Abstract
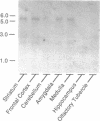
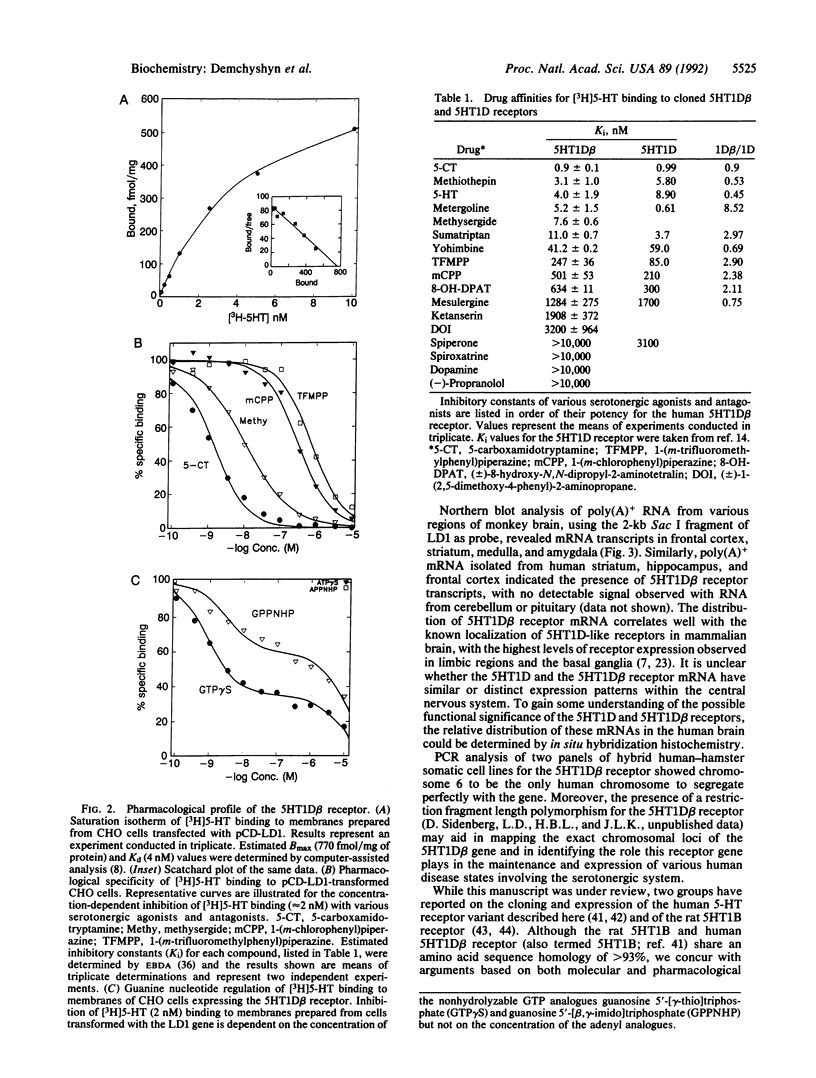
An intronless gene encoding a serotonin receptor (5HT1D beta) has been cloned and functionally expressed in mammalian fibroblast cultures. Based on the deduced amino acid sequence, the gene encodes a 390-amino acid protein displaying considerable homology, within putative transmembrane domains (approximately 75% identity) to the canine and human 5HT1D receptors. Membranes prepared from CHO cells stably expressing the receptor bound [3H]serotonin with high affinity (Kd 4 nM) and displayed a pharmacological profile consistent, but not identical, with that of the characterized serotonin 5HT1D receptor. Most notably, metergoline and serotonergic piperazine derivatives, as a group, display 3- to 8-fold lower affinity for the 5HT1D beta receptor than for the 5HT1D receptor, whereas both receptors display similar affinities for tryptamine derivatives, including the antimigraine drug sumatriptan. Northern blot analysis revealed an mRNA of approximately 5.5 kilobases expressed in human and monkey frontal cortex, medulla, striatum, hippocampus and amygdala but not in cerebellum, olfactory tubercle, and pituitary. The 5HT1D beta gene maps to human chromosome 6. The existence of multiple neuronal 5HT1D-like receptors may help account for some of the complexities associated with [3H]serotonin binding patterns in native membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adham N., Romanienko P., Hartig P., Weinshank R. L., Branchek T. The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1D beta receptor. Mol Pharmacol. 1992 Jan;41(1):1–7. [PubMed] [Google Scholar]
- Albert P. R., Zhou Q. Y., Van Tol H. H., Bunzow J. R., Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990 Apr 5;265(10):5825–5832. [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Regan J. W., Cotecchia S., Lefkowitz R. J., Caron M. G. Effector coupling mechanisms of the cloned 5-HT1A receptor. J Biol Chem. 1989 Sep 5;264(25):14848–14852. [PubMed] [Google Scholar]
- Fowler P. A., Thomas M., Lacey L. F., Andrew P., Dallas F. A. Early studies with the novel 5-HT 1-like agonist GR43175 in healthy volunteers. Cephalalgia. 1989;9 (Suppl 9):57–62. doi: 10.1111/J.1468-2982.1989.TB00074.X. [DOI] [PubMed] [Google Scholar]
- Hamblin M. W., Metcalf M. A. Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor. Mol Pharmacol. 1991 Aug;40(2):143–148. [PubMed] [Google Scholar]
- Hartig P., Kao H. T., Macchi M., Adham N., Zgombick J., Weinshank R., Branchek T. The molecular biology of serotonin receptors. An overview. Neuropsychopharmacology. 1990 Oct-Dec;3(5-6):335–347. [PubMed] [Google Scholar]
- Herrick-Davis K., Maisonneuve I. M., Titeler M. Postsynaptic localization and up-regulation of serotonin 5-HT1D receptors in rat brain. Brain Res. 1989 Mar 27;483(1):155–157. doi: 10.1016/0006-8993(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K., Titeler M. Detection and characterization of the serotonin 5-HT 1D receptor in rat and human brain. J Neurochem. 1988 May;50(5):1624–1631. doi: 10.1111/j.1471-4159.1988.tb03052.x. [DOI] [PubMed] [Google Scholar]
- Heuring R. E., Peroutka S. J. Characterization of a novel 3H-5-hydroxytryptamine binding site subtype in bovine brain membranes. J Neurosci. 1987 Mar;7(3):894–903. doi: 10.1523/JNEUROSCI.07-03-00894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D. Molecular pharmacology and biology of 5-HT1C receptors. Trends Pharmacol Sci. 1988 Mar;9(3):89–94. doi: 10.1016/0165-6147(88)90174-5. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Schoeffter P. 5-HT receptors: subtypes and second messengers. J Recept Res. 1991;11(1-4):197–214. doi: 10.3109/10799899109066399. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Schoeffter P., Waeber C., Palacios J. M. Serotonin 5-HT1D receptors. Ann N Y Acad Sci. 1990;600:168–182. doi: 10.1111/j.1749-6632.1990.tb16880.x. [DOI] [PubMed] [Google Scholar]
- Julius D., Huang K. N., Livelli T. J., Axel R., Jessell T. M. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A. 1990 Feb;87(3):928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Julius D. Molecular biology of serotonin receptors. Annu Rev Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Frielle T., Collins S., Yang-Feng T., Kobilka T. S., Francke U., Lefkowitz R. J., Caron M. G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987 Sep 3;329(6134):75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Leonhardt S., Herrick-Davis K., Titeler M. Detection of a novel serotonin receptor subtype (5-HT1E) in human brain: interaction with a GTP-binding protein. J Neurochem. 1989 Aug;53(2):465–471. doi: 10.1111/j.1471-4159.1989.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. A practical computer-based approach to the analysis of radioligand binding experiments. Comput Programs Biomed. 1983 Aug-Oct;17(1-2):107–113. doi: 10.1016/0010-468x(83)90031-4. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Nguyen T., Tirpak A., Jarvie K. R., Israel Y., Seeman P., Niznik H. B. Cloning of two additional catecholamine receptors from rat brain. FEBS Lett. 1990 Mar 12;262(1):8–12. doi: 10.1016/0014-5793(90)80140-e. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Waeber C., Hoyer D., Mengod G. Distribution of serotonin receptors. Ann N Y Acad Sci. 1990;600:36–52. doi: 10.1111/j.1749-6632.1990.tb16871.x. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. 5-Hydroxytryptamine receptor subtypes. Annu Rev Neurosci. 1988;11:45–60. doi: 10.1146/annurev.ne.11.030188.000401. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. Cortical and striatal variations in drug competition studies with putative 5-hydroxytryptamine1D binding sites. Brain Res. 1991 Jul 12;553(2):206–210. doi: 10.1016/0006-8993(91)90826-h. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol. 1979 Nov;16(3):687–699. [PubMed] [Google Scholar]
- Pritchett D. B., Bach A. W., Wozny M., Taleb O., Dal Toso R., Shih J. C., Seeburg P. H. Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO J. 1988 Dec 20;7(13):4135–4140. doi: 10.1002/j.1460-2075.1988.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J. R., Fargin A., Middleton J. P., Graff J. M., Haupt D. M., Caron M. G., Lefkowitz R. J., Dennis V. W. The human 5-HT1A receptor expressed in HeLa cells stimulates sodium-dependent phosphate uptake via protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21943–21950. [PubMed] [Google Scholar]
- Schmidt A. W., Peroutka S. J. 5-Hydroxytryptamine receptor "families". FASEB J. 1989 Sep;3(11):2242–2249. doi: 10.1096/fasebj.3.11.2673898. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Waeber C., Palacios J. M., Hoyer D. The 5-hydroxytryptamine 5-HT1D receptor subtype is negatively coupled to adenylate cyclase in calf substantia nigra. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):602–608. doi: 10.1007/BF00175784. [DOI] [PubMed] [Google Scholar]
- Sunahara R. K., Guan H. C., O'Dowd B. F., Seeman P., Laurier L. G., Ng G., George S. R., Torchia J., Van Tol H. H., Niznik H. B. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991 Apr 18;350(6319):614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991 Apr 18;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Voigt M. M., Laurie D. J., Seeburg P. H., Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J. 1991 Dec;10(13):4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C., Schoeffter P., Palacios J. M., Hoyer D. Molecular pharmacology of 5-HT1D recognition sites: radioligand binding studies in human, pig and calf brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):595–601. doi: 10.1007/BF00175783. [DOI] [PubMed] [Google Scholar]
- Weinshank R. L., Zgombick J. M., Macchi M. J., Branchek T. A., Hartig P. R. Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1D alpha and 5-HT1D beta. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3630–3634. doi: 10.1073/pnas.89.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witz P., Amlaiky N., Plassat J. L., Maroteaux L., Borrelli E., Hen R. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8940–8944. doi: 10.1073/pnas.87.22.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]