A 51-year-old woman was admitted to the emergency department with fever and acute behavioral changes. She had a history of benign ovarian cyst 10 years earlier. Three days before admission, she became agitated and confused but did not have any focal neurologic symptoms. Her CSF was reactive with 43 white blood cells/mm3 (predominantly lymphocytes), protein of 43 mg/dL, and normal glucose.
She was treated with IV acyclovir for herpetic meningoencephalitis. Despite treatment, on day 5 of admission, she became mute and catatonic and developed convulsive status epilepticus the next day requiring intensive care admission for seizure control. Herpes simplex virus PCR was negative in CSF as were the bacterial cultures. The remainder of her infectious and inflammatory screen was also negative. Brain MRI was normal.
On day 12 of admission, the anti-NMDA receptor antibodies (anti-NMDAR Abs) in CSF (titer: 1:10, indirect immunofluorescence of transfected cells, Euro-immunn) and plasma (titer: 1:100) were positive, and she was diagnosed with anti-NMDAR Ab encephalitis. She was initially treated with methylprednisolone, IV immunoglobulins, and rituximab IV. Pelvic MRI revealed left ovarian vein thrombosis. Considering the premenopausal status of the patient, the multidisciplinary team opted for laparoscopic bilateral ovariectomy; the pathologic analysis did not show any evidence of a teratoma.
During the following 4 months, she received IV immunoglobulins monthly. Throughout this period, the mutism and responsiveness to simple orders improved, her abnormal movement (purposeless lifting movement of the arms and legs) as well as catatonia disappeared. At 3 months, her NMDAR Abs became negative in plasma but persisted in CSF (titer: 1:50).
At the end of the fourth month, an [18F]-fluorodeoxyglucose (FDG)-PET scan was performed and detected a unique hypermetabolic pancreatic body tumor, previously underdiagnosed on the abdominal CT scans (figure).
Figure. FDG-PET and CT scans of the patient with pancreatic tumor.
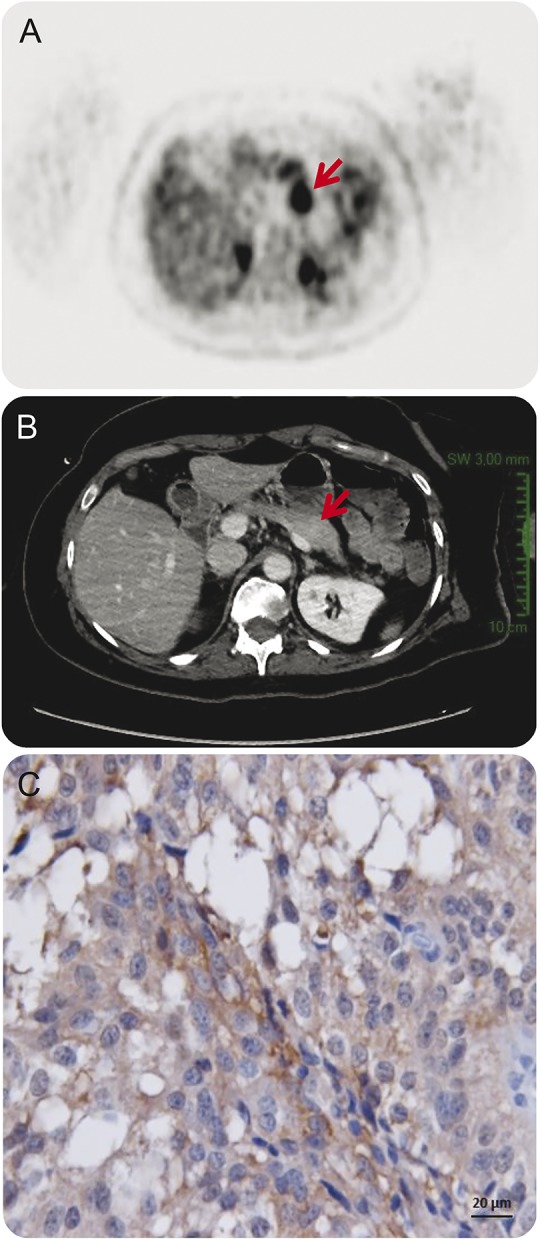
(A) The FDG-PET scan revealed a 4-cm-diameter tumor localized in the pancreatic body (arrow), (B) underdiagnosed on previous abdominal CT scan (arrow). (C) The pancreatic tumor of the patient immunolabeled with a specific antibody for the GluN1 subunit of the NMDA receptor (original magnification, ×400). FDG = [18F]-fluorodeoxyglucose.
Caudal pancreatectomy was delayed in view of concerns regarding high risk of postoperative complications and was eventually performed 2.5 months following the diagnosis (6.5 months following the presentation). Pathologic analysis revealed the well-differentiated grade 2 neuroendocrine tumor. The tumor cells express the NMDAR detected by immunostaining using commercial antibody directed against the GluN1 subunit of the NMDAR (Thermo-Fisher [clone R1JHL]) (figure).
Cognitive rehabilitation focused on executive function and short memory. Behavioral management, family psycho-education, and an integrated holistic multidisciplinary team and community approach were proposed. She made a slow but continuous improvement in her cognitive skills. At 1 and 3 months following tumor resection, she achieved 14/30 and 20/30 points on the Mini-Mental State Examination and 13/18 and 15/18 points on the Frontal Assessment Battery, respectively.
Discussion.
We report a rare case of anti-NMDAR Ab encephalitis associated with a pancreatic neuroendocrine tumor. Of note, the tumor expressed NMDARs detected using a GluR1 (encoding the NR1 subunit) commercial antibody, suggesting that other tumor types in addition to teratomas may have a role in the pathobiology of this disease. The presence of NMDARs on uterine carcinosarcoma with neuroendocrine differentiation was previously reported in one case.1 In a large cohort of patients with anti-NMDAR encephalitis, identification of tumors occurred in 220 of 577, which was age- and sex-dependent with only 9 of 220 tumors presenting in patients older than 45 years.2 Teratomas were the predominant tumor type, identified in 205 of 220 (94%). Previously, one other case of anti-NMDAR encephalitis associated with pancreatic cancer and 3 with neuroendocrine tumor were reported.1–3
The presence of a tumor associated with the anti-NMDAR Ab encephalitis has a direct influence on the management and outcome of patients with good outcome in the paraneoplastic group following prompt tumor removal. Although patients with tumors are more frequently admitted to the intensive care unit,2 tumor removal within 4 months from the onset of the disease speeds recovery.4 Early after tumor removal, our patient resumed a slow clinical improvement becoming capable of neuropsychological assessment. Screening strategy for rare tumors associated with anti-NMDAR Ab encephalitis is not well established,5 and whole-body FDG-PET scanning is not recommended as first-line screening. As FDG-PET is not sensitive for mature cystic teratomas (which have no or little uptake of FDG), this should be reserved for patients negative for ovarian tumor, using conventional screening examinations such as CT or MRI.
Conclusion.
This case highlights that in patients presenting at age 45 years or older, screening for malignancies should include imaging modalities to exclude other tumors, beyond ovarian teratomas. In particular, it is important to screen for neuroendocrine tumors, which may also express NMDARs. Tumor removal seemed to improve the neuropsychological outcome in this patient.
Footnotes
Author contributions: Vadim Afanasiev: manuscript concept, acquisition of data, critical revision of manuscript for intellectual content, edited the manuscript for nonintellectual content. Marie-Laure Brechemier: interpretation and acquisition of data. William Boisseau: acquisition of data. Romain Ducoudray: acquisition of data. Marie-Eve Mayeur: acquisition of data. David Meyronet: acquisition of data. Anne Peskine: critical revision of the manuscript for intellectual content. Virginie Desestret: critical revision of the manuscript for intellectual content, edited the manuscript for nonintellectual content. Dimitri Psimaras: critical revision of the manuscript for intellectual content.
Study funding: No targeted funding reported.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Hara M, Morita A, Kamei S, et al. Anti-N-methyl-D-aspartate receptor encephalitis associated with carcinosarcoma with neuroendocrine differentiation of the uterus. J Neurol 2011;258:1351–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol 2011;18:19–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


