Abstract
Previous investigations on photosynthesis have been performed on leaves irradiated from the adaxial surface. However, leaves usually sway because of wind. This action results in the alternating exposure of both the adaxial and abaxial surfaces to bright sunlight. To simulate adaxial and abaxial surfaces alternant irradiation (ad-ab-alt irradiation), the adaxial or abaxial surface of leaves were exposed to light regimes that fluctuated between 100 and 1,000 μmol m−2 s−1. Compared with constant adaxial irradiation, simulated ad-ab-alt irradiation suppressed net photosynthetic rate (Pn) and transpiration (E) but not water use efficiency. These suppressions were aggravated by an increase in alternant frequency of the light intensity. When leaves were transferred from constant light to simulated ad-ab-alt irradiation, the maximum Pn and E during the high light period decreased, but the rate of photosynthetic induction during this period remained constant. The sensitivity of photosynthetic gas exchange to simulated ad-ab-alt irradiation was lower on abaxial surface than adaxial surface. Under simulated ad-ab-alt irradiation, higher Pn and E were measured on abaxial surface compared with adaxial surface. Therefore, bifacial leaves can fix more carbon than leaves with two “sun-leaf-like” surfaces under ad-ab-alt irradiation. Photosynthetic research should be conducted under dynamic conditions that better mimic nature.
Bifacial leaves are heterogeneous. They have two distinct surfaces: the adaxial surface and the abaxial surface. It was widely believed that the adaxial surface is the main contributor to carbon gain because of its higher photosynthetic capacity and the preferential irradiation of the adaxial surface1,2. The adaxial surface intercepts light more efficiently than the abaxial surface because the palisade mesophyll (PM) cells of the adaxial surface are packed more closely than the spongy mesophyll (SM) cells near the abaxial surface3. The highest photosynthetic rates are observed in the middle and lower palisade layers4,5,6, which have higher electron transport activity and more photosynthetic proteins7,8. The adaxial surface also has a stronger photoprotection mechanism and an increased resistance to photoinhibition9. The adaxial surface is considered to have sun-leaf-like characteristics and the abaxial surface is considered to have shade-leaf-like characteristics10.
In addition, it is often empirically believed that the adaxial surface is exposed to more direct radiation, whereas the abaxial surface is shaded by the leaf itself and receives weaker light that is transmitted through the mesophyll and reflected from the surroundings1. However, leaves usually sway because of external forces such as wind. As a result, the adaxial and abaxial surfaces are alternately exposed to bright sunlight (Fig. 1). This phenomenon is more prevalent in tall trees and plants in windy areas11.
Figure 1.
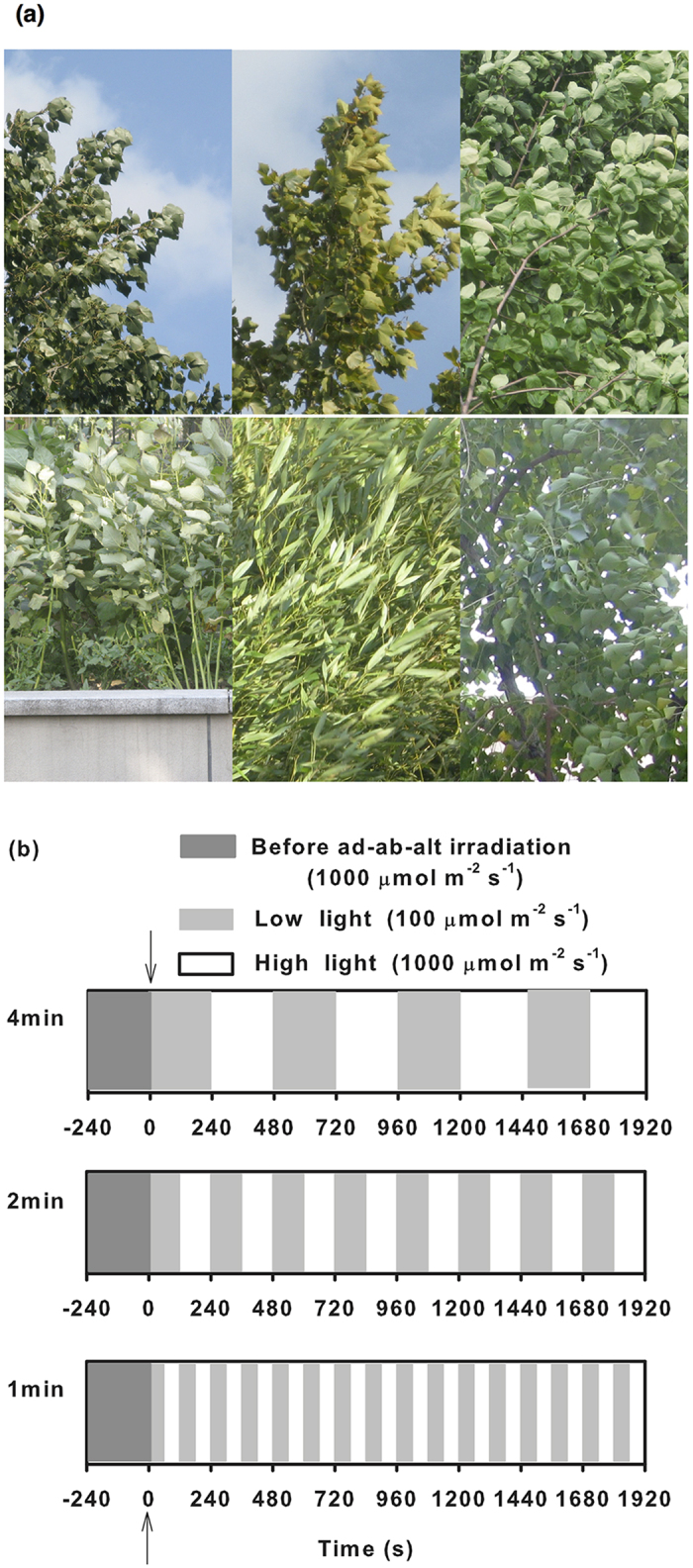
Photographs showing the abaxial surface exposed to the sun (a) and a sketch map of the fluctuating light regimes used in this study (b). In plot (a), the species in the top row are Populus ussuriensis Kom., Platanus orientalis, and Magnolia denudata Desr. (from left to right), and the species in the bottom row are Agastache rugosa, Bambusoideae species and Populus cathayana Rehd. (from left to right). In plot (b), the leaves were adapted under high light (1,000 μmol m−2 s−1) for 20–40 min until Pn and E stabilized (dark grey). The leaves were exposed to ad-ab-alt irradiation at 0 s (marked with an arrowhead). During the simulated ad-ab-alt irradiation, the irradiation was switched between high light (1,000 μmol m−2 s−1) and low light (100 μmol m−2 s−1) every 240 s, 120 s or 60 s. The high light periods (white segments) were used to calculate the integrated gas exchange.
Consequently, to understand photosynthesis under natural conditions, we need to understand photosynthesis under adaxial and abaxial surfaces alternant irradiation conditions (ad-ab-alt irradiation). Unfortunately, previous studies examining the photosynthetic performance of leaves were performed using adaxial surface irradiation12,13,14,15,16,17. Less attention has been paid to the photosynthetic performance of leaves under abaxial surface irradiation conditions9,18, and no attention has been given to investigating the photosynthetic gas exchange of leaves under ad-ab-alt irradiation.
Most of the photosynthetically active radiation (PAR, 400-700 nm; over 90% blue light and 70% red light) is absorbed by the surface facing the incident light. Only green light that has low efficiency in photosynthesis can be received by the other surface19,20. Therefore, both the adaxial surface and the abaxial surface of swaying leaves experience fluctuating light conditions between high and low light, similar to a sunfleck. It has been proved that the photosynthetic performance of leaves under sunfleck conditions and constant irradiation are different21,22. The deactivation of Rubisco23 and stomatal closure24,25 under low light will result in the depression of photosynthetic gas exchange during the following high light sunfleck period. We propose that photosynthetic gas exchange, such as CO2 fixation and transpiration, should be repressed with ad-ab-alt irradiation. In addition, due to the different protein and pigment distributions between the adaxial and abaxial surfaces5,8, we expect differential responses to ad-ab-alt irradiation between the adaxial and abaxial surfaces of an individual leaf.
To test these hypotheses, we examined dynamic gas exchange in the leaves of two trees (Platanus orientalis L. and Melia azedarach L.) and one herb (Solanum lycopersicum L.) under simulated ad-ab-alt irradiation or constant irradiation.
Results
Photosynthetic gas exchange and absorptance of leaves under constant light
The steady-state photosynthetic gas exchange of the leaves was measured when the adaxial or abaxial surface was exposed to constant high light (1,000 μmol m−2 s−1; ad-con irradiation or ab-con irradiation). The steady-state net photosynthetic rate (PnS), transpiration rate (ES) and water use efficiency (WUES) of the three species under ad-con irradiation were significantly higher than under ab-con irradiation. In Solanum lycopersicum L. and Platanus orientalis L. leaves, the ES was similar under ad- and ab-con irradiation (Supplementary Fig. S1). These results support the hypothesis that the adaxial and abaxial surfaces of bifacial leaves demonstrate the respective characteristics of sun and shade leaves3,26,27. The PAR absorptance of the leaves was similar under ad- and ab-con irradiation (Supplementary Fig. S1). This finding suggests that the differences in photosynthetic gas exchange under adaxial or abaxial surface irradiation were independent of the absorptance.
Gas exchange of leaves under simulated ad-ab-alt irradiation
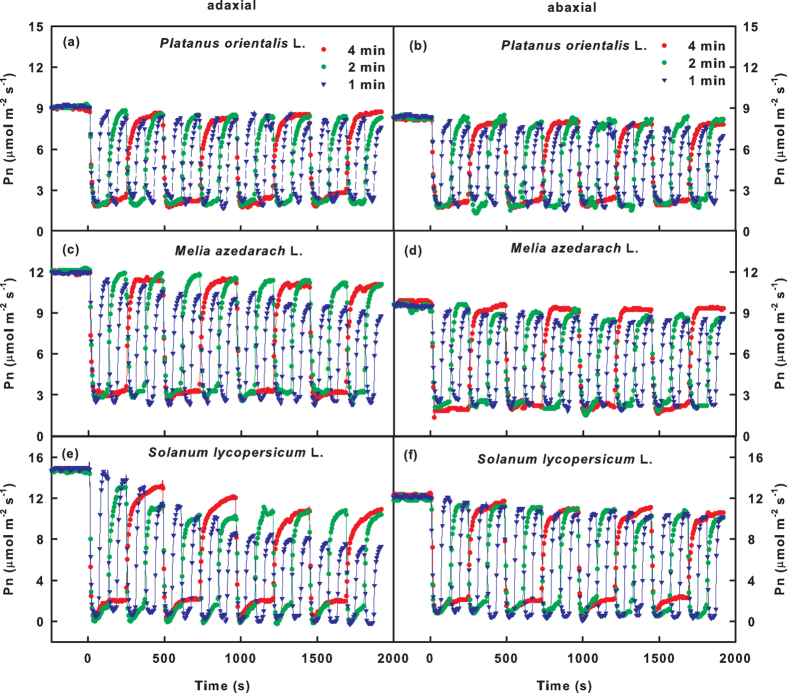
To simulate the ad-ab-alt irradiation, the adaxial or abaxial surface was irradiated by fluctuating light (Fig. 1). Under the fluctuating light condition, low light (100 μmol m−2 s−1) and high light (1,000 μmol m−2 s−1) were alternated every 60 s, 120 s or 240 s (low, medium or high frequency fluctuating light, respectively; Fig. 1). The representative traces of dynamic gas exchange in the leaves of the three species are shown in Fig. 2 and Supplementary Fig. S2.
Figure 2.
The time course of the net photosynthetic rates (Pn) of leaves of Platanus orientalis L. (a,b), Melia azedarach L. (c,d) and Solanum lycopersicum L. (e,f) when the adaxial (a,c,e) or abaxial surface (b,d,f) was irradiated under fluctuating light. The irradiation was switched between high light (1,000 μmol m−2 s−1) and low light (100 μmol m−2 s−1) every 240 s, 120 s or 60 s.
When the light intensity was switched from high light to low light, Pn immediately decreased by 80–95% and slightly increased in the following 2–3-min time frame (Fig. 2). This occurred because in plants that are irradiated under photorespiratory conditions are shifted to low light or darkness, CO2 fixation is immediately attenuated or stops; however, the CO2 released in photorespiration continues until all of the metabolic intermediates in the pathway are used28.
When the light intensity was switched from low light to high light, the Pn could not recover immediately. It gradually increased and stabilized within 1–2 min (Fig. 2). In Platanus orientalis L. leaves, the maximum Pn during the high light period (Pnmax) was similar to the PnS and was maintained at a constant level under fluctuating light (Fig. 2, Supplementary Fig. S3). In Melia azedarach L. leaves, the Pnmax was similar to the PnS under the low and medium light fluctuation frequencies (the light intensity changed every 4 min or 2 min, respectively), whereas the Pnmax decreased gradually under the high light fluctuation frequency (the light intensity changed every 1 min). Solanum lycopersicum L. leaves were more sensitive to fluctuating light than the leaves of the other species. The Pnmax of Solanum lycopersicum L. leaves decreased under fluctuating light, and the decrease was more severe under the high frequency fluctuating light. The minimum Pn during the high light period (Pnmin) remained constant under fluctuating light for all species (Fig. 2, Supplementary Fig. S3).
To our surprise, the decrease in the Pnmax under fluctuating light was only observed for the leaves whose adaxial surface was irradiated. The Pnmax of leaves that received abaxial surface irradiation remained constant under fluctuating light (Fig. 2, Supplementary Fig. S3). This result was independent of species and the frequency of the fluctuating light.
Transpiration (E) was similar to Pn for the leaves under fluctuating light. However, E decreased more slowly than Pn during the low light period (Supplementary Fig. S2). Both the maximum and minimum E during the high light period (Emax, Emin) remained constant in Platanus orientalis L. leaves. These values decreased gradually in Melia azedarach L. and Solanum lycopersicum L. leaves (Supplementary Figs S2 and S3). The reductions in the Emax and Emin were aggravated with the increased frequency of fluctuating light. Reductions in the Emax and Emin were observed under both abaxial and abaxial surface irradiation, but the reductions were much lower under abaxial surface irradiation (Supplementary Figs S2 and S3).
The WUE dramatically decreased during the low light period and increased during the high light period because E changed less and at a slower rate than Pn when the light intensity changed (Supplementary Fig. S2). In contrast to the behaviour of Pnmax and Emax, the WUEmax remained constant or increased under fluctuating light (Supplementary Figs S2 and S3). The WUEmin also remained constant under fluctuating light for all leaves.
Integrated gas exchange under simulated adaxial and abaxial surfaces alternant irradiation
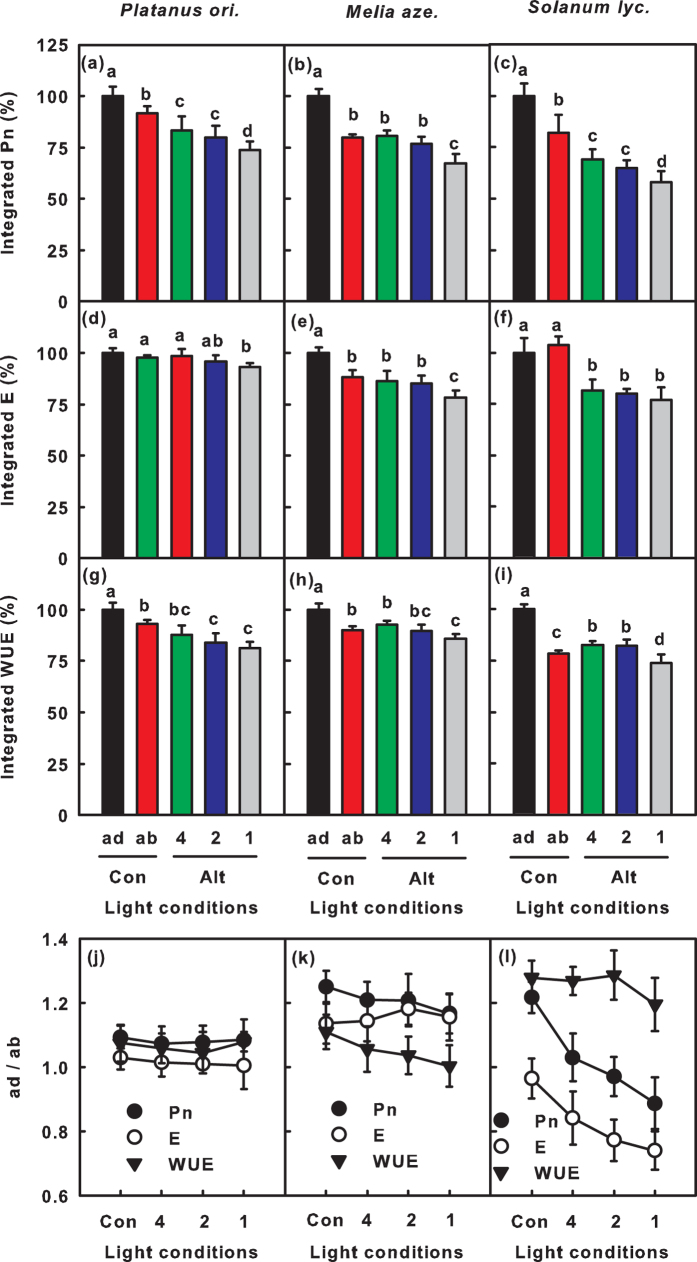
Integrated whole leaf gas exchange (the calculation method was showed in Methods) under simulated ad-ab-alt irradiation was compared to that resulting from ad-con and ab-con irradiation. The integrated Pn, E and WUE values under simulated ad-ab-alt irradiation were lower than those resulting from ad-con and ab-con irradiation (Fig. 3). This difference increased with an increase in fluctuation frequency. In Solanum lycopersicum L. leaves, the integrated Pn and E under high frequency fluctuating light were 42% and 23% lower than those under ad-con irradiation and were 30% and 26% lower than those under ab-con irradiation (Fig. 3).
Figure 3. Integrated gas exchange of the whole leaves and the ratio of integrated parameters for the adaxial and abaxial surfaces.
(a–i) The integrated net photosynthetic rate (Pn, a–c), transpiration rate (E, d–f) and water use efficiency (WUE; g–i) of Platanus orientalis L. (a,d,g), Melia azedarach L. (b,e,h) and Solanum lycopersicum L. (c,f,i) under constant irradiation (1,000 μmol m−2 s−1) or simulated ad-ab-alt irradiation. (j–l) The ratio of the integrated parameter of the adaxial and abaxial surfaces. To simulate alternant irradiation, the adaxial or abaxial surface was irradiated under fluctuating light. The irradiation was switched between high and low light every 4 min, 2 min or 1 min. To calculate the integrated gas exchange under simulated ad-ab-alt irradiation, the values measured during the high light periods of fluctuating light under abaxial and adaxial surface irradiation were summed. The integrated gas exchange under constant irradiation was 100%, whereas the integrated values under simulated ad-ab-alt irradiation were expressed as a percentage of the former value. The reported values are the mean (±SE), n = 5.
We also compared the contribution of the adaxial and abaxial surfaces to the gas exchange (ad/ab ratio) under simulated ad-ab-alt irradiation. The ratio between the gas exchange under ad-con and ab-con irradiation served as the control. The ad/ab ratios of gas exchange under simulated ad-ab-alt irradiation and constant irradiation were similar in Platanus orientalis L. leaves (Fig. 3). However, the ad/ab ratios of Pn and WUE in Melia azedarach L. leaves as well as the ad/ab ratios of Pn and E in Solanum lycopersicum L. leaves under simulated ad-ab-alt irradiation were lower than those under constant irradiation and showed a gradual decrease with an increase in fluctuation frequency (Fig. 3).
Gas exchange induction phase of leaves under high light
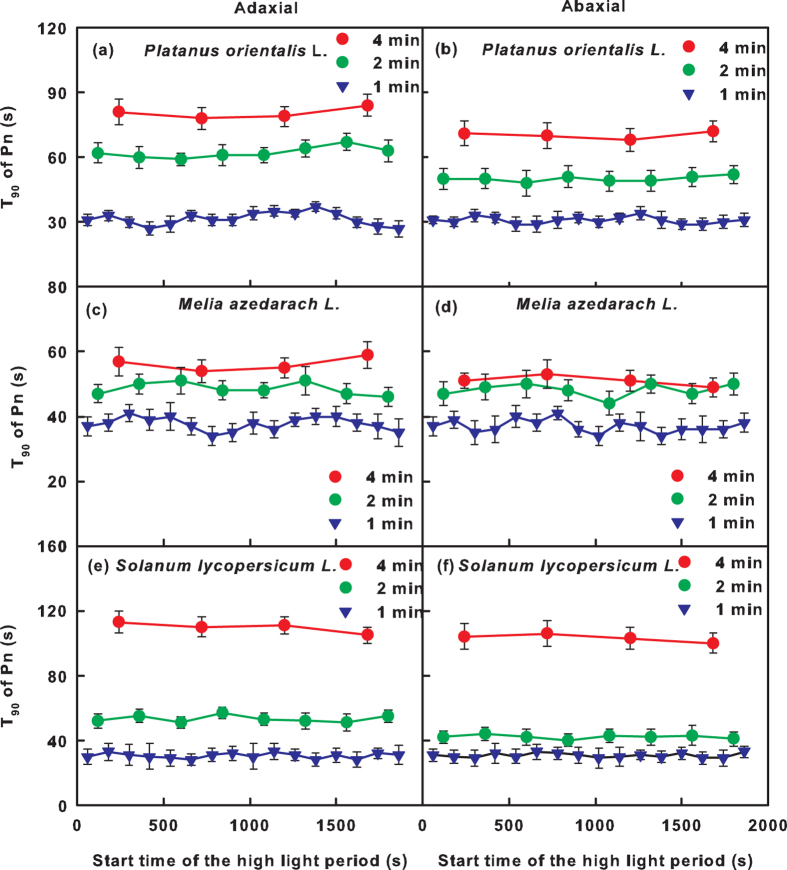
The time required to reach 90% of the maximum Pn, E and WUE (T90) when the light intensity was switched from low light to high light was used to calculate the rates of gas exchange induction. In the leaves from all three species, the T90 was constant during simulated ad-ab-alt irradiation but significantly decreased with an increase in fluctuation frequency (Fig. 4, Supplementary Fig. S4). The T90 of leaves under abaxial surface irradiation was similar or lower than that of leaves under adaxial surface irradiation (Fig. 4, Supplementary Fig. S4).
Figure 4. The time required to reach 90% of the maximum net photosynthetic rate (T90 of Pn) after the irradiation was changed from low light (100 μmol m−2 s−1) to high light (1,000 μmol m−2 s−1).
During the measurement, the adaxial (a,c,e) or abaxial surfaces (b,d,f) of Platanus orientalis L. (a,b), Melia azedarach L. (c,d) and Solanum lycopersicum L. (e,f) were irradiated by fluctuating light. The irradiation was switched between high and low light every 4 min, 2 min or 1 min. The x-axis indicates the start time of the high light period (as shown in Fig. 1b). Values are the mean (±SE), n = 5.
Stomatal limitation and nonstomatal limitation
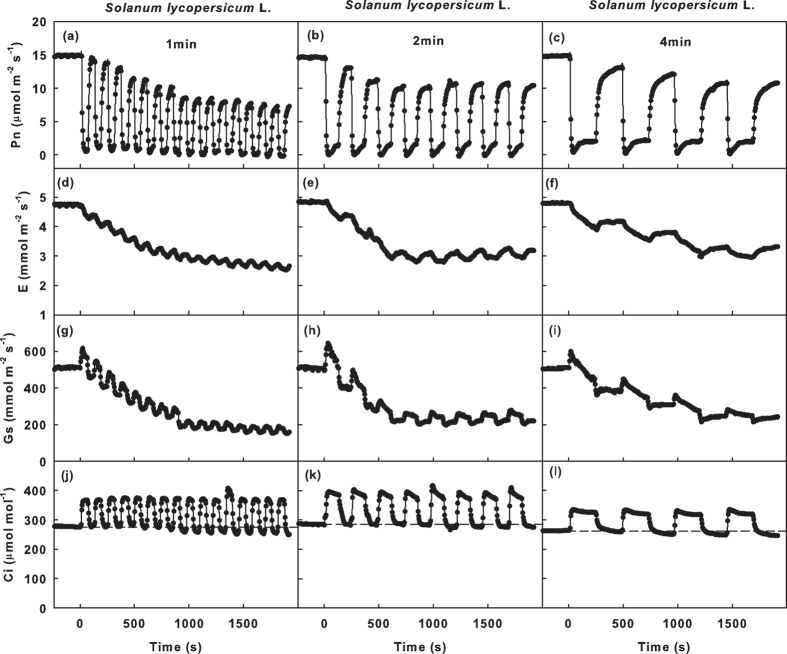
To clarify the role of stomata in the decrease of Pnmax and the delay of Pn induction under simulated ad-ab-alt irradiation, we analysed the Gs, Ci and Pn of Solanum lycopersicum L. leaves. When the light intensity switched from low light to high light, the E and Gs increased more slowly than the Pn, and the Ci decreased (Fig. 5). The minimum Ci during the high light period (Cimin) decreased with the decrease in Pnmax and Emax under simulated ad-ab-alt irradiation (Fig. 5).
Figure 5. Time course for Pn, E, Gs, Ci in Solanum lycopersicum L. leaves under simulated ad-ab-alt irradiation.
Time course for the net photosynthetic rates (Pn; a–c), transpiration rate (E; d–f), stomatal conductance (Gs; g–i) and intercelluar CO2 pressure (Ci; j–i) in Solanum lycopersicum L. leaves when the adaxial or abaxial surfaces of leaves were irradiated by fluctuating light, in which the irradiation was switched between high light (1,000 μmol m−2 s−1) and low light (100 μmol m−2 s−1) every 1 min (a,d,g,j) or 2 min (b,e,h,k) or 4 min (c,f,i,l). The dotted line shows the Ci under ad-con irradiation.
Sun leaves and shade leaves
The above results demonstrate that the abaxial surface has a lower sensitivity to fluctuating light compared with the adaxial surface. The adaxial surface has characteristics that resemble a classic sun leaf, whereas the abaxial surface has properties that are consistent with shade leaves3,26,27. Therefore, we hypothesized that shade leaf characteristics may contribute to the increased adaptability of the abaxial surface to fluctuating light. To test this hypothesis, we compared the gas exchange of a sun leaf and a shade leaf in response to fluctuating light. The sensitivity of gas exchange to fluctuating light on the adaxial surface of the shade leaf was lower than that of the sun leaf but was still higher than that of the abaxial surface of the sun leaf (Fig. 6). This result demonstrates that the characteristics of a shade leaf contribute to the increased adaptability of the abaxial surface to fluctuating light.
Figure 6. The time course of the net photosynthetic rates (Pn) of Solanum lycopersicum L. leaves under high or low light.
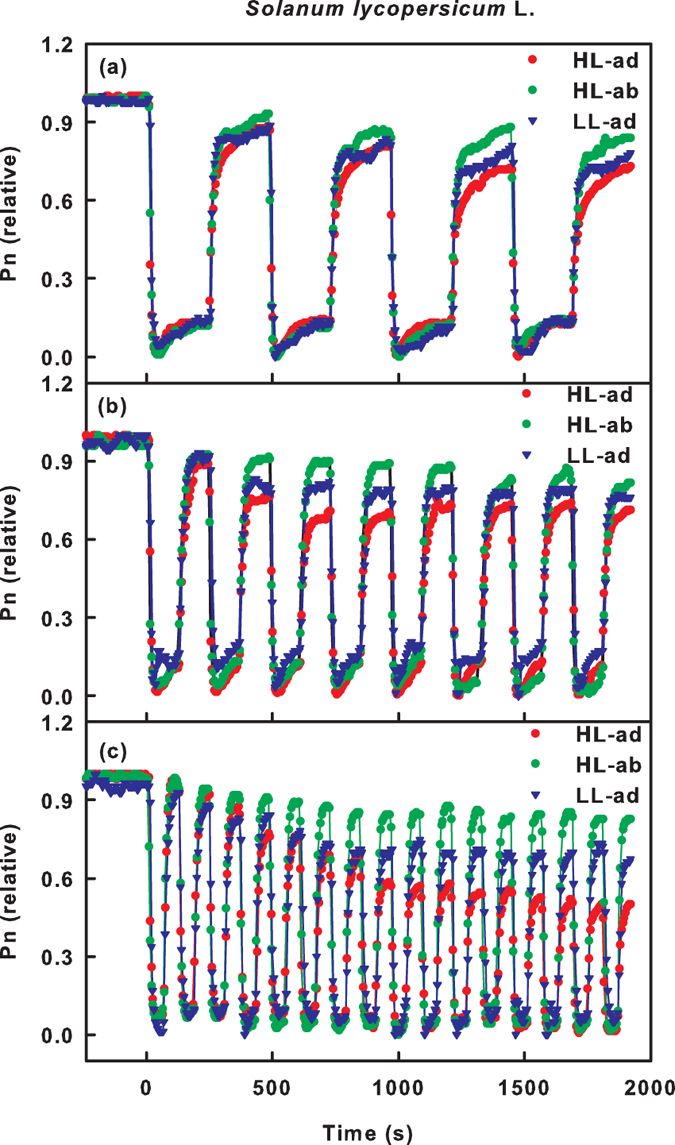
The adaxial or abaxial surfaces were irradiated by fluctuating light. The irradiation was switched between high light (1,000 μmol m−2 s−1) and low light (100 μmol m−2 s−1) every 4 min (a), 2 min (b) or 1 min (c). The initial values of Pn under constant irradiation were considered as 1; other values were calculated as a proportion of the initial values.
Discussion
Although some recent reports have investigated dynamic photosynthesis under fluctuating irradiance22, the majority of studies were performed on leaves that were irradiated on the adaxial surface12,13,14,15,16,17. Abaxial surface irradiation and ad-ab-alt irradiation, which is ubiquitous in the field, have been neglected11. Here, we report the first systematic research focused on the gas exchange of leaves under simulated ad-ab-alt irradiation. The results from this study support the following hypotheses: (1) photosynthetic gas exchange is repressed under simulated ad-ab-alt irradiation and (2) the abaxial surface of the leaf adapts to simulated ad-ab-alt irradiation better than the adaxial surface.
Previous reports regarding dynamic light conditions were focused on sunflecks21. Sunflecks provide additional light energy to shaded plants; consequently, sunfleck photosynthesis has always been compared with photosynthesis under shade21. However, the total PAR absorbed by the leaves was identical under ad-ab-alt irradiation and ad-con irradiation. The only differences between these two irradiation conditions are the spatial and temporal distributions of light. Therefore, photosynthetic gas exchange under ad-ab-alt irradiation should be compared with that under ad-con irradiation. This study demonstrated that simulated ad-ab-alt irradiation resulted in considerable losses of photosynthetic CO2 fixation (Fig. 3).
The loss of CO2 fixation under ad-ab-alt irradiation results from: (1) the reduction in Pnmax (region 1 in Figs 7 and 2) the induction requirement of CO2 assimilation when the light intensity switches from low light to high light (region 2 in Fig. 7). A previous report suggested that the Pnmax under sunflecks could reach a level identical to the steady state Pn under constant irradiation29. However, the current study demonstrated that substantial variation exists between species with respect to gas exchange response to a change in light intensity (Figs 2 and 3). In addition, the fluctuating frequency of light intensity critically influences the behaviour of Pn.
Figure 7. Sketch map of the time course of the net photosynthetic rates (Pn) in response to fluctuating light.
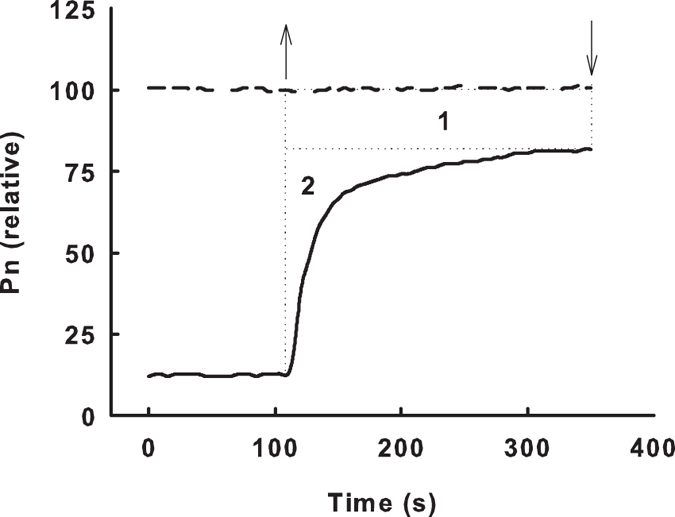
The upward arrow shows the leaves were transferred from low light to high light, and the downward arrow shows the leaves were transferred from high light to low light.
The Pnmax depends on the balance between the deactivation of the photosynthetic apparatus during low light periods and its reactivation in the following high light periods. It was observed that the T90 of Pn remained constant during the 32 min of fluctuating irradiation (Fig. 4). This finding indicated that the rate of photosynthetic activation is steady during high light periods. Consequently, the decreases in Pnmax during the simulated ad-ab-alt irradiation occurred because of the deactivation of the photosynthetic apparatus during the low light periods rather than the attenuation of activation during the high light periods.
A reduction in the fluctuation frequency of light intensity results in an extension of both the low light period and the high light period. The extension of the low light period aggravates the deactivation of the photosynthetic apparatus, and the extension of the high light period enhances the activation of the photosynthetic apparatus. The decreases in Pnmax were most obvious in leaves exposed to high frequency fluctuating irradiation (Fig. 2, Supplementary Fig. S3). This result suggests that the initial activation rate during the high light period was lower than the initial deactivation rate in the low light periods. However, a reduction in the fluctuation frequency lessened the decrease in Pnmax (Fig. 2, Supplementary Fig. S3), which indicates that the balance between the deactivation and activation of the photosynthetic apparatus was related to the duration of the high and low light periods, and the deactivation was more sensitive to the duration compared with the activation.
The photosynthetic activation during the high light periods was reduced with a reduction in the fluctuation frequency, which was reflected by the increase in the T90 of Pn (Fig. 4). This result demonstrated that the duration of the low light period not only affects the activation state but also the activation rate of the photosynthetic apparatus in the following high light periods. Previous studies demonstrated that both the deactivation in low light periods and the activation in high light periods consist of two phases. The rapid phase lasts approximately 1 min and is due to the depletion or accumulation of intermediate metabolites30. The slow phase, which is in the range of several minutes or more, occurs because light activation of the enzymes in the RuBP regeneration pathway, especially chloroplast fructose-1,6-bisphosphatase (FBPase) and sedoheptulose-1,7-bisphosphatase (SBPase)21,22, which deactivates more rapid under low light compared with Rubisco23,30. Under high frequency fluctuating light, the rapid phases of deactivation and activation are dominant. A decrease in the fluctuation frequency results in the occurrence of the slow phase, with a consequent deceleration in photosynthetic activation in the high light periods. Because of the more obvious decrease in Pnmax under high frequency fluctuating light (Fig. 2, Supplementary Fig. S3), we suggest that the imbalance between the activation and deactivation was more extreme in the rapid compared with the slow phase.
The gas exchange reached a steady state approximately 20 min after the leaves were exposed to simulated ad-ab-alt irradiation, which was reflected by the steady Pnmax (Fig. 2). In this state, the activation and deactivation of the photosynthetic apparatus in the low and high light phases were uniform. It is unclear how this steady state is achieved. It may be due to the adjustment of extra mechanisms or the characteristics of the photosynthetic apparatus. The specific mechanism requires further investigation.
Pn is restricted by mesophyll factors and stomatal factors25,31. When the light intensity switched from low to high light, Pn and E increased and Ci decreased (Fig. 5). This result suggests that the delay in stomatal opening limited the increase in Pn. In addition, Cimin decreased with a reduction in Pnmax and Emax (Fig. 5), indicating that the stomatal limitation contributed to the decrease in Pnmax under simulated ad-ab-alt irradiation. Thus, the suppression of Pn under simulated ad-ab-alt irradiation was at least partially due to stomatal limitation. In addition, because E was less sensitive than Pn to fluctuating light, the WUE of leaves under simulated ad-ab-alt irradiation was lower than that under constant irradiation (Fig. 2). The decrease in WUE under simulated ad-ab-alt irradiation was entirety due to the delay in induction (Fig. 2, Supplementary Fig. S3); however, the WUEmax remained steady or increased slightly (Fig. 2, Supplementary Fig. S3). The above factors demonstrate that the leaves attempted to balance the optimization of Pn and WUE by regulating the stomata under simulated ad-ab-alt irradiation, and the optimization of the WUE was more critical than the maximization of Pn for leaves under simulated ad-ab-alt irradiation. It should be particularly critical for plants in arid and semi-arid areas.
This study also demonstrated that the abaxial surface used fluctuating light more efficiently than the adaxial surface, which was reflected by the fact that the ad/ab ratios of Pn under ad-ab-alt irradiation were higher than the ratio of steady state Pn measured under ad-con and ab-con irradiation (Figs 2 and 3, Supplementary Fig. S3). In particular, when the CO2 assimilation of the adaxial surface declined severely under the high frequency fluctuating light, the abaxial surface contributed even more to CO2 assimilation than the adaxial surface (Fig. 3l).
Compared with the adaxial surface, the abaxial surface exhibited more rapid photosynthetic induction and maintained a higher Pnmax under simulated ad-ab-alt irradiation (Figs 2 and 4). It was also observed that the shade leaves maintained a higher Pnmax than the sun leaves under fluctuating light (Fig. 6). Previous studies showed that the rapid induction of photosynthesis during high light periods and the highly activated state of photosynthesis, which is maintained during low light periods, are critical for maximizing light energy under fluctuating light conditions, especially in undergrowth plants16,21,22. Consequently, we suggested that the adaptability of the abaxial surface to fluctuating light occurred because of the shade-leaf-like characteristics of the spongy mesophyll (SM) near the abaxial surface. Indeed, the abaxial surface has characteristics similar to those of shade leaves. It has been reported that plants grown under low-light conditions usually have faster rates of photosynthetic induction, and the loss of the activation state of the photosynthetic apparatus is slower under low light compared with plants grown in high light environments32,33. Therefore, it is possible that the abaxial surface of the leaf can maintain a higher activation state and more intermediate metabolites under a low level of fluctuating light compared with the adaxial surface.
In the classical view, the SM cells near the abaxial surface contain lower amounts of photosynthetic pigments and proteins7,8. Therefore, CO2 assimilation in SM cells is lower than that in PM cells regardless of which side of the leaf is irradiated4,5,6. However, the shade-leaf-like characteristic enables the abaxial surface to assimilate more CO2 under a fluctuating light condition. Consequently, the evolution of a bifacial leaf resulted not only from a resource economy perspective but also because the bifacial leaf could fix more carbon than a leaf with two “sun-leaf-like” surfaces under field conditions.
However, consideration of only the shade-leaf-like characteristic is not sufficient to explain the higher adaptability of the abaxial surface to fluctuating light. This is because the adaptability of the adaxial surface of the shade leaf was lower than that of the abaxial surface of the sun leaf (Fig. 6), even though the adaxial surface of the shade leaf and the abaxial surface of the sun leaf experience similar light environments. In addition, there is substantial variation between species in the adaptability of gas exchange to ad-ab-alt irradiation. Platanus orientalis L. is a typical light-demanding upper canopy species. However, contrast to our expectations, both the adaxial and abaxial surfaces of leaves of Platanus orientalis L. exhibited perfect adaptability to simulated ad-ab-alt irradiation (Fig. 2). Therefore, the adaptation mechanisms of gas exchange in leaves to ad-ab-alt irradiation remain to be studied further. Moreover, in this study, some factors that may affect the gas exchange of leaves under ad-ab-alt irradiation were neglected, such as the thickness of the leaf and temperature fluctuations. An ad-ab-alt irradiation condition that more closely approximates natural conditions should be considered in future studies.
Methods
Plant materials
Two-year-old Platanus orientalis L. and Melia azedarach L. seedlings and three-month-old Solanum lycopersicum L. seedlings were used in this study. The seedlings were grown in 10-L polythene pots containing fertile soil. The seedlings were cultivated in the field, and sufficient water and nutrients were supplied. The maximum light intensity at noon was 1200–1500 μmol m−2 s−1, and the maximum temperature at noon was 30–33 °C. Half of the Solanum lycopersicum L. seedlings were covered with black plastic velamen. The maximum light intensity below the velamen was approximately 100–150 μmol m−2 s−1. The youngest fully developed leaves were used for the experiments.
Absorptance
According to a previous description9, spectrum (400–700 nm) measurements were performed using a portable Unispec SC spectrometer (PP Systems, USA). Leaf reflectance was measured with a standard bifurcated fibre optic cable, standard leaf clip and the halogen lamp in the spectrometer. To calculate the reflectance, the leaf spectral radiance was divided by the radiance of a 99% reflective white reference standard (Spectralon, Labsphere, North Dutton, NH, USA). Leaf transmittance was measured using two standard straight-fibre optics, a custom-made device and the halogen lamp in the spectrometer. The absorptance was calculated as absorptance = 1 − reflectance − transmittance. The integrated absorptance was the average of the absorptance at different wavelengths between 400 nm and 700 nm.
Leaf gas exchange measurements
The net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (E), substomatal CO2 concentration (Ci) and water use efficiency (WUE = Pn / E) were measured using a CIRAS-3 portable photosynthesis system (PP Systems, Amsbury, MA, USA). Data were automatically recorded by the CIRAS-3 every 5 s. The CO2 concentration (380 μmol mol−1), relative humidity (60%) and leaf temperature (28 °C) were maintained using an automatic control device on the CIRAS-3. Red-blue light (90%: 10%) was provided by the LED light unit in the CIRAS-3.
The CIRAS-3 cannot irradiate both the adaxial and abaxial surfaces simultaneously. Therefore, we simulated ad-ab-alt irradiation by exposing the abaxial or adaxial surface to fluctuating light separately (Fig. 1b). Before the onset of data collection, the leaves were adapted under high light (1,000 μmol m−2 s−1) for 20–40 min until Pn and E stabilized. The leaves were then irradiated with constant light or fluctuating light for 32 min. The fluctuating light conditions consisted of a low light (100 μmol m−2 s−1) and high light (1,000 μmol m−2 s−1) period that was repeated every 60 s, 120 s or 240 s (Fig. 1b).
The area below the time course curve of the gas exchange represents the integrated gas exchange. The integrated whole leaf gas exchange under simulated ad-ab-alt irradiation was calculated by summing the area for the high light periods under the abaxial and adaxial surface irradiation (the white segments in Fig. 1b).
Statistical analysis
The absorptance measurements were repeated on 15 different leaves. The gas exchange measurements were repeated using 5 different leaves. The LSD (least significant difference) was used to analyse differences between the different treatments using SPSS 11 (SPSS Inc., Chicago, IL, USA). Different letters indicate significant differences at P < 0.05 (Tukey’s test).
Additional Information
How to cite this article: Zhang, Z.-S. et al. Characterization of photosynthetic gas exchange in leaves under simulated adaxial and abaxial surfaces alternant irradiation. Sci. Rep. 6, 26963; doi: 10.1038/srep26963 (2016).
Supplementary Material
Acknowledgments
This work was financially supported by the China Postdoctoral Science Foundation (2015M572068), the National Natural Science Foundation of China (31370276) and the State Key Basic Research and Development Plan of China (2015CB150105).
Footnotes
Author Contributions Z.-S.Z., H.-Y.G. and Q.-W.M. designed the research; Z.-S.Z., Y.-T.L. and C.Y. performed the research; Z.-S.Z. and C.Y. analysed the data; Z.-S.Z., Y.-T.L. and H.-Y.G. wrote the article; all authors read and approved the final manuscript.
References
- Wang Y., Noguchi K. O. & Terashima I. Distinct light responses of the adaxial and abaxial stomata in intact leaves of Helianthus annuus L. Plant Cell Environ. 31, 1307–1316 (2008). [DOI] [PubMed] [Google Scholar]
- Wang Y., Noguchi K. & Terashima I. Photosynthesis-dependent and-independent responses of stomata to blue, red and green monochromatic light: differences between the normally oriented and inverted leaves of sunflower. Plant Cell Physiol. 52, 479–489 (2011). [DOI] [PubMed] [Google Scholar]
- Clements E. S. The relation of leaf structure to physical factors. Trans. Am. Microsc. Soc. 26, 19–102 (1905). [Google Scholar]
- Nishio J. N., Sun J. & Vogelmann T. C. Carbon fixation gradients across spinach leaves do not follow internal light gradients. Plant Cell 5, 953–961 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. R. & Vogelmann T. C. Profiles of 14C fixation through spinach leaves in relation to light absorption and photosynthetic capacity. Plant Cell Environ. 26, 547–560 (2003). [Google Scholar]
- Evans J. R. & Vogelmann T. C. Photosynthesis within isobilateral Eucalyptus pauciflora leaves. New Phytol. 171, 771–782 (2006). [DOI] [PubMed] [Google Scholar]
- Terashima I. & Inoue Y. Palisade tissue chloroplasts and spongy tissue chloroplasts in spinach: biochemical and ultrastructural differences. Plant Cell Physiol. 26, 63–75 (1985). [Google Scholar]
- Sun J. & Nishio J. N. Why abaxial illumination limits photosynthetic carbon fixation in spinach leaves? Plant Cell Physiol. 42, 1–8 (2001). [DOI] [PubMed] [Google Scholar]
- Li P. M., Fang P., Wang W. B., Gao H. Y. & Peng T. The higher resistance to chilling stress in adaxial side of Rumex K-1 leaves is accompanied with higher photochemical and non-photochemical quenching. Photosynthetica 45, 496–502 (2007). [Google Scholar]
- Terashima I. & Evans J. R. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 29, 143–155 (1988). [DOI] [PubMed] [Google Scholar]
- Steyn W. J., Wand S. J. E., Holcroft D. M. & Jacobs G. Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol. 155, 349–361 (2002). [DOI] [PubMed] [Google Scholar]
- Hurry V., Tobiaeson M., Krömer S., Gardeström P. & Öquist G. Mitochondria contribute to increased photosynthetic capacity of leaves of winter rye (Secale cereale L.) following cold‐hardening. Plant Cell Environ. 18, 69–76 (1995). [Google Scholar]
- Krause G. H., Schmude C., Garden H., Koroleva O. Y. & Winter K. Effects of solar ultraviolet radiation on the potential efficiency of photosystem II in leaves of tropical plants. Plant Physiol. 121, 1349–1358 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S. et al. The solar action spectrum of photosystem II damage. Plant Physiol. 153, 988–993 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste S., Roggy J. C., Schimann H., Epron D. & Dreyer E. A cost–benefit analysis of acclimation to low irradiance in tropical rainforest tree seedlings: leaf life span and payback time for leaf deployment. J. Exp. Bot. 62, 3941–3955 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F., Jin L. Q., Zhang Z. S. & Gao H. Y. Systemic signalling in photosynthetic induction of Rumex K-1 (Rumex patientia × Rumex tianschaious) leaves. Plant Cell Environ. 38, 685–692 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang Z. S. et al. Water status related root-to-shoot communication regulates the chilling tolerance of shoot in cucumber (Cucumis sativus L.) plants. Sci. Rep. 5, 13094 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cordeiro A. S., Driscoll S. P., Arrabaça M. C. & Foyer C. H. Dorsoventral variations in dark chilling effects on photosynthesis and stomatal function in Paspalum dilatatum leaves. J. Exp. Bot. 62, 687–699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann T. C. & Evans J. R. Profiles of light absorption and chlorophyll within spinach leaves from chlorophyll fluorescence. Plant Cell Environ. 25, 1313–1323 (2002). [Google Scholar]
- Oguchi R., Douwstra P., Fujita T., Chow W. S. & Terashima I. Intra‐leaf gradients of photoinhibition induced by different color lights: implications for the dual mechanisms of photoinhibition and for the application of conventional chlorophyll fluorometers. New Phytol. 191, 146–159 (2011). [DOI] [PubMed] [Google Scholar]
- Way D. A. & Pearcy R. W. Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiol. 32, 1066–1081 (2012). [DOI] [PubMed] [Google Scholar]
- Kaiser E. et al. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 66, 2415–2426 (2015). [DOI] [PubMed] [Google Scholar]
- Mott K. A. & Woodrow, I. E. Modelling the role of Rubisco activase in limiting non‐steady‐state photosynthesis. J Exp. Bot. 51 (suppl 1), 399–406 (2000). [DOI] [PubMed] [Google Scholar]
- Tausz M., Warren C. R. & Adams M. A. Dynamic light use and protection from excess light in upper canopy and coppice leaves of Nothofagus cunninghamii in an old growth, cool temperate rainforest in Victoria, Australia. New Phytol. 165, 143–156 (2005). [DOI] [PubMed] [Google Scholar]
- Tomimatsu H. & Tang Y. Elevated CO2 differentially affects photosynthetic induction response in two Populus species with different stomatal behavior. Oecologia 169, 869–878 (2012). [DOI] [PubMed] [Google Scholar]
- Terashima I., Sakaguchi S. & Hara N. Intra-leaf and intracellular gradients in chloroplast ultrastructure of dorsiventral leaves illuminated from the adaxial or abaxial side during their development. Plant Cell Physiol. 27, 1023–1031 (1986). [Google Scholar]
- Lambers H., Chapin F. S. III & Pons T. L. [Photosynthesis, respiration, and long-distance transport] Plant physiological ecology. [Lambers H., Chapin III F. S. & Pons T. L. (ed.)] [10–153] (Springer, New York, 1998) [Google Scholar]
- Kebeish R. et al. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nature biotechnol. 25, 593–599 (2007). [DOI] [PubMed] [Google Scholar]
- Leakey A. D. B., Press M. C. & Scholes J. D. High‐temperature inhibition of photosynthesis is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant Cell Environ. 26, 1681–1690 (2003). [Google Scholar]
- Sassenrath-Cole G. F. & Pearcy R. W. The role of Ribulose-1,5- Bisphosphate regeneration in the induction requirement of photosynthetic CO2 exchange under transient light conditions. Plant Physiol. 99, 227–234 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. L., Chen C. W., Huang H. W. & Weng J. H. Using combined measurements for comparison of light induction of stomatal conductance, electron transport rate and CO2 fixation in woody and fern species adapted to different light regimes. Tree Physiol. 32, 535–544 (2012). [DOI] [PubMed] [Google Scholar]
- Tang Y., Hiroshi K., Mitsumasa S. & Izumi W. Characteristics of transient photosynthesis in Quercus serrata seedlings grown under lightfleck and constant light regimes. Oecologia 100, 463–469 (1994). [DOI] [PubMed] [Google Scholar]
- Urban O., Kosvancova M., Marek M. V. & Lichtenthaler H. K. Induction of photosynthesis and importance of limitations during the induction phase in sun and shade leaves of five ecologically contrasting tree species from the temperate zone. Tree Physiol. 27, 1207–1215 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.