Abstract
CD8+ encephalitis (CD8+E) is an emerging and incompletely understood HIV-associated neurological syndrome, typically presenting as a steroid-responsive subacute encephalopathy with prominent white matter changes in patients with apparently well-controlled HIV infection. Some cases can be associated with the phenomenon of ‘viral escape’ (disproportionate replication within the cerebrospinal fluid), but the most important pathophysiology of CD8+E is thought to involve an attack on HIV-infected CD4+ lymphocytes by autoreactive CD8+ cells. We report a case of CD8+E where the initial positive response to steroid treatment was followed by several relapses on withdrawal. This led to the use of mycophenolate mofetil (MMF) as a long-term steroid-sparing agent, which is the first time this approach has been reported in the literature. The patient has now been on treatment with MMF for 10 months and it has been possible to taper the steroids down to a minimal maintenance dose without further relapse.
Background
CD8+ encephalitis (CD8+E) is increasingly being recognised as part of the spectrum of HIV-associated neurological complications. Untreated CD8+E is often associated with a fatal outcome. Our case highlights the importance of prompt recognition and treatment of CD8+E. We report a case of CD8+E in a young patient who continued to relapse on withdrawal of steroids. This is a first description of using mycophenolate mofetil (MMF) as a steroid-sparing agent to treat CD8+E. As the clinical phenotype of CD8+E expands, we might consider drugs such as MMF as an alternative form of immunosuppression to long-term use of steroids. There is a clinical presentation overlap between CD8+E and central nervous system (CNS) HIV viral escape, so we advocate looking for HIV viral resistance and switch combination antiretroviral therapy (cART) early to achieve better viral suppression in the CNS and prevent further relapses.
Case presentation
A 34-year-old woman was first identified to be infected with HIV in 2006 during an intensive care admission with severe colitis. At the time of diagnosis, CD4 count was 25/mm3 and plasma HIV viral load was 511 000 copies/mL (wild type on resistance testing). She started cART, achieving a good response within 16 months, the CD4 count rising to 226/mm3.
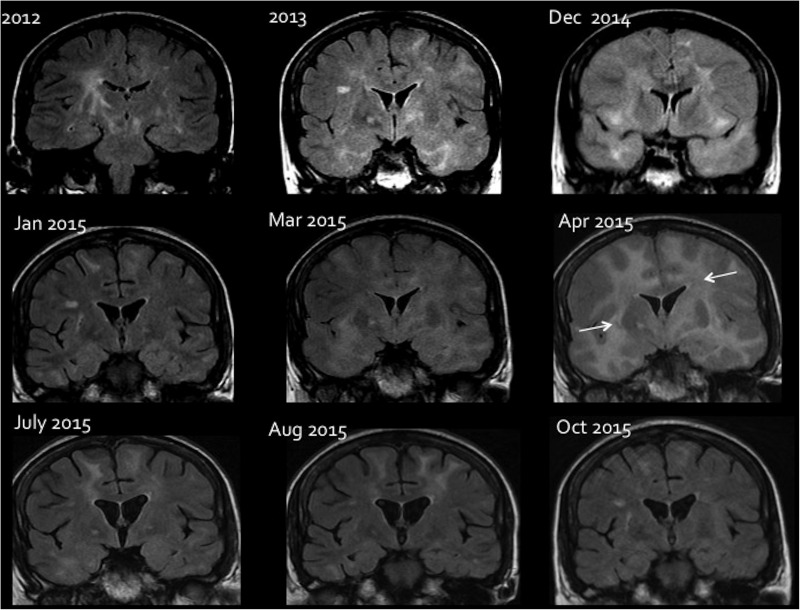
Between 2006 and 2014, she experienced repeated episodes of encephalopathy characterised by additional features of headache, ataxia and seizures, on each occasion preceded by a prodromal viral-type systemic illness. MRI showed bilateral diffuse non-enhancing white matter changes with mass effect (figure 1).
Figure 1.
Sequential MRI changes during treatment of CD8+E. Coronal FLAIR images showing the brain parenchymal changes and response to different treatment. In 2012, an abnormal high signal is seen in the basal ganglia bilaterally with signal change in the white matter in keeping with encephalitis. Image taken from 2013 shows a marked worsening of white matter signal change more extensive within the temporal lobes. In 2014, there is a bilateral white matter signal change with diffuse brain swelling and effacement of all cortical sulci. Following steroid initiation in December 2014, there is significant improvement in the signal change as well as mass effect on the January 2015 image. A worsening in March 2015 and a more pronounced deterioration in April 2015 (arrows), after reduction in steroids. A subsequent increase in steroids shows a remarkable improvement in July 2015. The patient was later started on mycophenolate mofetil in July 2015; this allowed a further amelioration of lesions as seen in August 2015 and October 2015, despite a tapering of steroids.
On each occasion, there was a convincing clinical and radiological response to high-dose steroid treatment. Extensive cerebrospinal fluid (CSF) investigations identified no evidence of opportunistic infection. Throughout this period, the patient had been adherent to cART. No evidence of resistance to this antiretroviral combination was identified on resistance mutation analysis of CSF-derived virus. After CSF and serum studies excluded common differential diagnoses of leucoencephalopathy in an HIV-positive individual (table 1), an initial presumptive diagnosis of HIV-related demyelination was made.
Table 1.
Possible differential diagnoses that were considered and discounted3
| Differential diagnosis | Exclusion by MRI evidence | Other tests |
|---|---|---|
| Toxoplasmosis | No focal enhancing parenchymal lesions | |
| Cryptococcus | No evidence of calcifications | Negative serum CRAG, negative India ink CSF stain |
| PML | The pattern of the white matter involvement atypical for PML, no abnormal DWI | JC-virus PCR in CSF/blood negative |
| Cerebral TB | No parenchymal focal or meningeal enhancement | Negative CSF TB cultures |
| Neurosyphilis | Unable to differentiate radiologically | Negative serum and CSF syphilis studies |
| HIV encephalitis HIV-associated vasculopathy IRIS |
Unable to differentiate radiologically Diffuse swelling and extensive parenchymal changes would be atypical for vasculopathy No focal enhancement |
No evidence of opportunistic infections |
| Primary cerebral lymphoma | No focal enhancing lesions | Negative CSF: Epstein-Barr PCR, normal cytology, CSF flow cytometry. stable CD4+ count |
| VZV vasculopathy | No evidence of vasculitis or stroke-like lesions | Negative CSF VZV PCR, no evidence of intrathecal VZV antibody synthesis |
CRAG, cryptococcal antigen; CSF, cerebrospinal fluid; DWI, diffusion weighted images; IRIS, immune reconstitution syndrome; PML, progressive multifocal leucoencephalopathy; TB, tuberculosis; VZV, varicella zoster virus.
In April 2015, following a prodromal viral gastroenteritic-type illness, she developed a more severe episode of encephalopathy including a period of status epilepticus. MRI demonstrated cerebral oedema and extensive diffuse white matter changes.
Her conscious level progressively dropped and she developed signs of raised intracranial pressure. On admission to a high dependency unit, she was treated with high-dose intravenous methylprednisolone and mannitol, and after 72 hours her clinical state had improved markedly.
Investigations
Given the history of relapsing steroid-responsive encephalitis, she was extensively investigated (tables 2 and 3).
Table 2.
Summary of blood-derived investigations in 2015
| Blood test | Results |
|---|---|
| Plasma HIV viral load | <40 copies/mL |
| White cell enzymes | Normal activity |
| MELAS/MERRF mutations | Negative |
| Notch 3 gene mutation for CADASIL | Negative |
| Anti-NMDAR/VGKC antibodies | Negative |
| Anti-TPO antibody | Negative |
| TSH | 0.13 mU/L (low) |
| p-ANCA/c-ANCA | Negative |
| Vasculitic screen including ENA, ANA, anti-dsDNA | Negative |
| Serum ACE | 38I U/L |
| Blood cultures | Nil growth |
| Cytomegalovirus serology | IgG positive, IgM negative |
| Epstein-Barr serology | IgG positive, IgM negative |
| JC/BK virus PCR | Negative |
CADASIL, cerebral autosomal-dominant arteriopathy with subcortical infarcts and leucoencephalopathy; dsDNA, double-stranded DNA; MELAS, mitochondrial encephalopathy, lactic acidosis and stroke-like episodes; MERRF, myoclonic epilepsy with ragged red fibres; NMDAR, N-methyl-D-aspartate receptor; TPO, thyroid peroxidase; VGKC, voltage gated potassium channel.
Table 3.
CSF and paired peripheral results from 2012 to 2015
| 2012 | 2014 | June 2015 | July 2015 | October 2015 | |
|---|---|---|---|---|---|
| Peripheral CD4+ count (cells/mm3) | 728 | 640 (39%) | NA | 531 (30%) | 1076 (35%) |
| Plasma HIV viral load (copies/mL) | NA | 189 | <40 | NA | 191 |
| CSF HIV load (copies/mL) | 1194 | 1188 | 3383 | NA | 724 |
| White cell count (cells/mm3) | 315 | 53 | NA | NA | 7 |
| Lymphocyte count (cells/mm3) | 310 | 50 | 5 | 20 | NA |
| CSF glucose (mmol/L) | 2.5 | NA | 2.3 | 2.8 | 2.9 |
| Plasma glucose (mmol/L) | 4.4 | NA | 4.2 | NA | 6.6 |
| CSF protein (g/L) | 1.1 | 0.89 | 0.5 | 0.69 | 0.54 |
| Bacterial culture | Negative | Negative | Negative | Negative | Negative |
| Flow cytometry | NA | NA | NA | CD4+:40% CD8+:47% |
CD4+:37% CD8+:55% |
| Viral PCRs (Epstein-Barr, cytomegalovirus, herpes simplex 1+2, varicella zoster, enterovirus, parechovirus) | Negative | Negative | Negative | NA | Negative (cytomegalovirus PCR NA) |
| JC virus PCR | Negative | Negative | NA | NA | Negative |
| CSF oligoclonal bands | CSF and serum weakly positive | 2 additional bands in CSF—suggestive of inflammatory response | CSF and serum positive—systemic IgG synthesis | NA | NA |
| Other tests | CSF India ink stain negative CSF TPPA, RPR negative |
CSF TPPA and RPR negative TB culture negative Serum CRAG negative |
CSF TPPA, RPR negative, syphilis PCR negative CSF ACE—1.28 μmol/min/L (mildly raised) |
Meningococcal PCR: negative No yeasts seen on culture TB culture negative CSF/intrathecal VZV IgG antibody not detected |
CRAG, cryptococcal antigen; CSF, cerebrospinal fluid; NA, not available; RPR, rapid plasma reagin test; TB, tuberculosis; TPPA, Treponema pallidum agglutination assay; VZV, varicella zoster virus.
With a background of possible thyroid disease, thyroid peroxidase antibodies were tested to exclude Hashimoto's encephalitis. No other autoimmune or infective cause was identified. The radiological appearances and history of migraine prompted testing for mitochondrial disorders, cerebral autosomal-dominant arteriopathy with subcortical infarcts and leucoencephalopathy and other causes (table 2).
Due to a raised intracranial pressure, lumbar puncture was deemed unsafe on admission. Five weeks post admission (while still on high-dose steroids), CSF was sent for investigations including flow cytometric studies, and demonstrated that 40% of the lymphocytes were CD4+ and 47% were CD8+ (table 3). The CSF viral load was detectable at 3383 copies/ml.
Varicella zoster virus (VZV) vasculopathy was initially considered in the differential diagnosis, but repeatedly negative CSF VZV PCR, no evidence of intrathecal VZV antibody synthesis and subsequent sustained response to steroids and MMF were very much against this chronic infection as the cause of the clinical syndrome.1 2
Treatment
With a history of multiple episodes of steroid-responsive encephalopathy with leucoencephalopathy, on each occasion responding dramatically to steroid treatment, and after extensive investigation to exclude alternative causes of the clinical syndrome, a clinical diagnosis of CD8+E was made. Although a definite diagnosis of this syndrome is only possible histologically, and is often only made at autopsy, the clinical picture in this case was considered convincing enough to avoid the significant risks of brain biopsy, and empirical treatment for CD8+E was considered appropriate. Although the clinical condition remained exquisitely steroid-sensitive, subsequent repeated attempts to reduce the dose of prednisolone below 20 mg daily led to a marked worsening of the ataxia, headache and encephalopathy. The patient began to develop increasingly intolerable side effects of long-term steroid therapy.
In this unusual situation, further treatment with a steroid-sparing immunosuppressant was considered. MMF was chosen due to its favourable CSF penetration, relatively benign side effect profile, absence of significant interaction with the patient's cART regimen and short half-life compared with some of the alternatives.
Outcome and follow-up
In the early stages of MMF treatment, while still on a low dose of 250 mg twice daily and during the relatively rapid withdrawal of prednisolone, the patient experienced a further relapse of her encephalopathy. During this relapse, the proportion of CD8+ lymphocytes in CSF increased to 55%. At this point, a new HIV resistance mutation (V28A) was identified in serum-derived virus. This prompted a change in cART to a regimen where the V28A mutation would not cause problems with ongoing resistance.
Since then, the patient has remained clinically well with no further clinical or radiological relapses. MMF has been tolerated without any side effects and, 10 months following initiation of treatment, it has been possible to reduce the dose of prednisolone down to a small maintenance dose of 5 mg daily. Plans are being made to complete the steroid withdrawal, followed by discontinuation of the MMF with ongoing close clinical, radiological and CSF surveillance.
Discussion
Although previous studies have demonstrated higher proportions of CD8+ lymphocytes in the CSF in patients with CD8+E, given the radiological appearances on MRI brain and clinical presentations, our patient was diagnosed with CD8+E.4 In addition, Ho et al5 found a higher proportion of CD8+ lymphocytes in the CSF of patients with HIV in comparison to healthy individuals.
Classical presenting features of CD8+E include headache, worsening confusion and seizures.4 Our patient exhibited most of these features during her numerous admissions, though slurred speech and ataxia seem to be much rarer clinical presentations of the syndrome.
CD8+E has similarities with the well-recognised ‘diffuse infiltrative lymphocytosis syndrome’ where CD8+ lymphocytes infiltrate the salivary glands, lung and sometimes peripheral nerves.6 The initial reports of CD8+E were uniformly severe and led to a fatal outcome, but subsequently the clinical spectrum has broadened to include milder cases of inflammatory leucoencephalopathy which respond favourably to steroid treatment.
In a case series of 14 patients with CD8+E, 9 patients had CSF flow cytometry, showing a percentage of CD8+ lymphocytes in the CSF of ∼65%.4 Most of these samples were taken during their initial acute presentation. However, our patient had her first CSF flow cytometry performed after almost 2 months of steroid therapy. Despite treatment with steroids and MMF, our patient had a higher percentage of CD8+ lymphocytes (55%) in the CSF in comparison with CD4+ lymphocytes (37%).
Our patient's viral load in the CSF falls within the range seen by Lescure et al4 (varying up to 36 242 copies/mL). This might suggest that, despite the relatively good peripheral control of HIV replication, the presence of detectable virus within the CNS will promote increased CD8+ lymphocyte infiltration in CD8+E.
Radiological hallmarks of CD8+E include diffuse high-intensity white matter signal and multiple punctate or linear lesions. Our patient demonstrated relapsing–remitting diffuse white matter changes on T2 MRI and cerebral oedema. Gray et al7 have shown diffuse CD8+ lymphocyte infiltration into perivascular spaces and within brain parenchyma. Brain biopsies on 10 of 14 patients studied showed that 2 had coexistent demyelination and evidence of histological inflammation.4
Lescure et al described four main triggers for CD8+E: viral escape, immune reconstitution syndrome (IRIS), another viral infection or an interruption of cART therapy. Our patient had no radiological or clinical evidence of IRIS and was compliant with cART. Although throughout her illness there were periodic ‘blips’ with rises in the plasma viral load, and during several of the episodes of encephalopathy HIV was detectable by PCR in the CSF, the virus in CSF and plasma remained sensitive to her cART until the first indication of a new viral resistance mutation arose in 2015, at which point the cART regimen was changed.
Steroid responsiveness appears to be characteristic in CD8+E.4 8 9 This patient responded well to early treatment with steroids and she repeatedly worsened after steroid discontinuation, developing a steroid-dependent neurological syndrome.
Prolonged use of steroids in a young HIV-positive patient presents difficulties with unwanted side effects and potential further immunosuppression. There is no clear guidance on the maintenance dose or recommended duration of steroid therapy, and no information available on the long-term management of patients who cannot be weaned off steroids. A trial of a steroid-sparing agent is a potential avenue that could be explored.
Preliminary studies have assessed MMF use in those with HIV and have shown additive antiviral benefits.10–12 MMF causes apoptosis of activated CD4+ lymphocytes in vitro with little effect on resting T cells and the lymphocytes of HIV-positive patients with uncontrolled viraemia are known to be sensitive to apoptosis.13 14 Initial studies have shown that combination of cART with MMF did not induce significant lymphocyte count suppression.10 11
CD8+E can be a life-threatening neurological complication of HIV that should be considered in the differential diagnosis of diffuse CNS white matter disease. Steroid treatment should be started promptly following the rapid exclusion of infections. In particular, VZV has a propensity for reactivation in immunocompromised individuals. VZV vasculopathy may need further investigation; additional studies with anti-VZV antibody IgG titres in serum and CSF should be considered, as they seem to be more sensitive than CSF VZV PCR. VZV vasculopathy carries significant mortality when untreated; the recommended treatment is with intravenous aciclovir. However, VZV vasculopathy tends to worsen with steroid treatment or further immunosuppression. Furthermore, the chronic relapsing nature of our patient's clinical picture is not typical of VZV vasculopathy.1 2
Viral escape as a main driver of the CD8+E should be considered early on in the disease course and even when there is no initial resistance noted, repeated viral resistance testing needs to be implemented as the early change of cART may help with the long-term management of CD8+E.
Learning points.
Include cerebrospinal (CSF) flow cytometry and CSF HIV viral load in HIV positive patients with neurological deterioration and diffuse white matter changes on imaging.
Explore possible triggers of CD8+E: viral illness, resistance to or interruption of combination antiretroviral therapy (cART), or immune reconstitution.
Start steroids promptly to prevent mortality/morbidity.
Look repeatedly for evidence of viral escape or cART resistance.
Further evidence for use of alternative steroid-sparing agents is required.
Footnotes
Contributors: SS was responsible for conception, writing of the article and revision of drafts. TM supervised the writing, edited the article and was the attending neurologist involved in care. DM supervised the writing and edited the article. AU contributed to conception, editing of the article and was the attending infectious diseases physician involved in care. RS interpreted the imaging and edited the radiological images used in the article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gilden D, Cohrs RJ, Mahalingam R et al. . Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2009;8:731–40. 10.1016/S1474-4422(09)70134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel MA. Varicella zoster virus vasculopathy: clinical features and pathogenesis. J Neurovirol 2014;20:157–63. 10.1007/s13365-013-0183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.http://radiopaedia.org/articles/white-matter-changes-in-hiv (September 2015).
- 4.Lescure FX, Moulignier A, Savatovsky J et al. . CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis 2013;57:101–8. 10.1093/cid/cit175 [DOI] [PubMed] [Google Scholar]
- 5.Ho EL, Ronquillo R, Altmeppen H et al. . Cellular composition of cerebrospinal fluid in HIV-1 infected and uninfected subjects. PLoS ONE 2013;8:e66188 10.1371/journal.pone.0066188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gherardi RK, Chrétien F, Delfau-Larue MH et al. . Neuropathy in diffuse infiltrative lymphocytosis syndrome: an HIV neuropathy, not a lymphoma. Neurology 1998;50:1041–4. 10.1212/WNL.50.4.1041 [DOI] [PubMed] [Google Scholar]
- 7.Gray F, Lescure FX, Adle-Biassette H et al. . Encephalitis with infiltration by CD8+ lymphocytes in HIV patients receiving combination antiretroviral treatment. Brain Pathol 2013;23:525–33. 10.1111/bpa.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulignier A, Savatovsky J, Polivka M et al. . CD8 T lymphocytes encephalitis mimicking brain tumor in HIV-1 infection. J Neurovirol 2013;19:606–9. 10.1007/s13365-013-0217-3 [DOI] [PubMed] [Google Scholar]
- 9.Moulignier A, Lescure FX, Savatovsky J et al. . CD8 transverse myelitis in a patient with HIV-1 infection. BMJ Case Rep 2014;2014:pii: bcr-2013-201073 10.1136/bcr-2013-201073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis DM, Kewn S, Coull JJ et al. . The addition of mycophenolate mofetil to antiretroviral therapy including abacavir is associated with depletion of intracellular deoxyguanosine triphosphate and a decrease in plasma HIV-1 RNA. J Acquir Immune Defic Syndr 2002;31:45–9. 10.1097/00126334-200209010-00006 [DOI] [PubMed] [Google Scholar]
- 11.Press N, Kimel G, Harris M et al. . Case series assessing the safety of mycophenolate as part of multidrug rescue treatment regimens. HIV Clin Trials 2002;3:17–20. 10.1310/B6T0-N98J-1J3M-EQPK [DOI] [PubMed] [Google Scholar]
- 12.Hossain MM, Coull JJ, Drusano GL et al. . Dose proportional inhibition of HIV-1 replication by mycophenolic acid and synergistic inhibition in combination with abacavir, didanosine, and tenofovir. Antiviral Res 2002;55:41–52. 10.1016/S0166-3542(02)00006-2 [DOI] [PubMed] [Google Scholar]
- 13.Coull JJ, Turner D, Melby T et al. . A pilot study of the use of mycophenolate mofetil as a component of therapy for multidrug-resistant HIV-1 infection. J Acquir Immune Defic Syndr 2001;26:423–34. 10.1097/00126334-200104150-00004 [DOI] [PubMed] [Google Scholar]
- 14.Cohn RG, Mirkovich A, Dunlap B et al. . Mycophenolic acid increases apoptosis, lysosomes and lipid droplets in human lymphoid and monocytic cell lines. Transplantation 1999;68:411–18. 10.1097/00007890-199908150-00014 [DOI] [PubMed] [Google Scholar]