Abstract
M032 is a second-generation oncolytic herpes simplex virus (oHSV) that selectively replicates in tumor cells. M032 kills tumor cells directly through oncolytic replication and then proceeds to infect tumor cells in proximity, continuing the process of tumor destruction. In addition to this direct oncolytic activity, the virus carries a therapeutic payload—thus acting as a gene therapy vector—and causes the tumor cell to synthesize and secrete the immunity-stimulating protein interleukin-12 (IL-12) before cell death.1 Human IL-12 is expressed and promotes an immune response against surviving tumor cells, increasing the antitumor effect of the therapy. IL-12 also produces an antiangiogenic effect, by interfering with the production of new tumor blood vessels necessary for tumor growth. Thus, M032 oHSV exerts antitumor effects through three distinct potential mechanisms. The virus has also been genetically engineered to minimize toxic effects for the patient. Preclinical animal models support the safety of intracranial inoculation with M032 in two relevant species (mouse and nonhuman primate). This clinical protocol outlines the dose-escalating phase I study for evaluation of M032 in patients with recurrent or progressive malignant glioma.
Introduction
Malignant gliomas and viral oncolytic therapy
Malignant gliomas are the most common primary brain tumors in humans, accounting for 30% of all primary central nervous system (CNS) tumors in adults.2 Of the malignant gliomas, those with the fewest treatment options and poorest outcomes include (1) anaplastic astrocytoma, (2) glioblastoma multiforme (GBM), and (3) gliosarcoma. Primary malignant brain tumors in the United States are estimated to occur at an incidence of 14.7 per 100,000 people, and 10,000–15,000 new cases are diagnosed annually.2,3 GBM, the most malignant type of brain tumor, has been refractory to improvements in treatment; the outcome of conventional treatments is poor, with a median survival just over one year. Gliomas develop from the unsuppressed growth of glioma progenitor cells, usually resulting in the first clinical symptoms such as memory loss, visual impairment, or seizures. Presently, the most common modalities for the treatment of brain tumors are surgical resection, chemotherapy, and/or radiation therapy, depending on tumor location, size, and pathological diagnosis. The major shortcoming associated with conventional treatment stems from tumor recurrence, neurologic disability, and, eventually, death.
Many vectors have been considered for neoplastic therapy, including naked DNA, liposomes, and viruses. Viral vectors include those derived from retrovirus, adenovirus (AdV), adeno-associated virus (AAV), and herpes simplex virus (HSV) type 1.
Recombinant HSV have been generated containing a variety of gene alterations (Fig. 1) to serve as oncolytic vectors. Their efficacy as antineoplastic agents has been examined in vitro and in vivo in numerous models of metastatic and primary tumors.4–7 These experiments have provided valuable safety information in various animal models. HSV vectors are neurotropic, and they have evolved to coexist within neuronal tissue. The discovery of viral genes essential for replication and virulence in the brain8,9 has opened new therapeutic avenues for currently incurable brain tumors. It has been clearly demonstrated that by altering these genes, either by deletion, insertion, or point mutation in the coding region, the virus will still replicate and kill dividing tumor cells but is otherwise avirulent after delivery into postmitotic brain tissue. Thus, it is possible to develop a genetically engineered virus with the ability to selectively replicate in glioma cells. This property is desirable when considering that, after resection, more than 109 tumor cells may remain in situ.
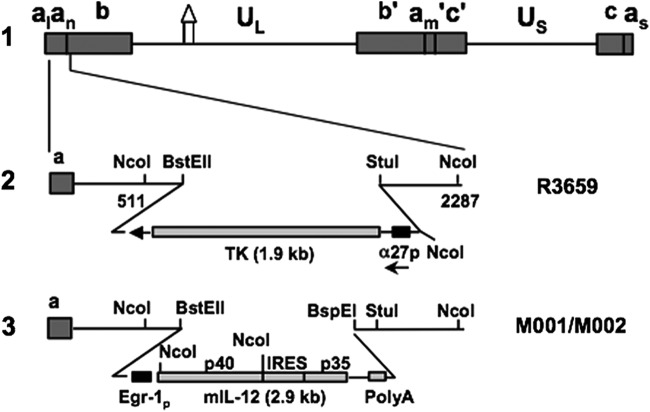
Figure 1.
Schematic representation of mIL-12-expressing HSV (M002). Line 1 illustrates the HSV-1 (F) Δ305 genome, which contains a 700 bp deletion within the tk gene, as indicated by the Δ symbol. UL and US represent the unique long and unique short sequences, respectively. The inverted repeat sequences are indicated by a, b, and c, with subscripts n and m representing variable numbers of a sequences. a1 and as represent the a sequences flanking the UL and US terminal repeats. Line 2 shows the sequence arrangement of the recombinant HSV R3659. The BstEII–StuI fragment within the γ134.5 gene was replaced by the chimeric α27-tk gene in the inverted sequences ab (shown above) and b′a′ (not shown) flanking the UL sequence. Line 3 shows the sequence arrangements of the relevant regions in the recombinant mIL-12-expressing HSV M001 (tk−) or M002 (tk+). NcoI restriction sites are indicated. (Copyright [2000] National Academy of Sciences, U.S.A. Reproduced with permission from PNAS [Parker et al.12]).
G207, a conditionally replicating HSV, has been studied after intracerebral administration in humans at doses up to 3 × 109 plaque-forming units (PFU) without evidence of vector-related toxicity.10 Additionally, selectively replicating recombinant HSVs can carry other recombinant genes and serve as a gene therapy vector. Therefore, opportunity exists to combine its direct oncolytic properties with gene therapy. The study agent, M032, takes advantage of the modified virus’ direct oncolytic activity in combination with the recruitment of a targeted inflammatory response through the production of interleukin-12 (IL-12) to kill tumor cells.
This “double-barrel” effect is accomplished by the expression of IL-12 in physiologically relevant concentrations from cells infected with recombinant HSV.11–13 Murine IL-12 is known to cross-react with the human IL-12 receptor and stimulate an immune response, but human IL-12 does not cross-react with the murine receptor. Thus, all in vivo preclinical efficacy studies have been performed in murine models using M002, a variant of the virus that expresses murine IL-12.11–13 IL-12 is a cytokine with potent antitumor qualities.14–16 By induction of interferon-γ production,17 IL-12 acts to enhance the cytolytic activity of natural killer cells and cytotoxic T lymphocytes.18 IL-12 also induces a TH-1-type immune response,19 which may provide a more durable antitumor effect than other cytokine antineoplastic approaches. Furthermore, IL-12 has also been demonstrated to have in vivo antiangiogenic activity, which may contribute to its antitumor effects.20,21 It has also been shown to produce important antiglioma activity in different rodent models.22,23 In preclinical models, M002, a similar oncolytic herpes simplex virus (oHSV) construct that expresses murine IL-12 instead of human, was found to be more effective than G207 or similar viruses as an inhibitor of tumor growth. Immunohistochemistry studies suggest that this increased efficacy may be because of an antitumor immune response induced by the secretion of IL-12 by the infected tumor cells.24 A potential advantage of IL-12 is its ability to stimulate an antitumor response in the absence of IL-2. This is important in patients with malignant glioma, who suffer from a decreased ability to respond to IL-2. However, the local immunomodulatory effects of IL-12 in the immunosuppressive milieu of human malignant gliomas is currently unknown, and will only be understood through a Phase I study designed to assess biologic activity.
Although early clinical studies of systemic IL-12 have produced evidence of toxicity,23 its effects when administered locally are not well known. Preclinical models support the safety of intracranial inoculation with M032 in two relevant species (mouse and nonhuman primate).
Preclinical data to support the efficacy and safety of the proposed study
M002 has been evaluated in vivo with animal models.11–13 mIL-12 has been shown to be expressed in physiologically relevant concentrations in various tumors of neuroectodermal origin (from 800 to 3200 pg per ml per 24 hr per 5 × 105 cells). Compared with either the parental virus R3659 that expresses no foreign genes or the clinically evaluated HSV G207, M002 produces improved survival in murine intracranial tumor models (Fig. 2) and superior tumor growth inhibition in subcutaneous models (Fig. 3).
Figure 2.
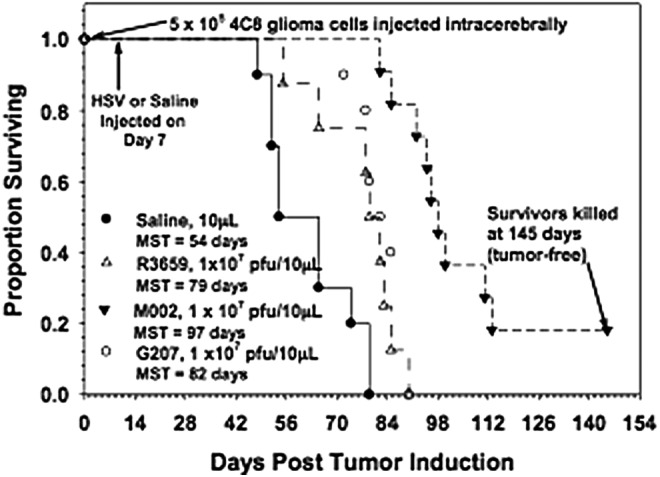
Immunocompetent C57BL/6xDBA/2 F1 hybrid mice were injected intracranially with 4C8 mouse glioma cells, followed in 7 days by saline or the viruses shown. Median survivals were determined by Kaplan–Meier plots and are shown on the figure. Log rank analyses of these survival values confirmed the significantly prolonged survival-afforded mice treated with M002, compared with R3659 (p = 0.00007) or G207 (p = 0.0003). In addition, about 20% of the M002-treated mice were apparently “cured” with no evidence of remaining 4C8 glioma cells histologically when the experiment was terminated. (Reproduced with permission from Oxford University Press [Hellums et al.11]).
Figure 3.
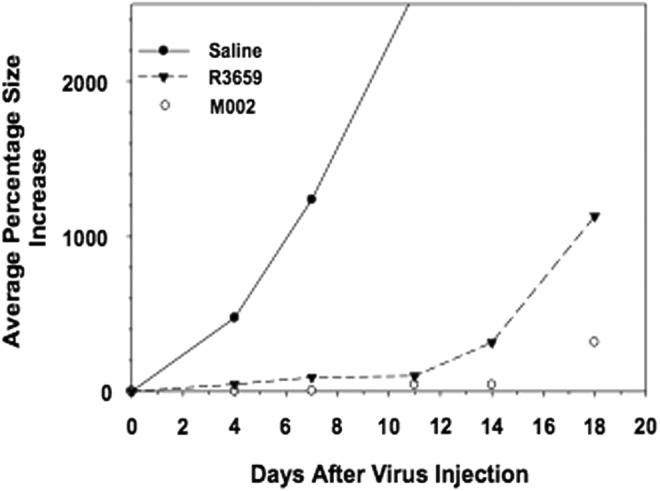
Immunocompetent BALB/c mice were injected subcutaneously with 1 × 105 neuro-2A cells. When tumors attained 200 mm3 in volume, they were injected with 50 liters of saline or 5 × 107 PFU of HSV R3659 or M002. Tumor volumes were measured every 3 days, and the average percentage increase in volume was calculated relative to the treatment date.
Preclinical studies have documented increased efficacy of IL-12-expressing oHSV over a parent γ134.5-deleted virus not expressing IL-12 in a syngeneic intracranial model of neuroblastoma and a syngeneic intracranial murine malignant glioma model.11 Further studies in both immunocompetent mice bearing syngeneic neuroblastomas and nude mice bearing human U87 malignant glioma xenografts have demonstrated the superiority of IL-12-expressing oHSV compared with G207. Immunohistochemistry reveals an enhanced inflammatory response, particularly CD4+ and CD8+ cells and macrophages, suggesting that an antitumor immune response may be partially responsible for the inflammatory response.11
Although M002 expresses murine IL-12 and has not been used in human clinical trials, the safety of M002 has been addressed through deliberate molecular engineering, described above, and in vivo testing in HSV-sensitive owl monkeys, Aotus nancymai. Murine IL-12 has been demonstrated to be active on Aotus lymphocytes, validating this as an appropriate model for preclinical safety testing (JM Markert, unpublished data). A purified extract (made according to GMP specifications) of M002 has been injected intracerebrally into three A. nancymai at titers up to 4.8 × 108 PFU with no adverse effects. Five and a half years after inoculation, one of the treated females, along with a cage mate, demonstrated a positive TB skin test that resulted in the intentional sacrifice of these animals. However, necropsy later revealed no evidence of tuberculosis infection or viral toxicity. Another of these Aotus was alive and well more than 10 years after inoculation. MRI analysis of the M002-treated monkeys showed no CNS abnormalities. An additional Aotus was injected with lower doses of M002 (nonpurified standard laboratory purifications of 1 × 107 and 1 × 108 PFU, respectively). This animal died while under anesthesia for a follow-up MRI. Necropsy demonstrated naturally occurring glomerulonephritis and bronchopneumonia that contributed to the anesthetic complication. No evidence of encephalitis or HSV-related toxicity was found.25
The biodistribution and toxicology studies of M032 were conducted by intracerebral injection of 100 μl of M032 HSV into 30 of 36 A. nancymae monkeys, distributed evenly by sex. The remaining 6 monkeys were injected with saline as a control. Two doses of HSV were tested—a low dose of 1 × 106 PFU and a high dose of 1 × 108 PFU. On a dose/gram of brain basis, these doses would correspond to human equivalent doses of 5 × 107 PFU and 5 × 109 PFU, respectively. Two male and two female monkeys in each of the low-dose and high-dose groups were killed at 3, 31, and 91 days and a complete necropsy was performed, including PCR analysis for presence and copy number of HSV in various organs and body sites. There were no serious adverse events or adverse events that could be attributed directly to M032.26
However, one animal was killed at 16 days because of a progressively moribund condition. This animal was losing weight, not eating, and became progressively lethargic without clear clinical evidence of viral encephalitis. A complete necropsy did not reveal any histopathological changes indicative of HSV encephalitis, and levels of HSV, as determined by quantitative PCR, in the brain and other organs were similar to levels detected in all other monkeys. Although there was nothing to support a diagnosis of HSV encephalitis or HSV infection in other organs, it could not be proven that the test article was not a contributing factor in this monkey's deteriorating condition.26 In summary, the HSV M032 that will be used in this clinical trial has been tested for safety and stability and has been determined to be safe for intracerebral administration in HSV-hypersensitive nonhuman primates at a per-kilogram dose, which far exceeds that we intend to employ in this study.
Study Objectives
The primary objective of this study is to determine the safety and tolerability of stereotactic intratumoral injections of escalating doses of M032 virus (NSC 733972), and to determine the maximum tolerated dose (MTD) of M032 in patients with recurrent or progressive malignant glioma who are not candidates for surgical resection.
The secondary objectives are (1) to obtain preliminary information about the potential benefit of M032 in the treatment of patients with recurrent malignant gliomas (progression-free survival, overall survival), (2) to delineate the local and systemic immune response to M032 administration, and (3) to characterize the in situ biologic activity of M032 after intratumoral inoculation.
Study Design
This study is a prospective medical investigation of human subjects designed to generate safety data. It is an open-label, dose-escalating phase I trial designed to determine the safety and tolerability of intratumoral injection of the M032 virus in patients with recurrent or progressive malignant gliomas. A single dose of M032 will be infused through a catheter into region(s) of tumor defined by MRI. Dosing will start at an initial dose level of 1 × 105 plaque-forming units of M032, and will then escalate by one log increments until the dose of 1 × 109 is completed or until the incidence of dose-limiting toxicities (DLTs) becomes unacceptable.
The primary endpoint is the MTD or maximally planned dose if no DLTs are observed for intratumoral injections of M032 virus. The safety and tolerability will be based on occurrence of adverse events. Although M032 has not previously been used in humans, the adverse events are expected to be similar to the adverse events observed in the phase I trials of a similar genetically engineered herpes simplex virus, G207.
Adverse events occurring after the administration of G207 included the following: asthenia, amnesia, nausea, somnolence, leukopenia, peripheral edema, fever, pneumonia, death, hemiplegia, confusion, headache, decreased consciousness, anemia, cachexia, varicella zoster infection, abnormal mentation, stroke, dysphasia, depression, deep vein thrombosis, seizure, abnormal erythrocytes, urinary tract infection, tumor progression, increased liver transaminases, and pseudoprogression. Other adverse events that might be expected based on the viral origin of M032 and on the inherent risks of malignant glioma include hematoma, encephalitis, hepatitis, disseminated HSV infection, allergic response to M032, nuchal rigidity, photophobia, and autoimmune response to CNS tissues.10,27,28 As a gene vector, M032 has the potential to produce IL-12-related toxicity. In the unlikely event that a cytokine storm is encountered, it will be treated with antiviral therapy (i.e., acyclovir), anti-inflammatory medications (i.e., corticosteroids), and close observation.
Any of the above adverse events will be considered a DLT, if attributable to M032 and if the severity of the adverse event is grade 3 or 4 as outlined in Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v 4.0) by the Cancer Therapy Evaluation Program (CTEP). However, preexisting neurologic symptoms from involvement of eloquent brain are common. Corticosteroids, radiation, and chemotherapies also produce preexisting symptoms in almost all cases. For all of these preexisting conditions, DLTs will be defined as progressive involvement of neurological dysfunction not attributable to tumor progression and/or the development of life-threatening symptomatology associated with the original baseline status. Neurologic deterioration will not be considered dose limiting if it resolves back to the patient's baseline within two weeks of completing treatment.
Enrollment will be stopped when the MTD is determined (as few as three but up to a maximum of six patients depending on the presence of any serious adverse events) at which time an additional six patients will be enrolled. Thus, a total of between 9 and 12 patients will be enrolled at the MTD.
The secondary endpoints include time to progression, survival, and biologic assessment of M032 in the patients with recurrent malignant gliomas. Preliminary information about the potential benefit of M032 will be gathered via the percentage of patients experiencing complete response, partial response, progressive disease, and stable disease based on tumor volume as assessed by MRI and clinical condition. Additionally, quality-of-life response will be analyzed via a Karnofsky Performance Status (KPS) score pre- and posttreatment for each patient. The local and systemic immune response to M032 administration will be evaluated via PCR and culture of serial serum and saliva samples to detect virus reactivation/shedding and ELISA to detect HSV antibody titers.
Follow-up evaluations will be performed using routine laboratory analyses and clinical measurements of neurological function and evidence of M032-related toxicity. Studies to evaluate the possibility of M032 shedding will also be conducted. Patients will be observed closely during the planned posttreatment hospitalization period, followed by outpatient evaluations at 10 days, and then months 1, 2, 3, 4, 5, 6, 9, and 12.
M032 dose and administration
Dosing will start at an initial dose level of 1 × 105 PFU of M032, and will then escalate by one log increments until the dose of 1 × 109 is completed or until the incidence of DLTs becomes unacceptable. If a DLT is encountered at any dose level, that cohort will be expanded to include six subjects. If two DLTs are encountered at any dose level, that will be defined as the MTD. Otherwise, escalation will proceed according to the dose escalation decision criteria (Table 1).
Table 1.
Dose escalation decision criteria
| Number of patients with DLT at a given dose level | Escalation decision rule |
|---|---|
| 0 out of 3 | Enter 3 patients at the next dose level |
| ≥2 | Dose escalation will be stopped. This dose level will be declared the maximally tolerated dose (highest dose administered). Three (3) additional patients will be entered at the next lowest dose level if only 3 patients were treated previously at that dose. |
| 1 out of 3 | Enter up to 3 more patients at this dose level. If 0 of these 3 patients experience DLT, proceed to the next dose level. If 1 or more of this group suffer DLT, then dose escalation is stopped, and this dose is declared the maximally administered dose. Three (3) additional patients will be entered at the next lowest dose level if only 3 patients were treated previously at that dose. |
| ≤1 out of 6 at highest dose level below the maximally administered dose | This is generally the recommended phase 2 dose. At least 6 patients must be entered at the recommended phase 2 dose. |
DLT, dose-limiting toxicity.
Adapted from Storer.29
Subject Selection and Withdrawal
Study subjects will include patients with recurrent or progressive malignant glioma in whom surgical resection is not considered feasible or is unlikely to afford benefit that would outweigh the risks of the operation. Eligible subjects will include men and women and members of all ethnic groups. Four to 24 patients with recurrent or progressive GBM, anaplastic astrocytoma, or gliosarcoma will be enrolled.
Inclusion criteria1
1. Patients must have histologically or cytologically confirmed GBM, anaplastic astrocytoma, or gliosarcoma.
2. Prior therapy: Patients must have failed external beam radiotherapy ≥5000 cGy to the brain and, if eligible and tolerated, undergone appropriate treatment with temozolomide. All radiation and chemotherapies must have been completed at least 4 weeks before and nitrosureas must have been completed at least 6 weeks before enrollment.
3. Age ≥19 years.
4. Karnofsky Performance Status ≥70%.
5. Life expectancy of greater than 4 weeks.
6. Patients must have normal organ and marrow function.
7. Residual lesion must be ≥1.0 cm in diameter as determined by MRI.
8. Since the effects of M032 on the developing human fetus are unknown, women of childbearing potential and men must agree to use adequate contraception before study entry and for the first 6 months after receiving M032.
9. Ability to understand and the willingness to sign a written informed consent document.
10. Females of childbearing potential must not be pregnant; this will be confirmed by a negative serum pregnancy test within 14 days before starting study treatment.
11. Steroid use is allowed as long as dose has not increased within 2 weeks of scheduled M032 administration. Whenever possible, the patient should be on a steroid dose that is equivalent to a dexamethasone dose of ≤2 mg daily at the time of treatment.
Exclusion criteria1
1. Patients who have had chemotherapy, cytotoxic therapy, immunotherapy within 4 weeks before entering the study (6 weeks for nitrosoureas), surgical resection within 4 weeks before entering the study, or have received experimental viral therapy or gene therapy at any time.
2. Patients who have not recovered from adverse events because of therapeutic interventions administered more than 4 weeks earlier.
3. Patients may not be receiving any other investigational agents.
4. History of allergic reactions attributed to compounds of similar biologic composition to M032 or to IL-12.
5. Tumor involvement, which would require ventricular, brainstem, basal ganglia, or posterior fossa inoculation or would require access through a ventricle in order to deliver treatment.
6. History of encephalitis, multiple sclerosis, or other CNS infection.
7. Required steroid increase within 2 weeks of scheduled M032 administration.
8. Active oral herpes lesion.
9. Concurrent therapy with any drug active against HSV.
10. Uncontrolled intercurrent systemic illness.
11. Pregnant women and immunocompromised patients excluded.
12. Patients with known history of allergic reaction to IV contrast material that is not amenable to pretreatment by UAB protocol.
13. Patients with pacemakers, ferro-magnetic aneurysm clips, metal infusion pumps, and metal or shrapnel fragments.
14. Receipt of gliadel therapy.
15. Receipt of bevacizumab (Avastin) therapy within four weeks of scheduled M032 administration.
Preexisting HSV immunity status will be considered in the safety and efficacy analyses; however, the numbers accumulated in this phase I dose escalation study will be too small to reach definitive conclusions or allow for a priori stratification.
Subject recruitment and screening
Recruitment will be performed by study personnel at the University of Alabama at Birmingham when a potential study subject is identified. Screening will take place within two weeks before the initiation of treatment. At that time, informed consent will be obtained, inclusion/exclusion criteria reviewed to ensure eligibility, and a comprehensive medical evaluation that includes study-specific laboratory testing and imaging will be performed. Additionally, all patients will be placed on anticonvulsants at the time of screening. This anticonvulsant will be continued a minimum of 28 days unless the patient experiences an adverse reaction. It is expected that this study will accrue approximately 10–12 patients per year.
Informed consent
The informed consent is included in the Supplementary Data (Supplementary Data are available online at www.liebertpub.com/humc). It will be obtained during the screening phase of the clinical protocol for each study subject.
Study Drug
M032 is a second-generation oHSV that selectively replicates in tumor cells and causes tumor cell to produce and express an immunity-stimulating protein called IL-12. The M032 virus will have direct oncolytic activity in addition to an indirect immune response enhancement and antiangiogenic effect via IL-12.
M032 will be provided for clinical study under an agreement between the NIH RAID program and the University of Alabama, Birmingham. HSV-M032 (NSC 733972; Lot # L0909005) is supplied in sterile, labeled 1.0 ml single-use glass vials containing 0.5 ml of HSV-M032 suspended in the storage buffer, Dulbecco's phosphate-buffered saline (D-PBS), 0.4 M NaCl, and 10% (w/v) glycerol, pH 7.4. The virus titer is 5.8 × 109 PFU/ml or 2.9 × 109 PFU/vial. The vials should remain frozen at −70°C or below until use. Before use, the frozen sample must be diluted in the excipient solution (Product Code 103382-50; Lot # 800612), which has been purchased from Alanza Inc. The dilution formulation is phosphate buffered saline and 10% (w/v) of glycerol, pH 7.0–7.4, and is supplied sterile in 50 ml aliquots in amber serum bottles with snap-cap butyl rubber stoppers.
Study Procedures
Treatment will be administered on an inpatient basis. On day 0, study subjects will be treated under monitored local anesthesia, or at the surgeon's discretion, under general anesthesia. The patients will then undergo a contrasted MRI scan to determine the site(s) for stereotactic biopsy and inoculation of the test agent. Patients will then undergo stereotactic biopsy of their tumor. The inoculation with M032 will proceed only if viable, recurrent, or progressive malignant glioma is identified on frozen section.
At least one cohort of three patients will be entered at each dosing level, given acceptable toxicities at previous dose levels. These patients will undergo placement of one to four stereotactically placed catheters, which will be primed with preservative-free sterile normal saline. The wound will be closed, the catheters aseptically exteriorized, and the subject allowed to recover overnight from the procedure in the neurosurgical ICU. If the patient's condition is stable, they may be transferred the next morning, day 1, to a clinical research unit bed for M032 administration and monitoring until discharge. After pathology results confirm recurrent tumor, and a CT confirms appropriateness of catheter placement, infusion of M032 may begin. If a catheter is determined to be misplaced by the investigator and cannot be properly positioned by simple partial withdrawal at the bedside, this/these catheter(s) will not be used for infusion. The catheters will be removed 6–18 hr after completion of the infusion.
Patients will then receive the entire M032 dose by intratumoral infusion over a 6 hr period (infusion may extend up to 12 hr if needed because of catheter malfunction). The total amount of M032, as defined by each patient's dose level, will be delivered in a total volume of ∼2.4 ml administered in up to four catheters at a total infusion rate of 400 μl/hr. The rate of administration through each individual catheter shall be determined by the following equation: 400 μl/hr/number of active catheters.
Dosing will start at an initial dose level of 1 × 105 PFU of M032, and will then escalate by one log increments until the dose of 1 × 109 is completed or until the incidence of DLT becomes unacceptable as outlined in dose escalation decision criteria (Table 1).
Each catheter will be placed in a different enhancing area of the tumor. If, in the surgeon's judgment, the tumor location or other properties are not suitable for all four catheters to be placed, fewer catheters will be placed. If delivery at the desired rate is not possible through an individual catheter, the rate of delivery through that catheter may be slowed and the rate of delivery through the remaining catheters increased as possible to attempt to maintain total delivery rate at 400 μl/hr.
After the initiation of the infusion, the subjects’ vital signs and neurologic examination will be monitored every hour for the first 12 hr, then every 2 hr for the next 12 hr, and then every 4 hr for the next 24 hr. Thereafter, the frequency of monitoring will be determined by the attending physician(s) based on the medical condition of the patient.
Patients are discharged home on day 3 after MRI confirms no evidence of encephalitis and clinical status is sufficiently stable. After discharge the subject will continue to be closely followed for evidence of adverse events, with outpatient follow-up evaluations scheduled at 1, 3, 6, 9, and 12 months, or more often if medically indicated. HSV detection will be performed at additional time points. Although viral shedding will be analyzed, because of the inability of the virus to replicate in normal tissues, its demonstrated lack of toxicity in hypersensitive primates, and the ability of acyclovir to halt replication of the virus, no quarantine period or limitation on viral shedding status has been required by the Food and Drug Administration or Internal Review Board. The collections at months 2, 4, and 5 will consist of sample collections and a study coordinator visit only. The study calendar is detailed in Table 2.
Table 2.
Study calendar
| Prestudy | Day 0 | Day 1 | Day 2 | Day 3 | Day 10 | Day 28 | Month 2 | Month 3 | Months 4 and 5 | Months 6, 9, and 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Informed consent | × | ||||||||||
| Inclusion/exclusion criteria | × | ||||||||||
| Demographics | × | ||||||||||
| Pregnancy test—serum | × | ||||||||||
| Medical history | × | ||||||||||
| Concurrent medications | × | × | × | × | × | × | × | × | × | × | × |
| Complete physical exam | × | × | × | × | × | × | × | × | |||
| Vital signs | × | × | × | × | × | × | × | × | × | ||
| KPS | × | × | × | × | × | × | |||||
| CBC | × | × | × | × | × | × | × | × | |||
| Serum chemistry, PT/INR, PTT | × | × | × | × | × | × | × | × | |||
| HIV serology | × | ||||||||||
| EKG | × | ||||||||||
| CXR | × | ||||||||||
| Urinalysis | × | ||||||||||
| Adverse event evaluation | × | × | × | × | × | × | × | × | × | × | |
| MRI | × | × | × | × | × | × | |||||
| Neurologic exam | × | × | × | × | × | × | × | × | × | ||
| HSV Ab titer | × | × | × | × | × | ||||||
| HSV detection | × | × | × | × | × | × | × | × | × | ||
| IL-12 detection | × | × | × | × | × | × | × | ||||
| Blood sample for LTA, ELISPOT | × | × | × | × | × | × | × | ||||
| INF gamma assay | × | × | × | × | × | × | × | ||||
| Blood stored for future immune studies | × | × | × | × | × | × | × | × | |||
| Head CT | × | ||||||||||
| Biopsy, catheter placement | × | ||||||||||
| Prophylactic AED | × | × | × | × | × | × | × | ||||
| M032 administration | × |
Statistical Plan
The primary endpoint of this study is to determine the safety and tolerability of a single stereotactic intracerebral infusion of escalating doses of M032 virus, and to determine the MTD of M032. The dose escalation scheme is outlined in Table 1.
Demographic and baseline characteristics will be summarized for each cohort using the statistics of number, mean, median, and range for continuous variables, and for discrete factors, values will be tabulated.
Descriptive statistics will be used in the reporting of adverse events. Adverse events will be recorded and frequency will be calculated. All events with a grade 3 toxicity or above will be recorded by event, as well as recording for all events related to the study drug. Analysis of laboratory data will consist of comparisons to baseline and to normal values for patients by dose group. Logarithmic transformations will be used as necessary. Standard errors and group means will be calculated for laboratory parameters. Concurrent illnesses will be listed and examined by univariate and multivariable analysis as possible confounders in the treatment–response relationship. Concurrent medications will also be listed. Effects of previous treatments for cancer will be examined by univariate and multivariable analysis; side effects potentially related to treatment will be evaluated.
Safety and Adverse Events Analyzed
Definitions
The CTEP has published CTCAE v 4 for the grading of adverse events experienced in clinical trials for antineoplastic agents. Any grade 3 or greater nonhematologic toxicity as defined by NCI CTCAE v 4, and considered as being possibly, probably, or likely related to M032 will be considered a DLT.
Reporting of serious adverse events and unanticipated issues
Serious adverse events (grade 3 or 4 toxicities) will be reported by fax, e-mail, or phone within 24 hr and a written expedited report filed within 7 days. Additionally, unexpected grade 2 or grade 3 toxicities will require a written expedited report within 7 days and grade 3 unexpected adverse events will also require a fax, e-mail, or phone call to the UAB IRB, the Neuro-Oncology CTMC, CCC CTRC, FDA, and OBA within 24 hr. Events that are related, unexpected, and serious will be reported to the FDA within 7 days of knowledge of the event.
Risk–Benefit Assessment
The primary objective of the study is to provide safety data on intratumoral injection of M032 in small cohorts of malignant glioma patients. Study risks include but are not limited to the previously mentioned adverse events seen with administration of G207, as well as risks associated with the viral origin of M032 and those inherent to malignant glioma. The primary benefit of this study will be the determination of the MTD of M032. This information can be used to design future trials that seek to determine the efficacy of intratumoral injection of M032 for the treatment of recurrent or progressive malignant glioma.
Acknowledgments
This research is supported in part by USPHS National Institutes of Health (P01 CA71933, P50 CA097247, P20 CA151129, and T32 CA091078) and by the University of Alabama at Birmingham.
Author Disclosure
Drs. Markert and Gillespie are founders of and own stock and stock options (<8% interest) in Aettis, Inc., a biotech company that has licensed M032 HSV from The Board of Trustees of the University of Alabama for the University of Alabama at Birmingham and is developing other oHSVs that are not the subject of this current investigation. Dr. Gillespie currently serves as one of five unpaid members of the Board of Directors for Aettis, Inc. Dr. Gillespie is a founder of and owns stock and stock options (<10%) in Maji Therapeutics, which is developing other HSVs that are not the subject of the current investigation. Drs. Markert and Gillespie were also founders of and owned stock and stock options (<8%) in Catherex Inc., a biotechnology company that had licensed additional intellectual property related to oHSV. Catherex, Inc., was sold to Amgen, Inc., on December 18, 2015, and they no longer participate in any decision making or have any control of any aspect of Catherex or Amgen, although they did receive proceeds from the sale of the company. Dr. Gillespie has served as a paid advisor to the Program Project at the Ohio State University that seeks to find improved methods for application of distinct oHSV to treat localized and metastatic cancers. This is generally, but not specifically, related to the subject matter of this investigation.
References
- 1.ClinicalTrials.gov. Genetically Engineered HSV-1 Phase 1 Study (M032-HSV-1). U.S. National Institutes of Health, 2014 [Google Scholar]
- 2.Andreansky SS, He B, Gillespie GY, et al. . The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci U S A 1996;93:11313–11318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine A. Principles and Practice of Oncology. (Lippincott Williams and Wilkins, New York: ). 1989 [Google Scholar]
- 4.Parker JN, Bauer DF, Cody JJ, et al. . Oncolytic viral therapy of malignant glioma. Neurotherapeutics 2009;6:558–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman GK, Pressey JG, Reddy AT, et al. . Herpes simplex virus oncolytic therapy for pediatric malignancies. Mol Ther 2009;17:1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markert JM, Parker JN, Buchsbaum DJ, et al. . Oncolytic HSV-1 for the treatment of brain tumours. Herpes 2006;13:66–71 [PubMed] [Google Scholar]
- 7.Shah AC, Benos D, Gillespie GY, et al. . Oncolytic viruses: Clinical applications as vectors for the treatment of malignant gliomas. J Neurooncol 2003;65:203–226 [DOI] [PubMed] [Google Scholar]
- 8.Chou J, Kern ER, Whitley RJ, et al. . Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 1990;250:1262–1266 [DOI] [PubMed] [Google Scholar]
- 9.Chou J, Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A 1992;89:3266–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markert JM, Medlock MD, Rabkin SD, et al. . Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther 2000;7:867–874 [DOI] [PubMed] [Google Scholar]
- 11.Hellums EK, Markert JM, Parker JN, et al. . Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol 2005;7:213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker JN, Gillespie GY, Love CE, et al. . Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A 2000;97:2208–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker JN, Meleth S, Hughes KB, et al. . Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther 2005;12:359–368 [DOI] [PubMed] [Google Scholar]
- 14.Caruso M, Pham-Nguyen K, Kwong YL, et al. . Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc Natl Acad Sci U S A 1996;93:11302–11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chua AO, Chizzonite R, Desai BB, et al. . Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. J Immunol 1994;153:128–136 [PubMed] [Google Scholar]
- 16.Toda M, Martuza RL, Kojima H, et al. . In situ cancer vaccination: An IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J Immunol 1998;160:4457–4464 [PubMed] [Google Scholar]
- 17.Bramson JL, Hitt M, Addison CL, et al. . Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum Gene Ther 1996;7:1995–2002 [DOI] [PubMed] [Google Scholar]
- 18.Wu CY, Demeure C, Kiniwa M, et al. . IL-12 induces the production of IFN-gamma by neonatal human CD4 T cells. J Immunol 1993;151:1938–1949 [PubMed] [Google Scholar]
- 19.Gately MK, Desai BB, Wolitzky AG, et al. . Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol 1991;147:874–882 [PubMed] [Google Scholar]
- 20.Manetti R, Parronchi P, Giudizi MG, et al. . Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med 1993;177:1199–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voest EE, Kenyon BM, O'Reilly MS, et al. . Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst 1995;87:581–586 [DOI] [PubMed] [Google Scholar]
- 22.Asselin-Paturel C, Lassau N, Guinebretiere JM, et al. . Transfer of the murine interleukin-12 gene in vivo by a Semliki Forest virus vector induces B16 tumor regression through inhibition of tumor blood vessel formation monitored by Doppler ultrasonography. Gene Ther 1999;6:606–615 [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi T, Joki T, Akasaki Y, et al. . Antitumor activity of interleukin 12 against interleukin 2-transduced mouse glioma cells. Cancer Lett 1999;135:47–51 [DOI] [PubMed] [Google Scholar]
- 24.Mikami T, Kurisu K, Kiya K, et al. . Antitumor effect of recombinant human lymphotoxin on a tumor line of human malignant glioma. Hiroshima J Med Sci 1989;38:103–107 [PubMed] [Google Scholar]
- 25.Markert JM, Cody JJ, Parker JN, et al. . Preclinical evaluation of a genetically engineered herpes simplex virus expressing interleukin-12. J Virol 2012;86:5304–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth JC, Cassady KA, Cody JJ, et al. . Evaluation of the safety and biodistribution of M032, an attenuated herpes simplex virus type 1 expressing hIL-12, after intracerebral administration to aotus nonhuman primates. Hum Gene Ther Clin Dev 2014;25:16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markert JM, Liechty PG, Wang W, et al. . Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther 2009;17:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markert JM, Razdan SN, Kuo HC, et al. . A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther 2014;22:1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storer BE. Design and analysis of phase I clinical trials. Biometrics 1989;45:925–937 [PubMed] [Google Scholar]