Abstract
Objective:
The aim of this study was to apply the HIV-associated neurocognitive disorders (HAND) criteria for diagnosing HAND in HIV-infected adults, in a cohort of HIV-infected youth to thus establish whether this system is able to detect a spectrum of neurocognitive disorders (ND) in HIV-infected youth.
Methods:
We used a comprehensive pediatric neurocognitive battery, an assessment of functional competence, and the American Academy of Neurology system for diagnosing ND in a cross-sectional study of HIV-infected youth (n = 86) and HIV-negative controls (n = 34) to establish whether this system could detect a spectrum of ND in HIV-infected youth (6–16 years).
Results:
Compared to a well-matched control group of HIV-negative youth, HIV-infected youth performed significantly more poorly on tests of Verbal IQ, Full Scale IQ, processing speed, finger tapping, verbal memory, expressive language, cognitive flexibility, and inhibition. HIV-infected youth were also more likely to have impaired total competence on the Child Behavior Checklist. Using the criteria for HAND, we found that 45.35% of the 86 HIV-infected youth could be diagnosed with an ND. Furthermore, youth with HIV encephalopathy (HIVE) were 9.4 times more likely to have a diagnosis of a major ND compared to HIV-infected youth without HIVE.
Conclusions:
The HAND criterion designed for adults was able to identify youth with important functional cognitive impairments who do not fit criteria for HIVE and would therefore not have been identified otherwise. This has major clinical implications regarding the importance of managing HIV-infected youth.
Because neurocognitive impairment (NCI) can have deleterious effects on children's ability to function, it is important for clinicians to be able to identify and manage these impairments. In South Africa, differences in treatment guidelines and differential access to antiretroviral treatment (ART) over the past 2 decades have resulted in great variability in disease severity and ART exposure among HIV-infected youth.1 As a result, a number of adolescents in South Africa would have initiated ART only after immune compromise or after the diagnosis of HIV encephalopathy (HIVE), resulting in neurocognitive deficits that remained permanent despite ART.1,2 However, other HIV-infected children have never initiated ART because they are slow progressors who have not yet met current clinical criteria to be eligible for ART.3
In the adult literature, there is growing understanding of the spectrum of HIV-associated neurocognitive disorders (HAND) and their reduction on ability to function.4 However, for children, there are still no diagnostic criteria for a spectrum of neurocognitive disorders (ND) secondary to HIV infection. The aim of this study was to apply the HAND criteria for diagnosing NCI in a cohort of HIV-infected youth and to thus establish whether this system was able to detect a spectrum of ND. A secondary outcome was to establish whether the HAND criteria could detect higher rates of major ND in HIV-infected youth with HIVE.
METHODS
Standard protocol approvals, registrations, and patient consents.
Ethical approval was obtained from the University of Cape Town's Faculty of Health Sciences Human Research Ethics Committee. Informed consent was obtained from parents and children aged 12 and older. Children provided assent at an age-appropriate level.
Participants.
From July 2011 until January 2014, 120 youth were recruited for the cross-sectional study. We recruited 86 perinatally HIV-infected youth (aged 6–16 years) from antiretroviral clinics at Groote Schuur Hospital, Red Cross War Memorial Children's Hospital, and community clinics in the Western Cape of South Africa.5 Neuroimaging data from this cohort have been published.5 Eligible youth and their parents or guardians were invited to participate. Eligible youth were those who were perinatally infected, had initial and confirmatory HIV tests, and who met the below criteria. Youth on ART were eligible if they were on antiretrovirals for a minimum of 6 months, as we wanted to include youth who were stable on treatment. We also included HIV-infected youth who were asymptomatic slow progressors and ART-naive.6 Youth who were ART-naive but not slow progressors were not included in the study. A control group of 34 HIV-negative youth, matched to the HIV infected group on age, sex, and ancestry, were recruited from the same schools as the HIV-infected youth to control for risk factors such as socioeconomic status, stressors in the community, and quality of education.5 Parents/caregivers of the control participants were asked about their HIV status and their response was recorded. Youth with a history of the following were excluded from participation: significant perinatal complications, drug or alcohol exposure during pregnancy; an unstable medical condition; active tuberculosis; an identified CNS condition; a history of traumatic head injury with loss of consciousness, skull fracture; or neurodevelopment disorder other than HIVE.5 The Beck Youth Inventory and the Conners Parents Rating Scale were used to exclude youth with clinically significant attention-deficit/hyperactivity disorder, depression, or anxiety that may have affected their performance on the neurocognitive battery.
Procedure.
HIV-infected youth underwent a full neurologic examination and were assessed for the diagnosis of HIVE by a pediatric neurologist. A diagnosis of HIVE was made on at least one of either of the 2 physical criteria in the 1994 Centers for Disease Control and Prevention diagnostic criteria: (1) acquired microcephaly as measured by sequential head circumference measurements, and (2) acquired symmetrical motor deficits manifested by 2 or more of the following: paresis, pathologic reflexes, ataxia, or gait disturbance.5,7 Neurodevelopmental criteria were not used as they may have confounded the neurocognitive assessment. MRI was conducted to exclude concurrent CNS illness, other than HIV infection, that could explain the findings. Blood samples were obtained from clinic records for viral load and CD4 count. Median income by area of residence was used as a measure of socioeconomic status. The median was taken from the City of Cape Town household income census data (2011) for small areas inside the city.
Neurocognitive assessment.
Each participant was assessed using a battery of standardized neurocognitive tests commonly used in pediatric neuropsychological assessment and research in South Africa. Tests were administered in the children's home language. Test instructions were translated and back-translated into Xhosa, and we took steps to ensure test administration maintained compliance with International Test Commission guidelines.8 For large test batteries with age adjustment and normative data, the scaled scores were used in the z score calculation. Scaled scores were calculated according to the normative scores provided in individual test manuals or using the appropriate software. For timed tests and all other tests, the raw scores from control participants were used in the z score calculation. General intellectual functioning was measured using the Wechsler Abbreviated Scale of Intelligence.9 The Fingertip Tapping subtest from the NEPSY-II (A Developmental Neuropsychological Assessment–Second Edition)10 and the Grooved Pegboard Test11 measured psychomotor speed and coordination. Subtests from the Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV)12 measured information processing speed. The Rey Complex Figure Test (RCFT)13 measured visuoconstructional ability. Expressive Language was measured using the Boston Naming Test–South African–short form, category and phonemic fluency.14 Learning and memory (visual and verbal) were measured using the immediate and delayed recall trials of the RCFT and the Hopkins Verbal Learning Test–Revised.15 Measures used to assess aspects of executive functioning included the WISC-IV Digit Span Backward subtest, the Color Trails Test 2, and the NEPSY-II Inhibition subtests.
Assessment of functional competence.
The Child Behavior Checklist (CBCL)16 Total Competence Index required a parent or caregiver to rate the child's competency at completing age-appropriate everyday activities successfully and independently. This instrument is suitable for use in children aged 6 to 18 years and has good cross-cultural validity.17 Higher scores on this scale indicate better functioning. A score of 36 to 40 (borderline range) indicates that the child requires assistance with what should be age-appropriate everyday activities. A score of 35 or less (clinical range) indicates significant impairment in social, school, or other everyday activities.
Statistical analyses.
Regarding sociodemographic characteristics, HIV-infected participants and HIV-negative participants were compared using independent-samples t tests (for continuous variables) and Pearson χ2 test (for categorical variables). In the creation of neurocognitive domains, we set 0.60 as the lower bound of Cronbach α. Composite neurocognitive scores were then created for each domain, using a method described by Ferrett et al.18 The composite scores for each domain were required to use the American Academy of Neurology (AAN) system for diagnosing HAND. Regarding neurocognitive and adaptive functioning outcome variables, the 2 groups were compared using independent-samples t tests.
Determination of ND status.
In 1991, the AAN detailed criteria for defining different levels of HAND.19 In 2007, a revised classification was proposed that aimed to categorize the presentation of neurocognitive disturbances in the post-HAART (highly active antiretroviral therapy) era.20 This system proposes 4 categories of HAND in adults: normal, asymptomatic neuropsychological impairment (ANI), mild ND, and HIV-dementia.20 In the absence of clinically significant functional impairment, the ANI category is used, while the mild ND and HIV-dementia categories are used when functional impairment is mild to moderate or severe.20,21 We used scores on the above-mentioned neurocognitive domains, together with an evaluation of functional competence, to classify youth into 1 of 4 HAND categories20: no impairment; ANI (diagnosed if the participant scored more than 1 SD below the mean on at least 2 cognitive domains); minor ND (diagnosed if the participant scored more than 1 SD below the mean on at least 2 cognitive domains and had a CBLC Total Competence Index score of ≤40); and major ND (diagnosed if the participant scored more than 2 SD below the mean on at least 2 cognitive domains and had a CBCL Total Competence Index score of ≤35).
RESULTS
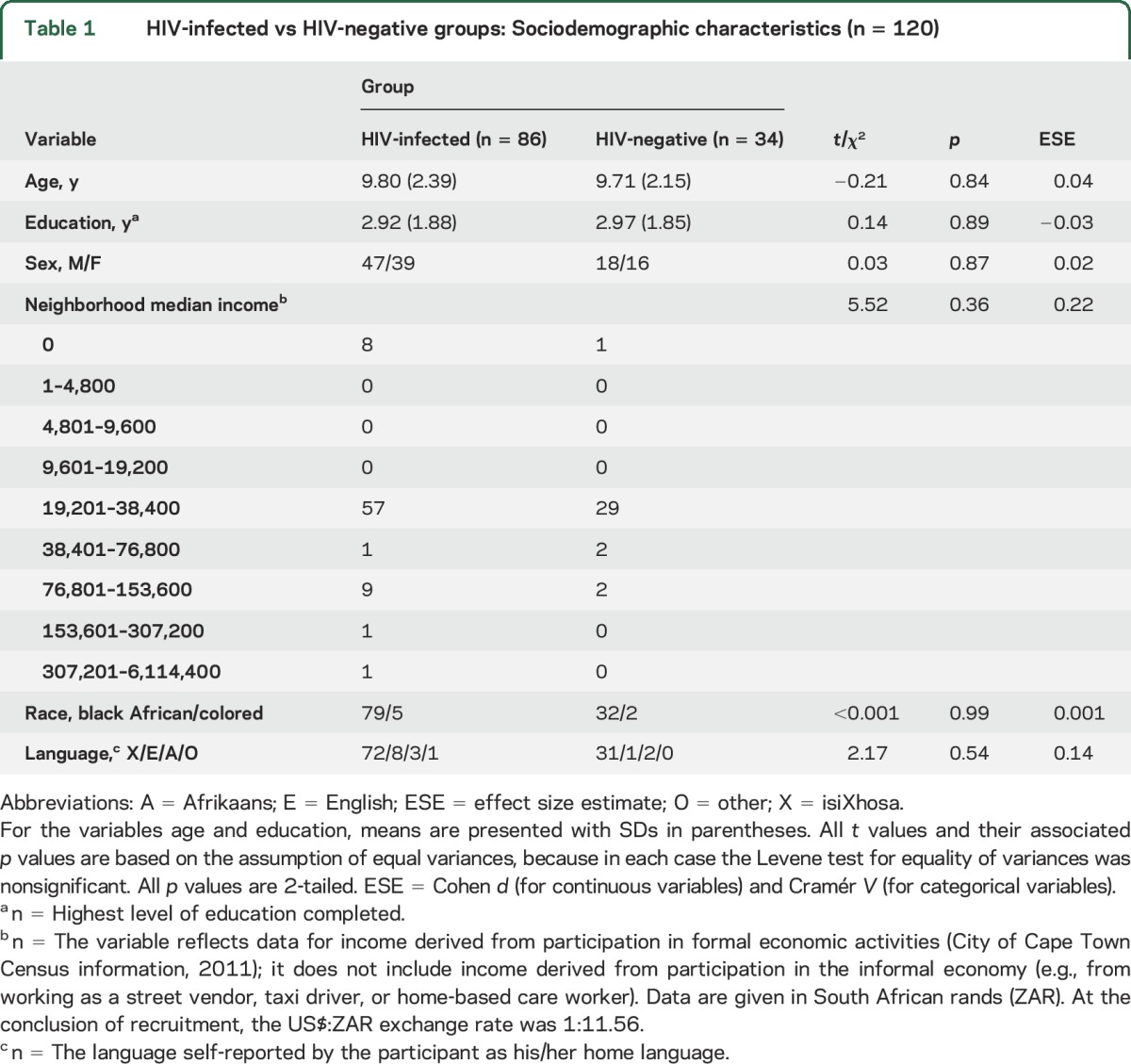
The 2 groups were well matched on sociodemographic characteristics (see table 1).
Table 1.
HIV-infected vs HIV-negative groups: Sociodemographic characteristics (n = 120)
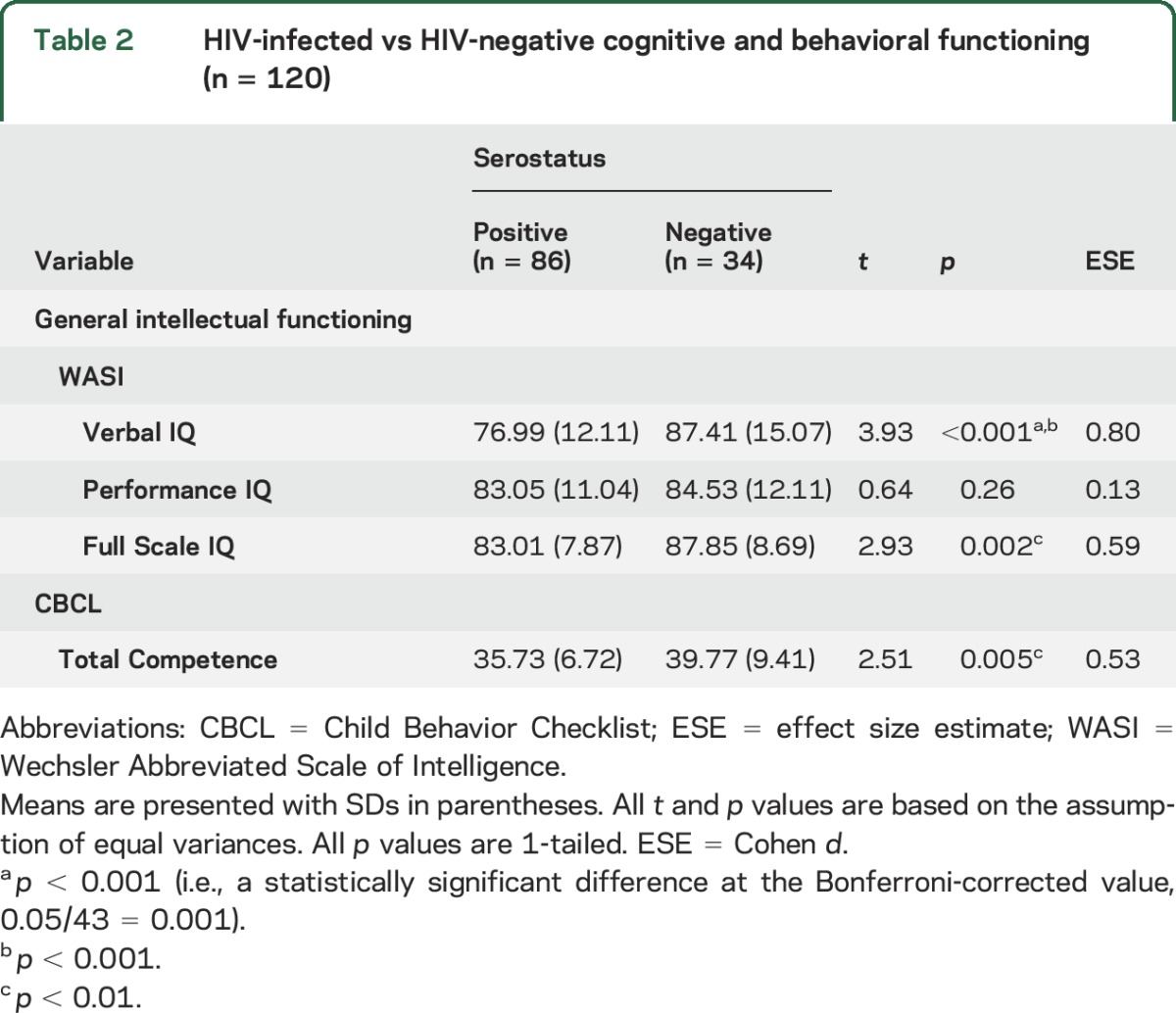
Compared to a well-matched control group of HIV-negative controls (n = 34), HIV-infected youth (n = 86) performed poorly on tests of verbal IQ and Full Scale IQ (see table 2). Regarding parental report on the CBCL Total Competence Index, HIV-infected youth showed lower levels of competence in completing age-appropriate, everyday activities.
Table 2.
HIV-infected vs HIV-negative cognitive and behavioral functioning (n = 120)
Eight neurocognitive domains were created; however, 3 of those domains (visuoconstructional ability, cognitive flexibility, and working memory) were each estimated by a single test (Rey-Osterrieth Complex Figure Test–Copy, Color Trails Test Part 2, and Digit Span Backward, respectively) (see table 3).
Table 3.
Neurocognitive test performance within composite cognitive domains (n = 120)
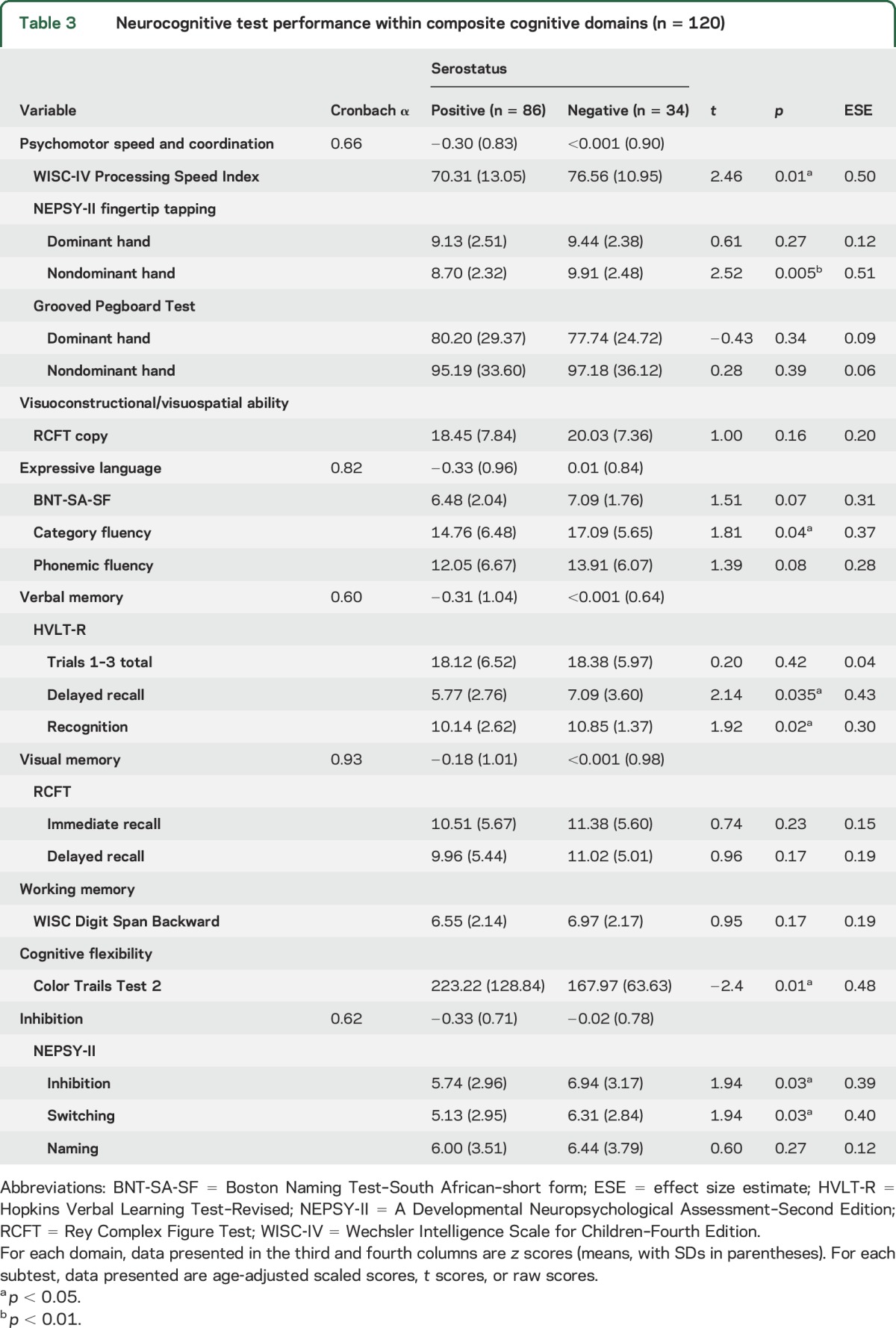
Regarding performance on the neurocognitive test battery, the analyses detected between-group differences (in favor of the HIV-negative controls) in the domains of psychomotor speed and coordination, verbal memory, expressive language, cognitive flexibility, and inhibition (see table 3).
Of the 86 HIV-infected youth, 18 youth stable on ART had a clinical diagnosis of HIVE, 45 were stable on ART with no diagnosis of HIVE, and 23 were ART-naive slow progressors (see table 4). Using the criteria for HAND, we found that 45.35% of the 86 HIV-infected youth could be diagnosed with an ND (ANI, minor ND, or major ND), 7.14% had a major ND, 32.59% had a minor ND, and 5.81% had ANI. Findings for ART-naive slow progressors (26%) and controls were similar when using the HAND criteria (29%) (table 4).
Table 4.
Cross-tabulation: HIV-associated neurocognitive disorders diagnosis and diagnosis via clinical subgroup (n = 120)
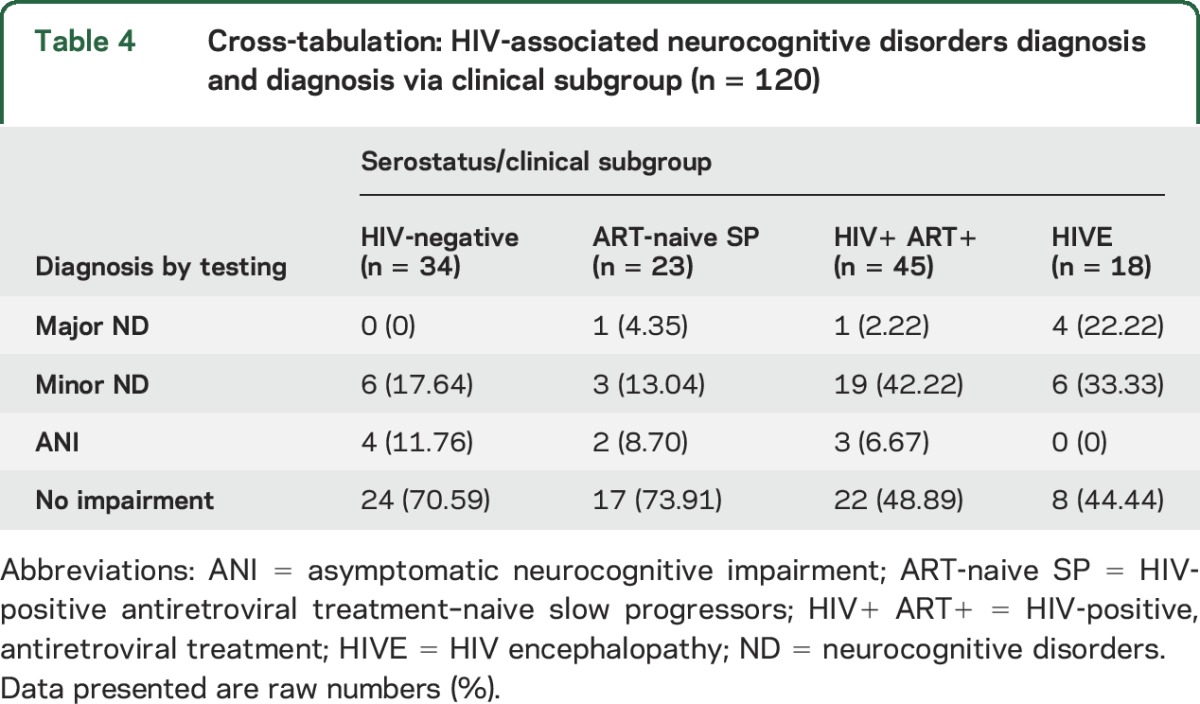
Analysis of the data presented in table 4 investigated the association, within the sample of HIV-infected youth (n = 86), between being diagnosed with HIVE and presenting with a major ND. Pearson χ2 test of contingency detected a significant association (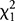 = 8.15, p = 0.004, Cramér V = 0.31). The odds ratio for this analysis was 9.42 (95% confidence interval = 1.57–56.62), suggesting that those HIV-infected youth with a diagnosis of HIVE were more than 9 times more likely to be observed as experiencing a major ND as those with no clinical diagnosis of HIVE.
= 8.15, p = 0.004, Cramér V = 0.31). The odds ratio for this analysis was 9.42 (95% confidence interval = 1.57–56.62), suggesting that those HIV-infected youth with a diagnosis of HIVE were more than 9 times more likely to be observed as experiencing a major ND as those with no clinical diagnosis of HIVE.
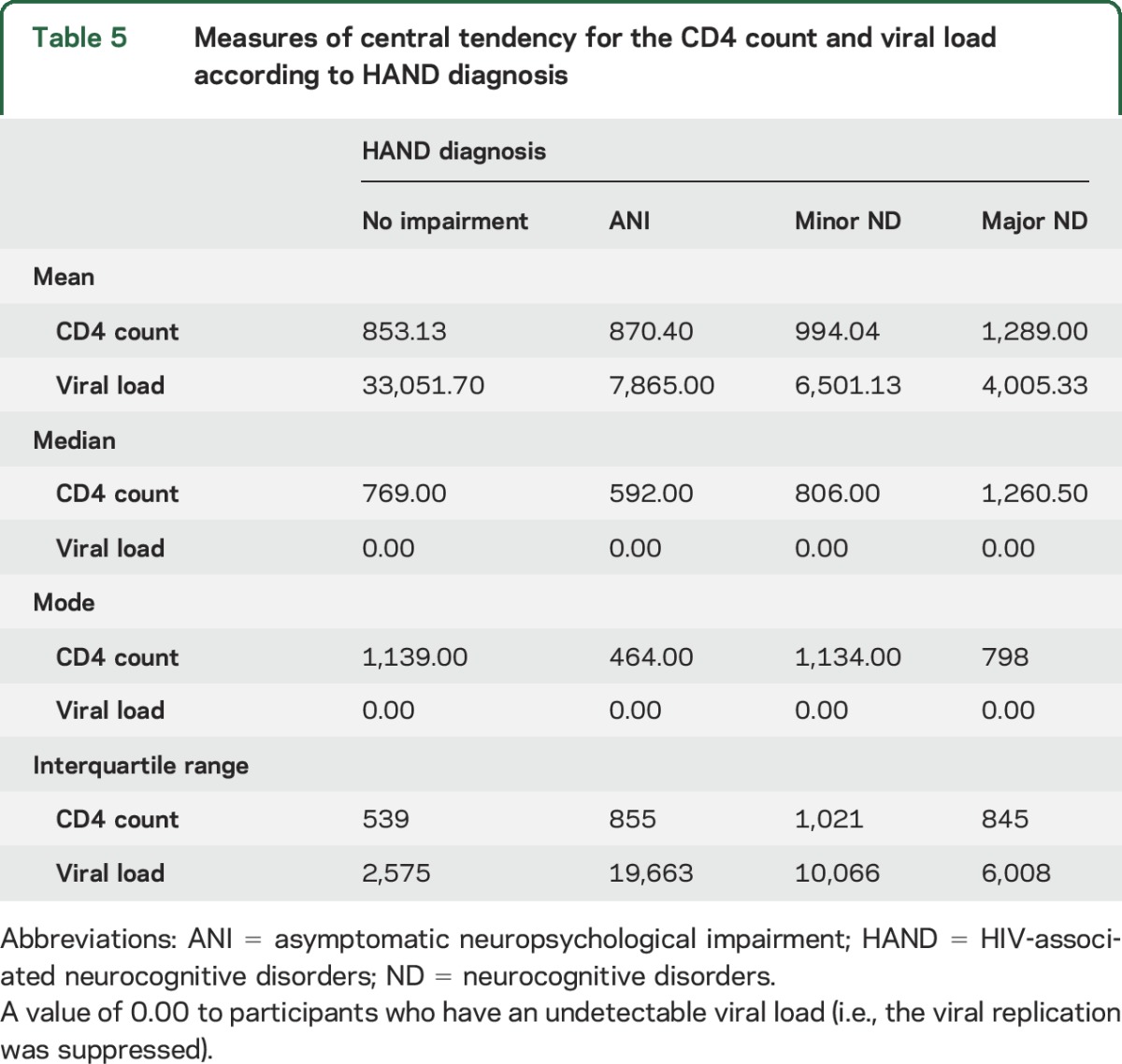
Current CD4 and viral load was not associated with severity of NCI. In fact, youth with major ND had higher mean CD4 and lower mean viral load than youth from other groups.
DISCUSSION
The youth included in this study were infected by perinatal transmission of the virus,22 and infection at this age can have a profound effect on neurocognitive function. HIV-infected youth perform poorly in general cognitive assessments compared to controls,23,24 although some studies have found no significant differences between groups.3,25 Our neurocognitive battery included a number of tests assessing specific cognitive domains, as global cognitive scores may overlook subtle deficits in one or more domains specific to HIV-infected children. In keeping with previous studies, HIV-infected children were found to perform significantly poorer in executive function tasks,24,25 particularly in cognitive flexibility and inhibition. Poor executive function could lead to significant difficulties with daily activities.26 HIV-infected youth in this study also had difficulties with processing speed, verbal memory, motor coordination, and expressive language, again in keeping with findings from previous studies.3,24,27 Processing speed has been associated with improved capacity for working memory, reasoning, and accuracy in solving arithmetic word problems, and consistently predicts performance on cognitive tasks.28 While lower scores on visual–spatial processing and visual memory have been described in HIV-infected children,29 this study did not find significant impairment compared to HIV-negative controls. As children transition to adolescence, language and reading skills are essential building blocks for literacy and future academic success.1 There is evidence that language is negatively affected in HIV-infected children,23,27,30 with our study finding significant impairment in expressive language. Poor executive functioning in adolescents having to cope with multiple environmental challenges could translate into significant functional impairment.26
Previous studies have reported on both subtle NCI in HIV-infected school-age children25 as well as the presence of HIVE,31 suggesting a spectrum of ND. Despite the use of ART and improved virologic control with immune reconstitution in this cohort, there are still a significant percentage of youth in this study who were found to have an ND. The prevalence of HIVE has reportedly decreased from 40% to 18% in the post-ART period,32 suggesting that ART is associated with improved neurocognitive outcomes in children with vertically acquired HIV infection.32 The HAND criteria were able to detect higher rates of ND in children with a diagnosis of HIVE; higher rates of HAND are to be expected in a group of children who have abnormal neurologic examinations. However, 44.4% of the children with HIVE were found to have no impairment. The diagnosis of HIVE was a clinical diagnosis based on the presence of at least 1 of the 2 physical criteria in the 1994 Centers for Disease Control and Prevention diagnostic criteria. It is possible that although the youth in this group have neurologic impairment, they do not have NCI. As HIVE is an umbrella diagnosis for the disease, damage, or malfunction of the brain caused by HIV, there will remain a group whose overall cognitive performance falls within the normal range despite an abnormal neurologic examination.7 Diffusion tensor imaging data from this cohort have been published. Youth with HIVE had poor white matter integrity compared to ART-treated children without encephalopathy.5 It is also possible that either the neurocognitive battery or the assessment of functional competence was not sensitive enough to detect more subtle deficits. It may be that an instrument such as the Vineland Adaptive Behavior Scale, which was not available to the team at the time of this study, is a more accurate measure of adaptive functioning. Current CD4 and viral load was not associated with severity of NCI. In fact, youth with major ND had higher mean CD4 and lower mean viral load than youth from other groups (table 5). Youth with major ND were more likely to have a diagnosis of HIVE. These children have more frequent contact with pediatric neurologic services and have improved adherence because of increased supervision.
Table 5.
Measures of central tendency for the CD4 count and viral load according to HAND diagnosis
Although higher rates of NCI were detected in the HIV-infected group, it is a concern that the HAND criteria also found NCI in the negative controls. Of the ART-naive slow progressors, who receive little attention from health services, 26.09% were found to have an ND. Significant motor and cognitive deficits have previously been found in HIV-infected, ART-naive Ugandan children with CD4 cell counts of >350 cells/μL.24 The ART-naive/slow progressors group differed little from the control group. A possible reason for these findings is that controls were recruited from families with low socioeconomic status. A large number of youth living in sub-Saharan Africa are not fulfilling their developmental potential. Many of these youth are exposed to multiple risks that may include poverty, malnutrition, poor health, compromised caregiving, and unstimulating home environments, which detrimentally affect their cognitive, motor, emotional, and social development.33 One review of early childhood development in low- and middle-income countries estimates that 15.7% of children are significantly delayed in their cognitive development.34 Parents/caregivers of the control participants were asked about their HIV status and their response recorded. Two of the parents of the controls reported when contacted that they were HIV-infected. HIV-uninfected youth born to HIV-infected mothers may exhibit differences in neurocognitive function compared to controls whose lives have not been affected by HIV. This could occur because of exposure to HIV and ART in utero and perinatally or the effect of having chronic stigmatizing illness in the environment in which they are raised.35
The neurologic and neurocognitive profile of HIV disease in youth has been considered to be distinct from the course of the disease in adults, and it may be that NCI in youth should be profiled differently. However, similar cognitive domains have been affected in South African HIV-infected adults.21 The application of a HAND model of HIV disease, developed with adult HIV/AIDS, to perinatal HIV disease in youth may not be ideal despite the AAN approval for such a model. However, this study serves as a starting point for this conversation. The importance of identifying a spectrum of NCI is valid in a low- to middle-income country where no systematic screening systems exist in standard practice for HIV-infected youth. The more acute “life-threatening conditions” tend to draw medical attention away from the chronic, more subtle, but very important issues such as neurocognitive function. Neurocognitive function in youth is becoming increasingly important as more severe physical manifestations of HIV become less common with improved accessibility to ART. It may be that a pediatric system based on modified HAND criteria could be developed.
Limitations of this study include the small sample size for the HIV-negative youth and the use of these youth across a wide age range to serve as a z score reference for nonnormed test measures; this may have prevented enough statistical power for some of the neurocognitive comparisons. However, we recruited HIV-negative controls from the same community, schools, and clinics as the HIV-infected youth. While applying an adult-based classification to HIV-infected youth may not be ideal, we used a pediatric neurocognitive battery to determine functioning in the various domains. The HAND criteria could not detect higher rates of ND in the ART-naive slow progressors.
Despite the rollout of ART in South Africa, the recognition of ND in nearly 46% of the HIV-infected group is an indication that these problems remain important to identify and are probably largely underrecognized. While applying an adult-based classification to HIV-infected youth may not be ideal, this study has started the process of identifying a spectrum of ND in HIV-infected youth. This is especially important in adolescents whose ability to function in school will directly affect their economic/social trajectory as adults. Further research into the impact of ART on neurocognitive function and the improvement stability or progression of HAND utilizing larger prospective cohort studies in sub-Saharan Africa is needed.
Supplementary Material
GLOSSARY
- AAN
American Academy of Neurology
- ANI
asymptomatic neuropsychological impairment
- ART
antiretroviral treatment
- CBCL
Child Behavior Checklist
- HAND
HIV-associated neurocognitive disorders
- HIVE
HIV encephalopathy
- NCI
neurocognitive impairment
- ND
neurocognitive disorders
- NEPSY-II
A Developmental Neuropsychological Assessment–Second Edition
- RCFT
Rey Complex Figure Test
- WISC-IV
Wechsler Intelligence Scale for Children–Fourth Edition
Footnotes
Editorial, page 17
AUTHOR CONTRIBUTIONS
Dr. Hoare was the principal investigator of the study. She conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Ms. Phillips designed the data collection instruments, and coordinated and supervised data collection and entry, critically reviewed the manuscript, and approved the final manuscript as submitted. Dr. Donald conducted all neurologic assessments of the participants, critically reviewed the manuscript, and approved the final manuscript as submitted. Prof. Stein, Prof. Paul, and Prof. Joska critically reviewed the manuscript, and approved the final manuscript as submitted. Prof. Thomas performed the statistical analyses, reviewed the manuscript, and approved the final manuscript as submitted.
STUDY FUNDING
All phases of the study were supported by the National Research Foundation (NRF) of South Africa, the Medical Research Council (MRC) of South Africa, and the Harry Crossley Foundation.
DISCLOSURE
J. Hoare, N. Phillips, J. Joska, and K. Donald report no disclosures relevant to the manuscript. D. Stein has received research grants and/or consultancy honoraria from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier, and Sun. K. Thomas reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc 2013;16:18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel K, Ming X, Williams PL, Robertson KR. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS 2009;23:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagenda D, Nassali A, Kalyesubula I, et al. Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics 2006;117:729–740. [DOI] [PubMed] [Google Scholar]
- 4.Rackstraw S. HIV-related neurocognitive impairment: a review. Psychol Health Med 2011;16:548–563. [DOI] [PubMed] [Google Scholar]
- 5.Hoare J, Fouche JP, Phillips N, et al. White matter micro-structural changes in ART-naive and ART-treated children and adolescents infected with HIV in South Africa. AIDS 2015;29:1793–1801. [DOI] [PubMed] [Google Scholar]
- 6.Hoare J, Fouche JP, Phillips N, Joska JA. Clinical associations of white matter damage in cART-treated HIV-positive children in South Africa. J Neurovirol 2015;21:120–128. [DOI] [PubMed] [Google Scholar]
- 7.Donald KA, Walker KG, Kilborn T, et al. HIV encephalopathy: pediatric case series description and insights from the clinic coalface. AIDS Res Ther 2015;12:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartram D. The development of international guidelines on test use: the International Test Commission Project. Int J Test 2001;1:33–53. [Google Scholar]
- 9.Axelrod BN. Validity of the Wechsler Abbreviated Scale of Intelligence and other very short forms of estimating intellectual functioning. Assessment 2002;9:17–23. [DOI] [PubMed] [Google Scholar]
- 10.Davis JL, Matthews RN. NEPSY-II review. J Psychoeduc Assess 2010;28:175–182. [Google Scholar]
- 11.Bryden PJ, Roy EA. A new method of administering the Grooved Pegboard Test: performance as a function of handedness and sex. Brain Cogn 2005;58:258–268. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler D. Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV). San Antonio: Harcourt Assessment; 2003. [Google Scholar]
- 13.Watanabe K, Ogino T, Nakano K, Hattori J, Kado Y. The Rey–Osterrieth complex figure as a measure of executive function in childhood. Brain Dev 2005;27:564–569. [DOI] [PubMed] [Google Scholar]
- 14.Roth C. Boston Naming Test. Encyclopedia Clin Neuropsychol 2011;2:430–433. [Google Scholar]
- 15.Benedict R, Schretlen D, Groninger L. Hopkins Verbal Learning Test–Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychologist 1998;12:43–55. [Google Scholar]
- 16.Achenbach TM, Dumenci L, Rescorla LA. Testing the 8-syndrome structure of the child behavior checklist in 30 societies. J Clin Child Adolesc Psychol 2007;36:405–417. [DOI] [PubMed] [Google Scholar]
- 17.Weisz JR, Sigman M, Weiss B, Mosk J. Parent reports of behavioral and emotional problems among children in Kenya, Thailand, and the United States. Child Dev 1993;64:98–109. [DOI] [PubMed] [Google Scholar]
- 18.Ferrett HL, Carey PD, Thomas KG, Tapert SF, Fein G. Neuropsychological performance of South African treatment-naïve adolescents with alcohol dependence. Drug Alcohol Depend 2010;110:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen RS, Cornblath DR, Epstein LG, Foa RP. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Neurology 1991;41:778–785. [DOI] [PubMed] [Google Scholar]
- 20.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joska JA, Westgarth-Taylor J, Myer L, et al. Characterization of HIV-associated neurocognitive disorders among individuals starting antiretroviral therapy in South Africa. AIDS Behav 2011;15:1197–1203. [DOI] [PubMed] [Google Scholar]
- 22.Wachsler-Felder JL, Golden CJ. Neuropsychological consequences of HIV in children: a review of current literature. Clin Psychol Rev 2002;22:443–464. [DOI] [PubMed] [Google Scholar]
- 23.Hoare J, Fouche JP, Spottiswoode B, et al. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naive “slow progressors.” J Neurovirol 2012;18:205–212. [DOI] [PubMed] [Google Scholar]
- 24.Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis 2012;54:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koekkoek S, de Sonneville LMJ, Wolfs TFW, Licht R, Geelen SPM. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol 2008;12:290–297. [DOI] [PubMed] [Google Scholar]
- 26.Poletti M. Adolescent brain development and executive functions: a prefrontal framework for developmental psychopathologies. Clin Neuropsychiatry 2009;6:155–156. [Google Scholar]
- 27.Smith R, Chernoff M, Williams PL, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J 2012;31:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kail RV, Ferrer E. Processing speed in childhood and adolescence: longitudinal models for examining developmental change. Child Dev 2007;78:1760–1770. [DOI] [PubMed] [Google Scholar]
- 29.Smith ML, King S, Fernandes-Penney A. Cognitive development in school-age children with vertically transmitted HIV infection. Dev Neuropsychol 2002;21:223–241. [DOI] [PubMed] [Google Scholar]
- 30.Abubakar A, Van Baar A, Van de Vijver FJR, Holding P, Newton CRJC. Paediatric HIV and neurodevelopment in sub-Saharan Africa: a systematic review. Trop Med Int Health 2008;13:880–887. [DOI] [PubMed] [Google Scholar]
- 31.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics 2008;122:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanbhag MC, Rutstein RM, Zaoutis T, Zhao H, Chao D, Radcliffe J. Neurocognitive functioning in pediatric human immunodeficiency virus infection: effects of combined therapy. Arch Pediatr Adolesc Med 2005;159:651–656. [DOI] [PubMed] [Google Scholar]
- 33.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boivin MJ, Kakooza AM, Warf BC, Davidson LL. Reducing neurodevelopmental disorders and disability through research and interventions. Nature 2015;527:155–160. [DOI] [PubMed] [Google Scholar]
- 35.Kerr SJ, Puthanakit T, Vibol U, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care 2014;26:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


