Abstract
Objective:
To compare daily sex hormone levels and rates of change between women with history of migraine and controls.
Methods:
History of migraine, daily headache diaries, and daily hormone data were collected in ovulatory cycles of pre- and early perimenopausal women in the Study of Women's Health Across the Nation. Peak hormone levels, average daily levels, and within-woman day-to-day rates of decline over the 5 days following each hormone peak were calculated in ovulatory cycles for conjugated urinary estrogens (E1c), pregnanediol-3-glucuronide, luteinizing hormone, and follicle-stimulating hormone. Comparisons were made between migraineurs and controls using 2-sample t tests on the log scale with results reported as geometric means.
Results:
The sample included 114 women with history of migraine and 223 controls. Analyses of within-woman rates of decline showed that E1c decline over the 2 days following the luteal peak was greater in migraineurs for both absolute rate of decline (33.8 [95% confidence interval 28.0–40.8] pg/mgCr vs 23.1 [95% confidence interval 20.1–26.6] pg/mgCr, p = 0.002) and percent change (40% vs 30%, p < 0.001). There was no significant difference between migraineurs and controls in absolute peak or daily E1c, pregnanediol-3-glucuronide, luteinizing hormone, and follicle-stimulating hormone levels. Secondary analyses demonstrated that, among migraineurs, the rate of E1c decline did not differ according to whether a headache occurred during the cycle studied.
Conclusions:
Migraineurs are characterized by faster late luteal phase E1c decline compared to controls. The timing and rate of estrogen withdrawal before menses may be a marker of neuroendocrine vulnerability in women with migraine.
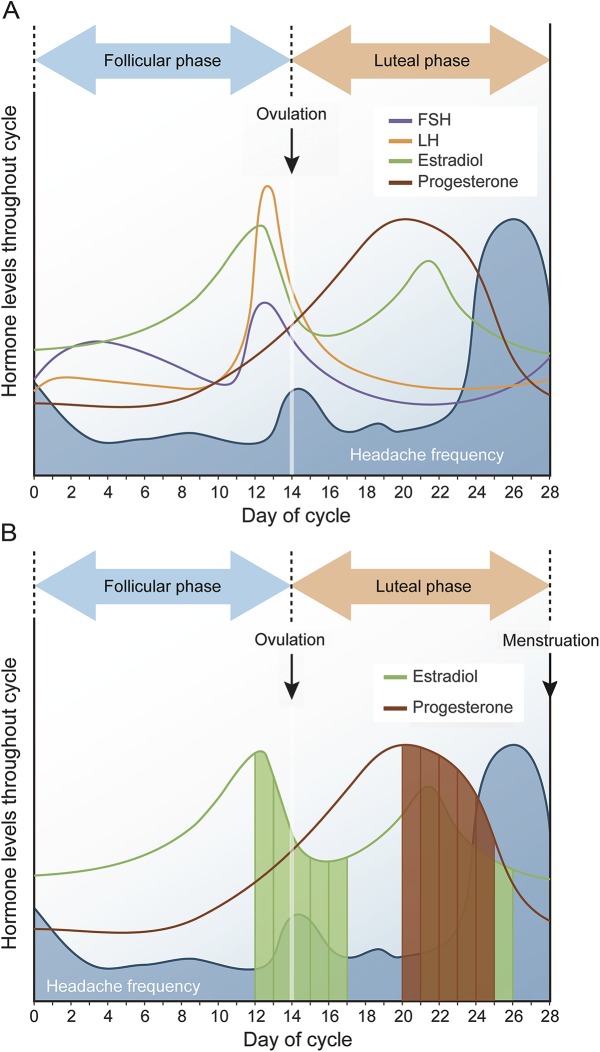
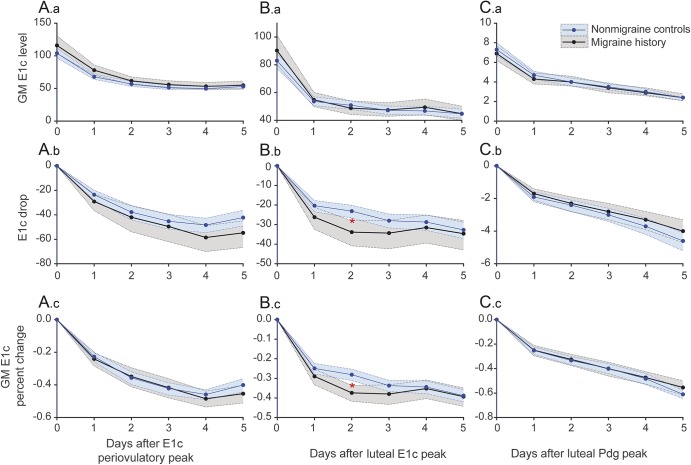
Migraine is predominantly a disorder of women that has long been linked with sex hormones. It poses a significant public health burden, particularly during the reproductive years.1–3 For the majority of women with migraine, headache attacks are more likely during the 2 days preceding the onset of menstrual bleeding and for the first 3 days of menses.4–7 These perimenstrual attacks are commonly labeled menstrual migraine and, according to the “estrogen withdrawal” migraine-triggering hypothesis,8–10 are thought to be attributable to estrogen decline in the late luteal phase (figure 1).9,10
Figure 1. Hormone levels and migraine frequency throughout the menstrual cycle.
(A) Pictorial depiction of menstrual cycle hormonal fluctuations and distribution of headache days (blue shading) in women with migraine based on literature.4–6 Migraines tend to peak during estrogen “withdrawal” in the late luteal phase, leading to perimenstrual migraine. Note that the periovulatory decline in estrogen does not appear to be associated with equally significant increase in migraine occurrence. (B) The focus of analyses of the manuscript, where changes in hormones 5 days post each hormone peak were examined (only estrogen [periovulatory and luteal peaks] and progesterone [midluteal peak] are presented in the figure. FSH and LH were examined in the same manner [not represented]). Represented in green are 5 days post periovulatory and late luteal estradiol peaks. In brown are the 5 days post midluteal progesterone peak. FSH = follicle-stimulating hormone; LH = luteinizing hormone.
Although the role of hormones in migraine has long been implicated, there are limited data regarding menstrual hormonal patterns in women with migraine10–14 and even fewer studies comparing hormone levels and patterns between women with history of migraine (MHx) and controls.11,14 Results of prior studies have been inconsistent and limited by small samples and lack of well-characterized daily hormone cycles.11,12,14 The one positive study reported elevated estradiol and progesterone levels in 12 women with migraine compared to 8 controls.11 Furthermore, prior studies have been limited to comparisons of group mean hormone levels and have not explored within-woman hormone change in relation to migraine.
The Study of Women's Health Across the Nation (SWAN) Daily Hormone Study (DHS) provided the opportunity to assess whether hormone levels and rates of change differ for women with migraine compared with controls. To explore the hypothesis that women with migraine have distinct hormone patterns, we compared peak and daily hormone levels (up to 5 days post peak) as well as mean within-woman daily rates of decline in ovulatory menstrual cycles for MHx and controls (figure 1). Secondary analyses within the migraine group examined whether hormonal patterns distinguished cycles in which acute headache occurred.
METHODS
Participants.
SWAN is a multisite, multiethnic, observational, longitudinal study designed to characterize biological and psychosocial changes occurring across the menopausal transition.15 Details of study design have been reported by Sowers et al.15: “baseline eligibility criteria included being aged 42–52 years, having an intact uterus and at least one ovary, not being pregnant or lactating, not using oral contraceptives or hormone therapy, and having a menstrual cycle in the 3 months before the baseline interview.” In 1996–1997, 3,302 women were enrolled and followed with annual clinic assessments.
The SWAN DHS included 848 women who were not using exogenous hormones and who completed a daily, first morning voided urine collection for an entire menstrual cycle ending in bleeding or for 50 days, whichever came first.16 They also completed a daily symptom diary that included headaches.
Analyses are based on the first ovulatory cycle with evidence of luteal activity (ELA) collected among pre- or early-perimenopausal women in the DHS. Of the 732 women with an ELA cycle, 627 had complete data for history of migraine and demographics and completed DHS daily diary headache data on at least 80% of the DHS collection days. The migraine group included 114 women with self-reported history of having ever been diagnosed with migraine before their first DHS ELA cycle. Potential controls were women without a history of diagnosed migraine at any SWAN visit before their first DHS ELA cycle (n = 513). Because migraine is underdiagnosed in the population,17 we excluded women who reported a severe headache in the DHS daily diary to avoid inclusion of undiagnosed migraineurs in the control group (n = 290). Thus, analyses include 337 women: 114 migraineurs and 223 controls. Peak hormone levels and patterns among women excluded from the controls were very similar to those of the included controls.
Standard protocol approvals, registrations, and patient consents.
SWAN protocols were approved by the institutional review board of each site. Written informed consent was obtained from each participant.
Measures.
Hormones.
Daily hormone levels were measured in first morning urine collections. Urinary estrogen (E2 urinary metabolites estrone conjugates [E1c]), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and the progesterone urinary metabolite pregnanediol-3-glucuronide (Pdg) were measured using chemiluminescent assays.18 Concentrations were normalized to creatinine excretion. Daily hormone profiles were categorized as ovulatory (ELA) by previously described algorithms.19,20 Previous studies have demonstrated that urinary levels of these hormones, collected and measured by these methods, mirror serum hormone patterns during the menstrual cycle in eumenorrheic control participants so closely that patterns of serum and urinary gonadotropins and sex steroids may be considered equivalent.21–23
The criteria for the occurrence of an E1c peak16 and for an LH surge24 are validated and previously reported. Visual inspection of daily levels plotted throughout the cycle was performed and in cases in which discrepancies occurred, algorithmic categories were overridden by consensus of 3 observers with expertise in reproductive endocrinology.
Migraine diagnosis.
At each visit, SWAN participants were asked, “Has a doctor, nurse practitioner, or other health care provider ever told you that you have migraine headaches?” Although this question does not meet the International Classification of Headache Disorders (ICHD)25 criteria for migraine, it is well accepted that self-reported medical diagnosis identifies migraine with high specificity but modest sensitivity.26,27 Therefore, nearly everyone with a reported medical diagnosis of migraine meets the ICHD case definition, but many with migraine are never diagnosed.17 As noted, to eliminate false negatives from the control group, potential controls who reported moderate to severe headaches in their DHS diaries were excluded.
Occurrence of headache during the DHS cycle.
Daily diaries covering 18 symptoms in the past 24 hours were completed at bedtime. Participants were asked to “think back over the last 24 hours and indicate whether or not you had a headache,” and were asked to rate it on a scale from 0 (no headache) to 4 (severe headache). A migraine during the DHS cycle was defined as at least 1 day with a headache rated as moderate to severe.
Menopause status and other covariates.
Primary race/ethnicity was self-identified as black or African American, non-Hispanic Caucasian, Chinese or Chinese American, Japanese or Japanese American, or Hispanic. Menopause status was based on self-reported menstrual irregularity, ascertained retrospectively at each visit. Premenopausal status was defined as the presence of menses within the past 3 months, with no decrease in cycle predictability. Early perimenopause was defined as the presence of menses within the past 3 months that had become less predictable in the past year or since last visit.28–30 Socioeconomic stress was defined by response to the question, “How hard is it for you to pay for the very basics like food, housing, medical care, and heating?” Weight was measured without shoes to the nearest 0.1 kg using a calibrated digital scale (Tanita BWB 800). Body mass index (BMI) was calculated from these measures: weight (kg)/height (m)2.
Statistical analysis.
The t test and χ2 statistics were used to compare characteristics of MHx and controls. Peak hormone levels were compared for MHx and controls using t tests for unadjusted differences and multivariable linear regression to adjust for covariates: age, continuous BMI, race/ethnicity, menopausal status, socioeconomic stress, years of education, and smoking history. Specifically, we compared peak hormone levels for the E1c periovulatory and luteal (premenstrual) peaks, LH and FSH, and the midluteal Pdg peak. We then examined group mean daily hormone levels over the 5 days following the peak for each hormone. Finally, we compared the mean within-woman absolute and percent daily changes in hormones over the 5 days post peak for MHx and controls.
Secondary analyses were conducted among the MHx to determine whether hormone patterns are related to headache occurrence within a cycle. MHx were divided into 2 groups, according to whether they reported a moderate to severe headache during the cycle studied. These groups were compared to each other and to control women using analysis of variance for unadjusted differences and multivariable linear regression to adjust for covariates listed above. Model assumptions/fit were assessed, including linear associations of outcomes with continuous predictors, and normally distributed residuals with constant variance. Hormone measures were analyzed on the log scale because of their skewed distribution. Results were reported as geometric and arithmetic means and 95% confidence interval (CI).
RESULTS
Characteristics of the sample.
Analyses include 337 women: 114 migraineurs and 223 controls. Table 1 shows demographic characteristics of migraineurs and controls. The majority of women in both groups were early perimenopausal. Migraineurs were more likely to be white or black and less likely to be Chinese or Japanese. Migraineurs were also more likely to have smoked and had higher mean BMI.
Table 1.
Characteristics of the cohort
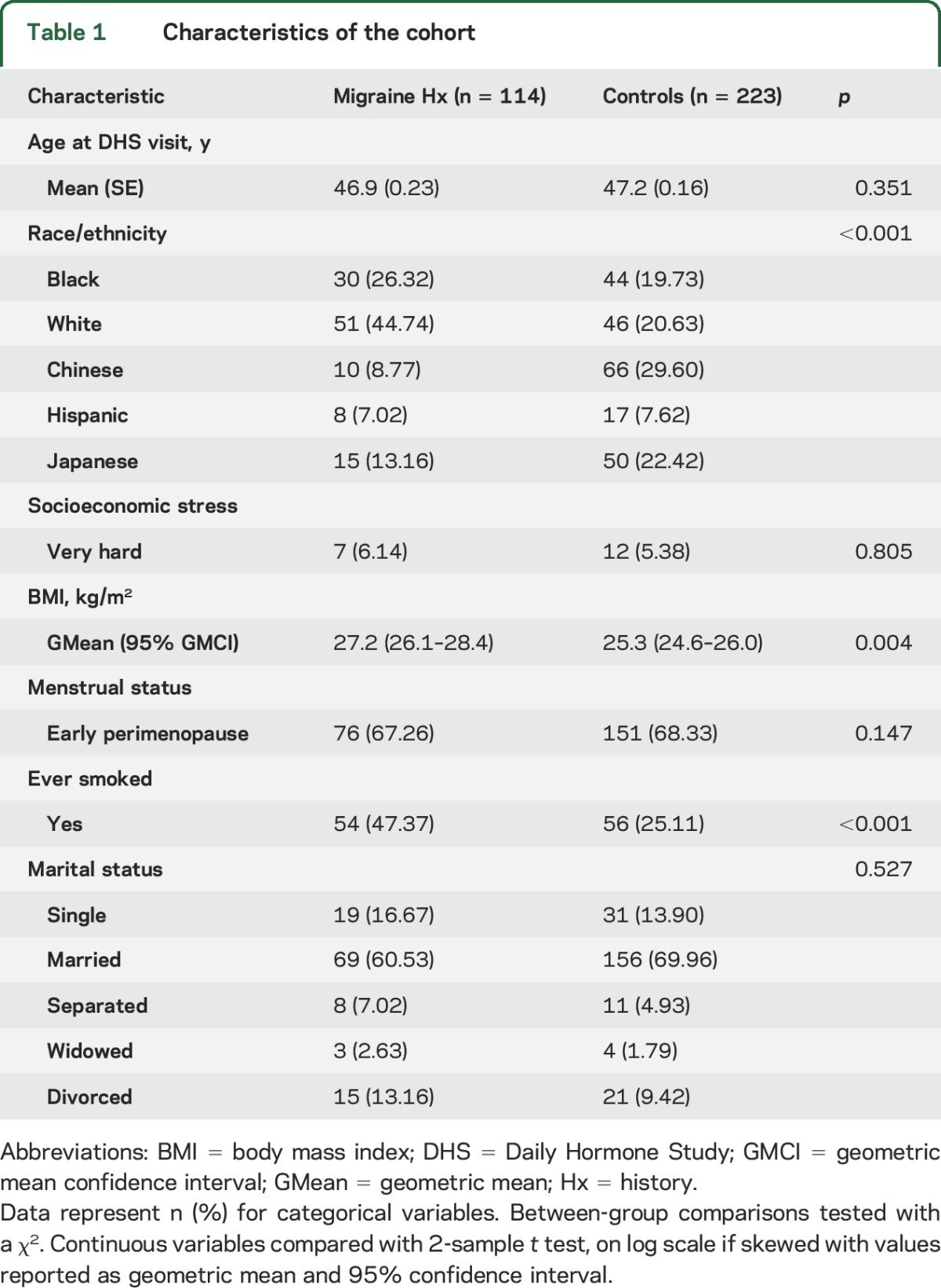
Peak and daily levels of estrogen and progesterone post peak.
Table 2 presents peak and daily levels of E1c over the 5 days following the periovulatory and luteal (premenstrual) peaks, and also peak and daily levels of Pdg following the midluteal progesterone peak. As expected, the absolute value of the periovulatory E1c peak was higher than the luteal (premenstrual) peak in both groups. Although both E1c peaks were slightly higher in migraineurs than in controls, differences were not statistically significant. Mean absolute daily E1c levels over the 5 days following the periovulatory and luteal estrogen peaks were not significantly different for migraineurs and controls. The Pdg midluteal peak was slightly higher in controls than in migraineurs, however the differences were not statistically significant. Figure 2 displays curves for mean E1c (panels A.a and B.a) and Pdg (panels C.a) on each day following the peaks and illustrates the overlap between migraineurs and controls.
Table 2.
Absolute peak and mean daily levels of estrogen and progesterone post peaks
Figure 2. Within-woman absolute and percent change for estrogen and progesterone post peaks.
No significant differences were observed in absolute levels and mean daily levels on each day post periovulatory E1c peak (A.a), luteal E1c (B.a), and midluteal Pdg (C.a) between women with history of migraine and controls. Absolute daily rates of decline from periovulatory E1c peak (A.b), late luteal E1c (B.b), and midluteal Pdg (C.b), and daily percent change (A.c, B.c, and C.c) for 5 days post peaks were calculated within woman. The significant difference was observed in E1c decline 2 days post luteal peak in women with history of migraine vs controls (B.b and B.c). Red asterisk marks the significant finding of second day post peak faster E1c decline in migraineurs. E1c = conjugated urinary estrogens; GM = geometric mean; Pdg = pregnanediol-3-glucuronide.
Absolute and percent daily change in estrogen and progesterone post peak.
Figure 2 represents average daily rates of decline (panels A.b, B.b, and C.b) and daily percent change (panels A.c, B.c, and C.c) for the 5 days following the E1c periovulatory (panel A) and luteal peaks (panel B) and the midluteal Pdg peak (panel C). There were significant differences in E1c decline in the late luteal phase. Migraineurs had a greater rate of E1c decline over the 2 days following the luteal peak than did controls for both absolute change (panel B.b) (33.8 [95% CI 28.0–40.8] pg/mgCr vs 23.1 [95% CI 20.1–26.6] pg/mgCr, p = 0.002) and percent change (panel C.b) (40% vs 30%, p < 0.001) (table e-1 on the Neurology® Web site at Neurology.org). This difference remained significant following adjustment for age; race/ethnicity; site; BMI; menopause, marital, and smoking status; and socioeconomic stress. No significant differences were observed following the midluteal Pdg decline between migraineurs and controls (table e-2).
Day-to-day levels and within-woman absolute and percent change of FSH and LH post peaks.
After covariate adjustments, there were no significant differences observed in FSH and LH (tables e-3 and e-4) peaks, daily levels, or within-women absolute or percent change over the 5 days following ovulatory FSH and LH peaks.
Analyses within migraineurs.
Figure 3 shows results of secondary analyses within the migraine group. Migraineurs were grouped according to presence of headache rated moderate to severe during the DHS cycle studied (n = 89 yes, n = 25 no) to determine whether, among migraineurs, hormone patterns are related to the occurrence of headache within a cycle. Hormone patterns for migraineurs were similar regardless of the occurrence of headache in the cycle, and the difference between migraineurs and controls was significant regardless of whether the migraineurs reported a headache.
Figure 3. Within-woman absolute and percent change in E1c following luteal peak in cycles with and without headaches.
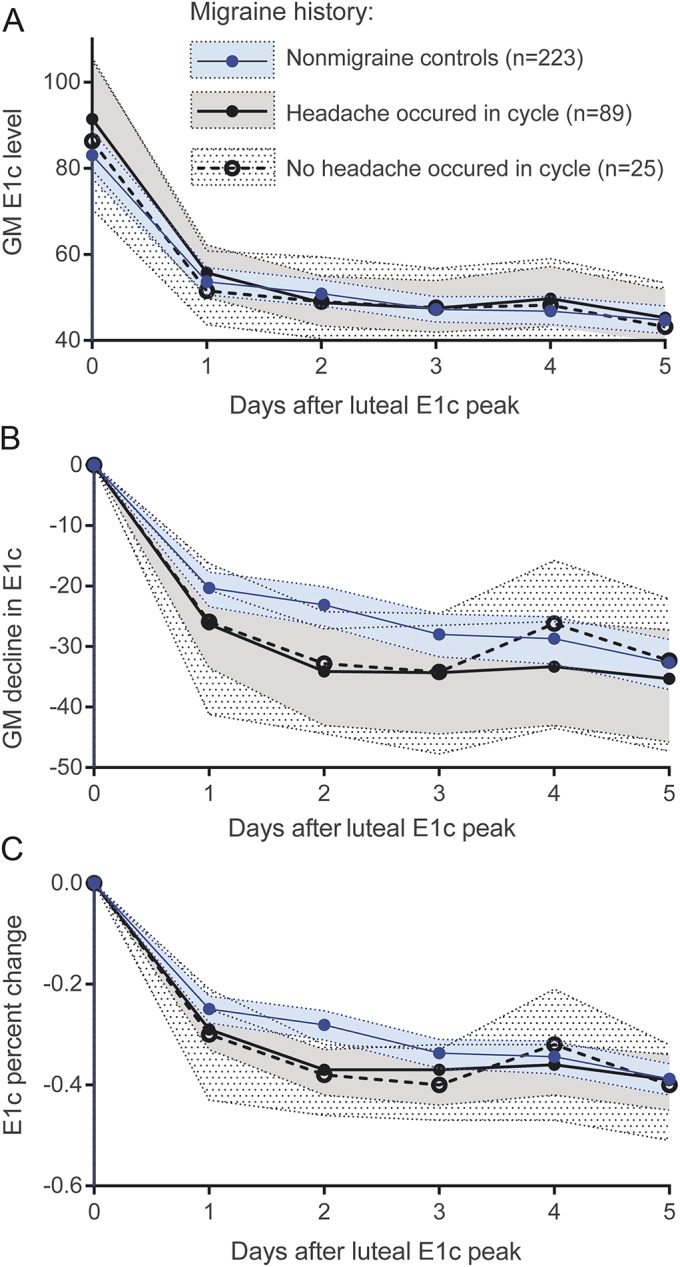
No difference in absolute levels on each day post luteal E1c (A) peak were observed when women with history of migraine were divided into those who reported headache(s) during the DHS cycle studied (n = 89) and those who were migraine free in the cycle studied (n = 25). Differences between migraineurs and controls were similar regardless of whether women with migraine reported headache in the cycle (B and C). Shading represents 95% confidence intervals. E1c = conjugated urinary estrogens.
DISCUSSION
We present a finding that migraineurs have distinct patterns of estrogen decline in the late luteal phase compared to controls. Our goal was to compare patterns of daily circulating sex hormone levels in women with and without history of migraine. We extend prior work in this area by examining not only group differences in daily and peak sex hormone levels, but also average within-woman daily rates of decline from peak. Our findings expand the “estrogen withdrawal hypothesis” of migraine triggering8,10,31 by offering the following observations: (1) there is no significant difference in estrogen peak levels or mean daily levels between migraineurs and controls; (2) however, there is a significant difference in the rate of estrogen decline specifically in the late luteal phase, while there is no difference in the rate of decline in the periovulatory phase; and (3) among migraineurs, the rate of estrogen decline does not distinguish cycles with and without an acute headache.
Our results show that differences in the rate of estrogen decline for migraineurs and controls are dependent on menstrual cycle phase and timing, specifically the rate of decline over the 2 days following the luteal E1c peak. These results shed light on long-standing questions regarding estrogen withdrawal and migraine, namely: why is the estrogen effect most pronounced in the perimenstrual period? Prior studies have consistently shown that migraine attacks are associated with estrogen withdrawal during the late luteal phase (premenstrual), while the association of migraine and estrogen decline in the follicular phase (periovulatory) has been controversial.4–6,32,33 One explanation that has been put forth to explain the menstrual cycle phase–dependent headache occurrence due to estrogen withdrawal is that a period of sustained high estrogen such as occurs in the late luteal phase is necessary for precipitation of headache by estrogen withdrawal.
Herein, presented findings offer a possible alternative explanation, showing that migraineurs have a faster rate of estrogen decline in the late luteal phase, while the rate of estrogen withdrawal following the periovulatory peak does not differ for migraineurs and controls. Furthermore, we show that migraineurs experience a faster rate of estrogen decline following the luteal estrogen peak regardless of whether they experience a severe headache during that cycle, offering an explanation for the clinical observation that women do not experience migraine with every menstrual cycle. Our results offer an expanded role of estrogen in perimenstrual migraine in that the estrogen withdrawal is not a direct trigger of migraine as commonly conceptualized. We propose a “two-hit” hypothesis of perimenstrual migraine initiation, whereby the more rapid estrogen decline is an endogenous trait of women with migraine that confers neuroendocrine vulnerability that may facilitate initiation of migraine attack(s) by common triggers, such as stress, disrupted sleep, foods, and wine.34
An endogenous difference in estrogen processing in women with migraine is further supported by a study of postmenopausal women in which estrogen decline following a single injection of estradiol triggered a migraine only in women with a premenopausal history of migraine associated with menstruation.35 This neurovulnerability to rapid estrogen decline may be due to the disruption of the serotonergic mechanisms involved in inhibition of pain leading to disruption of the trigeminovascular system.14,36,37 Furthermore, other hormones, and particularly progesterone, may modulate the effects of estrogen on migraine. While the late luteal estrogen decline is accompanied by progesterone decline, the effect of periovulatory estrogen decline on migraine may be counteracted by the rising progesterone, thus leading to a decrease in migraine occurrence.
Few studies have compared daily sex hormone levels between controls and migraineurs, and results are conflicting. A small study in the 1970s showed higher estrogen levels in migraineurs vs controls.11 In contrast, a recent study reported lower estradiol levels in women with menstrually related migraine during days 19 to 21 of the menstrual cycle.14 As this generally precedes the luteal peak, it is unclear how these findings relate to the present work. In our significantly larger and ethnically diverse study population, peak estrogen levels in migraineurs were not statistically significantly different from those in controls.
Prior studies focusing only on women with migraine have failed to identify a critical threshold or rate of estrogen change required to precipitate migraine, and have proposed that falling levels and prewithdrawal priming are more important than absolute levels and rate of change.10,38 It is critical to note that these studies examined only group mean hormone levels and did not examine within-woman rates of change in hormones. Our results underscore the need for analyses of within-woman changes in hormones. We observed no differences in group mean hormone peaks and daily hormone levels for migraineurs and controls. However, the within-woman analyses revealed a distinct pattern of faster premenstrual estrogen decline among migraineurs. That is, when we examined the levels of E1c, no differences between migraineurs and controls were observed. But when within-woman change in E1c was examined, a migraine history–related difference was observed.
This study has several limitations. Migraine diagnosis was based on self-report. Prior work has shown that almost everyone with a medical diagnosis of migraine meets an ICHD-based case definition, but many people with migraine are never diagnosed.17,39 Thus, we are confident that migraineurs in this study have the disorder although some with migraine may have been excluded. While we excluded controls who reported moderate to severe headaches in the DHS, it is possible that some with migraine who have not received physician diagnoses and are currently experiencing only mild headaches were included in the control group. However, this misclassification would have diluted the observed differences. Furthermore, the control group had proportionately more Chinese and Japanese women, while the migraineur group had more white and black women. Racial/ethnic difference in sex hormones have been reported,40 with significantly lower E1c in black, Chinese, and Japanese women compared with white and Hispanic women. These differences could be contributing to the observed associations between migraineurs and controls. However, we found that hormone patterns, but not absolute hormone levels, were related to history of migraine, and furthermore, adjustment for race/ethnicity did not change our results. Although analyses included only ovulatory cycles, these cycles were primarily among early perimenopausal women who may have somewhat elevated E1c levels relative to premenopausal women.21 Finally, as participation in the DHS is demanding, the generalizability of the results from this highly motivated group may be somewhat limited.
The strengths of this study are the careful characterization of menstrual cycles and the ability to examine daily sex hormone fluctuations in those cycles. Furthermore, the size of the study sample and the availability of hormone levels over complete menstrual cycles are unprecedented in the available literature.
Overall, we demonstrated that while absolute peak and day-to-day endogenous sex hormone levels were similar for migraineurs and controls, there were significant differences in the rate of estrogen withdrawal that were phase-specific (late luteal) and time-specific (2 days post peak). Furthermore, these differences occurred irrespective of whether migraineurs experienced headache within the cycle studied, suggesting neuroendocrine vulnerability in women with migraine. Future studies are needed to examine the day-to-day occurrence of headache in relation to daily hormone changes and to explore potential mechanisms that determine the phase- and time-dependent rates of estrogen decline in women with migraine.
Supplementary Material
ACKNOWLEDGMENT
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, principal investigator (PI) 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston—Joel Finkelstein, PI 1999–present, Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present, Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011, Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, PA—Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD—Winifred Rossi 2012–present, Sherry Sherman 1994–2012, Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers. Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, PA—Maria Mori Brooks, PI 2012–present, Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair. The authors thank the study staff at each site and all the women who participated in SWAN.
GLOSSARY
- BMI
body mass index
- DHS
Daily Hormone Study
- E1c
conjugated urinary estrogens
- ELA
evidence of luteal activity
- FSH
follicle-stimulating hormone
- ICHD
International Classification of Headache Disorders
- LH
luteinizing hormone
- MHx
women with history of migraine
- Pdg
pregnanediol-3-glucuronide
- SWAN
Study of Women's Health Across the Nation
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Amanda Allshouse and Nanette Santoro had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J.M.P. conceived of the study and participated in its design, interpretation of the data, coordination, drafting and editing of the manuscript. A.A.A. performed the statistical analysis and interpretation of the data and helped revise it for the intellectual content. N.F.S. conceived of the DHS, participated in the acquisition and interpretation of the data, coordination, and editing of the manuscript. S.L.C. participated in the design, statistical analyses, interpretation of the data, and editing of the manuscript. R.C.T. and G.S.N.-P. participated in interpretation of the data and revising of the manuscript. R.B.L. participated in the conception of the study, interpretation of the data, and revising of the manuscript. C.A.D. participated in the design of the study and interpretation of the data, coordination, and helped revise the manuscript for the intellectual content. All authors read and approved the final manuscript.
STUDY FUNDING
The Study of Women's Health Across the Nation (SWAN) has grant support from the NIH, DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women's Health (ORWH) (grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
DISCLOSURE
J. Pavlović has received honoraria from Allergan Inc. A. Allshouse reports no disclosures relevant to the manuscript. N. Santoro has received grant support from the NIH, funding from Bayer, Inc., for investigator-initiated grant support, and has stock options with MenoGeniX. S. Crawford receives grant support from the NIH. R. Thurston receives grant support from the NIH. G. Neal-Perry receives grant support from the NIH. R. Lipton has received grant support from the NIH, the National Headache Foundation, and the Migraine Research Fund. He serves as consultant, advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Boston Scientific, Bristol-Myers Squibb, CogniMed, CoLucid, Eli Lilly, Endo Pharmaceuticals, eNeura Therapeutics, GlaxoSmithKline, MAP, Merck, Novartis, NuPathe, and Pfizer. C. Derby receives grant support from the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol 2006;5:148–157. doi: 10.1016/S1474-4422(06)70348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States: relation to age, income, race, and other sociodemographic factors. JAMA 1992;267:64–69. [PubMed] [Google Scholar]
- 3.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–657. [DOI] [PubMed] [Google Scholar]
- 4.Johannes CB, Linet MS, Stewart WF, Celentano DD, Lipton RB, Szklo M. Relationship of headache to phase of the menstrual cycle among young women: a daily diary study. Neurology 1995;45:1076–1082. [DOI] [PubMed] [Google Scholar]
- 5.Stewart WF, Lipton RB, Chee E, Sawyer J, Silberstein SD. Menstrual cycle and headache in a population sample of migraineurs. Neurology 2000;55:1517–1523. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology 2004;63:351–353. [DOI] [PubMed] [Google Scholar]
- 7.Silberstein SD, Elkind AH, Schreiber C, Keywood C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology 2004;63:261–269. [DOI] [PubMed] [Google Scholar]
- 8.Welch KM, Darnley D, Simkins RT. The role of estrogen in migraine: a review and hypothesis. Cephalalgia 1984;4:227–236. [DOI] [PubMed] [Google Scholar]
- 9.Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology 1972;22:355–365. [DOI] [PubMed] [Google Scholar]
- 10.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 2006;67:2154–2158. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 11.Epstein MT, Hockaday JM, Hockaday TD. Migraine and reproductive hormones throughout the menstrual cycle. Lancet 1975;1:543–548. [DOI] [PubMed] [Google Scholar]
- 12.Horth CE, Wainscott G, Neylan C, Wilkinson MI. Proceedings: progesterone, oestradiol and aldosterone levels in plasma during the menstrual cycle of women suffering from migraine. J Endocrinol 1975;65:24P–25P. [PubMed] [Google Scholar]
- 13.Martin VT, Wernke S, Mandell K, et al. Defining the relationship between ovarian hormones and migraine headache. Headache 2005;45:1190–1201. doi: 10.1111/j.1526-4610.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahimi K, van Oosterhout WPJ, van Dorp W, et al. Reduced trigeminovascular cyclicity in patients with menstrually related migraine. Neurology 2015;84:125–131. doi: 10.1212/WNL.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 15.Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, editors. Menopause: Biology and Pathology. New York: Academic Press; 2000:175–188. [Google Scholar]
- 16.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 17.Lipton RB, Stewart WF, Celentano DD, Reed ML. Undiagnosed migraine headaches: a comparison of symptom-based and reported physician diagnosis. Arch Intern Med 1992;152:1273–1278. [DOI] [PubMed] [Google Scholar]
- 18.Santoro N, Crawford SL, Allsworth JE, et al. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab 2003;284:E521–E530. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 19.Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect 1996;104:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol 1998;147:1071–1080. [DOI] [PubMed] [Google Scholar]
- 21.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 22.Saketos M, Sharma N, Adel T, Raghuwanshi M, Santoro N. Time-resolved immunofluorometric assay and specimen storage conditions for measuring urinary gonadotropins. Clin Chem 1994;40:749–753. [PubMed] [Google Scholar]
- 23.O'Connor KA, Brindle E, Holman DJ, et al. Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research. Clin Chem 2003;49:1139–1148. [DOI] [PubMed] [Google Scholar]
- 24.Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril 2007;88:684–690. doi: 10.1016/j.fertnstert.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 25.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 26.Stewart WF, Lipton RB, Simon D, Liberman J, Von Korff M. Validity of an illness severity measure for headache in a population sample of migraine sufferers. Pain 1999;79:291–301. [DOI] [PubMed] [Google Scholar]
- 27.Lipton RB, Cady RK, Stewart WF, Wilks K, Hall C. Diagnostic lessons from the spectrum study. Neurology 2002;58(9 suppl 6):S27–S31. [DOI] [PubMed] [Google Scholar]
- 28.Brambilla DJ, McKinlay SM, Johannes CB. Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol 1994;140:1091–1095. [DOI] [PubMed] [Google Scholar]
- 29.Dudley EC, Hopper JL, Taffe J, Guthrie JR, Burger HG, Dennerstein L. Using longitudinal data to define the perimenopause by menstrual cycle characteristics. Climacteric 1998;1:18–25. [DOI] [PubMed] [Google Scholar]
- 30.Research on the menopause in the 1990s: report of a WHO scientific group. World Health Organ Tech Rep Ser 1996;866:1–107. [PubMed] [Google Scholar]
- 31.Somerville BW. Estrogen-withdrawal migraine: I: duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology 1975;25:239–244. [DOI] [PubMed] [Google Scholar]
- 32.Kibler JL, Rhudy JL, Penzien DB, et al. Hormones, menstrual distress, and migraine across the phases of the menstrual cycle. Headache 2005;45:1181–1189. doi: 10.1111/j.1526-4610.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 33.Calhoun AH. “The mystery of figure 2”: a case for ovulatory migraine? Headache 2007;47:1229–1230. doi: 10.1111/j.1526-4610.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 34. Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB. Trigger factors and premonitory features of migraine attacks: summary of studies. Headache 54:1670–1679. doi: 10.1111/head.12468. [DOI] [PubMed] [Google Scholar]
- 35.Lichten EM, Lichten JB, Whitty A, Pieper D. The confirmation of a biochemical marker for women's hormonal migraine: the depo-estradiol challenge test. Headache 1996;36:367–371. [DOI] [PubMed] [Google Scholar]
- 36.Martin VT, Lee J, Behbehani MM. Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: implications for menstrual migraine. Headache 2007;47:552–563. doi: 10.1111/j.1526-4610.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol 2012;8:89–99. doi: 10.3988/jcn.2012.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Somerville BW. Estrogen-withdrawal migraine: II: attempted prophylaxis by continuous estradiol administration. Neurology 1975;25:245–250. [DOI] [PubMed] [Google Scholar]
- 39.Lipton RB, Stewart WF, Simon D. Medical consultation for migraine: results from the American Migraine Study. Headache 1998;38:87–96. [DOI] [PubMed] [Google Scholar]
- 40.Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: the Study of Women's Health Across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab 2004;89:2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.