Abstract
Mild cognitive impairment (MCI) and Alzheimer's disease (AD) dementia are preceded by a phase of disease, referred to as ‘preclinical AD’, during which cognitively normal individuals have evidence of AD pathology in the absence of clinical impairment. This study examined whether a magnetic resonance imaging (MRI) measure of cortical thickness in brain regions, collectively known as ‘AD vulnerable’ regions, predicted the time to onset of clinical symptoms associated with MCI and whether cortical thickness was similarly predictive of clinical symptom onset within 7 years post baseline versus progression at a later point in time. These analyses included 240 participants from the BIOCARD study, a cohort of longitudinally followed individuals who were cognitively normal at the time of their MRI (mean age = 56 years). Participants have been followed for up to 18 years (M follow-up = 11.8 years) and 50 participants with MRIs at baseline have developed MCI or dementia over time (mean time to clinical symptom onset = 7 years). Cortical thickness in AD vulnerable regions was based on the mean thickness of eight cortical regions. Using Cox regression models, we found that lower mean cortical thickness was associated with an increased risk of progression from normal cognition to clinical symptom onset within 7 years of baseline (p = 0.03), but not with progression > 7 years from baseline (p = 0.30). Lower cortical thickness was also associated with higher levels of phosphorylated tau, measured in cerebrospinal fluid at baseline. These results suggest that cortical thinning in AD vulnerable regions is detectable in cognitively normal individuals several years prior to the onset of clinical symptoms that are a harbinger of a diagnosis of MCI, and that the changes are more likely to be evident in the years proximal to clinical symptom onset, consistent with hypothetical AD biomarker models.
Keywords: Alzheimer's disease, Preclinical AD, Cortical thickness, Magnetic resonance imaging, Cerebrospinal fluid, Phosphorylated tau
Highlights
-
•
Examined cortical thickness in relation to onset of clinical symptoms of MCI.
-
•
Cortical thickness was associated with clinical symptom onset within 7 years.
-
•
Cortical thickness was associated with levels of CSF p-tau, but not CSF amyloid.
-
•
Changes in cortical thickness may be evident during preclinical AD.
1. Introduction
Evidence indicates that the neuropathological changes underlying Alzheimer's disease (AD) begin many years before the manifestation of clinical symptoms (Sperling et al., 2011). A number of magnetic resonance imaging (MRI) studies demonstrate alterations in selected regions within the medial temporal lobe (MTL) that are thought to be an indirect reflection of neuronal injury during this preclinical phase of AD. For example, there is evidence that measures of the volume and thickness of MTL regions, such as the entorhinal cortex and hippocampus, are associated with the time to diagnosis of mild cognitive impairment (MCI) or time to onset of clinical symptoms associated with MCI (e.g., Csernansky et al., 2005, Soldan et al., 2015). A greater rate of atrophy in MTL regions has also been demonstrated among cognitively normal individuals who subsequently progress to MCI (e.g., Jack et al., 2004, Miller et al., 2013, Pacheco et al., 2015) compared to individuals who remain cognitively normal.
It is currently hypothesized that structural brain changes are also evident outside of the MTL during the preclinical phase of AD, though most of this work is based on cross-sectional or short-term longitudinal studies. Several groups have identified ‘AD vulnerable’ or ‘AD signature’ regions comprised of cortical areas thought to be particularly sensitive to the effects of early AD pathology (e.g., Dickerson et al., 2009, Sabuncu et al., 2011, Wang et al., 2015). Although the regions identified as ‘AD vulnerable’ or ‘AD signature’ have differed across studies, they predominantly include parts of inferior and anterior temporal lobe, inferior and superior parietal lobe, and posterior cingulate cortex. For example, a larger percentage of cognitively normal individuals with reduced cortical thickness in AD vulnerable regions showed cognitive decline over a three-year follow-up (Dickerson and Wolk, 2012), and reduced thickness in these regions was associated with time to diagnosis of dementia (Dickerson et al., 2011). Additionally, studies that have compared cognitively normal individuals who subsequently progress to MCI or AD dementia to those who remain cognitively normal have demonstrated between-group differences in baseline MRI volumes (Smith et al., 2007) or atrophy measures (Pacheco et al., 2015) in a subset of cortical regions, including parietal and temporal areas.
A number of cross-sectional studies have also examined cortical thickness differences between cognitively normal individuals with versus without biomarker profiles consistent with AD pathology, as determined by amyloid imaging or cerebrospinal fluid (CSF). These studies have demonstrated associations between biomarker positivity and reduced mean cortical thickness of AD vulnerable regions (Dickerson et al., 2009, Sabuncu et al., 2011) or similar cortical regions (e.g., Becker et al., 2011, Doré et al., 2013).
Although prior studies, taken together, suggest that reductions in cortical thickness are predictive of time to progression from normal cognition to symptom onset of MCI, this question has not been directly examined previously, to our knowledge. The present study was designed to address this gap. Utilizing MRI data from the BIOCARD study, whose participants were cognitively normal when first enrolled, we examined whether mean cortical thickness of AD vulnerable regions is associated with time to onset of clinical symptoms of MCI.
The long follow-up of the cohort (mean = 11.8 years, max = 18.2 years), the substantial size (N = 240), and the availability of CSF measures in the same individuals allowed us to extend prior work in a number of ways. First, we examined the timing of cortical thinning in AD vulnerable regions relative to the onset of symptoms of MCI. To do so, we tested whether cortical thickness is similarly predictive of the risk of clinical symptom onset for progression within a relatively short time frame (less than 7 years from baseline) versus progression at a later point in time, i.e., more than 7 years from baseline. This analysis was motivated by current AD biomarker models that hypothesize structural MRI measures of brain atrophy become abnormal more proximal to clinical symptom onset than measures of amyloid accumulation, which are thought to become abnormal earlier during the preclinical phase of AD (Jack et al., 2013). Second, we examined whether cortical thickness of AD vulnerable regions was associated with CSF biomarkers of neuronal injury (i.e., tau, phosphorylated tau) or a CSF biomarker of β-amyloid. Lastly, previous studies on this general topic have tended to include individuals in their 70′s, so it remains unclear whether AD-related cortical thickness changes during preclinical AD can be identified at younger ages. The present study was able to address this issue since it included a large cohort of individuals who were primarily middle-aged at their baseline MRI scan (mean (M) age = 56 years).
2. Method
2.1. Study design and participant selection
The study from which these data were drawn is known as the BIOCARD study, which was designed to recruit and follow a cohort of cognitively normal individuals who were primarily in middle age at baseline. By design, approximately 75% of the participants had a first degree relative with dementia of the Alzheimer type. The overarching goal was to identify variables among cognitively normal individuals that could predict the subsequent development of mild to moderate symptoms of AD. Recruitment procedures, baseline evaluations, and annual clinical and cognitive assessments have been described in detail elsewhere (Albert et al., 2014). Briefly, the study was initiated at the National Institutes of Health (NIH) in 1995, with recruitment conducted by the staff of the Geriatric Psychiatry Branch of the intramural program of the National Institute of Mental Health, beginning in 1995 and ending in 2005. After providing written informed consent, a total of 349 individuals were enrolled in the study. Participants were administered a comprehensive neuropsychological battery and clinical examination annually, and MRI scans, CSF, and blood specimens were obtained approximately every two years. In 2005, the study was stopped for administrative reasons, and in 2009, a research team at the Johns Hopkins School of Medicine was funded to re-establish the cohort, continue the annual clinical and cognitive assessments and evaluate the previously acquired MRI scans, CSF, and blood specimens. In 2015, the collection of both MRI and CSF biomarkers was reinitiated, and amyloid imaging was begun.
2.2. Clinical assessments and consensus diagnoses
Since the study has been conducted at Johns Hopkins, annual clinical assessments have included the following: a physical and neurologic examination, record of medication use, behavioral and mood assessments (Cummings et al., 1994, Yesavage et al., 1982), family history of dementia, history of symptom onset, and a Clinical Dementia Rating (CDR) based on a semi-structured interview (Hughes et al., 1982, Morris, 1993). Clinical assessments given at the NIH covered similar domains. Annual cognitive assessments consist of a neuropsychological battery covering all major cognitive domains (for comprehensive details, see Albert et al., 2014).
The consensus diagnosis procedures implemented by the Johns Hopkins team have been comparable with those used in the National Institute on Aging Alzheimer's Disease Centers program: (1) clinical data pertaining to the medical, neurologic, and psychiatric status of the participant were examined; (2) reports of changes in cognition by the participant and collateral sources were reviewed; and (3) decline in cognitive performance, based on review of longitudinal testing from multiple domains, was established. These three sources of data were used to determine whether a participant was impaired. If a participant was impaired, the likely etiology of the impairment was identified. Then, the age at which the clinical symptoms began was estimated, based primarily on the reports of the participant and collateral source derived from the CDR. This same diagnostic process was retrospectively applied to participants who had become cognitively impaired while the study was being conducted at the NIH.
2.3. MRI assessments, image processing and regions of interest
The baseline MRI scans included in the present study were acquired at the NIH. Scans were obtained using a standard multimodal protocol using a GE 1.5T scanner. The scanning protocol included localizer scans, axial Fast Spin Echo sequence (repetition time (TR) = 4250, echo time (TE) = 108, field of view (FOV) = 512 × 512, thickness/gap = 5.0/0.0 mm, flip angle = 90, 28 slices), axial Flair sequence (TR = 9002, TE = 157.5, FOV = 256 × 256, thickness/gap = 5.0/0.0 mm, flip angle = 90, 28 slices), coronal Spoiled Gradient Echo (SPGR) sequence (TR = 24, TE = 2, FOV = 256 × 256, thickness/gap = 2.0/0.0 mm, flip angle = 20, 124 slices), sagittal SPGR sequence (TR = 24, TE = 3, FOV = 256 × 256, thickness/gap 1.5/0.0 mm, flip angle = 45, 124 slices).
Cortical reconstruction and estimation of cortical thickness was performed on the coronal SPGR scans using FreeSurfer (version 5.1), an automated image processing pipeline that is documented and freely available online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these methods have been described in prior publications (e.g., Dale et al., 1999, Fischl and Dale, 2000, Fischl et al., 1999a, Fischl et al., 1999b, Fischl et al., 2004b). Briefly, processing includes removal of non-brain tissue (Segonne et al., 2004), segmentation of the subcortical white matter and deep gray matter structures (Fischl et al., 2002, Fischl et al., 2004a), tessellation of the gray matter-white matter boundary, and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders (Dale et al., 1999, Dale and Sereno, 1993, Fischl and Dale, 2000). Parcellation of the cerebral cortex into units based on gyral and sulcal structure (Desikan et al., 2006, Fischl et al., 2004b) allows for representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex (Fischl and Dale, 2000). The procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003, Salat et al., 2004), and FreeSurfer morphometric procedures have been demonstrated to show good reliability and validity (Desikan et al., 2006, Han et al., 2006).
Following completion of the automated FreeSurfer pipeline, all scans were reviewed to assess the quality of skull stripping and ensure that cortical surfaces followed the gray and white matter boundaries. Where needed, manual edits were performed to improve segmentation and parcellation accuracy, which primarily included the correction of pial surface misplacement (e.g., the inclusion of non-brain tissue) and errors in white matter segmentation. This editing procedure was conducted by operators blinded to the diagnostic status of the subjects on follow-up.
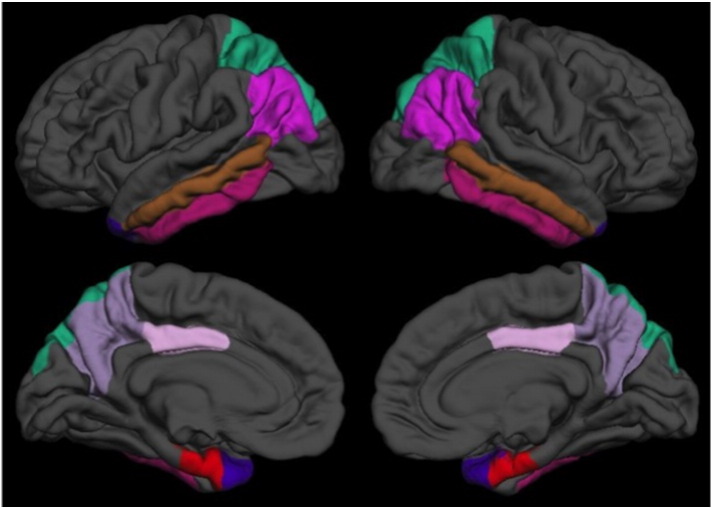
The present study examined cortical thickness measures in eight FreeSurfer-labeled regions of interest that were classified as ‘AD vulnerable’ cortical regions by at least two previous studies' (i.e., cross-study overlap includes Dickerson et al., 2009, Sabuncu et al., 2011, Wang et al., 2015). These regions included the entorhinal cortex, temporal pole, inferior temporal gyrus, middle temporal gyrus, inferior parietal cortex, superior parietal cortex, precuneus, and posterior cingulate cortex (averaged over the left and right hemispheres) (see Fig. 1). Note that we did not adjust the thickness measures for intracranial cavity size, as per standard procedure when evaluating cortical thickness (e.g., Westman et al., 2013). For each participant, the eight cortical thickness measures were averaged to create a measure of mean thickness of AD vulnerable regions, which served as the primary dependent variable in the below analyses.
Fig. 1.
Map of the eight of AD vulnerable regions based on FreeSurfer cortical labels, shown on the pial surface of the left (left) and right (right) hemispheres for lateral (top) and medial (bottom) views.
2.4. MRI scans used in the analyses
Baseline MRI scans were obtained from 331 participants in the study; of these, 62 scans were excluded, either because coronal SPGRs were not available (n = 22) or FreeSurfer surface reconstructions were deemed unreliable (e.g., motion artifact or low contrast resulted in poor scan quality or regions were missing from surfaces, n = 40). Of the 269 with useable FreeSurfer data, data from an additional 29 were not considered for analysis for reasons unrelated to scan quality (n = 22 have not yet re-enrolled or withdrawn from the study and n = 7 had clinical symptom onset at or prior to baseline, based on the estimated age of onset established during the consensus diagnosis procedure). The analyses reported below therefore include 240 participants who were cognitively normal at their baseline MRI scan (M duration of follow-up = 11.8 years, SD = 3.7).
2.5. Cerebrospinal fluid assessments
CSF measures were available for 170 of the participants who underwent lumbar puncture within 6 months of their baseline MRI scan (M gap time = 5.2 days between CSF draw and MRI, SD = 19.6). CSF specimens were analyzed using the same protocol employed in the Alzheimer's Disease Neuroimaging Initiative. As reported in Moghekar et al. (2013), this protocol used the xMAP-based AlzBio3 kit (Innogenetics, Ghent, Belgium) run on the Bioplex 200 system. The kit contains monoclonal antibodies specific for Aβ1–42 (4D7A3), total tau (t-tau) (AT120), and phosphorylated tau181p (p-tau) (AT270), each chemically bonded to unique sets of color-coded beads, and analyte-specific detector antibodies (HT7 and 3D6). Calibration curves were produced for each biomarker using aqueous buffered solutions that contained the combination of 3 biomarkers at concentrations ranging from 25 to 1555 pg/mL for recombinant tau, 54–1799 pg/mL for synthetic Aβ1–42 peptide, and 15–258 pg/mL for a tau synthetic peptide phosphorylated at the threonine 181 position (i.e., the p-tau181p standard). Each participant had all samples (run in triplicate) analyzed on the same plate. See Moghekar et al. (2012) for additional details regarding these procedures. The CSF specimen analyses were blinded to subject diagnostic status on follow-up.
2.6. Statistical analyses
Group differences in descriptive statistics were compared with t-tests for continuous variables or chi-square tests for dichotomous variables. We used Cox regression models (i.e., proportional hazard models) to determine whether mean baseline cortical thickness of AD vulnerable regions was associated with time (in years) to clinical symptom onset. To examine the issue of timing more carefully, we also examined whether cortical thickness was associated with clinical symptom onset for transitions occurring closer in time to baseline (i.e., transitions more proximal to the MRI scan) relative to transitions further in time from baseline. To test this, we ran an additional Cox model that included an indicator term for time (within 7 years) as part of the coefficient for cortical thickness. This is equivalent to treating thickness as a composition of two time-dependent variables, one only having an effect on risk of progression within 7 years, the other after 7 years (Fisher and Lin, 1999), with coefficients estimated using these corresponding methods. Thus, for each individual, this model allowed the coefficient for cortical thickness on risk of progression to be time-dependent and different within and after 7 years from baseline, therefore allowing us to examine the association between cortical thickness and risk of clinical symptom onset for progression within 7 years of baseline versus after 7 years from baseline. Seven years was selected as the cut-point since this is the mean time to clinical symptom onset in the data presented here (see Section 3.1). All Cox regression models included data from two groups: (1) participants who were cognitively normal at baseline and at their last visit (n = 190) and (2) participants were cognitively normal at baseline, but who received a diagnosis of MCI or dementia due to AD at their last visit (n = 50). All measures were standardized (i.e., z-scored) before model fitting and models were adjusted for age at scan and gender. These Cox regression models were also re-run with apolipoprotein E (APOE) genotype included as an additional dichotomous covariate, given that APOE-4 genetic status is a genetic risk factor for sporadic AD (Farrer et al., 1997). In these analyses, APOE-4 carriers were coded as 1, whereas non-carriers were coded as 0 (n = 8 individuals with one APOE-2 and one APOE-4 allele were excluded).
We calculated the hazard ratio (HR; i.e., relative hazard) for each thickness measure, which indicates the change in relative risk of progression for each one unit change in the predictor. A HR of 0.51, for example, means the hazard of clinical symptom onset is reduced by a factor of 0.51 (i.e., by 49%) for each standard deviation increase in cortical thickness. In contrast, a HR of 1.70 means the hazard of clinical symptom onset is increased by a factor of 1.70 (i.e., by 70%) for each standard deviation increase in cortical thickness.
Linear regression models were used to examine the association between AD vulnerable regions and CSF measures acquired within 6 months of an individual's scan date. Separate models were run for Aβ1–42, tau, and p-tau. For each model, the CSF measure, age, and gender served as predictors of mean thickness of the AD vulnerable regions. All analyses were run in R, version 3.2.2.
3. Results
Table 1 shows the comparison of the subjects who remained normal over time (n = 190) compared to those who progressed to MCI on follow-up (n = 50). At baseline, individuals who have since progressed to clinical symptoms of MCI were significantly older than those who have remained normal. Table 1 also includes baseline demographic characteristics for the entire BIOCARD cohort, for purposes of comparison. Of note, individuals with a diagnosis of ‘Impaired not MCI’ were included in the group of cognitively normal subjects; results were comparable when these subjects were excluded. Results for the associations between cortical thickness of AD vulnerable regions and time to clinical symptom onset were comparable when APOE-4 genetic status was included as an additional dichotomous covariate (data not shown). See the Supplementary Materials section for additional information about the characteristics of the subjects in the analyses.
Table 1.
Baseline participant characteristics for the entire BIOCARD cohort and participants in the analyses; characteristics for participants in the analyses are also stratified by clinical outcome. Values reflect means (standard deviations) unless otherwise indicated.
| Participants in analyses |
||||
|---|---|---|---|---|
| Entire BIOCARD cohort | All in analyses | Remained normal | Progressed to MCI or AD | |
| N | 349 | 240 | 190 | 50 |
| Age, years | 57.3 (10.4) | 56.0 (9.8) | 54.4 (8.8) | 62.2 (11.2)⁎ |
| Gender, females (%) | 57.6% | 61.7% | 64.7% | 50.0% |
| Ethnicity, Caucasian (%) | 97.1% | 97.9% | 99.5% | 92.0% |
| ApoE-4 carriers (%) | 33.6% | 29.2% | 32.1% | 36.0% |
| Education, years | 17.0 (2.4) | 17.0 (2.4) | 17.1 (2.3) | 16.7 (2.6) |
| MMSE score | 29.5 (0.9) | 29.6 (0.8) | 29.7 (0.7) | 29.3 (1.0)⁎ |
| Thickness of AD vulnerable regions, mm | – | 2.77 (0.13) | 2.78 (0.12) | 2.74 (0.15) |
| CSF Aβ1–42, pg/ml | – | 406.2 (98.0) [n = 170] |
414.0 (92.4) [n = 137] |
373.6 (114.5) [n = 33] |
| CSF t-tau, pg/ml | – | 69.0 (28.0) [n = 170] |
67.2 (27.4) [n = 137] |
76.4 (29.6) [n = 33] |
| CSF p-tau, pg/ml | – | 36.6 (15.6) [n = 170] |
35.1 (11.2) [n = 137] |
42.6 (22.4) [n = 33] |
Significant difference between individuals who have remained cognitively normal vs. progressed to MCI or AD dementia, p < 0.05.
3.1. Relationship between mean cortical thickness of AD vulnerable regions and time to onset of clinical symptoms
Mean baseline cortical thickness of AD vulnerable regions did not differ between individuals who remained cognitively normal compared to those who subsequently received a diagnosis of MCI or dementia due to AD (p = 0.12). The mean time from baseline to clinical symptom onset was 7.1 years (SD = 3.5; range 0.7–14.4). In the Cox regression model, there was no association between mean cortical thickness across all AD vulnerable regions and time to clinical symptom onset, when the length of time between the baseline scan and clinical symptom onset was not considered, HR = 0.77, p = 0.11, 95% CI [0.57, 1.06].
Next, we examined whether cortical thickness was associated with clinical symptom onset for progression more proximal to baseline (i.e., within 7 years) versus after 7 years from baseline. The mean time from baseline to clinical symptom onset for individuals who progressed within 7 years of their baseline scan was 3.9 years (SD = 2.2, range = 0.7–6.9; n = 22), versus 9.6 years (SD = 2.0, range = 7.1–14.4; n = 28) for those who progressed > 7 years from their baseline scan. With progression modeled differently within versus after 7 years from baseline (see Section 2.6 for model details), mean cortical thickness of AD vulnerable regions was significantly associated with clinical symptom onset within 7 years of baseline, but not after 7 years. Specifically, each standard deviation increase in mean thickness of AD vulnerable regions was associated with a reduced relative risk of clinical symptom onset for progression at time points within 7 years of the baseline scan, HR = 0.50, p = 0.03, 95% CI [0.28, 0.92], but not > 7 years from the baseline scan, HR = 1.08, p = 0.70, 95% CI [0.74, 1.57]. Results for the individual regions of interest are shown in Table 2 for reference.
Table 2.
Hazard ratios [95% confidence intervals] and p-values for cortical thickness of AD vulnerable regions in relation to clinical symptom onset, stratified by progression within vs. after 7 years from baseline.
| ≤ 7 years from baseline |
> 7 years from baseline |
|||
|---|---|---|---|---|
| HR | p-value | HR | p-value | |
| Average of AD vulnerable regions | 0.50 [0.28, 0.92] | 0.03 | 1.08 [0.74, 1.57] | 0.70 |
| Entorhinal cortex | 0.79 [0.52, 1.19] | 0.26 | 0.92 [0.63, 1.34] | 0.65 |
| Temporal pole | 0.61 [0.37, 1.02] | 0.058 | 0.82 [0.51, 1.31] | 0.41 |
| Inferior temporal gyrus | 0.48 [0.28, 0.81] | 0.006 | 1.30 [0.91, 1.87] | 0.16 |
| Middle temporal gyrus | 0.42 [0.22, 0.80] | 0.008 | 1.07 [0.72, 1.58] | 0.75 |
| Inferior parietal cortex | 0.71 [0.43, 1.16] | 0.17 | 1.40 [1.00, 1.96] | 0.05 |
| Superior parietal cortex | 0.73 [0.48, 1.11] | 0.14 | 1.10 [0.77, 1.59] | 0.60 |
| Precuneus | 0.58 [0.34, 0.98] | 0.04 | 1.16 [0.81, 1.66] | 0.42 |
| Posterior cingulate cortex | 0.87 [0.55, 1.37] | 0.55 | 1.15 [0.75, 1.78] | 0.52 |
3.2. Relationship between mean cortical thickness of AD vulnerable regions and CSF
Mean baseline CSF biomarker levels are shown in Table 1. Linear regression analyses revealed a significant negative association between the mean thickness of AD vulnerable regions and CSF p-tau (Fig. 2), such that individuals with smaller mean cortical thickness demonstrated higher levels of p-tau, β = − 0.20, p = 0.006. In contrast, the mean thickness of AD vulnerable regions was not associated with CSF Aβ1–42 (β = 0.01, p = 0.90) or t-tau (β = − 0.05, p = 0.51). See the Supplementary Materials section for auxiliary analyses of the CSF data, using Cox regression models comparable to those described above.
Fig. 2.
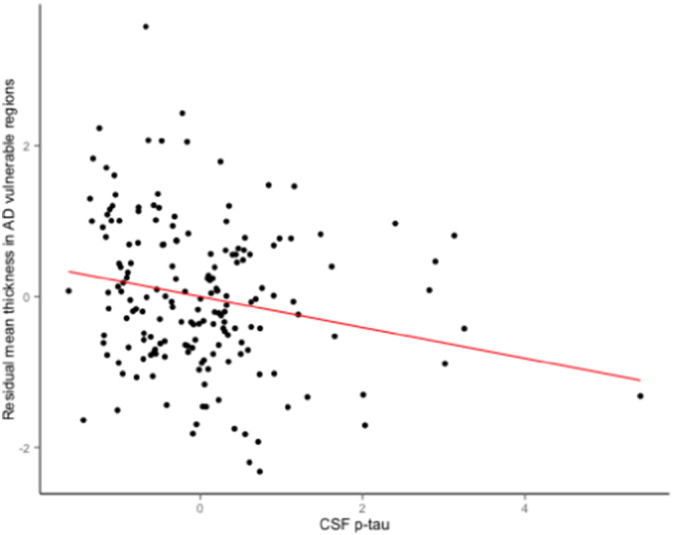
Scatterplot showing the partial correlation between mean thickness of AD vulnerable regions and standardized CSF p-tau levels (adjusted for age and gender).
4. Discussion
In the present study, we examined the association between mean cortical thickness across eight ‘AD vulnerable’ regions and time to onset of clinical symptoms in a cohort of well-characterized, longitudinally followed individuals who were cognitively normal at baseline, a subset of whom subsequently developed MCI or dementia due to AD (n = 50). We also examined whether mean thickness in these regions was related to cerebrospinal fluid biomarkers of AD pathology, including biomarkers of neuronal injury (tau and p-tau) and β-amyloid. Mean cortical thickness of the AD vulnerable regions was significantly associated with time to onset of clinical symptoms for progression within 7 years of baseline, but not for progression more than 7 years from baseline. This temporally dependent effect was evident when time to onset was modeled differently for progression within versus after 7 years from baseline, which corresponds to the mean time to clinical symptom onset across the 50 individuals who progressed to MCI or dementia over time. We also found that mean cortical thickness of AD vulnerable regions was inversely associated with levels of CSF p-tau, but not CSF total tau or Abeta.
These findings extend prior research in a number of ways. First, the cohort's extensive longitudinal follow-up allowed us to examine the timing of cortical thickness changes during preclinical AD, with results suggesting that these changes are more likely to be evident in the years proximal to clinical symptom onset. These results therefore extend previous work that used time to diagnosis of dementia as the outcome (Dickerson et al., 2011). Second, we defined AD vulnerable regions by examining cortical areas that had been identified as altered during AD by several different groups of investigators (Dickerson et al., 2009, Sabuncu et al., 2011, Wang et al., 2015), suggesting these regions are sensitive to AD-related changes across multiple cohorts. Third, the BIOCARD cohort was primarily middle aged at baseline. This allowed us to examine cortical thickness measures in a younger group than has been done previously. Moreover, we used an earlier diagnostic time point (i.e., time to clinical symptom onset), which is generally earlier than the date of diagnosis of MCI.
These MRI results are in line with studies finding that individuals who progress from normal cognition to MCI or dementia due to AD demonstrate greater atrophy in cortical regions outside the MTL, relative to those who remain normal, in both sporadic (Pacheco et al., 2015, Smith et al., 2007) and familial AD (Fox et al., 2001). To our knowledge, only one prior study has examined the mean thickness of AD vulnerable regions in cognitively normal individuals in relation to time to a clinical outcome. Dickerson et al. (2011) found a significant association between cortical thickness and time to diagnosis of dementia among 15 individuals who were diagnosed with dementia due to AD an average of 8 years later. Although these findings suggest we should have found a significant association between the MRI thickness measures and time to symptom onset for those individuals who transitioned to MCI after 7 years, it's difficult to compare our results with those of Dickerson et al. because they did not provide information about the onset of symptoms of MCI relative to the timing of the MRI scan (i.e., the outcome in the present study).
It is noteworthy that we previously reported that baseline entorhinal cortex thickness and hippocampal volume (particularly in the right hemisphere) were significantly associated with time to onset of clinical symptoms for the cohort taken as a whole (i.e., the Cox regression models in these prior analyses did not include a 7-year indicator) (Soldan et al., 2015). Although the results in the present study were in the expected direction, entorhinal cortex thickness derived from FreeSurfer was not significantly associated with time to onset of clinical symptoms within or after 7 years. This discrepancy between our prior and current results may be due to differences in the method of entorhinal cortex assessment. The entorhinal cortex is challenging to measure (Feczko et al., 2009) and semi-automated methods such as LDDMM may be better at detecting subtle changes in a region that is affected by multiple anatomical factors, including small size, collateral sulcus variability, and the presence of dura adjacent to the pial surface (Feczko et al., 2009). Taken together, our prior findings and the current ones suggest that MTL regions atrophy first, with atrophy in other AD vulnerable cortical regions occurring later in the progression of preclinical AD. These findings may have implications for the use of MRI biomarkers in clinical trials among cognitively normal individuals at risk for AD. Additionally, this interpretation is consistent with neuropathological studies showing that neurofibrillary tangles initially accumulate in MTL regions before spreading to other regions, including adjacent temporal and parietal areas (Braak and Braak, 1991).
It is also noteworthy that mean cortical thickness of AD vulnerable regions, hypothesized to be a marker of neuronal injury, was associated with levels of CSF phosphorylated tau, but not CSF Abeta. Wang et al. (2015) found an association between mean cortical thickness of AD vulnerable regions and CSF total tau, but not Abeta (cf. Sabuncu et al., 2011), in cognitively normal individuals who were tau positive (though their study did not include measures of p-tau). Unlike Wang et al. (2015), we did not find a relationship between cortical thickness and total tau in the present study, though this may be due in part to participant characteristics. Participants in the study by Wang et al. were in their early 70′s and, as there are age-related changes in tau, may have harbored more tau pathology than the primarily middle-aged participants included here. Additionally, subtle differences in CSF assays across sites could underlie the reason we found a relationship with p-tau but not tau. In future analyses, it will be important to assess the rate of change in AD vulnerable regions in relation to clinical symptom onset, as well as in relation to the rate of change in other biomarkers including CSF tau and p-tau.
These findings, taken as a whole, are in agreement with hypothetical models of preclinical AD that suggest structural MRI abnormalities occur more proximal to symptom onset than do changes in measures of amyloid and tau accumulation (Jack et al., 2013, Sperling et al., 2011). In line with this, we previously demonstrated that other AD biomarkers, including baseline cerebrospinal fluid (Moghekar et al., 2013) and medial temporal lobe volumetric MRI measures (Soldan et al., 2015) in this same cohort, were significantly associated with time to onset of clinical symptoms of MCI. In these prior studies, the mean time from biomarker assessment to symptom onset ranged from 5 to 6 years. Importantly, these prior analyses did not include a cut-point indicator in the Cox regression models, such as that used here. This suggests that CSF and medial temporal lobe MRI volumetric measures may be predictive of progression over a longer time frame than measures of cortical thickness.
This study must be interpreted within the context of its limitations. First, the BIOCARD cohort is a convenience sample and consists of well-educated, primarily Caucasian participants, the majority of whom have a family history of AD. These factors may limit generalization to the population at large. Second, although the brain regions examined here were selected on the basis of prior publications by other groups, we used cortical parcellations derived from FreeSurfer, which may be larger in size than the regions identified by some previous studies, and thus not directly comparable. Lastly, it is important to note that in these analyses, the cut-point for the indicator variable was selected on the basis of the mean time from baseline to clinical symptom onset for the specific measure under evaluation. We are currently applying change point models to a broad set of data which incorporates each domain of assessment obtained in this cohort (e.g., MRI, CSF, cognitive test scores) in order to examine the timing of changes in these measures in relation to one another and in relation to symptom onset during preclinical AD.
Disclosure statement
Dr. Pettigrew reports no disclosures.
Dr. Soldan reports no disclosures.
Ms. Zhu reports no disclosures.
Dr. Wang reports no disclosures.
Dr. Moghekar reports no disclosures.
Mr. Brown reports no disclosures.
Dr. Miller owns a significant equity share in “Anatomy Works”. This arrangement is being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Dr. Albert is an advisor to Eli Lilly.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (U19-AG03365, P50-AG005146, and T32-AG027668). The BIOCARD Study consists of 7 Cores with the following members: (1) the Administrative Core (Marilyn Albert, Barbara Rodzon); (2) the Clinical Core (Ola Selnes, Marilyn Albert, Anja Soldan, Rebecca Gottesman, Ned Sacktor, Guy McKhann, Scott Turner, Leonie Farrington, Maura Grega, Gay Rudow, Daniel D'Agostino, Scott Rudow); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Timothy Brown, Hayan Chi, Anthony Kolasny, Kenichi Oishi, Thomas Reigel, Laurent Younes); (4) the Biospecimen Core (Abhay Moghekar, Richard O′Brien, Abby Spangler); (5) the Informatics Core (Roberta Scherer, David Shade, Ann Ervin, Jennifer Jones, Matt Toepfner, Lauren Parlett, April Patterson, Aisha Mohammed); (6) the Biostatistics Core (Mei-Cheng Wang, Qing Cai, Yuxin Zhu); and (7) the Neuropathology Core (Juan Troncoso, Barbara Crain, Olga Pletnikova, Gay Rudow, and Karen Fisher). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs. John Cernansky, David Holtzman, David Knopman, Walter Kukull, and John McArdle, and Drs. Neil Buckholtz, John Hsiao, Laurie Ryan, and Jovier Evans, who provide oversight on behalf of the National Institute on Aging and the National Institute of Mental Health (NIMH), respectively. The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs. Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski.
The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principle investigator: Dr. Trey Sunderland). The authors are particularly indebted to Dr. Karen Putnam, who has provided ongoing documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.06.010.
Appendix A. Supplementary data
Supplementary material. Additional information about the characteristics of the subjects in the analyses.
References
- Albert M., Soldan A., Gottesman R., McKhann G., Sacktor N., Farrington L.…The BIOCARD Research Team. R Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr. Alzheimer Res. 2014;11:773–784. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.A., Hedden T., Carmasin J., Maye J., Rentz D.M., Putcha D.…Johnson K.A. Amyloid-β associated cortical thinning in clinically normal elderly. Ann. Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Csernansky J.G., Wang L., Swank J., Miller J.P., Gado M., McKeel D.…Morris J.C. Preclinical detection of Alzheimer's disease: hippocampal shape and volume predict dementia onset in the elderly. NeuroImage. 2005;25:783–792. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Sereno M.I. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cogn. Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D.…Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson B.C., Wolk D.A. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Bakkour A., Salat D.H., Feczko E., Pacheco J., Greve D.N.…Buckner R.L. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Stoub T.R., Shah R.C., Sperling R.A., Killiany R.J., Albert M.S.…Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré V., Villemagne V.L., Bourgeat P., Fripp J., Acosta O., Chetélat G.…Rowe C.C. Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013;70:903–911. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R.…van Duijn C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. J. Am. Med. Assoc. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Feczko E., Augustinack J.C., Fischl B., Dickerson B.C. An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiol. Aging. 2009;30:420–431. doi: 10.1016/j.neurobiolaging.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C.…Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J., Makris N., Segonne F., Quinn B.T., Dale A.M. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H.…Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fisher L.D., Lin D.Y. Time-dependent covariates in the Cox proportional-hazards regression model. Annu. Rev. Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- Fox N., Crum W., Scahill R., Stevens J. Imaging of onset and progression of Alzheimer’ s disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:8–11. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S.…Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jack C.R., Shiung M.M., Gunter J.L., O'Brien P.C., Weigand S.D., Knopman D.S.…Petersen R.C. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S.…Trojanowski J.Q. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg G.R., Broome M.R., McGuire P.K., David A.S., Eddy M., Ozawa F.…Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Miller M.I., Younes L., Ratnanather J.T., Brown T., Trinh H., Postell E.…Albert M. The diffeomorphometry of temporal lobe structures in preclinical Alzheimer's disease. Neuroimage Clin. 2013;3:352–360. doi: 10.1016/j.nicl.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A., Goh J., Li M., Albert M., O'Brien R.J. Cerebrospinal fluid Aβ and tau level fluctuation in an older clinical cohort. Arch. Neurol. 2012;69:246–250. doi: 10.1001/archneurol.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A., Li S., Lu Y., Li M., Wang M.-C., Albert M., O'Brien R. CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology. 2013;81:1753–1758. doi: 10.1212/01.wnl.0000435558.98447.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Pacheco J., Goh J.O., Kraut M.A., Ferrucci L., Resnick S.M. Greater cortical thinning in normal older adults predicts later cognitive impairment. Neurobiol. Aging. 2015;36:903–908. doi: 10.1016/j.neurobiolaging.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas H.D., Liu A.K., Hersch S., Glessner M., Ferrante R.J., Salat D.H.…Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Sabuncu M.R., Desikan R.S., Sepulcre J., Yeo B.T.T., Liu H., Schmansky N.J.…Fischl B. The dynamics of cortical and hippocampal atrophy in Alzheimer disease. Arch. Neurol. 2011;68:1040–1048. doi: 10.1001/archneurol.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat D.H., Buckner R.L., Snyder A.Z., Greve D.N., Desikan R.S., Busa E.…Fischl B. Thinning of the cerebral cortex in aging. Cereb. Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Segonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Smith C.D., Chebrolu H., Wekstein D.R., Schmitt F.A., Jicha G.A., Cooper G., Markesbery W.R. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- Soldan A., Pettigrew C., Lu Y., Wang M.-C., Selnes O., Albert M.…Miller M.I. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer's disease. Hum. Brain Mapp. 2015;36:2826–2841. doi: 10.1002/hbm.22810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M.…Phelps C.H. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Benzinger T.L., Hassenstab J., Blazey T., Owen C., Liu J.…Ances B.M. Spatially distinct atrophy is linked to B-amyloid and tau in preclinical Alzheimer disease. Neurology. 2015;84:1254–1260. doi: 10.1212/WNL.0000000000001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman E., Aguilar C., Muehlboeck J.-S., Simmons A. Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer's disease and mild cognitive impairment. Brain Topogr. 2013;26:9–23. doi: 10.1007/s10548-012-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M., Leirer V.O. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. Additional information about the characteristics of the subjects in the analyses.