Abstract
Objective:
To evaluate the onset of efficacy of TEV-48125, a monoclonal antibody against calcitonin gene-related peptide, recently shown to be effective for the preventive treatment of chronic migraine (CM) and high-frequency episodic migraine.
Methods:
A randomized placebo-controlled study tested once-monthly injections of TEV-48125 675/225 mg or 900 mg vs placebo. Headache information was captured daily using an electronic headache diary. The primary endpoint was change from baseline in the number of headache hours in month 3. Herein, we assess the efficacy of each dose at earlier time points.
Results:
The sample consisted of 261 patients. For headache hours, the 675/225-mg dose separated from placebo on day 7 and the 900-mg dose separated from placebo after 3 days of therapy (p = 0.048 and p = 0.033, respectively). For both the 675/225-mg and 900-mg doses, the improvement was sustained through the second (p = 0.004 and p < 0.001) and third (p = 0.025 and p < 0.001) weeks of therapy and throughout the study (month 3, p = 0.0386 and p = 0.0057). For change in weekly headache days of at least moderate intensity, both doses were superior to placebo at week 2 (p = 0.031 and p = 0.005).
Conclusions:
TEV-48125 demonstrated a significant improvement within 1 week of therapy initiation in patients with CM.
Classification of evidence:
This study provides Class II evidence that for patients with CM, TEV-48125 significantly decreases the number of headache hours within 3 to 7 days of injection.
Chronic migraine (CM) is characterized by headaches occurring on at least 15 days per month, with at least 8 days of migraine per month.1 It affects approximately 1% of the adult population2,3 and is the most frequently seen headache syndrome at major headache clinics and neurology specialty centers.4,5 On the basis of ictal disability alone, migraine was ranked sixth highest among specific causes of disability globally.6,7 Migraine-related disability is classified by the World Health Organization as more burdensome than paraplegia, deafness, or angina, and at the same level as psychosis and quadriplegia.8 Furthermore, relative to individuals with episodic migraine or without headaches, those with CM are significantly more likely to be unemployed or employable but not actively working for pay.9 Individuals with CM are also significantly more likely to be divorced and to have psychological comorbidities.9
Despite its enormous burden, CM is undertreated. Effective treatment, at a minimum, requires consultation with a physician, an accurate diagnosis, and receiving appropriate treatment. In the United States, less than 5% of persons with CM are able to traverse all 3 of these hurdles, and only a third of those with CM receive preventive medications.10 Furthermore, 1-year adherence to labeled or off-label migraine preventive medication among individuals with CM occurs in less than 20% of patients. The most important reasons for discontinuation of preventive medications among individuals with CM appear to be incomplete efficacy, as well as slow time to reach meaningful efficacy, and poor tolerability.10,11 It has been suggested that fast onset of efficacy of migraine drugs may have significant implications for patients, since it would favor compliance and improve long-term outcomes.12
TEV-48125 is a fully humanized monoclonal antibody that potently and selectively binds to calcitonin gene-related peptide (CGRP).13 Its efficacy in the preventive treatment of CM was demonstrated in a large phase 2b study, where both tested doses separated from placebo after 1 month of therapy for primary, secondary, and exploratory endpoints.14 Since statistically significant effects were seen very early in that trial, herein we conducted post hoc analyses to evaluate the efficacy of 2 doses of subcutaneous TEV-48125 within the first few weeks of therapy in patients with CM.
METHODS
Study design and patients.
The current study represents post hoc analyses conducted as part of a phase 2b trial assessing the efficacy of TEV-48125 in the preventive treatment of CM in adults.14 The randomized, double-blind, placebo-controlled, phase 2b study was conducted at 62 sites in the United States (headache centers, neurology clinics, and primary care facilities) and an independent clinical research organization, NCGS, monitored the study, assessing for appropriate patient eligibility, protocol adherence, and completeness and accuracy of case report entries. Eligible study participants were men or women aged 18 to 65 years with a history of CM as per the International Classification of Headache Disorders, 3rd edition (beta version).1 Headache and migraine frequencies were confirmed during a prospective 28 days run-in period. Participants could continue to use up to 2 different standard migraine preventive medications, if on stable doses for at least 3 months before study onset. They were allowed to treat their acute migraine attacks as usual and had to show higher than 80% compliance in completing the electronic headache diary during the 28-day run-in phase to participate in this study. Patients were excluded if they had received onabotulinumtoxinA during the 6 months before study entry or if 3 or more preventive medications failed because of lack of efficacy.
Standard protocol approvals, registrations, and patient consents.
The study was conducted in accordance with the principles of Good Clinical Practice and the US Food and Drug Administration guidelines for safety monitoring. All patients provided written informed consent before enrollment. The study protocol was approved by the institutional review boards for each site, and the trial is registered at clinicaltrials.gov (NCT02021773).
Randomization and treatment procedures.
After the run-in period, participants were randomized (1:1:1) via an electronic interactive web response system, which was accessible through the eClinical Operating System Portal. Randomization was stratified independently by sex and preventive medication use. The randomization sequence was developed centrally by staff at NCGS who had no further role in the study. Study sites had 2 blinded study coordinators at clinic visits, one for clinical assessments and one for treatment administration, and participants were masked to treatment allocations. Participants randomized to the 900-mg arm received 4 active injections of 225 mg/1.5 mL once monthly. Those in the 675/225-mg arm received an initial loading dose of 675 mg (3 active injections of 225 mg and one placebo injection), followed by maintenance doses of 225 mg (one active and 3 placebo injections) for the second and third monthly treatments. Patients receiving placebo received 4 placebo injections monthly. Adverse events, laboratory findings, ECG, and concomitant drugs were captured monthly at every visit.
Outcomes.
As described previously,14 the primary endpoint for the study was the mean change from baseline in the number of headache hours of any severity during the 28-day posttreatment period ending with month 3. The secondary endpoint was the mean change from baseline in the number of headache days of at least moderate severity during month 3. Here, we analyzed the weekly cumulative headache hours and headache days of at least moderate severity in the first month by week and further analyzed accumulative headache hours in the first 7 days after the first dose of study medication.
Statistical analyses.
Similar to the analyses conducted for the primary and secondary endpoints, the mixed-effects model repeated measurement analysis method was used for the post hoc analyses reported herein of the weekly change-from-baseline values for the number of headache hours and moderate/severe headache days in first 4 weeks. We used analysis of covariance for the post hoc intraweekly assessments. For each given period (e.g., first week after treatment, or 3 days after treatment), baseline values were calculated using the original monthly baseline value multiplied by a factor to match the time period (in the examples given above, first week or first 3 days, baseline value was the original monthly baseline multiplied by 7/28, or 3/28). In additional post hoc analyses, we calculated the absolute risk reduction and the number needed to treat (NNT) in the proportion of patients with at least a 50% reduction from baseline headache hours and moderate/severe headache days in the first few weeks.
All statistical tests were 2-sided at α level of 0.05. All efficacy variables were analyzed by the intent-to-treat principle, which included all randomized participants who received at least one dose of study drug and provided at least one endpoint measurement. Overall compliance for baseline and first month posttreatment was 92%. For the analyses presented herein, all p values presented are nominal without multiplicity adjustment. Analyses were conducted with SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
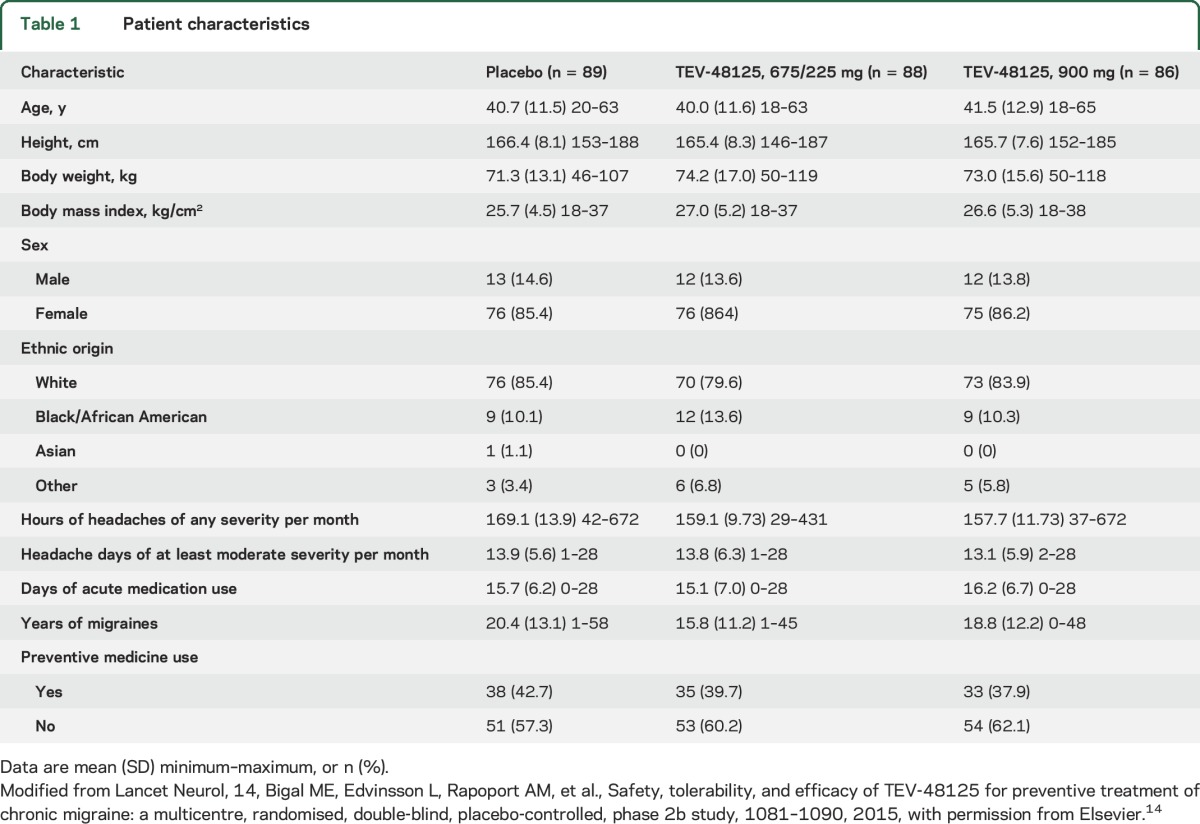
Eligibility screening for the phase 2 study began in January 2014 and the last patient visit occurred in December 2014. The sample consisted of 261 patients randomized to receive placebo (n = 89), 675/225 mg (n = 87), or 900 mg (n = 85) (figure 1). Demographics and clinical disease characteristics were similar across groups and are described in table 1. Mean overall age was 41 years, 86% of participants were women, 83% were white, and 40% of participants used preventive medications at the time of the study.
Figure 1. Patient disposition.
*Patients were considered screen failures for the chronic migraine study if they (1) had fewer than 15 days of headaches per month for 3 months before screening, (2) they had at least 15 days of headache per month for 3 months before screening but did not have 15 days of headaches or evidence of migraines during the 28-day run-in period, (3) they met the headache criteria but did not qualify for one or more of the other inclusion/exclusion criteria, (4) they were less than 80% compliant with diary entry during the 28-day run-in period and other miscellaneous reasons such as site closure, subject withdraws consent, and the subject was lost to follow-up. AE = adverse event. Modified from Lancet Neurol, 14, Bigal ME, Edvinsson L, Rapoport AM, et al., Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study, 1081–1090, 2015, with permission from Elsevier.14
Table 1.
Patient characteristics
Planned analyses recap.
A priori analyses have been published.14 In brief, at baseline, participants had a mean of 162 headache hours per month and a mean of 22 headache days and 17 migraine days per month. For the primary endpoint, least square mean (LSM) change from baseline to month 3 in the number of headache hours was −37.1 (SE 8.4) for placebo, −59.8 (8.6) for 675/225 mg (p = 0.039, LSM difference −22.7, and 95% confidence interval [CI]: −44.28 to −1.21), and −67.5 (8.6) for 900 mg (p = 0.006, LSM difference −30.4, and 95% CI: −51.88 to −8.95). At 1 month of therapy, the number of headache hours decreased from baseline for placebo was −18.1 (7.1), 675/225 mg = −44.1 (7.3; p = 0.003); 900 mg = −56.82 (7.3; p < 0.001). At 2 months of therapy, the number of hours decreased from baseline for placebo was −34.1 (8.0), 675/225 mg = −58.3 (8.1; p = 0.018); and 900 mg = −66.2 (8.1; p = 0.002).
Early time points: Headache hours.
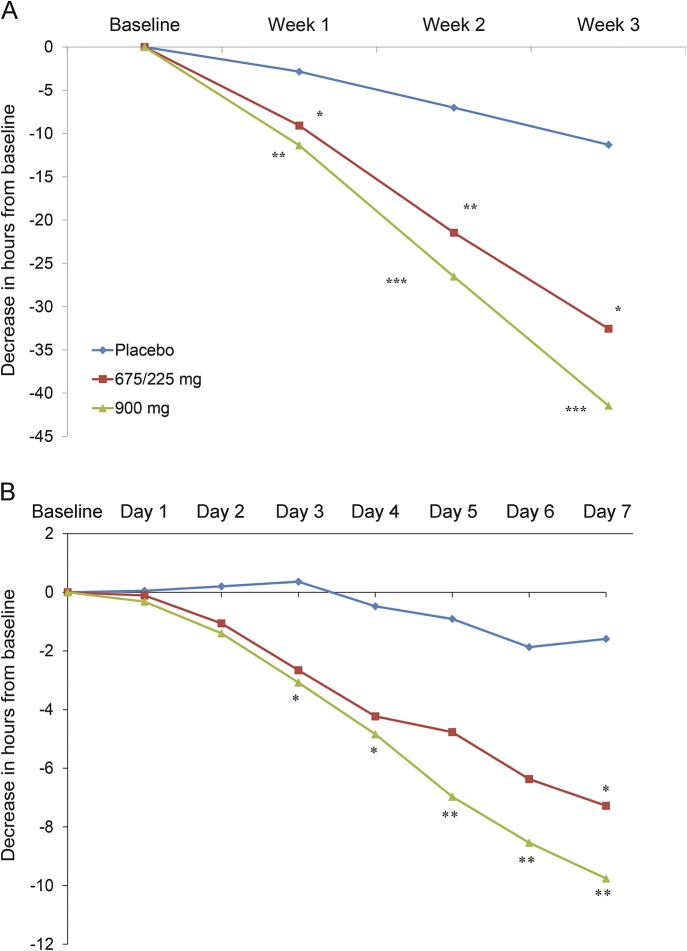
There were significant decreases in the mean number of headache hours after 1 week of therapy for both treatment doses relative to placebo. The LSM change from baseline to 1 week was −2.85 (2.21) hours for placebo, −9.08 (2.25) for 675/225 mg (p = 0.031, LSM difference −6.22, and 95% CI: −11.86 to −0.59), and −11.37 (2.26) for 900 mg (p = 0.003, LSM difference −8.52, and 95% CI: −14.27 to −2.87), a benefit that was extended through the second and third weeks of therapy (figure 2A). The 900-mg dose first separated from placebo after 3 days of therapy (−3.08 hours vs +0.36 for placebo, p = 0.0331). The lower dose separated from placebo on day 7 (placebo = −1.59 hours, 675/225 mg = −7.28, p = 0.0486, 900 mg = −9.76, p = 0.0048) (figure 2B).
Figure 2. Effect of TEV-48125 at early time points.
(A) Change in number of headache hours in the first 3 weeks of TEV-48125 treatment. (B) Change in headache hours in the first 7 days of TEV-48125 treatment. *p < 0.05, **p < 0.01, ***p < 0.001.
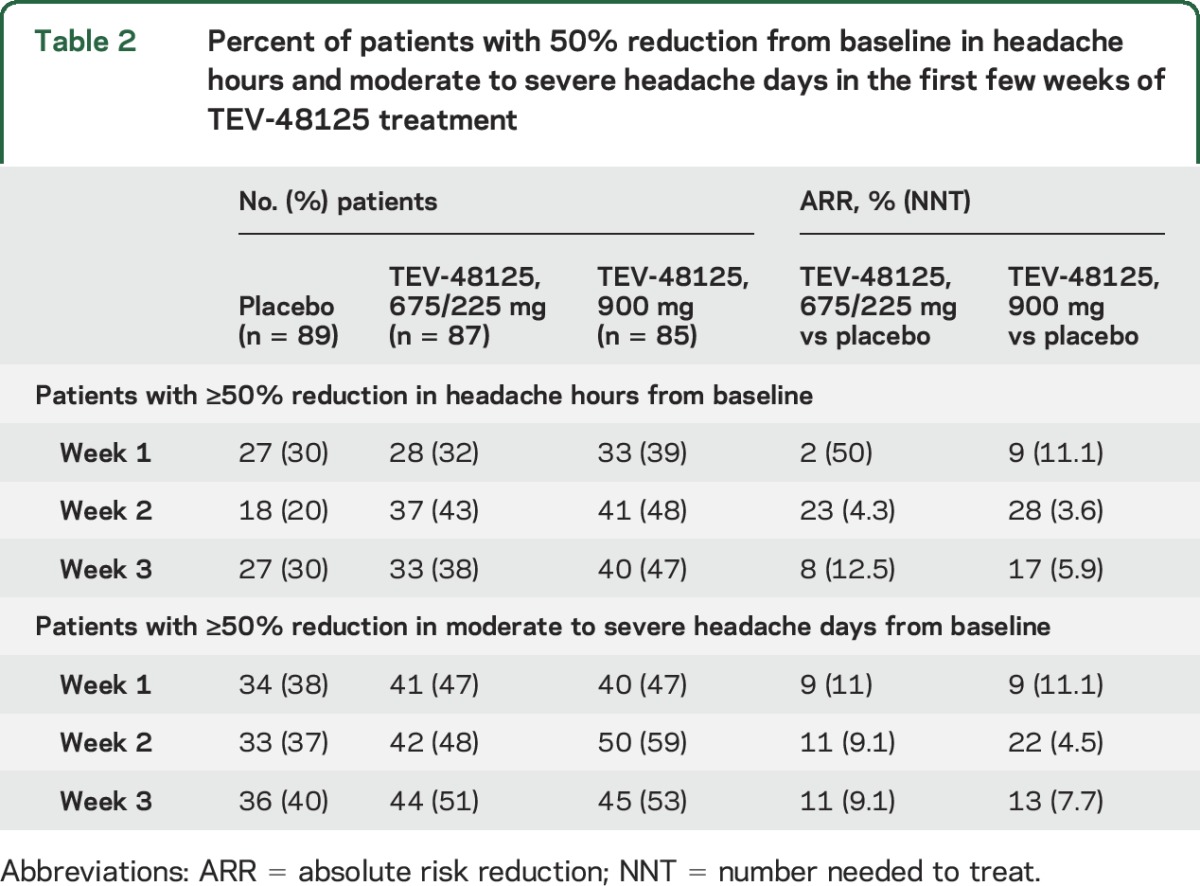
As shown in table 2, the percent of patients with a 50% reduction from baseline in the number of headache hours increased in the TEV-48125 groups relative to the placebo group in weeks 1 to 3, although the NNTs at these early time points were high.
Table 2.
Percent of patients with 50% reduction from baseline in headache hours and moderate to severe headache days in the first few weeks of TEV-48125 treatment
Early time points: Moderate to severe headache days.
For moderate to severe headache days, the lower dose, 675/225 mg, nonsignificantly reduced the number of days with moderate severity headaches in week 1 and week 3 (LSM change from baseline −1.12 vs placebo −0.77, p = 0.167 for week 1, and −1.13 vs −0.74, p = 0.142 for week 3). The 900 mg showed separation after 1 week (−1.26 vs −0.77 for placebo, p < 0.054). Both doses separated from placebo after 2 weeks' LSM change from baseline (SE): −0.79 (0.19) for placebo, −1.34 (0.20) for 675/225 mg (p = 0.031, LSM difference −0.55, and 95% CI: −1.06 to −0.05), and −1.51 (0.20) for 900 mg (p = 0.005, LSM difference 0.73, and 95% CI: −1.23 to −0.22) (p = 0.005) (figure 3). The 900-mg dose continued to separate from the placebo group in week 3 (−1.39 vs −0.74, p = 0.016).
Figure 3. Change from baseline in the number of headache days of at least moderate severity.
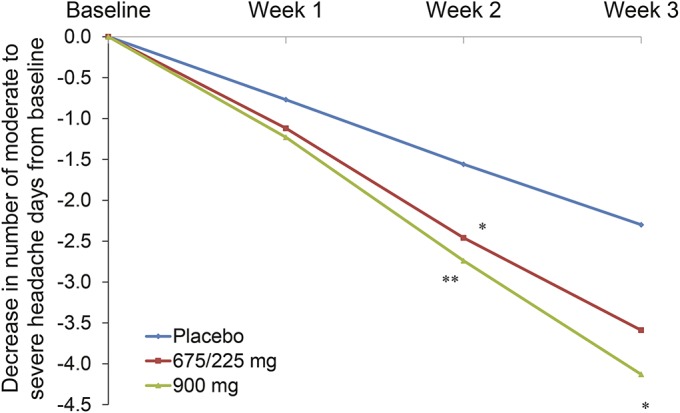
*p < 0.05, **p < 0.01.
The percent of patients with a 50% reduction from baseline in the number of moderate to severe headache days also increased in the TEV-48125 groups during weeks 1 to 3, although the NNTs, as seen for headache hours, were also high (table 2).
DISCUSSION
It has been previously demonstrated that both doses of TEV-48125 were superior to placebo in the preventive treatment of CM, validating for the first time CGRP as a therapeutic target in this disease. Since benefit was seen as early as 1 month after starting therapy, we explored the earliest time point at which efficacy began. The new analysis demonstrated a significant decrease in the number of headache hours starting as soon as 3 days after the highest dose (900 mg) was given, and 7 days after the lower dose (675/225 mg) was given. For moderate or severe headache days, a significant decrease was seen during the second week of treatment for the 675/225-mg and 900-mg doses. These data offer a glimpse of how quickly preventive treatment effects may occur for CGRP monoclonal antibodies in CM.
The new analysis of the data demonstrates that TEV-48125 can have an effect in some patients within a week of therapy initiation. Regarding clinical meaningfulness, it is not the purpose of summary measures to provide such information, but rather to offer the insight that some patients may benefit relatively rapidly from a new therapy. The early onset of effect is certainly of interest for at least 2 reasons. First, perceived early efficacy may be a reinforcing factor for compliance to therapy, especially in the context of well-tolerated medications. Second, the timing of the onset of action provides important insights on the relevance of CGRP in the pathophysiology of migraine.
Fast onset of headache improvement is a highly desirable attribute for migraine medications.15 Oral preventive medications must be titrated over weeks to effective doses, and then administered daily for approximately 3 months to establish efficacy.16,17 Although some patients respond quickly to onabotulinumtoxinA, the only approved CM preventive treatment, response is often delayed.18 Clinical experience also suggests that in many cases, adverse events with migraine preventive medications are perceived nearly immediately while efficacy requires time to be noticed. Early onset of efficacy may provide positive reinforcement for migraineurs and increase adherence to therapy.
Other CGRP monoclonal antibodies when studied in episodic migraine have shown fast onset of efficacy.19,20 It is known that circulating CGRP levels are increased in CM relative to episodic migraine and in episodic migraine relative to controls.21 CGRP-containing peripheral nerve cells in the trigeminal ganglion act as polymodal nociceptors, innervating peripheral tissues and in response to stimuli, release CGRP sending primary afferent sensory transmissions to neurons in dorsal horn of the spinal cord, the trigeminal nucleus caudalis (TNC), and the nucleus of the solitary tract. These neurons in turn project sensory inputs to the amygdala, hypothalamus, brainstem, and thalamus, which relay these inputs to the insular cortex.22–25 Monoclonal antibodies are large molecules that mostly do not cross the blood–brain barrier with immunoglobulin G plasma to CSF ratio of 0.1%.26 As a result, it has been suggested that modulation of CGRP outside the blood–brain barrier induces nearly immediate modulation of central pathways. This probable mechanism is supported by previous work suggesting that in humans, IV administration of CGRP, which does not cross the blood–brain barrier, induces migraine attacks in individuals with migraine.27 Antibodies could bind to the CGRP released at trigeminal nerve endings, thereby avoiding the peripheral events of migraine and consequent sensory transmission to central second-order neurons in the TNC, thus avoiding the secondary central sensitization that would follow.28 Reduced afferent input into central second-order neurons within the TNC could modulate neuronal activity and subsequent central trigeminal sensory transmission.
The study has important limitations that should be considered. First, the analyses reported in this article had not been a priori defined. Nonetheless, post hoc analyses have an important role in further defining the benefits of any drug, including subsets of patients experiencing particular benefit29,30 or, as in our case, providing preliminary evidence for future rigorous assessments. Second, and most important, we have not interviewed patients to check whether the effect size at early time points was clinically meaningful, and we do not suggest that they were for the early time points, although they certainly are for what is seen after 1 month of therapy, as the therapeutic gain (placebo-subtracted difference) seems to suggest so. In the pooled analyses of the onabotulinumtoxinA pivotal trials, the therapeutic gain for moderate or severe headache days after 6 months of therapy was −1.9.31 In the present study, after 1 month of therapy, 900-mg and 675/225-mg doses yielded a therapeutic gain of values of respectively −2.8 and −2.0 days. Since clinical benefit may be a function of absolute response rather than placebo-adjusted response, future studies should incorporate patients' subjective assessment of improvement.
Supplementary Material
GLOSSARY
- CGRP
calcitonin gene-related peptide
- CI
confidence interval
- CM
chronic migraine
- LSM
least square mean
- NNT
number needed to treat
- TNC
trigeminal nucleus caudalis
AUTHOR CONTRIBUTIONS
M.E.B. designed the study, interpreted data, and drafted, edited, and submitted the final article. D.W.D., A.V.K., J.H.V.P., S.J.T., E.A., and P.J.G. contributed to overseeing the data, discussed contents of the article, and participated in the writing of the article. Y.M. created the statistical analysis plan for the study and analyzed data. P.S.L. interpreted data, prepared tables and figures, and participated in writing and editing of the article.
STUDY FUNDING
The protocol was designed and the study was conducted by the funder with input from all authors. The funder was responsible for data collection, data analysis, data interpretation, and writing the article. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
DISCLOSURE
M. Bigal is an employee of Teva Pharmaceuticals Research and Development team. D. Dodick within the past 3 years has served on advisory boards and/or has consulted for Allergan, Amgen, Alder, Alcobra, Arteaus, Pfizer, CoLucid, Merck, ENeura, NuPathe, Eli Lilly and Company, Autonomic Technologies, Ethicon J&J, Zogenix, Supernus, Labrys, Boston Scientific, MAP, Novartis, Tonix, Teva, and Trigemina and has received funding for travel, speaking, editorial activities or royalty payments from IntraMed, SAGE Publishing, Sun Pharma, Allergan, Oxford University Press, HealthLogiX, Universal Meeting Management, WebMD, UpToDate, Starr Clinical, Decision Resources, and Synergy. A. Krymchantowski and J. VanderPluym report no disclosures relevant to the manuscript. S. Tepper received grants/research support from Alder, Allergan, Amgen, ATI, ElectroCore, eNeuro, GSK, Teva, Pernix, and OptiNose/Avanir/Otsuka. These grants do not go to him personally. S.T. has served as a consultant for Acorda, Allergan, Amgen, ATI, Avanir, Depomed, ElectroCore, Impax, Pfizer, Scion Neurostim, Teva, and Zosana and has been on the speakers bureau for Allergan, Depomed, Impax, Pernix, and Teva. In the last 12 months, he served on advisory boards for Allergan, Amgen, ATI, Avanir, Dr. Reddy's, Merck, Pfizer, and Teva. He is editor-in-chief of Headache Currents, American Headache Society, receives royalties for books published by University of Mississippi Press and Springer, and has stock options in ATI. E. Aycardi is an employee of Teva Pharmaceuticals Research and Development team. P. Loupe is an employee of Teva Pharmaceuticals Research and Development team. Y. Ma is an employee of Teva Pharmaceuticals Research and Development team. P. Goadsby reports grants and personal fees from Allergan, eNeura, Autonomic Technologies Inc., Amgen, and personal fees from AlderBio, Pfizer, Dr. Reddy's, Zosano, CoLucid, Eli Lilly, Avanir, Gore, Heptares, NuPathe, Teva, Cipla, Ajinomoto, Akita, Wells Fargo, Ethicon, EMKinetics, Promius, Medico-Legal, UpToDate, and Journal Watch. In addition, Dr. Goadsby has a patent magnetic stimulation for headache pending. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 2.Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache 2012;52:1456–1470. [DOI] [PubMed] [Google Scholar]
- 3.Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia 2010;30:599–609. [DOI] [PubMed] [Google Scholar]
- 4.Bigal ME, Sheftell FD, Rapoport AM, Lipton RB, Tepper SJ. Chronic daily headache in a tertiary care population: correlation between the International Headache Society diagnostic criteria and proposed revisions of criteria for chronic daily headache. Cephalalgia 2002;22:432–438. [DOI] [PubMed] [Google Scholar]
- 5.Meletiche DM, Lofland JH, Young WB. Quality-of-life differences between patients with episodic and transformed migraine. Headache 2001;41:573–578. [DOI] [PubMed] [Google Scholar]
- 6.Steiner TJ; World Headache Alliance. Lifting the burden: the global campaign against headache. Lancet Neurol 2004;3:204–205. [DOI] [PubMed] [Google Scholar]
- 7.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harwood RH, Sayer AA, Hirschfeld M. Current and future worldwide prevalence of dependency, its relationship to total population, and dependency ratios. Bull World Health Organ 2004;82:251–258. [PMC free article] [PubMed] [Google Scholar]
- 9.Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008;71:559–566. [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the Second International Burden of Migraine Study (IBMS-II). Headache 2013;53:644–655. [DOI] [PubMed] [Google Scholar]
- 11.Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine–preventive medications among patients with chronic migraine. Cephalalgia 2015;35:478–488. [DOI] [PubMed] [Google Scholar]
- 12.Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm 2014;20:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigal ME, Bronson B, Walter S, Sudworth M, Huggins JP, Garzone P. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: results of the phase 1 program. Cephalgia 2013;34:483–492. [DOI] [PubMed] [Google Scholar]
- 14.Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015;14:1081–1090. [DOI] [PubMed] [Google Scholar]
- 15.D'Amico D, Tepper SJ. Prophylaxis of migraine: general principles and patient acceptance. Neuropsychiatr Dis Treat 2008;4:1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:754–762. [DOI] [PubMed] [Google Scholar]
- 17.Evers S, Afra J, Frese A, et al. EFNS guidelines on the drug treatment of migraine-revised report of an EFNS task force. Eur J Neurol 2009;16:968–981. [DOI] [PubMed] [Google Scholar]
- 18.Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010;50:921–936. [DOI] [PubMed] [Google Scholar]
- 19.Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014;13:1100–1107. [DOI] [PubMed] [Google Scholar]
- 20.Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014;13:885–892. [DOI] [PubMed] [Google Scholar]
- 21.Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013;81:1191–1196. [DOI] [PubMed] [Google Scholar]
- 22.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010;6:573–582. [DOI] [PubMed] [Google Scholar]
- 23.Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol 2004;142:1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benarroch EE. CGRP: sensory neuropeptide with multiple neurologic implications. Neurology 2011;77:281–287. [DOI] [PubMed] [Google Scholar]
- 25.Summ O, Charbit AR, Andreou AP, Goadsby PJ. Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain 2010;133:2540–2548. [DOI] [PubMed] [Google Scholar]
- 26.Felgenhauer K. Protein size and cerebrospinal fluid composition. Klin Wochenschr 1974;52:1158–1164. [DOI] [PubMed] [Google Scholar]
- 27.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 2002;22:54–61. [DOI] [PubMed] [Google Scholar]
- 28.Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache 2013;53:1230–1244. [DOI] [PubMed] [Google Scholar]
- 29.Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci 2013;331:48–56. [DOI] [PubMed] [Google Scholar]
- 30.Diener HC, Dodick DW, Goadsby PJ, et al. Utility of topiramate for the treatment of patients with chronic migraine in the presence or absence of acute medication overuse. Cephalalgia 2009;29:1021–1027. [DOI] [PubMed] [Google Scholar]
- 31.Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache 2011;51:1358–1373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.