinal motor function is probably linked to both sex hormones: high progesterone levels accompanied by a reduction of estradiol. The major innovation is obtaining data in vivo using harmless techniques during several reproductive stages in the same animal. New studies can be exploited to refine and extend this idea toward clinical practice.
Applications
Traditional physiological aspects need to be revisited to draw general conclusions, due to methodological issues and different approaches. Thus, in vivo data are provided on the relationship between hormone level and motility, which is very important and has imminent clinical value. ACB is able to evaluate gastrointestinal contractility and transit in vivo. Besides, this approach allows analyzing the influence of hormone levels on motility parameters in an intact system.
Terminology
Gastrointestinal motility includes transit (displacement of ingested material between gastrointestinal segments) and contractility (rhythmical variation of the smooth muscle and gastrointestinal wall). Both can be registered by AC Biosusceptometry through ingested or fixed (serous) magnetic material.
Peer-review
The authors have attempted to determine changes in GI motility in rats during various phases of the reproductive cycle and pregnancy using non-invasive novel methods. It is important to recognize that this study is not a validation of these novel methods. That was done in prior studies already and this study uses these previously validated tools to study differences in motility.
Footnotes
Supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp), No. 2010/14845-4 and No. 2015/14923-9; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), No. 150456/2009-3.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Bioscience Institute/UNESP Ethics Committee on Use of Animals (CEUA Process 411).
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 8, 2016
First decision: April 14, 2016
Article in press: June 2, 2016
P- Reviewer: Arora Z S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Bonapace ES, Fisher RS. Constipation and diarrhea in pregnancy. Gastroenterol Clin North Am. 1998;27:197–211. doi: 10.1016/s0889-8553(05)70353-8. [DOI] [PubMed] [Google Scholar]
- 2.Chiloiro M, Darconza G, Piccioli E, De Carne M, Clemente C, Riezzo G. Gastric emptying and orocecal transit time in pregnancy. J Gastroenterol. 2001;36:538–543. doi: 10.1007/s005350170056. [DOI] [PubMed] [Google Scholar]
- 3.Hogan AM, Collins D, Baird AW, Winter DC. Estrogen and its role in gastrointestinal health and disease. Int J Colorectal Dis. 2009;24:1367–1375. doi: 10.1007/s00384-009-0785-0. [DOI] [PubMed] [Google Scholar]
- 4.Verrengia M, Sachdeva P, Gaughan J, Fisher RS, Parkman HP. Variation of symptoms during the menstrual cycle in female patients with gastroparesis. Neurogastroenterol Motil. 2011;23:625–e254. doi: 10.1111/j.1365-2982.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- 5.Caballero-Plasencia AM, Valenzuela-Barranco M, Martín-Ruiz JL, Herrerías-Gutiérrez JM, Esteban-Carretero JM. Are there changes in gastric emptying during the menstrual cycle? Scand J Gastroenterol. 1999;34:772–776. doi: 10.1080/003655299750025697. [DOI] [PubMed] [Google Scholar]
- 6.Güal O, Bozkurt A, Deniz M, Sungur M, Yeğen BC. Effect of sex steroids on colonic distension-induced delay of gastric emptying in rats. J Gastroenterol Hepatol. 2004;19:975–981. doi: 10.1111/j.1440-1746.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Zheng TZ, Li W, Qu SY, He DY. Action of progesterone on contractile activity of isolated gastric strips in rats. World J Gastroenterol. 2003;9:775–778. doi: 10.3748/wjg.v9.i4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Ann Intern Med. 1993;118:366–375. doi: 10.7326/0003-4819-118-5-199303010-00008. [DOI] [PubMed] [Google Scholar]
- 9.Chang J, Streitman D. Physiologic adaptations to pregnancy. Neurol Clin. 2012;30:781–789. doi: 10.1016/j.ncl.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Speranzini LB, Lopasso PP, Laudanna AA. Progesterone, estrogen and pregnancy do not decrease colon myoelectric activity in rats: an in vivo study. Gynecol Obstet Invest. 2008;66:53–58. doi: 10.1159/000119643. [DOI] [PubMed] [Google Scholar]
- 11.Liu CY, Chen LB, Liu PY, Xie DP, Wang PS. Effects of progesterone on gastric emptying and intestinal transit in male rats. World J Gastroenterol. 2002;8:338–341. doi: 10.3748/wjg.v8.i2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bratten J, Jones MP. New directions in the assessment of gastric function: clinical applications of physiologic measurements. Dig Dis. 2006;24:252–259. doi: 10.1159/000092878. [DOI] [PubMed] [Google Scholar]
- 13.Szarka LA, Camilleri M. Stomach dysfunction in diabetes mellitus: emerging technology and pharmacology. J Diabetes Sci Technol. 2010;4:180–189. doi: 10.1177/193229681000400123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quini CC, Américo MF, Corá LA, Calabresi MF, Alvarez M, Oliveira RB, Miranda JR. Employment of a noninvasive magnetic method for evaluation of gastrointestinal transit in rats. J Biol Eng. 2012;6:6. doi: 10.1186/1754-1611-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahid M. [Alpha thalassemia] J Med Liban. 1971;24:571–583. [PubMed] [Google Scholar]
- 16.Ramsey PL. The changing signs of congenital hip dislocation. J Pediatr Surg. 1977;12:437–441. doi: 10.1016/0022-3468(77)90022-7. [DOI] [PubMed] [Google Scholar]
- 17.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 18.Shah S, Hobbs A, Singh R, Cuevas J, Ignarro LJ, Chaudhuri G. Gastrointestinal motility during pregnancy: role of nitrergic component of NANC nerves. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1478–R1485. doi: 10.1152/ajpregu.2000.279.4.R1478. [DOI] [PubMed] [Google Scholar]
- 19.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol. 1995;268:G171–G176. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- 20.Corá LA, Américo MF, Romeiro FG, Oliveira RB, Miranda JR. Pharmaceutical applications of AC biosusceptometry. Eur J Pharm Biopharm. 2010;74:67–77. doi: 10.1016/j.ejpb.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Marques RG, Americo MF, Spadella CT, Corá LA, Oliveira RB, Miranda JR. Different patterns between mechanical and electrical activities: an approach to investigate gastric motility in a model of long-term diabetic rats. Physiol Meas. 2014;35:69–81. doi: 10.1088/0967-3334/35/1/69. [DOI] [PubMed] [Google Scholar]
- 22.Calabresi MF, Quini CC, Matos JF, Moretto GM, Americo MF, Graça JR, Santos AA, Oliveira RB, Pina DR, Miranda JR. Alternate current biosusceptometry for the assessment of gastric motility after proximal gastrectomy in rats: a feasibility study. Neurogastroenterol Motil. 2015;27:1613–1620. doi: 10.1111/nmo.12660. [DOI] [PubMed] [Google Scholar]
- 23.Andreis U, Américo MF, Corá LA, Oliveira RB, Baffa O, Miranda JR. Gastric motility evaluated by electrogastrography and alternating current biosusceptometry in dogs. Physiol Meas. 2008;29:1023–1031. doi: 10.1088/0967-3334/29/9/002. [DOI] [PubMed] [Google Scholar]
- 24.Spornitz UM, Socin CD, Dravid AA. Estrous stage determination in rats by means of scanning electron microscopic images of uterine surface epithelium. Anat Rec. 1999;254:116–126. doi: 10.1002/(SICI)1097-0185(19990101)254:1<116::AID-AR15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Coşkun T, Sevinç A, Tevetoğlu I, Alican I, Kurtel H, Yeğen BC. Delayed gastric emptying in conscious male rats following chronic estrogen and progesterone treatment. Res Exp Med (Berl) 1995;195:49–54. doi: 10.1007/BF02576773. [DOI] [PubMed] [Google Scholar]
- 26.Wald A, Van Thiel DH, Hoechstetter L, Gavaler JS, Egler KM, Verm R, Scott L, Lester R. Effect of pregnancy on gastrointestinal transit. Dig Dis Sci. 1982;27:1015–1018. doi: 10.1007/BF01391748. [DOI] [PubMed] [Google Scholar]
- 27.Faccio L, Da Silva AS, Tonin AA, França RT, Gressler LT, Copetti MM, Oliveira CB, Sangoi MB, Moresco RN, Bottari NB, et al. Serum levels of LH, FSH, estradiol and progesterone in female rats experimentally infected by Trypanosoma evansi. Exp Parasitol. 2013;135:110–115. doi: 10.1016/j.exppara.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Bruce LA, Behsudi FM. Differential inhibition of regional gastrointestinal tissue to progesterone in the rat. Life Sci. 1980;27:427–434. doi: 10.1016/0024-3205(80)90192-7. [DOI] [PubMed] [Google Scholar]
- 29.Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186:S198–S203. doi: 10.1067/mob.2002.122598. [DOI] [PubMed] [Google Scholar]
- 30.Cullen G, O’Donoghue D. Constipation and pregnancy. Best Pract Res Clin Gastroenterol. 2007;21:807–818. doi: 10.1016/j.bpg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Ryan JP, Bhojwani A. Colonic transit in rats: effect of ovariectomy, sex steroid hormones, and pregnancy. Am J Physiol. 1986;251:G46–G50. doi: 10.1152/ajpgi.1986.251.1.G46. [DOI] [PubMed] [Google Scholar]
- 32.Tsubouchi T, Saito T, Mizutani F, Yamauchi T, Iwanaga Y. Stimulatory action of itopride hydrochloride on colonic motor activity in vitro and in vivo. J Pharmacol Exp Ther. 2003;306:787–793. doi: 10.1124/jpet.102.048603. [DOI] [PubMed] [Google Scholar]
- 33.Carlin A, Alfirevic Z. Physiological changes of pregnancy and monitoring. Best Pract Res Clin Obstet Gynaecol. 2008;22:801–823. doi: 10.1016/j.bpobgyn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Chang FY, Lee SD, Yeh GH, Lu CC, Wang PS, Wang SW. Disturbed small intestinal motility in the late rat pregnancy. Gynecol Obstet Invest. 1998;45:221–224. doi: 10.1159/000009971. [DOI] [PubMed] [Google Scholar]
- 35.Bani D, Baccari MC, Quattrone S, Nistri S, Calamai F, Bigazzi M, Bani Sacchi T. Relaxin depresses small bowel motility through a nitric oxide-mediated mechanism. Studies in mice. Biol Reprod. 2002;66:778–784. doi: 10.1095/biolreprod66.3.778. [DOI] [PubMed] [Google Scholar]
- 36.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1546–R1554. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]


