Abstract
This study developed probabilistic models to predict Salmonella growth in processed meat products formulated with varying concentrations of NaCl and NaNO2. A five-strain mixture of Salmonella was inoculated in nutrient broth supplemented with NaCl (0%, 0.25%, 0.5%, 0.75%, 0.5%, 1.0%, 1.25%, and 1.75%) and NaNO2 (0, 15, 30, 45, 60, 75, 90, 105, and 120 ppm). The inoculated samples were then incubated under aerobic and anaerobic conditions at 4°C, 7°C, 10°C, 12°C, and 15°C for up to 60 days. Growth (assigned the value of 1) or no growth (assigned the value of 0) for each combination was evaluated by turbidity. These growth response data were analyzed with a logistic regression to evaluate the effect of NaCl and NaNO2 on Salmonella growth. The results from the developed model were compared to the observed data obtained from the frankfurters to evaluate the performance of the model. Results from the developed model showed that a single application of NaNO2 at low concentrations did not inhibit Salmonella growth, whereas NaCl significantly (p<0.05) inhibited Salmonella growth at 10°C, 12°C, and 15°C, regardless of the presence of oxygen. At 4°C and 7°C, Salmonella growth was not observed in either aerobic or anaerobic conditions. When NaNO2 was combined with NaCl, the probability of Salmonella growth decreased. The validation value confirmed that the performance of the developed model was appropriate. This study indicates that the developed probabilistic models should be useful for describing the combinational effect of NaNO2 and NaCl on inhibiting Salmonella growth in processed meat products.
Keywords: Salmonella, NaNO2, NaCl, Probabilistic Model
INTRODUCTION
Salmonella are facultative and intracellular pathogens, and they cause salmonellosis with the consumption of contaminated food (Jantsch et al., 2011). In the EU, salmonellosis was the second highest zoonotic disease in 2011, following campylobacteriosis (EFSA and ECDC, 2013). Nontyphoidal Salmonella infections are estimated to affect 1.4 million people annually in the United States, resulting in 15,000 hospitalizations and 400 deaths (Voetsch et al., 2004). Salmonellosis is mostly caused byanimal foods such as poultry, eggs, pork, beef, and processed meat products (Meyer et al., 2010; EFSA and ECDC, 2013). Salmonella contamination of meat products occurs frequently at processing plants through cross contamination (Beery et al., 1988; Izat et al., 1989). According to a study by Busani et al. (2005), Salmonella were isolated from 9.9% of raw poultry, 4.9% of raw pork, 0.1% of ice cream, and 5.3% of processed meats product.
NaNO2 is commonly used to fix a red color, to improve flavor, and as an antioxidant in processed meat, increasing the shelf-life of meat products (Jira, 2004). NaCl is also used to enhance flavor and as a protein extractant in processed meat (Aguilera and Karel, 1997; Munasinghe et al., 2004; Kostick, 2010). However, high NaNO2 and NaCl intake is related to cancer and cardiovascular diseases (Maekawa et al., 1982; Perry and Beevers, 1992; Frolich, 1999). Because of the associated health issues, consumers prefer low concentrations of NaNO2 and NaCl in meat products, and thus, these products may allow the growth of foodborne pathogens, especially Salmonella, which is commonly found in fresh meat. Therefore, the combinational effects of lower NaNO2 and NaCl concentrations on Salmonella growth need to be evaluated and predictive models need to be developed to evaluate the effects.
A probabilistic model can be used to predict the interface between the growth and no growth of bacteria or pathogens as a function of various factors (Ratkowsky and Ross, 1995; Schaffner and Labuza, 1997; Lee et al., 2013). For instance, Yoon et al. (2009) suggested a combination of minimum level of lactic acid, dipping time, and storage temperatures to inhibit Listeria monocytogenes growth in frankfurters using probabilistic model, and the model could be used to select conditions in meat products. Therefore, the objective of this study was to evaluate the combinational effect of low NaCl and NaNO2 concentration on inhibiting Salmonella growth in processed meat products with probabilistic models.
MATERIALS AND METHODS
Inoculum preparation
Salmonella Typhimurium NCCP10812, Salmonella Agona NCCP12231, Salmonella Enteritidis NCCP12243, Salmonella Enterica KACC11595, Salmonella Montevideo NCCP10141 were cultured in 10 mL nutrient broth (NB; Becton, Dickinson and Company, Sparks, MD, USA) at 35°C for 24 h. Subsequently, 0.1-mL aliquots of the cultures were subcultured in 10 mL NB at 35°C for 24 h. The subcultures of five strains were mixed, and the mixed subcultures were centrifuged (1,912×g, 15 min, 4°C). The pellets were then washed twice with phosphate buffered saline (PBS, pH 7.4; 0.2 g of KH2PO4, 1.5 g of Na2HPO4·7H2O, 8.0 g of NaCl, and 0.2 g of KCl in 1 L distilled water). The suspension was serially diluted with PBS to obtain 4 log CFU/mL of inoculum.
Media preparation and inoculation
Twenty five microliter aliquot of the inoculum was inoculated into 225 μL NB supplemented with combinations of NaCl (0%, 0.25%, 0.5%, 0.75%, 1.0%, 1.25%, 1.5%, and 1.75%) and NaNO2 (0, 15, 30, 45, 60, 75, 90, 105, and 120 ppm) in a 96-well microtiter plate. Two hundred fifty microliter aliquot of NB was used as a negative control, and 225 μL of NB inoculated with 25 μL of the cell suspension was used as a positive control. The microtiter plates were then incubated at 4°C, 7°C, 10°C, 12°C, and 15°C for up to 1,440 h, 1,440 h, 1,152 h, 384 h, and 168 h, respectively. For the aerobic conditions, the microtiter plates were sealed with plastic paraffin film (Parafilm M; Bemis Company Inc., Neenah, WI, USA). For the anaerobic conditions, the microtiter plates sealed with paraffin film were placed into tightly sealed plastic containers with Anaerogen (Oxoid Ltd., Basingstoke, Hampshire, UK). The Anaerogens were replaced every 24 h.
Model development
In this study, the combinations of NaCl (0%, 0.25%, 0.5%, 0.75%, 1.0%, 1.25%, 1.5%, and 1.75%), NaNO2 (0, 15, 30, 45, 60, 75, 90, 105, and 120 ppm), storage temperature (10°C, 12°C, and 15°C), and storage time (up to 1,440 h) were prepared. Each combination had eight observations (n = 8), and growth response was evaluated every 24 h during storage. If a well became turbid, it was assigned 1 (growth), otherwise it was assigned 0 (no growth). The growth response data were then analyzed by logistic regression using SAS (Version 9.3; SAS Institute Inc., Cary, NC, USA). The following equation was derived by the logistic regression and a stepwise selection method was used to select significant parameters (p<0.05). The selected parameters in the equation were used to describe the combinational effect of NaCl and NaNO2 on Salmonella growth by producing growth/no growth interfaces for Salmonella at probabilities of 0.1, 0.5, and 0.9.
where P is the probability of growth, ai are estimates, NaCl is the concentration of NaCl, NaNO2 is the NaNO2 concentration, and Time is the storage time.
Validation
To evaluatethe performance of the developed model, the predicted growth probabilities were compared to the results obtained from the frankfurters used as model processed meat products. To prepare the frankfurters, the formulation described by Kim et al. (2010) was used as follows: fat (20%) and lean meat (60%) were well-mixed with ice (20%), isolated soy protein (1.0%), phosphate (0.3%), spice (0.5%), potassium sorbate (0.2%), sugar (0.5%), NaNO2 (0 or 10 ppm), and NaCl (1.0%, 1.25%, or 1.5%). The mixtures were stored at 4°C for 1 h, followed by stuffing them into collagen casings (ca., 30 g/each) using an automatic sausage can filler (Konti A50; Frey, Herbrechtingen, Germany). The frankfurters were subsequently cooked at 75°C for 40 min in a smokehouse (MAXI 3501; Kerres, Backnang, Germany). After cooling, the sausages were vacuum-packaged, and then heated for a second time at 80°C for 15 min, followed by dipping into ice water for 10 min and storage at 4°C overnight. The frankfurters were cut into 25-g samples. Thirty frankfurters were immersed in 500 mL inoculum (3 log CFU/mL) in a sterilized plastic container for 2 min. The samples were air-dried under a laminar flow cabinet for 15 min to allow Salmonella attachment then transferred into vacuum bags (Gwak et al., 2015). The bags were sealed for aerobic storage or vacuum packaged for anaerobic storage using a vacuum packager (HFV-500; Fujee Inc., Hwaseong, Korea). The packaged samples were incubated at 4°C, 10°C, and 15°C for up to for 1,440 h. During incubation, Salmonella were enumerated on xylose lysine deoxycholate agar (XLD; Beckton, Dickinson, and Company) at the appropriate time interval (6 to 8 times), depending on the incubation temperature. If the Salmonella cell counts increased above 1 log CFU/g compared to the cell counts on day 0, it was considered as “growth” and if the cell counts decreased or increased less than 1 log CFU/g, it was considered “no growth” (Koutsoumanis et al., 2004; Yoon et al., 2009).
RESULTS AND DISCUSSION
The estimates of significant parameters are listed in Table 1. These estimates were used to predict the growth and no growth interfaces at probabilities of 0.1, 0.5, and 0.9. For both aerobic and anaerobic conditions, Salmonella growth was not observed at 4°C or 7°C (data not shown). Salmonella growth was initiated earlier in aerobic conditions than in anaerobic conditions at 10°C to 15°C (Figures 1 to 6). Møller (2012) reported a Tmin (theoretical minimum temperature for growth) for Salmonella in pork at 2.33°C and the Tmin for Salmonella suggested by Pin et al. (2011) was 4.27°C in broth media, which are both lower than the approximate lowest temperature (7°C) for Salmonella growth evaluated in this study. Fares (2007) and University of Nebraska Cooperative Extension (2005) also suggested 6°C to 7°C as the minimum temperature for Salmonella. Because Tmin is a theoretical lower temperature limit of growth below which the calculated growth rate is close to zero (Ratkowsky et al., 1991), Tmin and the minimum observed temperature in food are different. Even though low NaNO2 and NaCl concentrations in processed meat products could be microbiologically unsafe, storage of the samples below 7°C can make the product safe. However, since NaNO2 is very closely related to the inhibition of Clostridium botulinum germination (Christiansen et al., 1973), use of low NaNO2 concentration for processed meat products should be considered very carefully.
Table 1.
Estimates of the parameters selected from the logistic regression analysis using a stepwise selection method to predict the interfaces between growth and no growth of Salmonella at desired probabilities
| Storage | Variables | Estimate | SE | p value |
|---|---|---|---|---|
| Aerobic | Interception | 312.5 | 5.6395 | <0.0001 |
| Temperature | 45.2212 | 0.8203 | <0.0001 | |
| NaNO2/10 | 1.1584 | 0.1178 | <0.0001 | |
| NaCl | 5.5105 | 0.7921 | <0.0001 | |
| Log(Time) | 7.5708 | 0.6221 | <0.0001 | |
| Temperature×NaNO2/10 | 0.0533 | 0.0054 | <0.0001 | |
| Temperature×NaCl | 0.1559 | 0.0362 | <0.0001 | |
| NaNO2/10×log(Time) | 0.1207 | 0.0277 | <0.0001 | |
| NaCl×log(Time) | 0.6743 | 0.1878 | 0.0003 | |
| Temperature2 | 1.6619 | 0.0304 | <0.0001 | |
| (NaNO2/10)2 | 0.0257 | 0.0020 | <0.0001 | |
| NaCl2 | 1.5059 | 0.0902 | <0.0001 | |
| (log(Time))2 | 7.3097 | 0.1890 | <0.0001 | |
| Anaerobic | Interception | 183.7 | 2.5665 | <0.0001 |
| Temperature | 24.6180 | 0.3562 | <0.0001 | |
| NaNO2/10 | 0.5442 | 0.0336 | <0.0001 | |
| NaCl | 1.1670 | 0.9812 | 0.2343 | |
| Log(Time) | 1.4953 | 0.5295 | 0.0047 | |
| Temperature*NaCl | 0.1759 | 0.0469 | <0.0001 | |
| NaNO2/10*log(Time) | 0.2190 | 0.0117 | <0.0001 | |
| NaCl *log(Time) | 0.5362 | 0.1988 | 0.0070 | |
| Temperature2 | 0.8589 | 0.0132 | <0.0001 | |
| (NaNO2/10)2 | 0.0165 | 0.0015 | <0.0001 | |
| NaCl2 | 0.7122 | 0.0685 | <0.0001 | |
| (log(Time))2 | 3.8334 | 0.1207 | <0.0001 |
Figure 1.
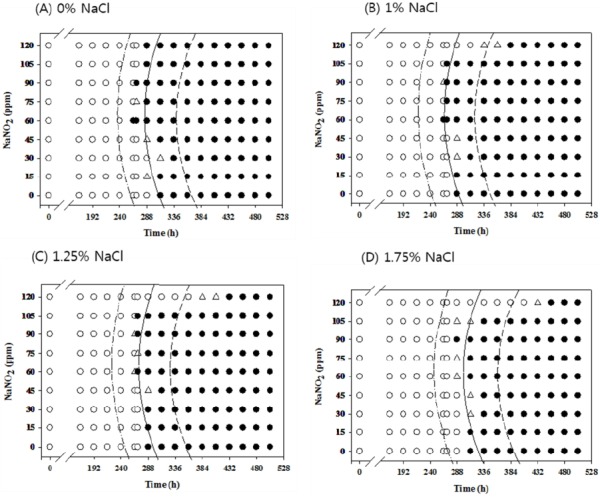
Growth/no-growth interfaces of Salmonella in nutrient broth at 10°C with respect to sodium nitrite concentration and storage time as a function of NaCl levels in aerobic conditions at growth probabilities of 0.1 (left line), 0.5 (middle line), and 0.9 (right line); no growth: ○, growth: ●, 50% growth: △; only representative data are presented.
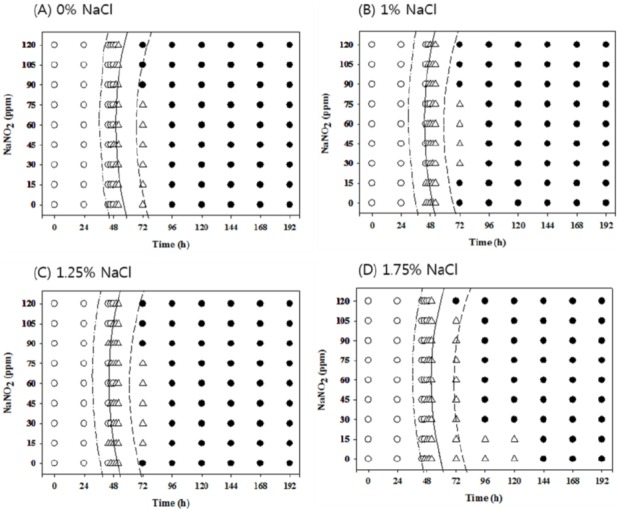
Figure 6.
Growth/no-growth interfaces of Salmonella in nutrient broth at 15°C with respect to sodium nitrite concentration and storage time as a function of NaCl levels in anaerobic conditions at growth probabilities of 0.1 (left line), 0.5 (middle line), and 0.9 (right line); no growth: ○, growth: ●, 50% growth: △; only representative data are presented.
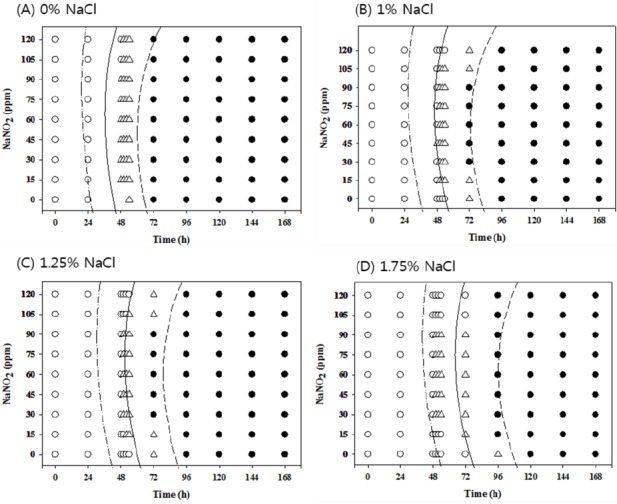
At 10°C under aerobic conditions, no antimicrobial effects from NaNO2 were observed at all concentrations with 0% NaCl, but 120 ppm NaNO2 showed obvious antimicrobial effects with more than 1% NaCl compared to other NaNO2 concentrations. The antimicrobial effect of NaNO2 became more obvious with 1.75% NaCl (Figure 1). However, at 10°C under anaerobic conditions, the antimicrobial activity of NaNO2 increased as the NaCl concentration increased and became more obvious with 1.75% NaCl (Figure 2). At 12°C, the antimicrobial activity of NaNO2 on Salmonella growth was not obvious with 0% NaCl, but NaCl with 0 ppm NaNO2 inhibited Salmonella growth (p<0.05) under aerobic conditions in a concentration-dependent manner. In addition, the combinational effect of NaNO2 and NaCl was not obvious under aerobic conditions, but the antimicrobial effect of NaNO2 increased as NaCl concentration increased under anaerobic conditions (Figures 3 and 4). At 15°C, the antimicrobial activity of NaNO2 was not observed with 0% NaCl under both aerobic and anaerobic conditions, and the combinational effect of NaNO2 and NaCl was not obvious under aerobic conditions at 12°C, but the antimicrobial effect of NaNO2 increased as NaCl concentration increased under anaerobic conditions (Figures 5 to 6). Jo et al. (2014) also developed probabilistic models to describe Pseudomonas growth responses in processed meat products formulated with NaNO2 and NaCl, and found that the antimicrobial effect of NaNO2 on Pseudomonas increased as NaCl concentration increased. In addition, Pelroy et al. (1994) reported increased NaNO2 antimicrobial activity against Listeria monocytogenes in salmon with high NaCl concentrations. These results suggest that NaNO2 should be combined with NaCl in processed meat products to inhibit Salmonella growth, especially in products with low NaNO2 concentrations.
Figure 2.
Growth/no-growth interfaces of Salmonella in nutrient broth at 10°C with respect to sodium nitrite concentration and storage time as a function of NaCl levels in anaerobic conditions at growth probabilities of 0.1 (left line), 0.5 (middle line), and 0.9 (right line); no growth: ○, growth: ●, 50% growth: △; only representative data are presented.
Figure 3.
Growth/no-growth interfaces of Salmonella in nutrient broth at 12°C with respect to sodium nitrite concentration and storage time as a function of NaCl levels in aerobic conditions at growth probabilities of 0.1 (left line), 0.5 (middle line), and 0.9 (right line); no growth: ○, growth: ●, 50% growth: △; only representative data are presented.
Figure 4.
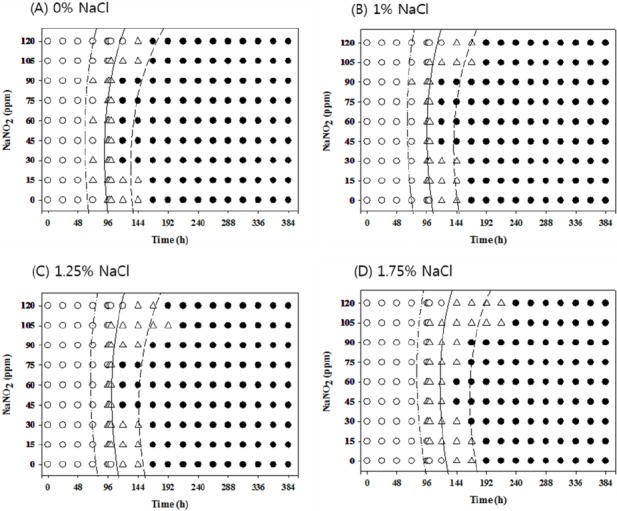
Growth/no-growth interfaces of Salmonella in nutrient broth at 12°C with respect to sodium nitrite concentration and storage time as a function of NaCl levels in anaerobic conditions at growth probabilities of 0.1 (left line), 0.5 (middle line), and 0.9 (right line); no growth: ○, growth: ●, 50% growth: △; only representative data are presented.
Figure 5.
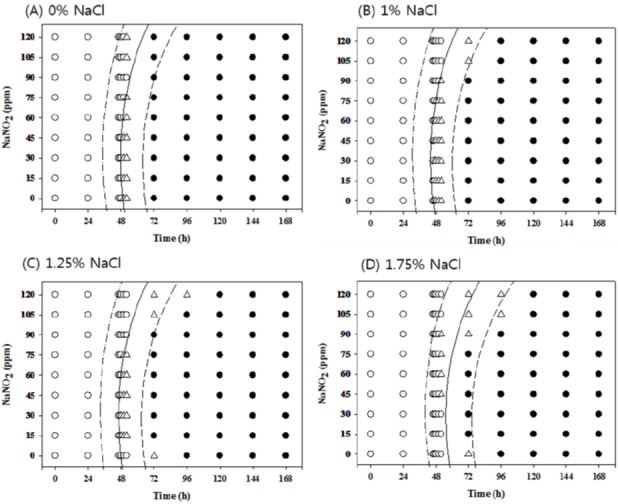
Growth/no-growth interfaces of Salmonella in nutrient broth at 15°C with respect to sodium nitrite concentration and storage time as a function of NaCl levels in aerobic conditions at growth probabilities of 0.1 (left line), 0.5 (middle line), and 0.9 (right line); no growth: ○, growth: ●, 50% growth: △; only representative data are presented.
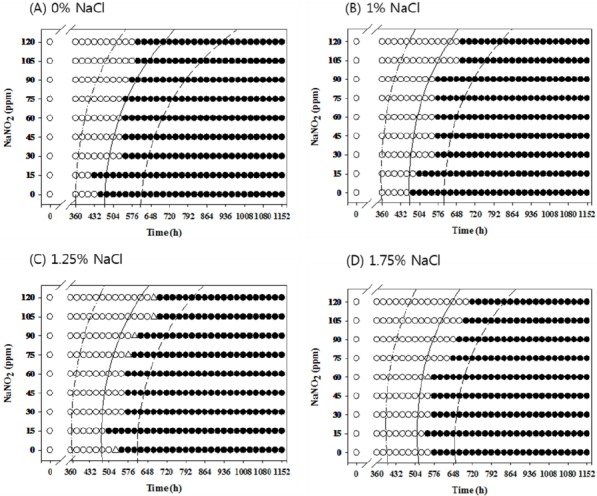
To evaluate the performance of the developed probabilistic model, Salmonella growth response data were collected from frankfurters formulated with various concentrations of NaCl and NaNO2, and the growth response data were compared to the results predicted from the probabilistic model under aerobic and anaerobic conditions; growth probability above 0.5 was considered growth (Koutsoumanis et al., 2004). The percentage of agreement between the observed and predicted growth response was approximately 81% (Table 2), indicating that the developed probabilistic model in this study was acceptable for evaluating the effect of low NaCl and NaNO2 concentrations on Salmonella growth.
Table 2.
Comparison between observed growth responses and the predicted growth responses of Salmonella under aerobic condition
| Temperature (°C) | NaNO2 (ppm) | NaCl (%) | Time (h) | Observed growth response | Predicted growth responses |
|---|---|---|---|---|---|
| 10 | 0 | 1 | 12 | NG | NG |
| 24 | NG | NG | |||
| 48 | NG | NG | |||
| 192 | G | NG | |||
| 288 | G | NG | |||
| 336 | G | G | |||
| 1.25 | 12 | NG | NG | ||
| 24 | NG | NG | |||
| 48 | NG | NG | |||
| 192 | G | NG | |||
| 288 | G | NG | |||
| 336 | G | G | |||
| 1.5 | 12 | NG | NG | ||
| 24 | NG | NG | |||
| 48 | NG | NG | |||
| 192 | G | NG | |||
| 288 | G | NG | |||
| 336 | G | G | |||
| 10 | 1 | 24 | NG | NG | |
| 96 | NG | NG | |||
| 144 | NG | NG | |||
| 192 | G | NG | |||
| 240 | G | G | |||
| 384 | G | G | |||
| 1.25 | 24 | NG | NG | ||
| 96 | NG | NG | |||
| 144 | NG | NG | |||
| 192 | G | NG | |||
| 240 | G | NG | |||
| 384 | G | G | |||
| 1.5 | 24 | NG | NG | ||
| 96 | NG | NG | |||
| 144 | NG | NG | |||
| 192 | G | NG | |||
| 240 | G | NG | |||
| 384 | G | G | |||
| 15 | 0 | 1 | 6 | NG | NG |
| 12 | NG | NG | |||
| 24 | NG | NG | |||
| 48 | G | G | |||
| 96 | G | G | |||
| 120 | G | G | |||
| 1.25 | 6 | NG | NG | ||
| 12 | NG | NG | |||
| 24 | NG | NG | |||
| 48 | G | G | |||
| 96 | G | G | |||
| 120 | G | G | |||
| 1.5 | 6 | NG | NG | ||
| 12 | NG | NG | |||
| 24 | NG | NG | |||
| 48 | NG | NG | |||
| 96 | G | G | |||
| 120 | G | G | |||
| 10 | 1 | 24 | G | NG | |
| 48 | G | G | |||
| 72 | G | G | |||
| 96 | G | G | |||
| 120 | G | G | |||
| 1.25 | 24 | NG | NG | ||
| 48 | G | G | |||
| 72 | G | G | |||
| 96 | G | G | |||
| 120 | G | G | |||
| 1.5 | 24 | NG | NG | ||
| 48 | G | NG | |||
| 72 | G | G | |||
| 96 | G | G | |||
| 120 | G | G |
In conclusion, processed meat products containing low concentrations of NaNO2 and NaCl should be stored below 7°C to inhibit Salmonella growth, regardless of the atmospheric conditions. However, if the products must be stored above 7°C, low NaNO2 concentrations should be combined with NaCl to inhibit Salmonella growth under both aerobic and anaerobic conditions. In addition, the developed probabilistic model is appropriate for predicting the growth probability of Salmonella in processed meat products formulated with NaNO2 and NaCl, which should be useful for describing the effect of NaNO2 and NaCl on Salmonella growth in processed meat products.
ACKNOWLEDGMENTS
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009237)” Rural Development Administration, Republic of Korea.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- Aguilera JM, Karel M. Preservation of biological materials under desiccation. Crit Rev Food Sci Nutr. 1997;37:287–309. doi: 10.1080/10408399709527776. [DOI] [PubMed] [Google Scholar]
- Beery JT, Hugdahl MB, Doyle MP. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl Environ Microb. 1988;54:2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busani L, Cigliano A, Taioli E, Caligiuri V, Chiavacci L, Di Bella C, Battisti A, Duranti A, Gianfranceschi M, Nardella MC, Ricci A, Rolesu S, Tamba M, Marabelli R, Caprioli A Italian Group of Veterinary Epidemiology (GLEV) Prevalence of Salmonella enterica and Listeria monocytogenes contamination in foods of animal origin in Italy. J Food Prot. 2005;8:1556–1775. doi: 10.4315/0362-028x-68.8.1729. [DOI] [PubMed] [Google Scholar]
- Christiansen LN, Johnston RW, Kautter DA, Howard JW, Aunan WJ. Effect of nitrite and nitrate on toxin production by Clostridium botulinum and on nitrosamine formation in perishable canned comminuted cured meat. Appl Environ Microbiol. 1973;25:357–362. doi: 10.1128/am.25.3.357-362.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. [Accessed August 1, 2015];EFSA J. 2013 11:3129. http://www.efsa.europa.eu/en/efsajournal/doc/3129.pdf. [Google Scholar]
- Fares A. Quantitative risk assessment model of human salmonellosis linked to the consumption of Camembert cheese made from raw milk. AgroPrisTech; 2007. [Acessed August 2, 2015]. https://tel.archives-ouvertes.fr/pastel-00003463/document. [Google Scholar]
- Frolich ED. Risk mechanisms in hypertensive heart disease. Hypertension. 1999;34:782–789. doi: 10.1161/01.hyp.34.4.782. [DOI] [PubMed] [Google Scholar]
- Gwak E, Oh M, Park B, Lee H, Lee S, Ha J, Lee J, Kim S, Choi K-H, Yoon Y. Probabilistic models to predict Listeria monocytogenes growth at low concentrations of NaNO2 and NaCl in frankfurters. Korean J Food Sci An. 2015;35:815–823. doi: 10.5851/kosfa.2015.35.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izat AL, Driggers CD, Colberg M, Reiber MA, Adams MH. Comparison of the DNA probe to culture methods for the detection of Salmonella on poultry carcasses and processing waters. J Food Prot. 1989;52:564–570. doi: 10.4315/0362-028X-52.8.564. [DOI] [PubMed] [Google Scholar]
- Jantsch J, Chikkaballi D, Hensel M. Cellular aspects of immunity to intracellular Salmonella enterica. Immunol Rev. 2011;240:185–195. doi: 10.1111/j.1600-065X.2010.00981.x. [DOI] [PubMed] [Google Scholar]
- Jira W. Chemical reactions of curing and smoking. Fleischwirtschaft. 2004;84:235–239. [Google Scholar]
- Jo H, Park B, Oh M, Gwak E, Lee H, Lee S, Yoon Y. Probabilistic models to predict the growth initiation time for Pseudomonas spp. in processed meats formulated with NaCl and NaNO2. Korean J Food Sci An. 2014;34:736–741. doi: 10.5851/kosfa.2014.34.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Lee ES, Jeong JY, Choi JH, Choi YS, Han DJ, Lee MA, Kim SY, Kim CJ. Effect of bamboo salt on the physicochemical properties of meat emulsion systems. Meat Sci. 2010;86:960–965. doi: 10.1016/j.meatsci.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Kostick DS. [Accessed December 23, 2014];Salt, US Geological Survey, 2008 Minerals Yearbook. 2010 http://minerals.usgs.gov/minerals/pubs/commodity/salt/myb1-2008-salt.pdf.
- Koutsoumanis KP, Kendall PA, Sofos JN. Modeling the boundaries of growth of Salmonella Typhimurium in broth as a function of temperature, water activity, and pH. J Food Prot. 2004;67:53–59. doi: 10.4315/0362-028x-67.1.53. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee H, Lee JY, Skandamis PN, Park BY, Oh MH, Yoon Y. Mathematical models to predict kinetic behavior and growth probabilities of Listeria monocytogenes on pork skin at constant and dynamic temperatures. J Food Prot. 2013;76:1868–1872. doi: 10.4315/0362-028X.JFP-13-197. [DOI] [PubMed] [Google Scholar]
- Maekawa A, Ogiu T, Onodera H, Furuta K, Matsuoka C, Ohmo Y, Odashima S. Carcinogenicity studies of sodium nitrite and sodium nitrate in F-344 rats. Food Chem Toxicol. 1982;20:25–33. doi: 10.1016/s0278-6915(82)80005-7. [DOI] [PubMed] [Google Scholar]
- Meyer C, Thiel S, Ullrich U, Stolle A. Salmonella in raw meat and by products from pork and beef. J Food Prot. 2010;73:1780–1784. doi: 10.4315/0362-028x-73.10.1780. [DOI] [PubMed] [Google Scholar]
- Møller COA. Ph D Thesis. Technical University of Denmark; Lyngby, Denmark: 2012. The Transfer and Growth of Salmonella Modelled during Pork Processing and Applied to a Risk Assessment for the Catering Sector. [Google Scholar]
- Munasinghe DM, Sakai T. Sodium chloride as a preferred protein extractant for pork lean meat. Meat Sci. 2004;67:697–703. doi: 10.1016/j.meatsci.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Pelroy G, Peterson M, Paranjpye R, Almond J, Eklund M. Inhinition of Listeria monocytogenes in cold-process (smoked) salmon by sodium nitrite and packaging method. J Food Prot. 1994;57:114–119. doi: 10.4315/0362-028X-57.2.114. [DOI] [PubMed] [Google Scholar]
- Perry IJ, Beevers DG. Salt intake and stroke: A possible direct effect. J Hum Hypertens. 1992;6:23–25. [PubMed] [Google Scholar]
- Pin C, Avendaño-Perez G, Cosciani-Cunico E, Gómez N, Gounadakic A, Nychas GJ, Skandamis P, Barker G. Modelling Salmonella concentration throughout the pork supply chain by considering grow th and survival in fluctuating conditions of temperature, pH and aw. Int J Food Microbiol. 2011;145:S96–S102. doi: 10.1016/j.ijfoodmicro.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Ratkowsky DA, Ross T, McMeekin TA, Olley J. Comparison of Arrhenius-type and Belehradek-type models for prediction of bacterial growth in foods. J Appl Microbiol. 1991;71:452–459. [Google Scholar]
- Ratkowsky DA, Ross T. Modelling the bacterial growth/no growth interface. Lett Appl Microbiol. 1995;20:29–33. [Google Scholar]
- Schaffner DW, Labuza TP. Predictive microbiology: Where are we, and where are we going? Food Technol. 1997;51:95–99. [Google Scholar]
- University of Nebrask Cooperative Extension. [Accessed August 2, 2015];Salmonella. 2005 http://www.foodsafety.unl.edu/pathogens/salmonella.html.
- Yoon Y, Kendall PA, Belk KE, Scanga JA, Smith GC, Sofos JN. Modeling the growth/no-growth boundaries of postprocessing Listeria monocytogenes contamination on frankfurters and bologna treated with lactic acid. Appl Environ Microbiol. 2009;75:353–358. doi: 10.1128/AEM.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voetsch AC, Van Gilder TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, Cieslak RR, Deneen VC, Tauxe RV Emerging Infections Program FoodNet Working Group. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis. 2004;38:S127–S134. doi: 10.1086/381578. [DOI] [PubMed] [Google Scholar]


