Abstract
Introduction
Poor healthcare access is a major barrier to receiving antenatal care and a cause of high maternal mortality in South Africa (SA). ‘Point-of-care’ (POC) diagnostics is a powerful emerging healthcare approach to improve healthcare access. This study focuses on evaluating the accessibility and utility of POC diagnostics for maternal health in rural SA primary healthcare (PHC) clinics in order to generate a model framework of implementation of POC diagnostics in rural South African clinics.
Method and analyses
We will use several research methods, including a systematic review, quasi-experiments, survey, key informant interviews and audits. We will conduct a systematic review and experimental study to determine the impact of POC diagnostics on maternal health. We will perform a cross-sectional case study of 100 randomly selected rural primary healthcare clinics in KwaZulu-Natal to measure the context and patterns of POC diagnostics access and usage by maternal health providers and patients. We will conduct interviews with relevant key stakeholders to determine the reasons for POC deficiencies regarding accessibility and utility of HIV-related POC diagnostics for maternal health. We will also conduct a vertical audit to investigate all the quality aspects of POC diagnostic services including diagnostic accuracy in a select number of clinics. On the basis of information gathered, we will propose a model framework for improved implementation of POC diagnostics in rural South African public healthcare clinics. Statistical (Stata-13) and thematic (NVIVO) data analysis will be used in this study.
Ethics and dissemination
The study protocol was approved by the Ethics Committee of the University of KwaZulu-Natal (BE 484/14) and the KwaZulu-Natal Department of Health based on the Helsinki Declaration (HRKM 40/15). Findings of this study will be disseminated electronically and in print. They will be presented to conferences related to HIV/AIDS, diagnostics, maternal health and strengthening of health systems.
Keywords: Point-of-care diagnostics, Maternal health outcomes, Primary health care
Strengths and limitations of this study.
This study will include relevant key stakeholders of point-of-care (POC) diagnostics in the study setting.
Evaluation will reveal barriers and challenges that need to be addressed before the adoption of new POC diagnostics or scaling up the current POC diagnostics services in the study setting.
To ensure reliability of results, the evaluation of POC test performance will only include PHC clinics that are within close proximity to the testing laboratory.
Introduction
South Africa (SA) has ∼6 million HIV-infected people, most of them women with 27% of pregnant women living with HIV.1 Of this, the highest infection rate at 10% higher than the national average as well as HIV prevalence and maternal death rates are seen in the KwaZulu-Natal (KZN) province.1 The South African National Strategic Plan (NSP) for HIV, tuberculosis and sexually transmitted infections 2012–2016 contains important recommendations linked to maternal health and highlights the importance of innovations such as point-of-care (POC) diagnostics for improvement of patient outcomes.2 Unicef reports the need to prioritise the improvement of quality of SA health services at primary care level, ensuring timely referral of patients to higher levels of the health system when necessary.2 There remains a lack of uniformity in SA primary health service distribution resulting in failure to meet the unmet needs of patients in resource-limited settings.3 Research studies have demonstrated the implications of poor access to healthcare services in SA rural communities4 5 and the usefulness of POC diagnostics for the improvement of healthcare access in rural and resource-limited communities in the developing world.6 7
A major advantage of POC diagnostics over standard laboratory testing is the ability to provide rapid results, permitting timely initiation of suitable therapy as well as facilitating linkages to care and referrals.8 POC diagnostics has the potential to improve healthcare services by enabling delivery of pathology testing in settings that have limited access to laboratory infrastructure.8 For these reasons, these diagnostics have the potential to play a major role in revolutionising the diagnosis, initiation and monitoring of treatment of major global diseases. The clinical impact of POC diagnostics has been shown in a variety of infectious diseases, including HIV/AIDS.8 Consequently, WHO called for new clinical diagnostics methods that are designed to function in settings with limited access to laboratory services9 leading to an increase in marketing, manufacturing and development of POC diagnostic instruments and reagents for clinical use.10
The use of POC diagnostics could prevent more than 1.2 million deaths from HIV/AIDS, its co-infections and malaria.11 However, previous research has demonstrated that the availability of health technologies in these settings does not always guarantee patient-centred outcomes.12 Implementation of diagnostic POC tests should be evaluated within a given context to ensure the utility of these novel technologies developed in high income countries for use in low income countries.13 14 Evaluations are needed in developing, low-resource settings where pregnant and postnatal HIV-positive mothers have poor access to quality healthcare facilities and clinical laboratories.15
Gaining insight into these barriers is useful for informing POC diagnostic developers, policymakers, clinicians and users to ensure the usefulness of POC diagnostics in reducing HIV-related maternal mortality in rural resource-limited settings. The overarching aim of this study is to evaluate the accessibility, availability and utility of POC diagnostic services for rural primary healthcare clinics in SA in order to develop an ideal model framework and recommendations for improved implementation of POC diagnostics in these settings. The following objectives will be outlined in order to address the aim of the study: first, to investigate the typology, supply chain POC diagnostics in rural PHC clinics in SA; second, to investigate the deficiencies and their causes for POC diagnostics in rural PHC clinics in SA; third, to investigate the quality management systems emplaced to ensure reliability of the HIV-related POC diagnostics for maternal health in their current setting; fourth, to determine the impact of HIV-related POC diagnostics on maternal mortality using an interrupted time series study; fifth, to evaluate whether introduction POC diagnostics into algorithms for diagnosing maternal patients, improves maternal health for HIV-infected women using a systematic review and finally to develop a model framework and recommendations for improved implementation of POC diagnostics on SA rural PHCs.
Methods
A summary of the methodology used by this multiphase/component study can be found in table 1. In this study, we will use several research methods to determine the impact of POC diagnostics on maternal health in rural KZN. We define rural as sparsely populated areas in which people farm or depend on natural resources, including the villages and small towns that are dispersed through these areas. In addition, they include the large settlements in the former homelands, created by the apartheid removals, which depend, for their survival, on migratory labour and remittances.
Table 1.
Summary of the methodology
| Objectives | Hypothesis | Study design | Recruitment/sampling | Independent variable | Dependent variable | Analysis | Outcome measure |
|---|---|---|---|---|---|---|---|
| To investigate the typology, supply chain of HIV-related and MH-related POC diagnostics | Improved accessibility of maternal health-related and HIV-related outcomes can improve effectiveness of maternal health services in rural PHCs | Cross-sectional | A total of 100 rural KZN PHC clinics using PPS, stratified into the following: 25 PHC clinics with high healthcare worker headcount; 25 PHC clinics with low healthcare worker headcount; 25 PHC clinics with high patient headcount; 25 PHC clinics with low patient headcount (list of PHC clinics will be obtained from the SA DHIS). | Coverage and usage of MH-related and HIV-related POCT: geographic location, district classification (NHI pilot site); access to tertiary healthcare | Number and percentage of POC diagnostics | Frequency distribution | Clinics with the highest and lowest POC tests availability in each of the 11 districts, clinic location, distance between the clinic and nearest town/city, distance between clinic and nearest referral hospital |
| Proportion of facilities that need HIV-related and MH-related POC diagnostics | Number of diagnostics used | Clinics with the highest and lowest POC tests need in each of the 11 KZN districts, list of HIV-related POC diagnostics in the clinic, list of HIV-related POC diagnostics needed in the clinic, laboratory test turnaround times | |||||
| Staff POC diagnostic knowledge and skill assessment | Knowledge of POCT used for diagnosis, monitoring and reduction of referrals | Clinic staff with the highest and lowest POC tests knowledge in each of the 11 KZN districts | |||||
| Health demand, availability, supply chain | Frequency of usage for HIV-related and MH-related POC diagnostics | List of HIV-related POC tests available in the clinic, most and least frequently used POCT in each of the 11 KZN districts, number of maternal health patients using the clinic, number of healthcare workers offering services in the clinics | |||||
| To investigate deficiencies and their causes in HIV-related and maternal health related POC diagnostics | Demonstrating the causes linked to poor accessibility of maternal health-related and HIV-related outcomes can help inform and guide implementers during scaling up of current POC services and adoption of new POC services | In-depth interviews | Healthcare workers from PHCs with low availability and usage of POC diagnostics; 11 public health officials from each (1 from each district) | Management; human resources; infrastructure; staff knowledge, skill and attitude; believes; stakeholder perception | Level of accessibility, availability and usage and of HIV and MH POCT for maternal health services | Thematic analysis | NA |
| To investigate the QMS emplaced to ensure reliability of the HIV-related and MH-related POC diagnostics in their current setting | Providing evidence on the reliability and sustainability of the QMS for PHC-based POCT can provide reassurance to implementers during scaling up of current POC services and adoption of new POC services | Vertical assessment/audit against SANAS ISO 15189:2012 and ISO 22870:2006 | Document review from PHCs with high availability and usage of POC diagnostics. | Infrastructure; QMS; operational time taken from results reporting to patient treatment for routine cases | Level of quality of service delivery | Correlation coefficient | Overall compliance with relevant ISO standards |
| Ease of use | User acceptability | Correlation coefficient | Overall compliance with relevant ISO standards | ||||
| Linkage to healthcare | Time from diagnosis to healthcare | Diagnostics turnaround times | Correlation coefficient | Overall compliance with relevant ISO standards | |||
| Efficacy test | Blood samples from maternal health patients who are receiving POC diagnostic services from PHCs with high quality of POC diagnostics service delivery. | Stability of the test under user conditions | Specificity; sensitivity; positive and negative likelihood ratios; PPV and NPV | 95% CIs and paired Z test to compare CIs of validation indices (sensitivity; specificity; NPV and PPV κ-statistic) between POC diagnostics results and laboratory results | Correlation between the laboratory and POCT results | ||
| The sample size at the required absolute precision level for sensitivity and specificity will be dependent on survey, vertical audit results and clinic size. It will can be calculated by Buderer's formula.16 | Reliability of the POCT results | Accuracy of results in comparison with gold standard (ELISA) | Correlation coefficient | Correlation between the laboratory and POCT results | |||
| To determine the impact of HIV-related and MH-related POC diagnostics on maternal mortality using quasi-experiment | Demonstrating the impact of HIV-related and MH-related POC tests on maternal mortality provides merit/worth for POC testing scale up in KZN maternal health clinics | Quasi-experimental, interrupted time series | DHIS data on MMR data from the PHC clinics with high quality of POC diagnostics service delivery. | Time aggregation: monthly level, facility level; facility-specific time of implementation of POC diagnostics (syphilis) will be used as a break point of in segmented regression | Change in maternal mortality rate | Segmented regression modelling | Reduction in maternal mortality post-POCT implementation. |
| To evaluate whether introduction POC diagnostics into algorithms for diagnosing maternal patients, reduces the maternal mortality rate in rural sub-Saharan Africa | Evidence from a systematic review (highest quality of evidence) indicating the impact of HIV-related POCT on maternal outcomes of HIV-infected mothers will show significance for POCT scale up in rural and resource-limited maternal health clinics | Systematic review and meta-analysis | Peer reviews literature fitting the inclusion and exclusion criteria | analysis of MMR data pre and post syphilis POC testing interventions | Improved maternal outcomes: maternal mortality; prevention of mother to child transmission of HIV and | Meta-analysis | Studies reporting a significant improvement of maternal outcomes |
| To develop a model framework and recommendations for improved implementation of POC diagnostics on SA rural PHC clinics | Developing local evidence-based frameworks and guidelines can improve the effectiveness of the services | KZN province | NA | Evidence-based guidelines (based on the evidence obtained from the above objectives) | Improved implementation of POC diagnostic services for rural SA | NA | NA |
CIs, confidence intervals; DHIS, District Health Information System; KZN, KwaZulu-Natal; MMR, maternal mortality rate; NA, not applicable; NHI, National Health Insurance; NPV, negative predictive value; PHC, primary healthcare; POC, point-of-care; PPS, probability proportional to size; PPV, positive predictive value; QMS, quality management systems; SA, South Africa; SANAS, South African National Accreditation System.
Overall design
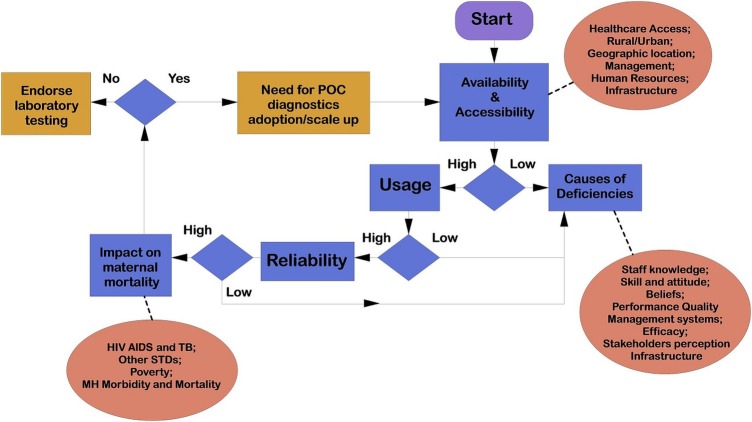
The programme evaluation theory is a promising approach to explain how a programme produces the desired effects.17–19 This theory argues that effective implementation of a programme requires gathering of evidence from relevant stakeholders.17 20 The programme evaluation theory involves three distinctive approaches: postpositivism, interpretivism and critical normative science paradigm.21 Adopting the postpostivism paradigm will enable us to rationally deduce research experience and interpret into concepts and knowledge.22 The interpretivism paradigm will enable us to contextualise subjective realities of study participants in terms of their experiences of POC diagnostics’ challenges and barriers and enable us to attach this to meaning and qualitative evidence.23 The critical normative science paradigm will enable a critical analysis of POC diagnostic services in order to determine its merit. Combining the programme evaluation theory paradigms, we develop a theoretical framework to guide this study (figure 1). We will use data triangulation to increase confidence and diversity regarding the research data.16 24 Data for this study will be collected from seven sources: surveys, interviews, record review, District Health Information Software (DHIS) routine data, peer-reviewed literature and audits.
Figure 1.
A theoretical framework underpinning this study, programme evaluation theory adapted to the local context. POC, point-of-care; STDs, sexually transmitted diseases; TB, tuberculosis.
Research team and study settings
The evaluation study will be carried out in KZN province, SA. KZN is located in the southeast of the country on the coast of the Indian Ocean, shares borders with three other provinces and the countries of Mozambique, Swaziland and Lesotho. KZN is the largest province in SA consisting of 11 districts and 52 municipalities and consists of a mix of urban, semiurban and rural areas. The province has a total population of ∼10 694 400, of which 86.8% are black Africans and Zulu speakers.25 A representative subset of maternal health primary healthcare clinics in rural KZN will be sampled for this study.
Data sources, sampling variables and analyses
The full data analysis plan for each objective/component can also be found in table 1. In this study, various methods of data collection and interpretation will be used as an integrated form to carry out methodological triangulation of sources for collecting quantitative and qualitative samples.22 23
Objective 1: To investigate the typology, supply chain POC diagnostics in rural PHC clinics in SA.
Data sources: Survey (survey tool has been provided as online supplementary material file). The cross sectional survey study protocol was developed a priori and was registered in the Clinical Trial.gov website. ClinicalTrials.gov Identifier: NCT02692274, available at: https://clinicaltrials.gov/ct2/show/NCT02692274.
bmjopen-2016-011155supp.pdf (801.9KB, pdf)
Sampling: We will conduct a stratified random sampling of PHC clinics to ensure generalisability (external validity). PHC clinic healthcare professionals responsible for the POC diagnostic services in the clinic will be requested to participate in the survey. A sample size of 100 primary units has been demonstrated to be an appropriate sample size for this type of facility-based survey.26 The most recent (2014) data on PHC headcount, professional nurse clinic workdays, annual nurse's estimate and average headcount per week was requested from the South African DHIS in order to assign sample strata. Four strata were created based on the above sets. A total of 25 facilities were sampled within each of these strata using probability proportional to size (PPS). Proportionate stratification was implemented to ensure that the sample size of each stratum is proportionate to the population size of the stratum among all 11 KZN districts. The sample size of each stratum is proportionate to the population size of the stratum. Strata sample sizes were determined by the following equation:
where nh is the sample size for stratum h, Nh the population size for stratum h, N the total population size and n is the total sample size. Table 2 shows the sampling frame per district.
Table 2.
Project sampling frame
| District code | District name | Sample number |
|---|---|---|
| B1 | Amajuba District Municipality | 6 |
| B2 | eThekwini Metropolitan Municipality | 7 |
| B3 | iLembe District Municipality | 7 |
| B4 | Harry Gwala District Municipality | 8 |
| B5 | Ugu District Municipality | 14 |
| B6 | uMgungundlovu District Municipality | 4 |
| B7 | uMkhanyakude District Municipality | 22 |
| B8 | uMzinyathi District Municipality | 5 |
| B9 | uThukela District Municipality | 6 |
| B10 | uThungulu District Municipality | 18 |
| B11 | Zululand District Municipality | 3 |
Objective 2: To investigate the deficiencies and their causes for POC diagnostics in rural PHC clinics in SA.
Data sources: In-depth interviews.
Sampling: Clinics with low POC diagnostic availability and usage based on the overall average level of availability and usage of POC diagnostics from the sampled clinics.
Variables: The interviews will be aimed at gaining rich data on patients-centred and staff-centred advantages, barriers, challenges of current POC diagnostic services and future service needs.
Objective 3: To investigate the quality management systems emplaced to ensure reliability of the HIV-related POC diagnostics for maternal health in their current setting.
Data sources: Audit and validation test.
Sampling: To determine the reliability of POC diagnostic services in clinics with high availability, accessibility and usability of POC diagnostics, the quality management systems implemented in the clinics will be assessed against relevant quality indicators as prescribed by the most recent WHO guidelines for POC diagnostics in resource-limited settings, through an audit.27 The audit will include evaluation of the performance, operational characteristics of the test and linkage to healthcare. To determine reliability of the results produced by PHC clinics WHO standards, a POC diagnostic validation test will be carried out. Full blood samples will be requested from consenting women who will be attending the clinic and receiving an HIV POC diagnostic test for laboratory testing. A validation test will be conducted against the gold standard test for HIV, the ELISA test. The sample size at the required absolute precision level for sensitivity and specificity will be dependent on the survey, vertical audit results and clinic size. It will be calculated by Buderer's formula16 which is demonstrated below:
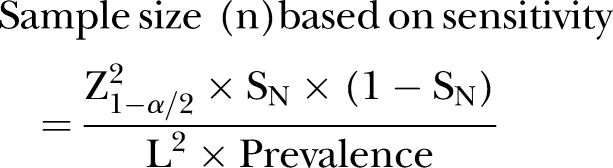 |
and
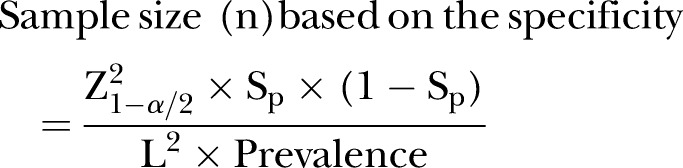 |
Here, n is the required sample size, SN the anticipated sensitivity, Sp the anticipated specificity, α the size of the critical region (1-α is the confidence level), Z1−α/2 the standard normal deviate corresponding to the specified size of the critical region (α) and L is the absolute precision desired on either side (half-width of the CI) of sensitivity or specificity.
Owing to lack of laboratory infrastructure in rural and resource-limited KZN and to ensure reliability of the POC performance validation test, only PHC clinics that are within 60 km to the testing laboratory will be included in the POC test evaluation. It is anticipated that sensitivity (or specificity) of a given POC test is 80% for detecting a given outcome against the laboratory gold standard, assuming an absolute precision of ±10% and the prevalence of outcome in the study population is 27%28 and based on the average patient headcount per week, then it will be necessary to sample and test 207 study subjects using both the POCT and the laboratory gold standard.
Objective 4: To determine the impact of HIV-related POC diagnostics on maternal mortality using an interrupted time series study.
Data sources: South African DHIS.
Sampling: Retrospective data on rural KZN maternal mortality rate from all KZN districts. The time of POC test implementation in KZN rural clinics will be obtained from the Department of Health archives.
Variables: The following is an explanation of the method of Wagner et al,29 applied to our practical analysis. Time series of maternal mortality rate will be assessed using segmented negative binomial regression analysis, which is a method of estimating changes in levels and trends in an outcome associated with an intervention (POC diagnostics). The time series regression equation for this model is as follows:
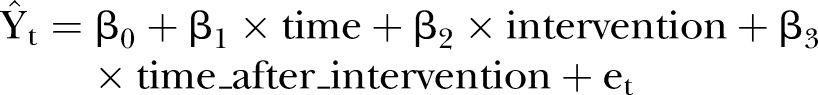 |
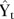 is the outcome (mean number of deaths per quarter), time indicates the number of quarters) from the start of the series, intervention is the dummy variable taking the values 0 in the preintervention segment and 1 in the postintervention segment, time_after_intervention is 0 in the preintervention segment and counts the quarters in the postintervention segment at time t. The coefficient β0 estimates the base level of the outcome (number of deaths) at the beginning of the series, β1 estimates the base trend, that is, the change in outcome per quarter in the preintervention segment, β2 estimates the change in level of deaths on the postintervention segment, β3 estimates the change in trend in deaths in the postintervention segment, et estimates the error.
is the outcome (mean number of deaths per quarter), time indicates the number of quarters) from the start of the series, intervention is the dummy variable taking the values 0 in the preintervention segment and 1 in the postintervention segment, time_after_intervention is 0 in the preintervention segment and counts the quarters in the postintervention segment at time t. The coefficient β0 estimates the base level of the outcome (number of deaths) at the beginning of the series, β1 estimates the base trend, that is, the change in outcome per quarter in the preintervention segment, β2 estimates the change in level of deaths on the postintervention segment, β3 estimates the change in trend in deaths in the postintervention segment, et estimates the error.
This model will be used to estimate the impact of HIV-related and MH-related POC diagnostics on maternal mortality in rural KZN.
Objective 5: To evaluate whether the introduction of POC diagnostics into algorithms for diagnosing maternal patients improves maternal health for HIV-infected women using a systematic review.
The systematic review protocol was developed a priori and was registered in the PROSPERO international prospective register of systematic reviews and in publication.30 PROSPERO record: CRD42014015439, available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014015439#.VSfoV-_GPug.
The systematic review will follow recommendations described in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement31 and the Cochrane Handbook for Intervention Reviews.32 The findings of the systematic review will be disseminated through publication in a peer-reviewed journal and will be formatted according to the specific journal publication guidelines.
Data source: The studies will be selected by evaluation of the inclusion and exclusion criteria. This will be carried out in duplicate and independently by two authors with agreement assessed using κ-statistics.
Objective 6: To develop a model framework and recommendations for improved implementation of POC diagnostics on SA rural PHCs.
Guided by the information gathered from the above objectives, we will propose a model framework for improved implementation of POC diagnostics in rural SA PHCs.
Analyses
Qualitative data analysis
Interviews will be conducted with consenting participants in English. Field notes from observations will be written down about each observation session. The observations will be used to contextualise the interview findings and confirm the validity of interpretations.
We will perform a verbatim transcription of all interviews and check transcripts with study participants to seek points of clarification in relation to issues arising from interviews. We will also perform an audit trial for assessing the entire research process. Thematic content analyses will be performed to identify the patterns of POC diagnostic key utility areas and deficiencies from respondent's interviews, using NVIVO software. First, participants' responses will be coded into categories which were then grouped into themes. The codes will be grouped into similar concepts that reflect the context about local factors that determine healthcare workers and patients' engagement with POC diagnostics. Finally, the identified themes will be validated by the study supervisor. Anticipated themes include: management, human resources, infrastructure, staff knowledge, skill, attitude, believes, relevant key stakeholders' perceptions on the quality of POC diagnostics and relevant key stakeholders’ perception on POC diagnostics scale-up.
Quantitative analysis
Quantitative data will be entered into a project-specific Microsoft Access database and extracted manually onto a categorised table. Data will be grouped into two levels: facility level and individual level. Facility-level data will include data from clinic audits and PHC clinic nurses. Inferential statistics will be used to determine significant differences in the availability of POC diagnostics from the sampled clinics. Standard Student's t-test and analysis of variance (ANOVA) will be used to compare means across groups while the Pearson χ2 test will be used for contingency tables. Factors such as distance to the nearest emergency hospital and clinic size in terms of patient volumes and staff numbers, frequency of use for the diagnostics and level of need for the diagnostics (list of POCT requested by the clinic staff during survey) will be taken into account. The reliability and accuracy of the POCT test results versus the laboratory gold standard will be estimated along with 95% CIs. Table 1 show variables and analysis for the data obtained from the audit. Systematic review analysis will follow the relevant PRISMA guidelines as stipulated in the published protocol.30
All quantitative data will be processed and analysed using Stata V.13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, Texas, USA: StataCorp L). Descriptive statistics that include frequency distribution, percentages and percentiles, means and SDs and cross-tabulations will be used to describe the characteristics of POC diagnostic service in rural KZN. A 95% CI will be constructed around point estimates given the sampling design.
Limitations
We will address any missing data using appropriate statistical methods.33 Data can be missed for many reasons, such as on occasions when a participant did not show up to participate in a study; or one group had more participants than another; or a device did not record the data correctly. The nature of missing data will determine the statistical analysis methods to be used.
We will undertake a careful and prolonged planning of the study to reduce or eliminate the potential sources of bias including sampling bias, recall bias and reporting bias. Recall bias can be introduced into the qualitative data collection stage of investigation, presenting a major threat to the internal validity and credibility of the study. To overcome this limitation in this study, participants will be provided with enough time before answering the question, to reflect and think through a sequence of events in their professional history. The risk of bias for (eg, internal validity) the studies included in a systematic review will be evaluated using the Cochrane Risk of Bias Tool.32
Ethics and dissemination
Ethical principles and patient informed consent
The study is being conducted in accordance with the Helsinki Declaration. Permission letters were obtained from all study site managers: KZN health district managers and KZN National Health Laboratory manager. Written consent authorisation will be obtained from all study participants prior to any study-related procedures being performed. Participants will be given a copy of the consent form for their record. The consent process will be documented. Participation will be voluntary and each participant will be able to drop out at any time for any reason. All discordant HIV test results will be reported to the clinic; relevant clinic staff will recall the patients involved for second sample collection and retest by the laboratory.
Legal principles
All personal data will be eliminated. All electronic data of the complete, coded documents will be saved on a protected server which can only be accessed by the members of the internal study team. The paper for the document will be stored in archives closed for external persons. Data will be kept anonymised during the study and will be kept strictly confidential in storage for 2-year after completion of the study. No identifying data will be published.
Dissemination of study findings
With this study, our aim is to influence rural PHC policy by translating research evidence to relevant stakeholders. Knowledge translation would be a key aspect of the study, where we intend to disseminate findings to contribute evidence to inform policymakers regarding guidelines for the adoption of new POC diagnostic devices and for possible scaling-up the use of POC diagnostic services, for improvement of HIV and maternal healthcare in rural resource-limited settings.
Study timeline
Table 3 depicts the study timeline for each of the study objectives.
Table 3.
Study timeline
| Objectives | Data collection | Data entry and cleaning | Data analysis | Reporting |
|---|---|---|---|---|
| Objective 1 | April to August 2015 | September 2015 to March 2016 | March 2016 to April 2016 | May 2016 |
| Objective 2 | October to December 2015 | January 2016 to March 2016 | April 2016 | July 2016 |
| Objective 3 | November to December 2015 | December 2015 to January 2016 | February to March 2016 | May 2016 |
| Objective 4 | April 2015 to April 2016 | May to June 2016 | July 2016 | September 2016 |
| Objective 5 | October 2015 | November 2015 | December 2015 to February 2016 | March 2016 |
| Objective 6 | May 2016 | June 2016 | July 2016 | October 2016 |
Acknowledgments
The authors acknowledge Rowan Mark Thompson (RMT) for his assistance with technical graphics. Open access publication of this article has been made possible through support from the Victor Daitz Information Gateway, an initiative of the Victor Daitz Foundation and the University of KwaZulu-Natal.
Footnotes
Contributors: TPM-T, BS and PKD conceptualised the design of the study. TPM-T produced the first draft of the manuscript. BS and PKD commented on this draft and contributed to the final version. All the authors read and approved the final manuscript.
Funding: University of KwaZulu-Natal (grant: College of Health Sciences Research Scholarship); South African Centre of Excellence for Epidemiology and Modelling Analysis (grant: PhD scholarhip); African Population and Health Research Centre (grant: 2015 African Doctorate Dissertation Fellowship).
Competing interests: None declared.
Ethics approval: The protocol was approved by the University of KwaZulu-Natal, Biomedical Research Ethics Committee (reference number: BE484/14) and the KwaZulu-Natal Department of Health Ethics Committee (reference number: HRKM 40/15).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cullinan K. South Africa far from targets to reduce maternal, infant mortality. 30 June 2014. http://wwwhealth-eorgza/2013/10/29/south-africa-far-targets-reduce-maternal-infant-mortality/
- 2.The National Strategic Plan (NSP) 2012 – 2016 in a nutshell. http:// sanacorgza/2013/07/01/the-national-strategic-plan-nsp-2012-2016-in-anutshell/ (accessed 18 Jun 2015).
- 3.McIntyre D, Gilson L, Wadee H et al. Commercialisation and extreme inequality in health: the policy challenges in South Africa. J Int Dev 2006;18:435–46. 10.1002/jid.1293 [DOI] [Google Scholar]
- 4.Woolman S, Sprague C, Black V. Why state policies that undermine HIV lay counsellors constitute retrogressive measures that violate the right of access to health care for pregnant women and infants. S Afr J Hum Rights 2009;25:102–25. [Google Scholar]
- 5.Dhai A. A health system that violates patients' rights to access health care. S Afr J Bioeth Law 2012;5:2–3. [Google Scholar]
- 6.Dinnes J, Deeks J, Kunst H et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007;11:1–196. 10.3310/hta11030 [DOI] [PubMed] [Google Scholar]
- 7.Shephard MDS, Mazzachi BC, Shephard AK et al. The impact of point of care testing on diabetes services along Victoria's Mallee Track: results of a community-based diabetes risk assessment and management program. Rural Remote Health 2005;5:371. [PubMed] [Google Scholar]
- 8.Pai NP, Vadnais C, Denkinger C et al. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med 2012;9:e1001306 10.1371/journal.pmed.1001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabey D, Peeling RW, Ustianowski A et al. Diagnostics for the developing world. Nat Rev Microbiol 2004;2:231–40. 10.1038/nrmicro841 [DOI] [PubMed] [Google Scholar]
- 10.The World Market for Point of Care (POC) Diagnostics http://wwwkaloramainformationcom/Point-Care-POC-9030928/ (accessed 18 Jun 2016).
- 11.Keeler E, Perkins MD, Small P et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 2006;444:49–57. 10.1038/nature05446 [DOI] [PubMed] [Google Scholar]
- 12.Scott L. A laboratorian's experience of implementing multiple point-of-care testing in HIV antiretroviral treatment clinics in South Africa. S Afr Med J 2013;103:883–4. [DOI] [PubMed] [Google Scholar]
- 13.Nabyonga J, Orem J. From knowledge to policy: lessons from Africa. Sci Transl Med 2014;6:240ed13 10.1126/scitranslmed.3008852 [DOI] [PubMed] [Google Scholar]
- 14.Drain PK, Hyle EP, Noubary F et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 2014;14:239–49. 10.1016/S1473-3099(13)70250-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng 2008;10:107–44. 10.1146/annurev.bioeng.10.061807.160524 [DOI] [PubMed] [Google Scholar]
- 16.Buderer NM. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med 1996;3:895–900. [DOI] [PubMed] [Google Scholar]
- 17.Cojocaru S. Clarifying the theory-based evaluation. Rev Cercet Intervenţie Socială 2009;26:76–86. [Google Scholar]
- 18.Mercer SH, Idler AM, Bartfai JM. Theory-driven evaluation in school psychology intervention research: 2007-2012. Sch Psychol Rev 2014;43:119–31. [Google Scholar]
- 19.Haji F, Morin M-P, Parker K. Rethinking programme evaluation in health professions education: beyond ‘did it work?’ Med Educ 2013;47:342–51. 10.1111/medu.12091 [DOI] [PubMed] [Google Scholar]
- 20.Lynch BK. Language assessment and programme evaluation. Edinburgh University Press, 2003. [Google Scholar]
- 21.Bickman L. The functions of program theory. New Directions for Program Evaluation 1987;1987:5–18. 10.1002/ev.1443 [DOI] [Google Scholar]
- 22.Ryan AB. Post-positivist approaches to research [Researching and writing your thesis: a guide for postgraduate students]. 2006:12–26. [Google Scholar]
- 23.Denzin NK, Lincoln YS. The SAGE handbook of qualitative research: Sage, 2011. [Google Scholar]
- 24.Adami MF, Kiger A. The use of triangulation for completeness purposes. Nurse Res 2005;12:19–29. [DOI] [PubMed] [Google Scholar]
- 25.Africa SS. Mid-year population estimates. Statistics South Africa Pretoria, 2013. [Google Scholar]
- 26.Valliant R, Dever JA, Kreuter F. Practical tools for designing and weighting survey samples. Springer, 2013. [Google Scholar]
- 27.Wu G, Zaman MH. Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. Bull World Health Organ 2012;90:914–20. 10.2471/BLT.12.102780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burd EM. Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev 2010;23:550–76. 10.1128/CMR.00074-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner AK, Soumerai SB, Zhang F et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 30.Mashamba-Thompson TP, Sartorius B, Thabane L et al. Impact of point-of-care diagnostics on maternal outcomes in HIV-infected women: systematic review and meta-analysis protocol. BMJ Open 2016;6:e008002 10.1136/bmjopen-2015-008002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley Online Library, 2008. [Google Scholar]
- 33.Howell DC. The analysis of missing data. In: Outhwaite W, Turner S. Handbook of Social Science Methodology. London: Sage, 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-011155supp.pdf (801.9KB, pdf)