Description
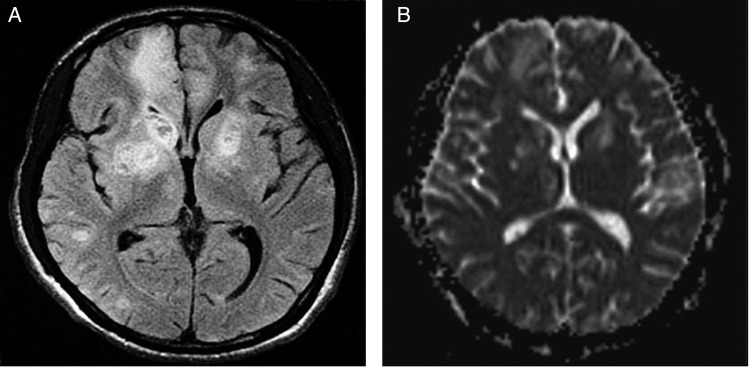
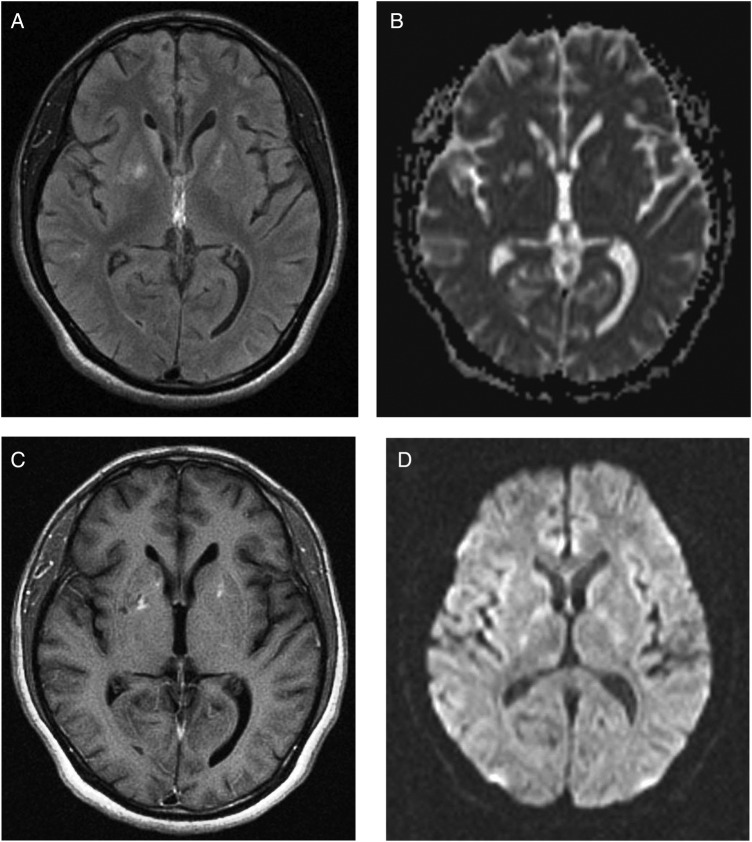
A previously well 22-year-old African man had cognitive decline for 1 month and involuntary movements for 10 days. Video in segment 1 showed right-sided choreoathetosis. Segment 2, day 6, after 5 days of treatment (table 1) demonstrated less choreoathetosis and brief dystonia of the right foot. Segment 3, on day 10 exhibited normality. The patient scored 5/30 on the Mini Mental State Examination (MMSE). Investigations showed HIV-AIDS associated with secondary syphilis and also a third condition, central nervous system (CNS) toxoplasmosis (table 2). MRI scan of the brain showed cystic and solid areas with hyperintensities and perilesional oedema in some areas on T2-weighted and fluid-attenuated inversion recovery sequences and low signal on T1-weighted imaging in the right parietal-temporal lobes and in the frontal regions, basal ganglia and thalamus bilaterally. The cystic lesions also demonstrated restricted diffusion on diffusion-weighted imaging/apparent diffusion coefficient-weighted images (figure 1A–D). Empirical antibiotic and antiretroviral treatment was, by the use of qualitative Bayesian probability, a necessity since consent for brain biopsy was declined.1 On highly active antiretroviral therapy, intravenous penicillin for the spirochate syphilis and the antiprotozoal drug, oral trimethoprim–sulfamethoxazole for cerebral toxoplasmosis, the patient gradually improved over the next 4 months, when the MMSE improved to 30/30 and the patient became fully ambulant. Repeat MRI (figure 2A–D), HIV viral load and CD4+ T cell count performed 12 weeks after treatment showed improvement. A significant fourfold rise in serum toxoplasmosis IgG confirmed cerebral toxoplasmosis (table 2).
Table 1.
Medical treatment
| Dosage | Period of treatment | |
|---|---|---|
| Intravenous drug | ||
| Trimethoprim–sulfamethoxazole | 160 mg/800 mg 3 times a day | 14 days |
| Fluconazole | 300 mg 2 times a day | 5 days |
| Aqueous crystalline penicillin | 4 million units every 4 hours | 10 days |
| Acyclovir | 800 mg 3 times a day | 14 |
| Haloperidol | 5 mg 3 times a day | 6 days |
| Intramuscular drug | ||
| Dexamethasone | 16 mg stat followed by 8 mg 3 times a day for 3 days and 4 mg 3 times a day for 3 days | 7 days |
| Oral drug | ||
| Lopinavir–ritonavir | 200/50 mg per tablet 2 tablets 2 times a day |
4 months |
| Tenofovir disoproxil-emtricitabine | 245/200 mg per tablet 1 tablet daily |
4 months |
| Paracetamol | 500 mg 3 times a day | 6 days |
| Pantoprazole | 40 mg 2 times a day | 6 days |
| Azithromycin | 500 mg daily for 1 week followed by 1 g weekly | 4 months |
| Trimethoprim–sulfamethoxazole | 80 mg/400 mg per tablet 2 tablets 2 times a day |
4 months |
| Fluconazole | 150 mg daily | 4 months |
| Carbamazepine | 200 mg 2 times a day | 6 days |
| Phenytoin | 100 mg 3 times a day | 4 months |
Table 2.
Medical investigations
| Blood test | Result | Reference range |
|---|---|---|
| White cell count | 18.4×109/L | 4.5–11.0×109/L |
| Venereal disease research laboratory on admission | Reactive | Non-reactive or reactive |
| Fluorescent Treponema pallidum antibody absorption on admission. | Positive | Positive or negative |
| Elisa for HIV | Reactive | Non-reactive or reactive |
| Western blot test for HIV | Positive | Positive or negative |
| HIV viral load on admission | 217 839 RNA copies/mL | 20–10 000 000 copies/mL |
| HIV viral load 1-month after admission | 48 477 RNA copies/mL | 20–10 000 000 copies/mL |
| CD4+ T cell count on admission | 3 cells/µL | 410–1590 cells/µL |
| CD4+ T cell count 4 months after admission | 121 cells/µL | 410–1590 cells/µL |
| Antistreptolysin O titer | 26 IU/mL | 0–200 IU/mL |
| T. gondii IgG antibodies on admission | 3.63 | Positive: >1.09 |
| T. gondii IgM antibodies on admission | 0.23 | Negative: <0.55 |
| T. gondii IgG antibodies 4 months after admission | >250 | Positive: >1.09 |
| T. gondii IgM antibodies 4 months after admission | 0.0 | Negative: <0.55 |
| Herpes virus 1 IgG antibodies | 5.00 | Index positive: >1.10 |
| Herpes virus 1 IgM antibodies | <0.9 | Index negative: <0.9 |
| Herpes virus 2 IgG antibodies | 1.30 | Index positive: >1.10 |
| Herpes virus 2 IgM antibodies | <0.9 | Index negative: <0.9 |
| Other tests | ||
| CSF test (lumbar puncture performed on day 3rd after admission) | ||
| Opening CSF pressure | 12 cm of H2O | 6–25 cm of water |
| Cell count | Nil | 0–5 cells/mm3 |
| Protein | 28.3 mg/dL | 5–40 mg/dL |
| Glucose | 60 mg/dL | 50–80 mg/dL |
| Culture | No bacterial growth | No bacterial growth or bacterial growth |
| Cytology | Negative for neoplastic cells | Negative or positive for neoplastic cells |
| India ink for Cryptococcus neoformans | Negative | Positive of negative |
| VDRL | No reactive | Non-reactive or reactive |
| FTA-ABS | Negative | Positive or negative |
| PCR for herpes virus, Cytomegalovirus, Epstein-Barr virus, Enterovirus, Parvovirus, Lymphocytic choriomeningitis virus and T. gondii | Tests not obtained | Negative or positive |
| Scalp EEG | Normal | Normal or abnormal |
| Brain/leptomeningeal biopsy | Consent was declined | |
CSF, cerebrospinal fluid; FTA-ABS, Fluorescent Treponema pallidum antibody absorption; T. gondii, Toxoplasma gondii; VDRL, Venereal Disease Research Laboratory test.
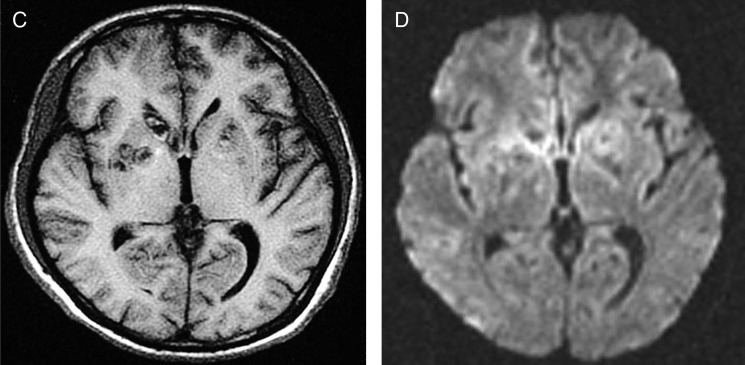
Figure 1.
(A) Axial T2-weighted fluid-attenuated inversion recovery MRI view with multiple abnormal hyperintense signals in basal ganglia and thalamus bilaterally, frontal regions and right parietal and temporal lobes suggestive of central nervous system (CNS) toxoplasmosis, but HIV encephalopathy and/or CNS lymphoma could not be ruled out. Some lesions showed mild perilesional oedema. (B) Axial diffusion-weighted imaging with restricted diffusion of the cystic lesions involving both cerebral hemispheres. (C) Axial T1-weighted MRI view with low signals located in basal ganglia and thalamus bilaterally, and in frontal regions and right parietal-temporal lobes. (D) Axial MRI apparent diffusion coefficient (ADC) map with reduced ADC values, which confirms restricted diffusion in the cystic lesions.
Figure 2.
(A) Axial T2-weighted fluid-attenuated inversion recovery MRI view with significant improvement of abnormal hyperintense signals and oedema throughout. Small abnormal hyperintense signals remain in basal ganglia bilaterally, and in frontal regions and right parietal-temporal lobes. (B) Axial diffusion-weighted imaging with restricted diffusion of the cystic lesions involving both cerebral hemispheres with significant improvement. (C) Axial T1-weighted MRI view with low signals located in basal ganglia and thalamus bilaterally, and in frontal regions, and right parietal and temporal lobes, with significant improvement. (D) Axial MRI apparent diffusion coefficient (ADC) map with reduced ADC values, which confirms restricted diffusion in the significantly decreased number of cystic lesions.
Figure 1.
Contined
Video 1.
Segment 1 showed right-sided choreoathetosis;Segment 2, day 6, after 5 days of treatment (table 1) demonstrated less choreoathetosis and brief dystonia of the right foot; Segment 3, on day 10 exhibited normality.
Damage to the thalamus, subthalamic areas, caudate and/or putamen nucleus and globus pallidus has been postulated as the pathogenic mechanism in movement disorders associated with HIV-AIDS.
To the best of our knowledge, serial imaging demonstrating movement disorders in a patient with HIV-AIDS of new onset, with positive serology for syphilis and confirmed CNS toxoplasmosis, has not been reported previously.2 3
Learning points.
HIV-AIDS related multiple neuropathology is a documented cause of abnormal movement disorders.
Damage to the thalamus, subthalamic areas, caudate and/or putamen nucleus, and globus pallidus has been postulated as the pathogenic mechanism in movement disorders associated with HIV-AIDS.
Constrained by the demand of patients for non-invasiveness, or due to unavailability of tests, aggressive empirical antibiotic and antiretroviral therapy is, by the use of Bayesian probability, a practical necessity that can prevent death and disability among patients with HIV-AIDS-related multiple neuropathology.
Acknowledgments
The authors would like to thank Dr Fidel Rampersad for assistance with the radiological aspects, Dr Shane Karim for preparation of the video and Ms Sharon Sealy for preparation of the images.
Footnotes
Contributors: AJR conceived the idea of this case report. All the authors contributed equally to preparation of the manuscript and approved the final contents.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Medow MA, Lucey CR. A qualitative approach to Bayes’ theorem. Evid Based Med 2011;16:163–7. 10.1136/ebm-2011-0007 [DOI] [PubMed] [Google Scholar]
- 2.Tse W, Cersosimo MG, Gracies JM et al. Movement disorders and AIDS: a review. Parkinsonism Relat Disord 2004;10:323–34. 10.1016/j.parkreldis.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Ramos JR, Factor SA, Weiner WJ et al. Hemichorea-hemiballismus associated with acquired immune deficiency syndrome and cerebral toxoplasmosis. Mov Disord 1989;4:266–73. 10.1002/mds.870040308 [DOI] [PubMed] [Google Scholar]