Abstract
A 70-year-old Caucasian woman was treated for Capnocytophaga canimorsus septicaemia. The source of bacteraemia was very likely to be her household pet, an Italian greyhound. The patient presented with a presumed complex partial seizure but deteriorated rapidly with sepsis and multiorgan dysfunction. Neither scratch nor bite was established, although close petting including licks was reported. Blood cultures grew Gram-negative rods, identified by molecular techniques as C. canimorsus—a bacterium frequently isolated in the oral cavities of dogs and cats. A full recovery was made following 2 weeks of intensive care support and broad-spectrum antibiotics. No underlying immune dysfunction was found.
Background
Capnocytophaga canimorsus is a rare yet significant cause of fulminant sepsis typically following a dog bite. Unusually, in this case, there was no evidence of inoculation, with neither bite nor scratch mark described or identified clinically.
This report highlights that infection can occur without overt scratch or bite injuries. It also reminds us that the elderly are at higher risk of infection, perhaps due to age-related immune dysfunction and increasing pet ownership. Microbiological identification of C. canimorsus is challenging, however, clinical suspicion can facilitate identification with optimal culture techniques.
Pet-related zoonoses are frequently missed diagnoses; we conclude with a table summarising the important infections transmitted by cats and dogs.
Case presentation
A 70-year-old Caucasian woman developed slurred speech and became unresponsive while on the telephone to a relative. Paramedics discovered the patient slumped in a chair, with decreased consciousness: Glasgow Coma Scale 7/15 (E1V1M5). A full recovery was made over the following 30 min en route to hospital.
On questioning, the patient's last recollection was the phone ringing. She denied ongoing symptoms but recalled a moderate intensity occipital headache the previous night and feeling generally fatigued. Her medical history comprised of transient ischaemic attacks (TIAs), epilepsy and asthma. The epilepsy was well controlled with sodium valproate. She was a non-smoker and rarely drank alcohol. She lived independently with her dog, an Italian greyhound.
Examination on arrival was unremarkable with no evidence of bites, scratch marks, broken skin or cellulitis. The patient was alert, afebrile, haemodynamically stable and displayed no evidence of meningism. Initial investigations revealed a profound hyponatraemia (121 mmol/L) and mild hyperkalaemia (5.6 mmol/L) with normal renal function. The hyperkalaemia resolved within 24 hours. Chest X-ray, urine dipstick, blood glucose and full blood count were normal. Inflammatory markers were not significantly elevated (C reactive protein (CRP) 11 mg/dL, white cell count 8.4×109/L). Further investigations for hyponatraemia suggested hypothyroidism as a contributing factor (thyroid-stimulating hormone 6.0 mU/L, free T4 4.0 μg/dL, random serum cortisol 439 nmol/L, urine osmolality 380 mOsm/L, serum osmolality 264 mOsm/L, urinary sodium 138 mmol/L). Unenhanced CT of the head demonstrated no acute intracranial pathology. The patient was admitted under the medicine for the elderly team with a provisional diagnosis of a partial complex seizure secondary to hyponatraemia on a background of epilepsy. She was managed with fluid restriction, thyroid hormone replacement, and daily monitoring of serum electrolytes and renal function. Sodium valproate was considered a potential contributor to the hyponatraemia and was held on admission and phenytoin started in its place.
On day 4 of admission, the patient developed new onset confusion, headache, diarrhoea and rigors, with a fever of 39.0°C. Biochemistry demonstrated an acute kidney injury with rising inflammatory markers (CRP 200 mg/dL) and a metabolic acidosis (lactate 5.8 mmol/L, base excess −11 mEq/L). Blood cultures were drawn and she was moved to intensive care, and started on intravenous cefuroxime and metronidazole for empirical treatment of severe sepsis of unknown origin.
On day 5, very thin Gram-negative rods were identified in aerobic and anaerobic blood culture bottles. The patient was subsequently switched to piperacillin/tazobactam and teicoplanin. On day 6, a second anaerobic blood culture bottle also grew Gram-negative rods and the teicoplanin was stopped. The organisms grew exceedingly slowly requiring specialist subculture delaying identification.
Meanwhile, urine culture, full stool culture, transthoracic echocardiogram and CT chest/abdomen/pelvis failed to demonstrate a clear source of infection. The admission was further complicated by disseminated intravascular coagulation, deranged liver function and respiratory failure requiring non-invasive ventilation.
Blood culture samples were sent to a reference laboratory, where ribosomal DNA amplification of subculture colonies confirmed the presence of C. canimorsus.
Outcome and follow-up
After 4 days of intravenous antibiotics, the patient started improving clinically and biochemically, with less confusion and decreasing inflammatory markers. She was stepped down from intensive care after 10 days and discharged from hospital following a total inpatient stay of 30 days. Her sodium level remained persistently low during admission and was 132 mmol/L on discharge. She was discharged with thyroid hormone replacement, levetiracetam and phenytoin, with a plan to wean the phenytoin in the outpatient setting. She remains well and symptom free 1 year following discharge.
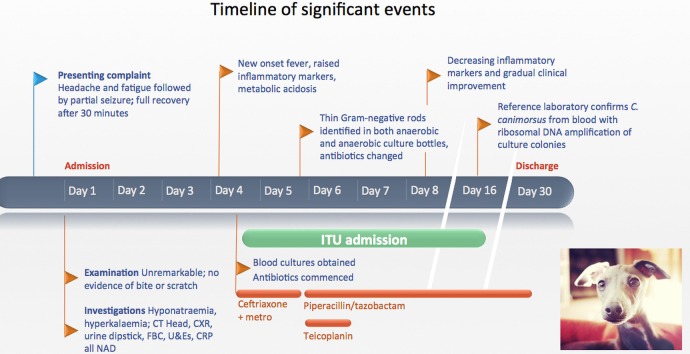
A full timeline of events is illustrated in figure 1.
Figure 1.
Timeline summary of events. Photograph credit: Italian greyhound by Kaleb Coleman, licensed under Creative Common BY 2.0 (http://creativecommons.org/licenses/by/2.0/). C. canimorsus, Capnocytophaga canimorsus; CRP, C reactive protein; CXR, chest X-ray; FBC, full blood count; ITU, intensive therapy unit; metro, metronidazole; NAD, no abnormality detected; U&Es, urea and electrolytes.
Discussion
First described in 1976,1 C. canimorsus is a Gram-negative rod and facultative anaerobe. It is one of several Capnocytophaga species inhabiting the oral cavities of dogs and cats, and a rare yet emerging cause of fulminant sepsis, with 13 cases reported in the UK since 1990.2 A recent review reports a mortality rate of 26%, with 60% of cases reporting a dog bite and 24% reporting other dog contact, typically scratches. Transmission has been reported from licks.3 4 The median time from exposure to sepsis is 3 days (range 1–8 days).2 5
Although most dog bites occur in children and young adults, C. canimorsus sepsis is reported across all age groups. The elderly are disproportionately susceptible to infection, which has been attributed to age-related immune dysfunction and increasing pet ownership. In all age groups, patients with asplenism and alcoholism are at higher risk of developing sepsis.2 6
Common presentations include sepsis associated with shock, disseminated intravascular coagulation and peripheral gangrene. Meningitis, endocarditis and abdominal symptoms including pain and diarrhoea have also been reported. In our case, we were unable to reliably exclude meningitis as cerebrospinal fluid analysis was not performed.
When associated with bites, the wounds may appear benign and may not cause concern to the patient. Wound site cultures seldom identify the bacterium. Blood culture is hindered by the organism's fastidious nature, however, molecular techniques are increasingly available for diagnosis.7
No consensus exists on optimal antibiotic choice or treatment duration. Owing to the slow-growing nature of the organism and difficulty in obtaining sensitivities, treatment is frequently empirical and guided by the identification of Gram-negative fusiform rods on microscopy. In vitro studies report susceptibility to a range of broad-spectrum antibiotics, including β-lactamase inhibitor combinations and third-generation cephalosporins.8
We have described a case of severe sepsis in an elderly patient caused by an uncommon household zoonosis. The occult means of acquisition demonstrates that severe zoonosis may occur in the absence of obvious bites or scratches and should always be considered in cases of severe sepsis in pet-owning elderly patients.
Table 1 reviews the important infections caused by bites, scratches and licks from dogs and cats.
Table 1.
Important infections caused by bites, scratches and licks from dogs and cats
| Disease | Aetiology | Transmission | Incidence | Severity | Presentation |
|---|---|---|---|---|---|
| Rabies | Lyssavirus spp. in saliva | Dog bites in developing world, bat bites in developed world. Rarely cats | Extremely rare in developed world | Near 100% mortality | 1–3-month incubation period followed by flu-like illness, hyperactivity, hydrophobia and reduced consciousness |
| Capnocytophaga sepsis | C. canimorsus in saliva | Dogs >cats saliva | Rare | Severe; 30% mortality | Fulminant sepsis. Occasionally meningitis and endocarditis. Rarely wound infection |
| Gram-positive/anaerobic infection | Various, including staph species, strep species and anaerobes | Contamination of wound by skin flora | Uncommon | Variable | Frequently self-limiting localised cellulitis, occasionally systemic sepsis, ie, MRSA, anaerobic sepsis in the immunocompromised |
| Pasteurellosis | Pasteurella spp. in saliva | Dog or cat bites, scratches or licks, often occult | Common: most frequent isolate from bites | Mild to moderate | Rapid-onset cellulitis, septic arthritis proximal to wound, osteomyelitis, occasionally pneumonia and severe sepsis |
| Brucellosis | Brucella spp (B. canis from dog saliva) | Exposure to body fluids from domestic animals (especially farm settings), consumption of unpasteurised dairy products | Rare in developed world, most common zoonosis worldwide | Mild | 1–4-week incubation; broad range of symptoms ranging from asymptomatic to fever, arthralgia and severe sepsis |
| Cat scratch disease | Bartonella henselae in saliva | Cat bites, scratches and licks. Occasionally flea bites | Common, especially in children and young adults | Mild | Inoculation site lesion followed by regional lymphadenopathy; occasionally disseminated disease |
MRSA, methicillin-resistant staphylococcus aureus; Staph, staphylococcus; Strep, streptococcus.
Learning points.
Capnocytophaga canimorsus is a rare but significant cause of fulminant sepsis in pet owners.
Identification of C. canimorsus is facilitated by clinical suspicion and requires close collaboration with microbiology colleagues.
Infection may occur following close contact with a dog and does not require overt scratch or bite injuries.
Increased pet ownership and age-related immune dysfunction are thought to confer higher risk in the elderly.
Bacterial zoonoses from common household pets are frequently missed diagnoses.
Footnotes
Twitter: Follow James Wilson at @jamesemaj
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bobo RA, Newton EJ. A previously undescribed Gram-negative bacillus causing septicemia and meningitis. Am J Clin Pathol 1976;65:564–9. 10.1093/ajcp/65.4.564 [DOI] [PubMed] [Google Scholar]
- 2.Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015;34:1271–80. 10.1007/s10096-015-2360-7 [DOI] [PubMed] [Google Scholar]
- 3.Low SC, Greenwood JE. Capnocytophaga canimorsus: infection, septicaemia, recovery and reconstruction. J Med Microbiol 2008;57(Pt 7):901–3. 10.1099/jmm.0.47756-0 [DOI] [PubMed] [Google Scholar]
- 4.Valtonen M, Lauhio A, Carlson P et al. Capnocytophaga canimorsus septicemia: fifth report of a cat-associated infection and five other cases. Eur J Clin Microbiol Infect Dis 1995;14:520–3. 10.1007/BF02113430 [DOI] [PubMed] [Google Scholar]
- 5.Popiel KY, Vinh DC. ‘Bobo-Newton syndrome’: an unwanted gift from man's best friend. Can J Infect Dis Med Microbiol 2013;24:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overall KL, Love M. Dog bites to humans—demography, epidemiology, injury, and risk. J Am Vet Med Assoc 2001;218:1923–34. 10.2460/javma.2001.218.1923 [DOI] [PubMed] [Google Scholar]
- 7.Woo PC, Ng KH, Lau SK et al. Usefulness of the MicroSeq 500 16S ribosomal DNA-based bacterial identification system for identification of clinically significant bacterial isolates with ambiguous biochemical profiles. J Clin Microbiol 2003;41:1996–2001. 10.1128/JCM.41.5.1996-2001.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jolivet-Gougeon A, Sixou JL, Tamanai-Shacoori Z et al. Antimicrobial treatment of Capnocytophaga infections. Int J Antimicrob Agents 2007;29:367–73. 10.1016/j.ijantimicag.2006.10.005 [DOI] [PubMed] [Google Scholar]