Abstract
The aim of this study was to evaluate the effect of replacing corn grain for sugar cane molasses (MO) or glucose syrup (GS) in the starter concentrate on performance and metabolism of dairy calves. Thirty-six individually housed Holstein male calves were blocked according to weight and date of birth and assigned to one of the starter feed treatments, during an 8 week study: i) starter containing 65% corn with no MO or GS (0MO); ii) starter containing 60% corn and 5% MO (5MO); iii) starter containing 55% corn and 10% MO (10MO); and iv) starter containing 60% corn and 5% GS (5GS). Animals received 4 L of milk replacer daily (20 crude protein, 16 ether extract, 12.5% solids), divided in two meals (0700 and 1700 h). Starter and water were provided ad libitum. Starter intake and fecal score were monitored daily until animals were eight weeks old. Body weight and measurements (withers height, hip width and heart girth) were measured weekly before the morning feeding. From the second week of age, blood samples were collected weekly, 2 h after the morning feeding, for glucose, β-hydroxybutyrate and lactate determination. Ruminal fluid was collected at 4, 6, and 8 weeks of age using an oro-ruminal probe and a suction pump for determination of pH and short-chain fatty acids (SCFA). At the end of the eighth week, animals were harvested to evaluate development of the proximal digestive tract. The composition of the starter did not affect (p>0.05) concentrate intake, weight gain, fecal score, blood parameters, and rumen development. However, treatment 5MO showed higher (p<0.05) total concentration of SCFAs, acetate and propionate than 0MO, and these treatments did not differ from 10MO and 5GS (p>0.05). Thus, it can be concluded that the replacement of corn by 5% or 10% sugar cane molasses or 5% GS on starter concentrate did not impact performance, however it has some positive effects on rumen fermentation which may be beneficial for calves with a developing rumen.
Keywords: By-product, Sucrose, Butyrate, Ruminal Development
INTRODUCTION
Corn is the main energy source used in the formulation of starters for calves, since it presents approximately 67% starch in its composition (Nocek and Tamminga, 1991). Starch is a non-structural carbohydrate with extensive and fast degradability generating high production of short chain fatty acid (SCFA) per unit time (Noziére et al., 2010). Fermentation on starch may result in increased lactic acid production, dramatically decreasing rumen pH, which decreases starter intake by dairy calves. However, the price of corn varies during the year, reaching the highest values in the offseason, burdening production costs. Because of this, alternatives for corn inclusion in starters may be interesting by the economic and production point of view.
Molasses is a by-product of the ethanol industry, consisting mainly of sugars quickly and extensively fermented in the rumen (Noziére et al., 2010), with approximately 93% of total digestible nutrients (TDN) of the corn (NRC, 2001). On the other hand, glucose syrup is a by-product of corn industry, composed of glucose and maltose, with 90.9% of TDN in dry matter (DM), higher than the corn, which lies close to 84.9% (NRC, 2001).
Molasses has been included in starter for milk-fed dairy calves not only for being a cheaper source of readily fermentable carbohydrate, but also by having positive impact on intake and assisting in particle agglutination in the concentrate (Hill et al., 2008). In addition, there are reports that molasses can increase the production of butyrate in the rumen (Kellogg and Owen, 1969a; Martel et al., 2011) and thus accelerate the ruminal development, since that SCFA has an important role in the growth of rumen papillae (Tamate et al., 1962). The rapid and efficient transition from pre-ruminant (liquid diet) to functional ruminant (only solid diet) has substantial economic importance to producers.
Few studies have been conducted to elucidate the effects of partial replacement of corn by sugarcane molasses in the concentrate for milk-fed calves. On the other hand, there are no published studies using glucose syrup in the starter feed for this animal class. Thus, the objective of this work was to evaluate different levels of sugar cane molasses and glucose syrup to replace corn in the starter feed on performance and metabolism of calves.
MATERIAL AND METHODS
Thirty-six newborn Holstein calves were fed approximately 6 L of colostrum, during the first 12 hours after birth, receiving milk-replacer thereafter. After the colostrum feeding period, calves were individually housed in wood hutches (1.00×1.45 m) distributed in a grass field, at the experimental calf facility of the Department of Animal Science of the “Luiz de Queiroz” College of Agriculture, University of Sao Paulo. Study was conducted from February to June of 2011, with calves born from February to April. All calves were individually fed and received 4 L of milk replacer daily (Sprayfo Violeta, 20% crude protein (CP), 16% ether extract (EE), 12.5% solids, Sloten of Brazil Ltd., Santos, SP, Brazil). Calves were fed milk replacer in two equal feedings at 0700 and 1700 h and had free access to water and starter feed. Calves were blocked by weight and date of birth and assigned to one of the four starter feed compositions (9 calves/treatment), during an 8 week study: 0MO (0% MO and 65% corn), 5MO (5% of MO and 60% corn), 10MO (10% of MO and 55% corn) and 5GS (5% of GS and 60% corn).
Corn grain was ground to reach particle size close to 2 mm and blended with the other ingredients of the concentrate diets using a horizontal mixer (Lucato, Limeira, Brazil), resulting in a coarsely ground physical form. All starter concentrates were formulated according to NRC (2001) to have the same crude protein (CP) and minerals concentration (Table 1). The starter feed was supplied ad libitum and every morning remains were weighed to obtain the daily starter feed intake. Weaning was abruptly performed at the 8th week of age, when trial ended. Animals were weighed weekly, before the morning milk feeding, on a mechanical scale (ICS-300, Coimma Ltd. Piracicaba, SP, Brasil), and measurements of withers height, heart girth and hip width were taken. Every morning animal’s feces were scored, as described by Larson et al. (1977), on a scale from 1 to 4 (1 = normal; 2 = loose; 3 = very loose, no watery separation; and 4 = very watery). To avoid variations a sole observer scored feces according to this scale, after being trained with the aid of pictures.
Table 1.
Ingredients and chemical composition of starter
| Items | Treatments1 | |||
|---|---|---|---|---|
|
| ||||
| 0MO | 5MO | 10MO | 5GS | |
| Ingredients (% DM) | ||||
| Corn | 65 | 60 | 55 | 60 |
| Soybean meal | 24 | 24 | 23 | 24 |
| Sugarcane molasses | 0 | 5 | 10 | 0 |
| Glucose syrup | 0 | 0 | 0 | 5 |
| Soybean hulls | 10 | 10 | 11 | 10 |
| Mineral/vitamin premix2 | 1 | 1 | 1 | 1 |
| Chemical composition | ||||
| Dry matter (% fed basis) | 88.0 | 87.4 | 87.8 | 87.9 |
| Ash (% DM) | 4.2 | 4.7 | 4.7 | 4.1 |
| Crude protein (% DM) | 19.6 | 19.9 | 19.3 | 19.5 |
| Ether extract (% DM) | 4.1 | 4.1 | 3.4 | 3.9 |
| NDF (% DM) | 16.4 | 18.8 | 18.7 | 16.9 |
| ADF (% DM) | 9.4 | 10.8 | 11.5 | 10.3 |
| N-NDF (% total N) | 12.2 | 8.6 | 10.1 | 7.9 |
| N-ADF (% total N) | 5.6 | 3.9 | 2.6 | 3.6 |
| Lignin (% DM) | 0.75 | 0.72 | 0.94 | 0.82 |
| NFC (% DM) | 55.7 | 52.5 | 53.9 | 55.6 |
| ME (Mcal/kg DM)3 | 3.00 | 3.00 | 2.98 | 3.00 |
| Net energy for gain (Mcal/kg DM)3 | 1.93 | 1.92 | 1.91 | 1.92 |
DM, dry matter; NDF, neutral detergent fiber; ADF, acid detergent fiber; N-NDF, nitrogen in NDF; N-ADF, nitrogen in ADF; NFC, non-fibrous carbohydrate; ME, metabolizable energy.
0MO, no molasses; 5MO, 5% molasses; 10MO, 10% molasses; 5GS, 5% glucose syrup.
Mineral/vitamin premix composition: Ca 16.8%; P 4.2%; S 2.3%; Na 11.6%; Cl 8.0%; Mg 2.4; Co 38.2 ppm; Cu 343 ppm; I 30.2 ppm; Fe 578.2 ppm; Mn 1,146.4 ppm; Se 15.5 ppm; Zn 1,176.2 ppm; Vit. A 68,760 UI/kg; Vit. E 764 UI/kg; Vit. D 57,300 IU/kg.
Value estimated by NRC (2001).
Ruminal fluid samples were collected at the 4th, 6th, and 8th week of age, using an oro-esophageal tube and a vacuum pump (TE-0581, Tecnal Ltd. Piracicaba, SP, Brazil), with the first fraction of fluid collected discarded to avoid saliva. Samples (50 mL) were filtered through appropriate cloth tissue (around 8 layers of surgical gauze) and pH was immediately measured (Tec-5, Tecnal Ltda., Brasil). Samples were frozen for latter analyses of SCFA. Samples were centrifuged at 15,000×g (Universal 320R, Hettich, Tuttlinger, Germany) for determination of SCFA, as described by Ferreira et al. (2009). Samples were analyzed by gas chromatograph (Hewlett Packard 5890 Series II GC, Wilmington, DE, USA) equipped with integrator (Hewlett Packard 3396 Series II Integrator, USA), and automatic injector (Hewlett Packard 6890 Series Injector, USA). A volume of 100 μL of the internal standard, 2-methylbutyric acid, 800 μL of sample and 200 μL sample of formic acid were pipette in a vial for gas chromatograph injection. A mixture of short-chain fatty acids of known concentration was used as external standard for calibration.
Blood samples were taken weekly, two hours after morning feeding, via jugular venipuncture by vacuum tubes containing sodium fluoride and potassium EDTA (Vacuette of Brazil, Campinas, SP, Brazil). Samples were centrifuged at 2,000×g, (20 min at 4°C) and plasma was stored until analysis. Specific enzymatic kits were used to analyzed plasma concentrations of glucose (Glicose HK Liquiform – Ref.: 85, Labtest Diagnóstica S.A., Lagoa Santa, MG, Brazil) and β-hydroxybutyrate (RANBUT – Ref.: RB1007, Randox Laboratories, Crumlin, UK) in an automatic biochemistry system (SBA-200, CELM, Barueri, SP, Brazil).
At weaning, with 8 weeks of age, animals were slaughtered by stunning and bleeding, to evaluate development of the upper digestive tract and rumen papillae. The compartments of the stomach (rumen, reticulum, omasum, and abomasum) were separated and weighed individually. Reticulum-rumen had its maximum volume measured by filling it with water. Samples from the ventral sac of the rumen were collected and preserved in 10% formaldehyde solution. Number, height and width of papillae (cm2) were measured as by Lesmeister and Heinrichs (2004), through a stereoscopic microscope equipped with a scale.
Samples of starter feed were periodically sampled and ground through a 1-mm mesh for dry matter (DM), ashes, and EE determination according to AOAC (1990); nitrogen was determined by combustion, according to the Dumas method, using an N analyzer by LECO, model FP-528 (St. Joseph, MI, USA) and CP was calculated by multiplying results by 6.25; free-ash neutral detergent fiber (NDF), acid detergent fiber (ADF) and lignin were determined, according to the method of Van Soest et al. (1991), using sodium sulfite and thermo stable amylase when required; and nitrogen in NDF and ADF were determined according to Licitra et al. (1996). The TDN values were calculated according to the equation proposed by Weiss (1993) and non-fiber carbohydrates (NFC) by the equation: NFC = 100 − (crude protein+ether extract+neutral detergent fibercp+ashes), expressed as g/kg DM, being NDFcp the NDF free of crude protein.
Data concerning concentrate intake, body weight, average daily gain, body measurements, as well as ruminal and plasma parameters were analyzed as repeated measurements using the PROC MIXED from SAS software according to the model (1). The best covariance structure was identified from different covariance structures (arma (1, 1), ar (1); arh (1); Toep; Toeph; UN; CS) by comparing the AICC statistic (Akaike Information Criteria Corrected). Differences were considered significant at p<0.05 unless otherwise stated. Data for morphometric measurements of the proximal digestive tract and development of the rumen (papillae) were performed by the model (2), using the general linear model from SAS. Significance was adopted for values of p<0.05 for all parameters.
| (1) |
| (2) |
Where, Yijk is the response variable, μ is the overall mean, Ti is the treatment effect, Bj is the block effect, Wk is the age effect, TiWk is the interaction of treatment and age effects, and Eijk is the residual effect.
RESULTS AND DISCUSSION
Starter intake was not affected (p>0.05) by the substitution of corn by molasses or glucose syrup, as well as by age or by the interaction of these factors (Table 2). All treatments resulted in concentrate intake higher than 700 g/d at weaning, considered suitable for Holstein calves subjected to early weaning (Quigley, 1996). It is recommended that calves present this level of intake at weaning, because it guarantees a minimum level of rumen development that allows calves to maintain weight gain after the interruption of liquid diet feeding. Concentrate intake is closely linked to production of SCFA, which are the main stimulators of development of rumen epithelium.
Table 2.
Effect of sugarcane molasses and glucose syrup as a replacement for corn in the starter feed of dairy calves on average starter intake, body weight, average daily gain and gain of body measurements
| Treatments1 | SEM | p2 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0MO | 5MO | 10MO | 5GS | T | A | T×A | ||
| Starter intake (g/d) | 261.8 | 396.0 | 443.8 | 323.9 | 68.88 | 0.54 | 0.16 | 0.57 |
| Body weight (kg) | 39.6 | 40.8 | 42.8 | 40.3 | 1.207 | 0.30 | <0.001 | 0.21 |
| Average daily gain (g) | 256.7 | 289.6 | 298.5 | 214.4 | 52.68 | 0.70 | <0.001 | 0.11 |
| Withers height gain (cm/wk) | 0.74 | 0.84 | 0.54 | 0.75 | 0.146 | 0.50 | <0.001 | 0.46 |
| Heart girth gain (cm/wk) | 1.18 | 1.38 | 1.56 | 1.24 | 0.213 | 0.58 | <0.001 | 0.12 |
| Hip width gain (cm/wk) | 0.30 | 0.37 | 0.37 | 0.27 | 0.057 | 0.51 | <0.001 | 0.67 |
SEM, standard error of the mean.
0MO, no molasses; 5MO, 5% molasses; 10MO, 10% molasses; 5GS, 5% glucose syrup.
T, treatment effect; A, age (week) effect; T×A, treatment and age interaction effect.
It was expected that the mean intake of starter containing sugar cane molasses treatments were higher than that observed for the control treatment, since this ingredient has been used to enhance palatability in the diet (Hill et al., 2008). Likewise, it was believed that the glucose syrup would also increase the starter intake, because it is basically composed of glucose and maltose, carbohydrates with fast ruminal fermentation and intestinal absorption. However, no significant differences for the starter intake were observed. Similar results to the present study were reported by Hill et al. (2008), who replaced the corn for molasses in the starter feed for Holstein calves weaned at 42 days and found no differences (p>0.05) in the consumption of calves receiving 5 (454 g/d) or 10% (381 g/d) of molasses. Lesmeister and Heinrichs (2005) evaluated the inclusion of molasses in the concentrate for calves from the first to the sixth week of age and observed a decrease in intake when inclusion was increased from 5% (509 g/d) to 12% (396 g/d). However, there was no difference (p>0.05) for weight gain and final body weight for all treatments (Table 2).
Starter composition had no effect (p>0.05) on daily gain at weaning and average daily gain. There was also no interaction between starter composition and age of calves for these variables (Table 2). However, there was an increase (p<0.01) in average daily gain as animals aged (Table 2). Weight loss was observed in all treatments between the first and second week, which may have been associated with low starter intake at this stage (Table 2) and the occurrence of diarrhea (Figure 1), associated with the low milk replacer feeding volume. According to Van Amburgh and Drackley (2005), calves fed 4 L of milk replacer requires the energy of 75% of total intake just for maintenance, which may affect body weight gain negatively, mainly at early ages as a result of the low starter intake during this period.
Figure 1.
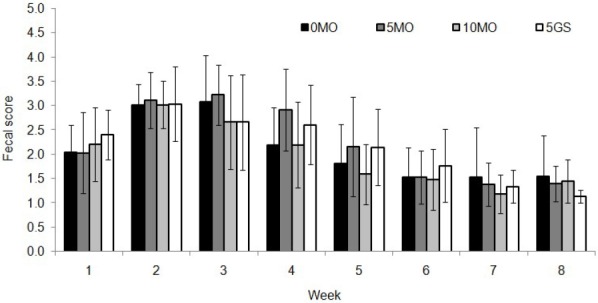
Fecal scores according to week of age, of calves with supplemented of sugarcane molasses or glucose syrup in replacing corn. 0MO, no molasses; 5MO, 5% molasses; 10MO, 10% molasses; 5GS, 5% glucose syrup.
Starter composition did not affect (p>0.05) initial, final or average weight (Table 2). However, there was an age effect (p<0.01), with increasing body weight over the course of weeks (Table 2). These results were expected, since the starter intake has increased considerably with the passage of weeks and did not differ among treatments during the experimental period.
Withers height, heart girth and hip width (Table 2) were not affected by the substitution of corn by molasses or glucose syrup, or by the interaction of this factor with age (p>0.05). However significant effects of animal age (p<0.001) for those body measurements were observed, increasing as animals aged. Withers height was lower than the standard for Holstein calves, which is 79.4 cm (±3.3 cm) (Heinrichs and Losinger, 1998). This may explain the lower withers height gain compared to that suggested by Hoffman (1997), which is between 1.3 and 1.4 cm per week until 2 months of age. However, other authors have reported similar values to those of the present study to assess the withers height of calves fed starter feed containing molasses (Hill et al., 2008) and animals with similar body frame (Ferreira and Bittar, 2010).
Fecal score was not affected (p>0.05) by the starter composition (Figure 1). Likewise, the interaction of starter composition and age was not significant (p>0.05). However, there was an age effect (p<0.001), with decreasing fecal score over the weeks. It was believed that the inclusion of molasses in the starter concentrate could increase the incidence of scores indicative of diarrhea, since there is no sucrase activity in calves up to 44 days (Huber et al., 1961), which could impair the use of sucrose with a consequent increase of osmotic pressure in the intestine.
Animals fed starter concentrate containing 5% or 10% molasses replacing corn tended (p<0.10) to have lower ruminal pH than others groups (Table 3). This result is probably due to the fast production of SCFA, since this ingredient has a high rumen fermentation rate (Oliveira et al., 2003). Thus, diets containing molasses can lead to a faster production of SCFA when compared to corn and, therefore, decreasing the pH of the rumen fluid. However, the same results were expected for calves consuming concentrate containing glucose syrup, since glucose and maltose, the main components of this ingredient, have high fermentation rate. The relatively low pH values found in this study may be related to the consumption of considerable amounts of concentrate. According to Quigley (1996), ruminal pH varies with the rate at which SCFA and ammonia are produced and absorbed by the rumen wall or other microorganisms. Since milk-fed calves do not have a fully developed rumen papillae, the absorption area is small and therefore, there may be an accumulation of SCFA in ruminal fluid, resulting in a drop in pH (NRC, 2001). Age and the interaction of age and starter composition presented a significant effect on rumen pH (Table 3). As animals aged and starter intake increased, rumen pH decreased because of higher fermentation end products.
Table 3.
Effect of sugarcane molasses and glucose syrup as a replacement for corn in the starter feed of dairy calves on mean values of pH and short chain fatty acids
| Treatments1 | SEM | p2 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0MO | 5MO | 10MO | 5GS | T | A | T×A | ||
| pH | 5.59 | 5.14 | 5.14 | 5.26 | 0.138 | 0.09 | 0.01 | 0.01 |
| Short chain fatty acids (mmol/L) | ||||||||
| Acetic | 57.63b | 68.00a | 63.66ab | 60.94ab | 2.515 | 0.04 | 0.01 | 0.17 |
| Propionic | 33.65b | 48.07a | 44.69ab | 38.20ab | 3.577 | 0.03 | 0.01 | 0.40 |
| Butyric | 7.81 | 10.66 | 12.77 | 11.90 | 1.376 | 0.07 | 0.01 | 0.06 |
| Total | 104.2b | 132.3a | 127.6ab | 116.8ab | 7.177 | 0.04 | 0.01 | 0.08 |
| Acetic:propionic | 2.11 | 1.60 | 1.53 | 1.89 | 0.184 | 0.11 | 0.01 | 0.53 |
| Ammonia-N | 18.03 | 16.18 | 19.30 | 16.36 | 1.889 | 0.58 | 0.01 | 0.39 |
SEM, standard error of the mean.
0MO, no molasses; 5MO, 5% molasses; 10MO, 10% molasses; 5GS, 5% glucose syrup.
T, treatment effect; A= age (week) effect; T×A = treatment and age interaction effect.
Lower letters in the same row differ for p<0.05.
Animals fed starter concentrate containing 5% molasses replacing corn had higher (p<0.05) molar total SCFA than those fed 0MO. However, due to the higher rate and extent of fermentation of sugarcane molasses and glucose syrup when compared to corn, higher values were expected for molar SCFA concentration in ruminal fluid of 10MO and 5GS fed animals. Lesmeister and Heinrichs (2005) reported higher plasma concentration of total SCFA in calves fed 12% of sugarcane molasses in the starter feed compared to those who received 5%. According to these authors, a higher plasma concentration of SCFA indicates higher metabolic activity of ruminal epithelium (absorptive rate) or increase in the production of these compounds in the rumen resulting from a more digestible diet.
Similar results were observed for individual SCFA molar concentrations (Table 3), with higher values (p<0.05) for calves fed 5MO than Control calves, with no differences among other treatments. A higher concentration of propionate was expected for animals fed sugarcane molasses or glucose syrup, because of the faster and more extensive fermentation of these ingredients. However, there may have been greater lactic acid production (not measured), which would explain the reduction in ruminal pH with increasing age of the animals. Ruminal butyrate molar concentration tended to be higher (p = 0.07) in animals receiving 10% molasses replacing corn in the starter concentrate as compared to animals fed 0MO (Table 3). This result is of great importance in the nutrition of calves, since this SCFA is primarily responsible for the growth of rumen papillae (Quigley, 1996). Increases in the concentration of butyrate concentration by adding molasses or sucrose are described in the literature for both in vitro (Kellogg and Owen, 1969b) and in vivo (Martel et al., 2011) studies.
Even though there was an age effect for total and individual SCFA rumen molar concentration, there was no significant interaction of age and concentrate composition (p<0.05). Similarly as for the total SCFA concentration, these results are a response to the increasing starter concentrate intake from the fourth to the sixth and the eighth weeks of age. Similar results are usually found in the literature, since SCFA are generated by the fermentation of organic matter present in the rumen, which increases as animals increase the consumption of solid feed.
The concentration of ammonia-N in rumen fluid was not affected (p>0.05) by the inclusion of sugarcane molasses or glucose syrup replacing corn in the starter concentrate (Table 3). Even though there was an age effect (p<0.01), with decreasing values as animals aged, there was no interaction between age and starter composition. As the rumen develops and is colonized by microorganisms and there is a proper supply of nutrients to ferment, there is an increase in the ability to use and recycle ammonia-N, resulting in decreased values of rumen ammonia-N concentration. The concentration of ammonia-N remained above 5 mg/dL in all samples, suggesting that there was sufficient nitrogen for proper microbial growth. According to Leng and Nolan (1984), 5 mg/dL is the minimum concentration required to satisfactory microbial protein synthesis.
Plasma concentrations of glucose and β-hydroxybutyrate (βHBA) were not affected by inclusion of co-products replacing corn in the concentrate, nor by the age effect or the interaction between age and starter composition (Table 4). Glucose plasma concentration usually decreases as animal’s age (Quigley et al., 1991; Haga et al., 2008) and rumen develops as a result of solid feed intake. According to Haga et al. (2008), glucose is the primary energy source for calves with an undeveloped rumen, with lactose from the liquid diet being the main supply. As animals increase starter intake and the rumen develops, there is a very low absorption of glucose from the diet, and most of plasma glucose has its origin on hepatic gluconeogenesis from propionate. From that point on, animals rely mostly on ketone bodies as energy source, mainly in peripheral tissues (Haga et al., 2008).
Table 4.
Effect of sugarcane molasses and glucose syrup as a replacement for corn in the starter feed of dairy calves on mean values of plasma glucose and β-Hydroxybutyric acid concentration
| Treatments1 | SEM | p2 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0MO | 5MO | 10MO | 5GS | T | A | T×A | ||
| Glucose (mg/dL) | 96.5 | 101.8 | 95.9 | 92.3 | 3.078 | 0.16 | 0.48 | 0.17 |
| βHBA (mmol/L) | 0.14 | 0.14 | 0.13 | 0.13 | 0.012 | 0.97 | 0.86 | 0.80 |
| Lactate (mg/dL) | 9.16 | 9.65 | 9.47 | 10.06 | 0.804 | 0.88 | 0.66 | 0.63 |
SEM, standard error of the mean; βHBA, β-hydroxybutyrate.
0MO, no molasses; 5MO, 5% molasses; 10MO, 10% molasses; 5GS, 5% glucose syrup.
T, treatment effect; A, age (week) effect; T×A, treatment and age interaction effect.
In addition, an increase in the concentration of βHBA with advancing age of the animals was expected, since there was an increase in the starter intake from the fourth week of age (Figure 1) and a consequent increase in the molar concentration of butyrate in ruminal fluid. The concentration of βHBA is highly correlated with the starter intake (Quigley et al., 1991) and has been used as a parameter for monitoring rumen development. β-hydroxybutyrate is the product of butyrate metabolism by rumen epithelial cells (ketogenesis), but also may undergo oxidative metabolism through β-oxidation and the citric acid cycle (Wiese et al., 2013).
According to Davis and Drackley (1998), propionic and butyric acids are the primary stimulators of growth of rumen tissue, in part because they are extensively metabolized by the rumen epithelium during absorption. This metabolism provides energy for growth of epithelial tissue and muscle contractions. However, even though there were some differences in SCFA concentrations (Table 3), no effects were observed for rumen development as a result of inclusion of sugarcane molasses replacing corn in the concentrate (Table 5). Higher molar concentration of total SCFA and propionic acid for animals fed 5MO (Table 3), as compared to animals fed 0MO, had no effect on rumen development. There was no effect on rumen development probably because the increase on total SCFA and propionic acid was accompanied by increased acetate molar concentration, with no effect on the acetate:propionate ratio (Table 3).
Table 5.
Effect of sugarcane molasses and glucose syrup as a replacement for corn in the starter feed of dairy calves on mean values of forestomach morphometrics
| Treatments1 | SEM | p | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0MO | 5MO | 10MO | 5GS | |||
| Total tract (g) | 1,153.9 | 1,433.0 | 1,306.5 | 1,161.1 | 106.92 | 0.25 |
| Rumen-reticulum (g) | 764.9 | 973.4 | 871.7 | 752.8 | 84.33 | 0.26 |
| % of total tract | 65.77 | 66.34 | 65.65 | 64.23 | 1.977 | 0.91 |
| Capacity (L) | 8.31 | 9.39 | 8.50 | 7.85 | 0.888 | 0.68 |
| Omasum (g) | 167.2 | 207.0 | 184.8 | 144.9 | 21.32 | 0.28 |
| % of total tract | 14.40 | 14.42 | 13.59 | 12.55 | 1.109 | 0.65 |
| Abomasum(g) | 226.7 | 260.2 | 250.1 | 263.3 | 11.80 | 0.18 |
| % of total tract | 20.26 | 19.66 | 20.79 | 23.12 | 1.850 | 0.62 |
| Papillae number (number/cm2) | 94.1 | 75.2 | 74.2 | 87.3 | 7.17 | 0.18 |
| Papillae height (mm) | 1.69 | 2.03 | 3.38 | 1.76 | 0.565 | 0.13 |
| Papillae width (mm) | 1.00 | 1.10 | 1.36 | 1.10 | 0.209 | 0.62 |
| Papillae area (cm2) | 1.93 | 2.57 | 5.25 | 2.00 | 1.258 | 0.21 |
SEM, standard error of the mean.
0MO, no molasses; 5MO, 5% molasses; 10MO, 10% molasses; 5GS, 5% glucose syrup.
Reticulum-rumen weight and its proportion of the total tract weight as well as its volume were also not affected by starter composition (p>0.05). Those values are higher than those found by Nussio et al. (2003) and Ferreira et al. (2009), with similar animals and experimental design, most likely due to the higher starter intake observed in the present study. According to Quigley (1996), the reticulum-rumen of a four-week-old calf should correspond to 60% of the total weight of the forestomach, the omasum to 13% and the abomasum to 27%. Values observed are higher for the reticulum-rumen and lower for the abomasum proportion, probably due to the higher age at slaughter and higher concentrate intake as compared to a four-week-old calf. Papillae measurements were not affected by inclusion of sugarcane molasses or glucose syrup as a replacement for corn in the concentrate (Table 5). Other researchers reported a trend of greater height and width of papillae of calves fed 12% molasses in the concentrate compared to those receiving 5% (Lesmeister and Heinrichs, 2005). Values observed are lower than those suggested by Huber (1969) for calves eight weeks old (5 to 7 mm).
CONCLUSION
Replacement of corn by 5% or 10% of sugarcane molasses, or by 5% glucose syrup in the starter concentrate had no effect on performance, fecal score or rumen development. However, feeding molasses increased total SCFA and propionic acid concentration, which may be beneficial for calves with a developing rumen. Therefore, these energy sources may be included in the starter concentrate, in these substitution rates, for dairy calves during the liquid-feeding phase.
ACKNOWLEDGMENTS
The authors wish to express their appreciation for the financial support provided by the CNPq and FAPESP.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- AOAC. Official Methods of Analysis. 18th edn. AOAC; Arlington, VA, USA: 1990. [Google Scholar]
- Davis CL, Drackley JK. The Development Nutrition and Management of the Young Calf. Iowa State University Press; Ames, Iowa, USA: 1998. [Google Scholar]
- Ferreira LS, Bittar CMM. Performance and plasma metabolites of dairy calves fed starter containing sodium butyrate, calcium propionate or sodium monensin. Animal. 2010;5:239–245. doi: 10.1017/S1751731110001965. [DOI] [PubMed] [Google Scholar]
- Ferreira LS, Bittar CMM, Santos VP, Mattos WRS, Pires AV. Effect of inclusion of sodium butyrate, calcium propionate or sodium monensin in the starter feed on ruminal parameters and forestomach development in dairy calves. Braz J Anim Sci. 2009;38:2238–2246. [Google Scholar]
- Haga S, Fujimoto S, Yonezawa T, Yoshioka K, Shingu H, Kobayashi Y, Takahasshi T, Otani Y, Katoh K, Obara Y. Changes in hepatic key enzymes of dairy calves in early weaning production systems. J Dairy Sci. 2008;91:3156–3164. doi: 10.3168/jds.2007-0853. [DOI] [PubMed] [Google Scholar]
- Heinrichs AJ, Losinger WC. Growth of Holstein dairy heifers in the United States. J Anim Sci. 1998;76:1254–1260. doi: 10.2527/1998.7651254x. [DOI] [PubMed] [Google Scholar]
- Hill TM, Baterman HG, II, Aldrich JM, Schlotherbeck RL. Effects of feeding different carbohydrates sources and amounts to young calves. J Dairy Sci. 2008;91:3128–3137. doi: 10.3168/jds.2007-0950. [DOI] [PubMed] [Google Scholar]
- Hoffman PC. Optimum body size of Holstein replacement heifers. J Anim Sci. 1997;75:836–845. doi: 10.2527/1997.753836x. [DOI] [PubMed] [Google Scholar]
- Huber JT. Development of the digestive and metabolic apparatus of the calf. J Dairy Sci. 1969;52:1303–1315. doi: 10.3168/jds.S0022-0302(69)86744-5. [DOI] [PubMed] [Google Scholar]
- Huber JT, Jacobson NI, Allen RS. Digestive enzyme activities in the young calf. J Dairy Sci. 1961;44:1494–1501. [Google Scholar]
- Kellogg DW, Owen FG. Relation of ration sucrose level and grain content to lactation performance and rumen fermentation. J Dairy Sci. 1969a;52:657–662. doi: 10.3168/jds.S0022-0302(69)86624-5. [DOI] [PubMed] [Google Scholar]
- Kellogg DW, Owen FG. Alterations of in vitro rumen fermentation patterns with various levels of sucrose and cellulose. J Dairy Sci. 1969b;52:1458–1460. [Google Scholar]
- Larson LL, Owen FG, Albright JL, Appleman RD, Lamb RC, Muller LD. Guidelines toward more uniformity in measuring and reporting calf experimental data. J Dairy Sci. 1977;60:989–991. [Google Scholar]
- Leng RA, Nolan JV. Nitrogen metabolism in the rumen. J Dairy Sci. 1984;67:1072–1089. doi: 10.3168/jds.S0022-0302(84)81409-5. [DOI] [PubMed] [Google Scholar]
- Lesmeister KE, Heinrichs AJ. Effects of corn processing on growth characteristics, rumen development, and rumen parameters in neonatal dairy calves. J Dairy Sci. 2004;87:3439–3450. doi: 10.3168/jds.S0022-0302(04)73479-7. [DOI] [PubMed] [Google Scholar]
- Lesmeister KE, Heinrichs AJ. Effects of adding extra molasses to a texturized calf starter on rumen development, growth characteristics, and blood parameters in neonatal dairy calves. J Dairy Sci. 2005;88:411–418. doi: 10.3168/jds.S0022-0302(05)72702-8. [DOI] [PubMed] [Google Scholar]
- Licitra G, Hernandez TM, Van Soest PJ. Standardization of procedures of nitrogen fractionation of ruminant feeds. Anim Feed Sci Technol. 1996;57:347–358. [Google Scholar]
- Martel CA, Titgemeyer EC, Mamedova LK, Bradfort BJ. Dietary molasses increases ruminal pH and enhances ruminal biohydrogenation during milk fat depression. J Dairy Sci. 2011;94:3995–4004. doi: 10.3168/jds.2011-4178. [DOI] [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirement in Dairy Cattle. 7th edn. National Academy of Science; Washington, DC, USA: 2001. [Google Scholar]
- Nocek JE, Tamminga S. Site of digestion of starch in the gastrointestinal tract of the dairy cows and its effects on milk yield and composition. J Dairy Sci. 1991;74:3598–3629. doi: 10.3168/jds.S0022-0302(91)78552-4. [DOI] [PubMed] [Google Scholar]
- Nozière P, Ortigues-Marty I, Loncke C, Sauvant D. Carbohydrate quantitative digestion and absorption in ruminants: from feed starch and fiber to nutrients available for tissues. Animal. 2010;4:1057–1074. doi: 10.1017/S1751731110000844. [DOI] [PubMed] [Google Scholar]
- Nussio CMB, Santos FAP, Zopollatto M, Pires AV, Morais JB, Fernandes JJR. Ruminal fermentation parameters and metric measurements of the rumen of dairy calves fed processed corn (steam-rolled vs. steam-flaked) and monensin. Braz J Anim Sci. 2003;32:1021–1031. [Google Scholar]
- Oliveira MVM, Vargas FM, Jr, Sanchez LMB, Paris W, Frizzo A, Haygert IP, Montagner D, Weber A, Cerdótes L. Ruminal degradability and intestinal digestibility of feeds by means of associated technical in situ and mobile nylon bag. Braz J Anim Sci. 2003;32:2023–2031. [Google Scholar]
- Quigley JD, III, Smith ZP, Heitmann RN. Changes in plasma volatile fatty acids in response to weaning and feed intake in young calves. J Dairy Sci. 1991;74:258–263. doi: 10.3168/jds.S0022-0302(91)78168-X. [DOI] [PubMed] [Google Scholar]
- Quigley JD., III Feeding prior to weaning. Proceedings of Calves, Heifers and Dairy Profitability National Conference; Harrisburg, PA, USA. 1996. pp. 245–255. [Google Scholar]
- Tamate H, McGilliard AD, Jacobson NL, Getty R. Effect of various dietaries on the anatomical development of the stomach in the calf. J Dairy Sci. 1962;45:408–420. [Google Scholar]
- Van Amburgh M, Drackley JK. Current perspectives on the energy and protein requirements of the pre-weaned calf. In: Gransworthy PC, editor. Calf and Heifer Rearing: Principles of Rearing the Modern Dairy Heifer from Calf to Calving. Nottingham University Press; Nottingham, UK: 2005. pp. 67–82. [Google Scholar]
- Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Weiss WP. Predicting energy values of feeds. J Dairy Sci. 1993;76:1802–1811. [Google Scholar]
- Wiese BI, Górka P, Mutsvangwa T, Okine E, Penner GB. Short communication: Interrelationship between butyrate and glucose supply on butyrate and glucose oxidation by ruminal epithelial preparations. J Dairy Sci. 2013;96:5914–5918. doi: 10.3168/jds.2013-6677. [DOI] [PubMed] [Google Scholar]


