Abstract
Objective
Obesity is associated with elevated C reactive protein (CRP) levels. The Ankylosing Spondylitis Disease Activity Score (ASDAS) combines patient-reported outcomes (PROs) and CRP. We evaluated the effect of body mass index (BMI) on CRP and on ASDAS, and studied if ASDAS can be used in obese axial spondyloarthritis (axSpA) patients to assess disease activity.
Methods
Baseline data of patients with chronic back pain of short duration included in the SPondyloArthritis Caught Early (SPACE) cohort were used. Collected data included BMI and ASDAS. Patients were classified according to the ASAS axSpA classification criteria and BMI (overweight ≥25 and obese ≥30). Correlation and linear regression analyses were performed to assess the relation between BMI and ASDAS. Linear regression models were performed to assess if age or gender were effect modifiers in the relation between BMI and CRP, and between BMI and ASDAS.
Results
In total, 428 patients were analysed (n=168 axSpA; n=260 no-axSpA). The mean age was 31.1 years, 36.9% were male, 26.4% were overweight and 13.3% obese, median CRP was 3 mg/L and the mean ASDAS was 2.6. Gender was the only factor modifying the relationship between BMI and CRP as BMI had an influence on CRP only in females (β=0.35; p<0.001). Correlations between BMI and CRP or PROs were generally weak, and only significant for CRP in female patients. BMI was not related to ASDAS in axSpA patients.
Conclusions
ASDAS is not affected by BMI in axSpA patients. Therefore, based on our data it is not necessary to take BMI in consideration when assessing disease activity using ASDAS in axSpA patients.
Keywords: Ankylosing Spondylitis, Spondyloarthritis, Disease Activity
Key messages.
What is already known about this subject?
Obesity is associated with elevated C reactive protein (CRP) levels. Together with four patients reported outcomes (PRO), CRP is used to calculate Ankylosing Spondylitis Disease Activity Scores (ASDAS) in axial spondyloarthritis (axSpA) patients. Perhaps ASDAS should not be used in patients with extremely high or low body mass indexes (BMIs).
What does this study add?
Correlations between BMI and CRP or PROs were generally weak, and only significant for CRP in female patients; however, BMI was not related to ASDAS in axSpA patients.
How might this impact on clinical practice?
In clinical practice, there is no need to take BMI into account when using ASDAS and CRP in axSpA patients.
Introduction
The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is the most widely used measure of disease activity in ankylosing spondylitis (AS) patients, but relies only on patient-reported outcomes (PROs).1 To overcome this limitation the Assessment in SpondyloArthritis international Society (ASAS) developed the Ankylosing Spondylitis Disease Activity Score (ASDAS), which combines PROs and C reactive protein (CRP) to assess disease activity in axial spondyloarthritis (axSpA).2 Although ASDAS was developed in patients with AS, it has also shown validity in patients with early axSpA, including non-radiographic axSpA.3
An increase in adipose tissue, which is considered a dynamic endocrine organ,4 is associated with an increased production of several proinflammatory cytokines and acute phase reactants,5 such as CRP.6 7 The association between obesity and elevated serum levels of CRP appears to be stronger in women than in men.8 Moreover, there are also data that PROs are higher in obese patients with chronic back pain.9
Therefore, it might be difficult to determine in axSpA patients with high BMI whether a high ASDAS score reflects a high disease activity or is (partly) a consequence of more adipose tissue. The aim of this study is to evaluate the effect of an increased BMI on CRP levels and on ASDAS, and to assess if ASDAS is a valid disease activity score in overweight axSpA patients.
Methods
Patients and data collection
For the present study, baseline data of the SPondyloArthritis Caught Early (SPACE) cohort, a multicenter observational cohort, were used. The SPACE-cohort is described extensively elsewhere;10 in brief, the cohort started in January 2009 and is an ongoing cohort including patients aged ≥16 years with short-term chronic back pain (≥3 months, but ≤2 years) with the onset at <45 years of age. Patients referred to the rheumatology outpatient clinics of five participating centres (three centres in the Netherlands, one in Norway and one in Italy) were included after signing informed consent. The local medical ethical committees of the participating sites approved the study.
Patients were classified according to their BMI in two groups (normal weight ≤24.9 and overweight ≥25). The group of ‘normal’ weight includes 3.3% patients who were underweight (BMI<18). Obesity was considered if BMI ≥30.11 CRP levels were collected, and elevated CRP was considered if ≥5 mg/L. Disease activity was measured by BASDAI1 and by ASDAS.2 BASDAI consists of six PROs, and has an overall score from 0 to 10; 0 represents inactive disease and 10 extremely active disease.
ASDAS was calculated using the following formula:
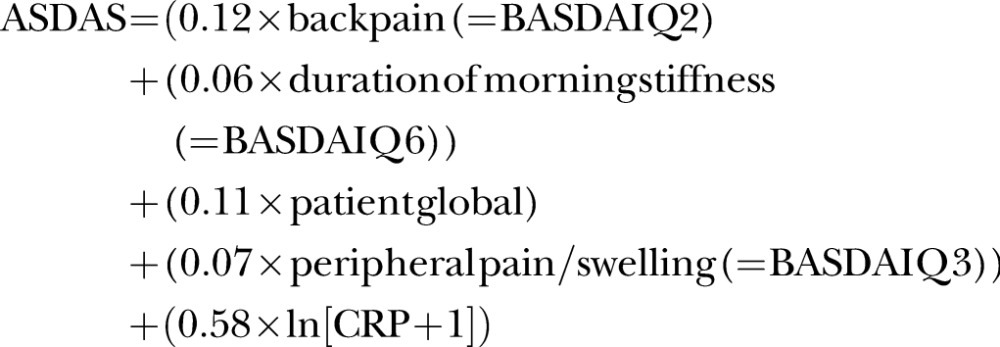 |
According to recommendations, a value of ‘2’ was used if CRP serum levels were below 3 mg/L.12
Statistical analyses
First, linear regression models were performed to analyse if age (dichotomised by the median age of 30.5 years in the total population) or gender might act as effect modifier in the relation between BMI and CRP in all patients, and age and gender in the relation between BMI and ASDAS in the axSpA group only. CRP and ASDAS (continuous) were the dependent variables and BMI (dichotomised by ≤24.9 and ≥25), the independent variable. Each interaction term was tested separately. We considered an effect modifier to be present if p<0.1 (two-tailed). As gender was an effect modifier, all analyses were performed for male and female patients separately. Descriptive statistics were used for the baseline characteristics. Patients were stratified according to ASAS axSpA classification criteria,13 and also stratified for BMI and gender within the groups of axSpA and no-axSpA patients. Results from categorical and dichotomous variables were expressed as frequencies and percentages, and continuous variables as means and SDs or medians and IQR, according to the distribution of the variables. χ2 Tests or Fisher's exact tests were performed for the comparison of categorical and dichotomous data, and the Mann-Whitney U test or student's t-test for the comparison of continuous variables, as appropriate. Spearman's rank correlation tests were performed to analyse whether BMI is correlated to ASDAS or to each of its five individual parameters, in axSpA and no-axSpA patients, and in males and females (in the whole population and in the axSpA group). The relation between BMI and ASDAS was analysed by univariable and multivariable linear regression analyses. p Values <0.05 (two-tailed) were considered statistically significant.
Analyses were performed using STATA SE V.14 (College Station, Texas: StataCorp LP, 2015).
Results
Baseline characteristics
A total of 428 patients with complete data were included to assess fulfilment of the ASAS axSpA criteria, and to calculate BMI and ASDAS; 168/428 (39.3%) fulfilled the ASAS classification criteria for axSpA (table 1). The axSpA group has a statistically significant lower prevalence of overweight and obesity (18.4% and 11.9%, respectively), compared with the no-axSpA group (31.5% and 14.2%, respectively) (table 1). CRP levels were not different between axSpA and no-axSpA patients; however, within both groups differences were seen when comparing the BMI subgroups (table 1). A higher percentage of patients with a high CRP was found in the overweight category compared with the normal weight category in both axSpA and no-axSpA patients (45.1% and 27.4% vs 42.9% and 27%, respectively) with statistical significance (p=0.02 and 0.01, respectively). In the no- axSpA group, eight patients had CRP serum levels higher or equal to 20, with most patients having inflammatory conditions such as uveitis, gout, peripheral SpA, and psoriasis. Every element included in ASDAS was higher in the overweight category compared with the normal weight category in the axSpA group, but only CRP reached statistical significance (p=0.01) (table 1). ASDAS and BASDAI were higher in the overweight category compared with the normal weight category in the axSpA group, but this difference was not statistically significant (table 1).
Table 1.
Baseline characteristics in patients with and without axSpA, stratified by BMI (cut-off of 25)
| axSpA N=168 |
no-axSpA N=260 |
|||||
|---|---|---|---|---|---|---|
| Total group | BMI ≤24.9 N=117 |
BMI ≥25 N=51 |
Total group | BMI ≤24.9 N=141 |
BMI ≥25 N=119 |
|
| Age, mean (SD), years | 30.2 (8.2) | 29.5 (8.4) | 31.9 (7.8) | 31.7 (8.4) | 29.3 (8.2)§ | 34.6 (7.7)§ |
| Gender (male), n (%) | 81 (48.2)§ | 55 (47) | 26 (51) | 77 (29.6)§ | 39 (27.7) | 38 (31.9) |
| HLA-B27+, n (%) | 156 (92.9)§ | 112 (95.7)ŧ | 44 (86.3)ŧ | 24 (9.3)§ | 16 (11.4) | 8 (6.8) |
| BMI, mean (SD) | 24.5 (5.6) | 21.8 (2.0)§ | 30.6 (6.3)§ | 25.4 (5.1) | 21.9 (1.9)§ | 29.7 (4.4)§ |
| Underweight (≤18.5), n (%) | 6 (3.6)ŧ | – | – | 8 (3.1)ŧ | – | – |
| Normal (18.5–24.9), n (%) | 111 (66.1)ŧ | – | – | 133 (51.2)ŧ | – | – |
| Overweight (25–29.9), n (%) | 31 (18.4)ŧ | – | – | 82 (31.5)ŧ | – | – |
| Obese (≥30), n (%) | 20 (11.9)ŧ | – | – | 37 (14.2)ŧ | – | – |
| CRP (mg/L) median (IQR), (range)* | 3 (3–7) (0–59) |
3 (2–5) (0.2–59)¤ |
4 (3–11) (0–41)¤ |
3 (3–6) (0–96) |
3 (3–5) (0–96)ŧ |
3 (3–6), (0.1–30)ŧ |
| CRP ≥5, n (%) | 55 (32.7) | 32 (27.4)ŧ | 23 (45.1)ŧ | 89 (34.2) | 38 (27)¤ | 51 (42.9)¤ |
| ESR (mm/h), median (IQR), (range) | 9 (5–15) (1–87) |
9 (5–15) (1–87) |
8.5 (4–18) (2–65) |
8 (2–14) (1–66) |
6 (2–10) (1–66)¤ |
9 (5–17) (1–50)¤ |
| Physician global, mean (SD) | 5.2 (2.4) | 5.3 (2.5) | 5.0 (2.2) | 4.9 (2.3) | 4.9 (2.2) | 4.9 (2.4) |
| Patient's global, mean (SD)* | 4.2 (2.6)§ | 4.2 (2.7) | 4.3 (2.5) | 5.2 (2.7)§ | 4.8 (2.7)ŧ | 5.6 (2.6)ŧ |
| Total back pain, mean (SD)* | 4.7 (2.7)ŧ | 4.7 (2.6) | 4.8 (2.7) | 5.4 (2.7)ŧ | 5.1 (2.7) | 5.7 (2.7) |
| Peripheral pain/swelling, mean (SD)* | 2.4 (2.8)¤ | 2.4 (2.7) | 2.6 (3.1) | 3.3 (3.0)¤ | 3.3 (3.1) | 3.3 (3.0) |
| Duration of morning stiffness, mean (SD)* | 3.8 (2.8) | 3.7 (2.8) | 4.1 (2.9) | 4.3 (3.0) | 4.0 (2.9) | 4.6 (3.0) |
| ASDAS, mean (SD) | 2.4 (0.9) | 2.3 (0.9)† | 2.6 (0.9)† | |||
| BASDAI, mean (SD) | 3.9 (2.1) | 3.9 (2.1) | 4.2 (2.1) | |||
§p<0.001, ¤p<0.01,ŧp<0.05.
*Parameters of ASDAS.
†ASDAS p value=0.15.
ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BMI, body mass index; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HLA-B27, human leucocyte antigen B27.
Since gender was an effect modifier in the relation between BMI and CRP (see linear regression analyses), we present the baseline data also stratified by gender for patients in the axSpA and no-axSpA group separately (table 2). In axSpA patients, every PRO was higher in female than in male patients. However, CRP levels were not different between male and female patients in axSpA and no-axSpA patients. In patients with axSpA, females had higher BASDAI and ASDAS scores than males, but this difference was only statistically significant for BASDAI (table 2).
Table 2.
Baseline characteristics in patients with and without axSpA, stratified by gender
| axSpA N=168 |
no-axSpA N=260 |
|||
|---|---|---|---|---|
| Male n=81 | Female n=87 | Male n=77 | Female n=183 | |
| Age, mean (SD), years | 29.9 (8.0) | 30.5 (8.5) | 30.7 (8.7) | 32.2 (8.3) |
| HLA-B27+, n (%) | 77 (95.1) | 79 (90.8) | 11 (14.5) | 13 (7.1) |
| BMI, mean (SD) | 24.6 (4.1)ŧ | 24.4 (6.7)ŧ | 24.9 (3.8) | 25.7 (5.5) |
| Underweight (≤18.5), n (%) | 2 (2.5) | 4 (4.6) | 3 (3.9)ŧ | 5 (2.7)ŧ |
| Normal (18.5–24.9), n (%) | 53 (65.4) | 58 (66.7) | 36 (46.7)ŧ | 97 (53)ŧ |
| Overweight (25–29.9), n (%) | 18 (22.2) | 13 (14.9) | 33 (42.9)ŧ | 49 (26.8)ŧ |
| Obese (≥30), n (%) | 8 (9.9) | 12 (13.8) | 5 (6.5)ŧ | 32 (17.5)ŧ |
| CRP (mg/L) median (IQR), (range)* | 3 (3–6) (0–59) |
3 (2–7) (0.2–46) |
3 (3–4) (0.4–96) |
3 (3–6) (0–46) |
| CRP ≥5, n (%) | 26 (32.1) | 29 (33.3) | 19 (24.7)ŧ | 70 (38.3)ŧ |
| ESR (mm/h), median (IQR), (range). | 6 (2–11) (1–67)§ |
10 (6–18) (2–87)§ |
2 (2–9) (1–65)§ |
9 (5–14) (2–66)§ |
| Physician global, mean (SD) | 5.1 (2.5) | 5.3 (2.4) | 5.0 (2.4) | 4.9 (2.3) |
| Patient's global, mean (SD)* | 3.8 (2.6) | 4.5 (2.6) | 4.9 (2.7) | 5.3 (2.7) |
| Total back pain, mean (SD)* | 4.5 (2.6) | 4.9 (2.7) | 4.5 (2.6)§ | 5.7 (2.7)§ |
| Peripheral pain/swelling, mean (SD)* | 2.1 (2.6) | 2.7 (3.0) | 2.4 (2.7)§ | 3.7 (3.1)§ |
| Duration of morning stiffness, mean (SD)* | 3.7 (2.9) | 3.9 (2.8) | 4.0 (3.1) | 4.4 (2.9) |
| ASDAS-CRP, mean (SD) | 2.3 (0.9)† | 2.5 (0.9)† | ||
| BASDAI, mean (SD) | 3.6 (2)ŧ | 4.3 (2.2)ŧ | ||
§p<0.001, ŧp<0.05.
*ASDAS-CRP p value=0.18.
†Parameters of ASDAS-CRP.
ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BMI, body mass index CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HLA-B27, human leucocyte antigen B27.
Linear regression analyses
Gender, but not age, appeared to be an effect modifier in the relation between BMI and CRP in the whole population (p-value for BMI x gender interaction=0.02), but not in the relation between BMI and ASDAS in axSpA patients (p value for BMI x gender interaction=0.88) (table 3).
Table 3.
Assessment of possible effect modifiers on the relation of BMI on CRP in the whole population (428 patients), and on the relation of BMI on ASDAS-CRP in axSpA patients (168 patients) by linear regression modelling
| CRP | ||||
|---|---|---|---|---|
| n=428 (whole population) | β | SE | 95% CI | p Value |
| BMI | 1.14 | 1.30 | −1.41 to 3.70 | 0.38 |
| Age | 0.78 | 2.52 | −4.16 to 5.73 | 0.76 |
| BMI x age | −1.02 | 1.72 | −4.41 to 2.36 | 0.55 |
| BMI | −6.12 | 2.86 | −11.74 to −0.50 | 0.03 |
| Gender | −5.49 | 2.50 | −10.40 to −0.58 | 0.03 |
| BMI x gender | 4.01 | 1.68 | 0.70 to 7.32 | 0.02 |
|
ASDAS-CRP | ||||
| n=168 (axSpA population) | β | SE | 95% CI | p Value |
| BMI | 0.31 | 0.22 | −0.12 to 0.74 | 0.16 |
| Age | 0.15 | 0.42 | −0.69 to 0.98 | 0.73 |
| BMI x age | −0.17 | 0.31 | −0.77 to 0.43 | 0.58 |
| BMI | 0.29 | 0.48 | −0.65 to 1.23 | 0.54 |
| Gender | 0.25 | 0.42 | −0.57 to 1.07 | 0.54 |
| BMI x gender | −0.05 | 0.30 | −0.64 to 0.55 | 0.88 |
p Value in bold: with statistical significance.
x=interaction term.
ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BMI, body mass index; CRP, C reactive protein; β, unstandardised β coefficient.
Consequently, all subsequent analyses are stratified by gender. As ASDAS can only be calculated in axSpA patients, data on BMI and ASDAS are presented in this subgroup only. BMI has an influence on CRP in females (β=0.35; p<0.001; R2=0.09) but not in males (β=−0.11; p=0.58; R2=0.00) (table 4). In female patients, CRP increases 0.35 mg/L for each increased BMI point.
Table 4.
Linear regression analyses explaining CRP by BMI according to gender in the whole population (n=428)
| β | SE | 95% CI | R-squared | p Value | |
|---|---|---|---|---|---|
| Male (n=158) | |||||
| BMI | −0.11 | 0.20 | −0.51 to 0.28 | 0.00 | 0.58 |
| Female (n=270) | |||||
| BMI | 0.35 | 0.07 | 0.21 to 0.49 | 0.09 | <0.001 |
p Value in bold with statistical significance.
β, unstandardised β coefficient; BMI, body mass index; CRP, C reactive protein.
For example, in a hypothetical case where a female patient would spectacularly gain 10 BMI points, and all the PROs included in ASDAS remain the same (eg, a score of 3) with a baseline CRP of 5 will mean that ASDAS score will increase by 0.3. Then, each increased BMI point will indicate an increase of 0.03 in ASDAS. In line with the observation that only very large changes in BMI affect CRP levels in a clinically meaningful way, in our analysis BMI is not statistically significantly related to ASDAS in axSpA patients (β=0.02; p=0.13; R2=0.03), independent of age and gender (table 5).
Table 5.
Linear regression analyses explaining ASDAS by BMI in the 168 axSpA patients
| β | SE | 95% CI | R-squared | p Value | |
|---|---|---|---|---|---|
| Without adjustments | |||||
| BMI | 0.02 | 0.01 | −0.01 to 0.04 | 0.01 | 0.18 |
| Adjusted for age and gender | |||||
| BMI | 0.02 | 0.01 | −0.01 to 0.04 | 0.03 | 0.13 |
ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; β, unstandardised β coefficient.
Correlation between ASDAS and BMI, and between the individual parameters and BMI
CRP shows a small positive but statistically significant correlation with BMI in axSpA and no-axSpA (r=0.27 and r=0.21, respectively) (see online supplementary table S1). Moreover, CRP shows a statistically significant positive correlation with BMI in female patients, but not in male patients in the whole population (r=0.35 and r=-0.03, respectively), and in axSpA patients (r=0.48 and r=0.00, respectively). We found very similar results after performing Pearson correlation by using CRP log transformed (data not shown). ASDAS did not show any correlation with BMI in patients with axSpA (r=0.09; p=0.24). BMI is not correlated to any of the PROs included in ASDAS.
Discussion
In this study we investigated whether CRP and ASDAS truthfully reflect disease activity in patients with axSpA with a high BMI. We found that gender modifies the relation between BMI and CRP. BMI has an influence on CRP only in female patients, independent of age. The stronger association found between BMI and CRP in females as compared with males has been shown in previous studies.8 14 15 In female patients, CRP increases 0.35 mg/L for each increased BMI point, which is not clinically relevant. Moreover, we found that BMI has no significant correlation with any PRO included in ASDAS in axSpA patients. As an expected consequence of these findings, we did not find a relation between BMI and ASDAS in axSpA patients. Therefore, it is not necessary to take BMI into consideration when assessing disease activity by ASDAS in overweight axSpA patients.
In this study we have shown that in the axSpA group, CRP is statistically significantly increased in the overweight category. Also the percentage of patients with an elevated CRP increased around 18% in the axSpA and no-axSpA group. PROs and ASDAS are slightly increased in the overweight axSpA patients, but this is not reaching statistical significance. Moreover, BMI has an influence on CRP in female patients only (β=0.35). In an extreme case where a female patient gains 15 BMI points, and all the PROs included in ASDAS remain the same (eg, a score of 3) with a baseline CRP of 5, it means that CRP will increase with 5.25 mg/L. Then, ASDAS will change from 2.1 to 2.5, implying a small change (remaining within the same disease state). This reasoning applies also in case there is a difference of 15 BMI points between two patients. As with every cut-off, some patients will change categories of disease activity state solely based on a high BMI. Therefore, in clinical practice it is important to keep in mind that for very overweight female axSpA patients a small part of the ASDAS can be explained by BMI.
The well-documented sex differences in body fat distribution and systemic sex hormone concentrations might explain the sex differences that we have observed. Sex hormones could increase CRP levels in women.16 Moreover, women have a higher fat percentage than men, and store more fat in the gluteal-femoral region and subcutaneous adipose tissue, whereas men have a higher visceral adipose tissue deposition.17 Importantly, visceral adipose tissue has the strongest impact on elevated CRP concentrations.18
A limitation of BMI is that it is unable to distinguish between fat and lean mass.19 It has been shown that BMI has a good specificity, but poor sensitivity in identifying excessive adiposity.20 Taking into account this limitation of BMI, and the fact that men store more visceral adipose tissue than women might explain why despite the fact that BMI has an influence on CRP in female patients only, CRP levels were not different between male and female patients in axSpA and no-axSpA patients in our population.
A limitation of our study is that there were only few overweight axSpA patients (n=51) included, which may have diminished the power of our study to find an association between BMI and ASDAS in the group of patients with axSpA. However, this does probably not play an important role, as we were able to confirm the relationship between CRP and female patients found in previous studies.8 17 An important strength of our study is that a control group (patients with chronic back pain not fulfilling the ASAS axSpA criteria) is included in SPACE, enabling us to compare the relation between BMI and CRP in axSpA and no-axSpA patients.
In summary, BMI has an influence on CRP only in female patients, but this influence is of only limited clinical relevance. In axSpA patients, BMI did not have a significant influence on any PRO included in ASDAS nor on ASDAS itself. In general, it is not necessary to take BMI into account when assessing disease activity by ASDAS in axSpA patients with high BMI, but there may be a slight increase in ASDAS in female patients with very high BMI. It might be interesting to further explore the influence of BMI on CRP in a larger sample size of overweight female axSpA patients, with a more accurate test to estimate fat mass (such as MRI and spectroscopy).21 In conclusion, ASDAS provides a reliable disease activity score in patients with axSpA, regardless of BMI.
Footnotes
Funding: This work was supported by the Spanish Society of Rheumatology (15AE01)
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The local medical ethical committees of the participating sites (3 centres in the Netherlands, 1 in Norway and 1 in Italy) approved the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Garrett S, Jenkinson T, Kennedy LG et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 2.Lukas C, Landewé R, Sieper J et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. 10.1136/ard.2008.094870 [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Espartero C, de Miguel E, Loza E et al. Validity of the ankylosing spondylitis disease activity score (ASDAS) in patients with early spondyloarthritis from the Esperanza programme. Ann Rheum Dis 2014;73:1350–5. 10.1136/annrheumdis-2012-202976 [DOI] [PubMed] [Google Scholar]
- 4.Smitka K, Marešová D. Adipose tissue as an endocrine organ: an update on pro-inflammatory and anti-inflammatory microenvironment. Prague Med Rep 2015;116:87–111. 10.14712/23362936.2015.49 [DOI] [PubMed] [Google Scholar]
- 5.Sopasakis VR, Nagaev I, Smith U. Cytokine release from adipose tissue of nonobese individuals. Int J Obes 2005;29:1144–7. [DOI] [PubMed] [Google Scholar]
- 6.Bednarek-Tupikowska G, Zdrojowy-Wełna A, Stachowska B et al. Accumulation of abdominal fat in relation to selected proinflammatory cytokines concentrations in non-obese Wrocław inhabitants. Endokrynol Pol 2014;65:449–55. 10.5603/EP.2014.0062 [DOI] [PubMed] [Google Scholar]
- 7.Calabro P, Chang DW, Willerson JT et al. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol 2005;46:1112–13. 10.1016/j.jacc.2005.06.017 [DOI] [PubMed] [Google Scholar]
- 8.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 2013;14:232–44. 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- 9.Vincent HK, Seay AN, Montero C et al. Functional pain severity and mobility in overweight older men and women with chronic low-back pain–part I. Am J Phys Med Rehabil 2013;92:430–8. 10.1097/PHM.0b013e31828763a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg R, de Hooge M, van Gaalen F et al. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology (Oxford) 2013;52:1492–9. 10.1093/rheumatology/ket164 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Obesity and overweight. http://www.who int/mediacentre/factsheets/fs311/en/ 2013; Updated January 2015.
- 12.Machado P, Navarro-Compán V, Landewé R et al. Calculating the ankylosing spondylitis disease activity score if the conventional c-reactive protein level is below the limit of detection or if high-sensitivity c-reactive protein is used: an analysis in the DESIR cohort. Arthritis Rheumatol 2015;67:408–13. 10.1002/art.38921 [DOI] [PubMed] [Google Scholar]
- 13.Rudwaleit M, van der Heijde D, Landewé R et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 14.Arena R, Arrowood JA, Fei D-Y et al. The relationship between C-reactive protein and other cardiovascular risk factors in men and women. J Cardpulm Rehabil 2006;26:323–7; quiz328–9 10.1097/00008483-200609000-00009 [DOI] [PubMed] [Google Scholar]
- 15.Lear SA, Chen MM, Birmingham CL et al. The relationship between simple anthropometric indices and C-reactive protein: ethnic and gender differences. Metabolism 2003;52:1542–6. 10.1016/j.metabol.2003.07.005 [DOI] [PubMed] [Google Scholar]
- 16.Salpeter SR, Walsh JME, Ormiston TM et al. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 2006;8:538–54. 10.1111/j.1463-1326.2005.00545.x [DOI] [PubMed] [Google Scholar]
- 17.Cartier A, Côté M, Lemieux I et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr 2009;89:1307–14. 10.3945/ajcn.2008.27030 [DOI] [PubMed] [Google Scholar]
- 18.Lemieux I, Pascot A, Prud'homme D et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 2001;21:961–7. 10.1161/01.ATV.21.6.961 [DOI] [PubMed] [Google Scholar]
- 19.Kontogianni MD, Panagiotakos DB, Skopouli FN. Does body mass index reflect adequately the body fat content in perimenopausal women? Maturitas 2005;51:307–13. 10.1016/j.maturitas.2004.08.014 [DOI] [PubMed] [Google Scholar]
- 20.Okorodudu DO, Jumean MF, Montori VM et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–9. [DOI] [PubMed] [Google Scholar]
- 21.Thomas EL, Fitzpatrick JA, Malik SJ et al. Whole body fat: content and distribution. Prog Nucl Magn Reson Spectrosc 2013;73:56–80. [DOI] [PubMed] [Google Scholar]


