Abstract
Background and Aim
The function of the common bile duct is to transport bile from the liver and the gall bladder to the duodenum. Since the bile duct is a distensible tube consisting mainly of connective tissue, it is important to obtain data on the passive mechanical wall properties. The aims of this study were to study morphometric and biomechanical wall properties during distension of the bile duct.
Methods
Ten normal porcine common bile ducts were examined in vitro. A computer-controlled volume ramp infusion system with concomitant pressure recordings was constructed. A video camera provided simultaneous measurement of outer dimensions of the common bile duct. Wall stresses and strains were computed.
Results
The common bile duct length increased by 25% from 24.4 ± 1.8 mm at zero pressure to 30.5 ± 2.0 mm at 5 kPa (p < 0.01). The diameter increased less than 10% in the same pressure range from 8.6 ± 0.4 mm to 9.3 ± 0.4 mm (p < 0.01). The stress-strain relations showed an exponential behavior with a good fit to the equation: σ = α . (exp(βε) - 1). The circumferential stress-strain curve was shifted to the left when compared to the longitudinal stress-strain curve, i.e. the linear constants (α values) were different (p < 0.01) whereas the exponential constants (β values) did not differ (p > 0.5).
Conclusion
The porcine bile duct exhibited nonlinear anisotropic mechanical properties.
Keywords: common bile duct, distension, morphometric parameters, stress-strain, wall stiffness
Background
The function of the common bile duct is to transport the bile from either the gall bladder or the liver to the duodenum by passing it through the sphincter of Oddi. The duct has been described as a passive conduit consisting mainly of connective tissue with a high collagen content and only few smooth muscle cells [1,2]. Contractions have been reported [3] but these may be retrograde projections of contractions from the sphincter of Oddi [1].
Contractions of the gall bladder and relaxation of the sphincter of Oddi facilitate bile flow [4,5]. Hence, the bile duct can be considered as a pressure vessel without the ability to generate active forces by itself. Since the biliary tract is a distensible pressure vessel, it is important to obtain data on the mechanical wall properties. The mechanical properties will determine the behavior of the duct during loading and is likely to change in diseases of the biliary system. Few papers have focused on the biomechanical and morphometric properties of the bile duct wall. The literature on bile duct mechanics mainly contains data on ducts tested uni-axially in vitro [6-8]. Uni-axial testing can not be done with preserved tri-dimensional structural integrity of the organ wall. Distension of intact segments provides a more physiological-like condition of testing. When the force of inflation is applied, the intact segment deforms [9]. This approach was used in two studies of the normal porcine bile duct [10,11]. However, these studies were limited to measurements only at high pressures or to circumferential tension-strain relations where tension was computed from the transmural pressure and radius but where the wall thickness was not measurable. The stress-strain relation is, however, a more valid measure of the biomechanical properties [12]. Stress is force per unit cross-sectional area. Strain refers to the resulting deformation of the material and is usually expressed as a fraction of the initial length. Strain is non-dimensional which favors comparison between different experiments. The proportionality constant between stress and strain for a linear relationship is called the elastic modulus and is a measure of wall stiffness [9,12]. For non-linear stress-strain relations, an incremental modulus can be computed or mechanical constants determined. In cylindrical tubes the normal stress and strain components are in radial, longitudinal and circumferential directions. If the wall is thin, then the radial component can be ignored and the mechanical problem can be reduced to a two-dimensional one.
The aims of this study were to provide morphometric measures of the wall changes during distension of the bile duct and to derive the circumferential and longitudinal stress-strain relations in order to determine the mechanical properties.
Material and methods
Anesthesia and blood samples
Ten four-months-old LY-strain female pigs (a mixture of Danish Country breed and Yorkshire) weighing 43.9 kg ± 0.7 kg were studied. They were fasted overnight and received as im. premedication 4.8 mg kg-1azaperone (Sedaperone®) and 0.6 mg kg-1midazolam (Dormicum®). Thirty minutes later 0.4 mg kg-1etomidate (Hypnomidate®) was administered iv. and the pigs were intubated and connected to the respirator. Anesthesia was maintained by continuous intravenous infusion of 10 mg kg-1 h-1ketaminol (Ketamine®), 0.6 mg kg-1 h-1midazolam and 0.12 mg kg-1 h-1parvulon (Pancuron Bromide®). A blood sample was taken for analysis of bilirubin, alanine transaminase and alkaline phosphatase to reduce the probability of the pigs having illnesses of the liver and biliary system. The values for bilirubin, alanine transaminase and alkaline phosphatase were 7.6 ± 0.99 μmol l-1, 48.4 ± 4.3 U/l and 247.8 ± 19.8 U/l, respectively. Thus, all pigs were considered normal and included in the study. The study complied with the Danish regulations for care and use of laboratory animals.
Isolation of the common bile duct
An upper midline abdominal incision was made and the bile duct was exposed. The pressure in the bile duct was measured by inserting a needle into the duct and then connecting it to a low compliance perfusion system using external pressure transducers (Baxter uniflow™). The pressure signal was analog-to-digital converted with a sampling frequency of 5 Hz and acquired on-line to a computer using dedicated software (SuperMingo™, Gatehouse Aps, Aalborg, DK). The mean pressure in the common bile duct was 0.81 ± 0.06 kPa. The pressure fluctuated with respiration with approximately ± 0.05 kPa. Next the bile duct was dissected from the adjacent tissue. Proximally, the duct was cut at the level where the cystic duct intersects with the hepatic duct to create the common bile duct. Distally, the duct was cut at the duodenal wall. The duct did not seem to change length during excision. The pig was euthanized by an intracardiac injection of KCl after the segment was removed.
In vitro procedures
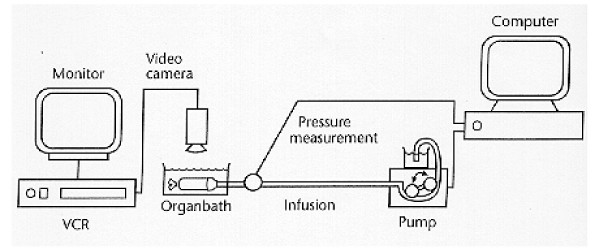
After excision the common bile duct was immediately transferred to an organ bath containing 22°C oxygenated (95% O2 and 5% CO2) calcium-free Krebs-Ringer solution with 95 mg l-1 EGTA and 60 g l-1 Dextran at pH = 7.40. EGTA was added to abolish muscle contractions. The pressure measuring system was calibrated and the zero pressure level was set with the transducer at the same level as the surface of the solution in the organ bath. The proximal end of the segment was ligated to a tube connected to an infusion system and the transducer. The other end was ligated as close to the sphincter of Oddi as possible. Air was removed from the lumen of the segment. The above-mentioned Krebs-Ringer solution was used for infusion into the segment after equilibrium to zero pressure. The data acquisition software also controlled a rollerpump with infusion and withdrawal functions (Ole Dick, Instrumentmakers Aps, Denmark). The pump was programmed to infuse or withdraw volume so that inflation to 5 kPa lasted approximately 1.5 min. The volume rate was between 0.5 and 1.5 ml min-1, depending on the size of the bile duct in each pig. When the pressure reached 5 kPa, the flow was reversed for the same time period as the infusion to assure that the withdrawn volume equaled the infused volume. The bile duct was preconditioned by six cycles of volume infusions and withdrawals up to 5 kPa pressure (the number of cycles determined by pilot studies). After the preconditioning procedure, the distension experiment was done as one more cycle to the same pressure level. A Sony CCD camera and a VCR provided recordings of the outer dimension of the bile duct under the pressure changes induced. The experimental set up is illustrated in figure 1. The segment was removed from the organ bath after the distension series and four rings from each segment were cut at 20, 40, 60 and 80% length with the proximal end of the segment being 0%. The rings were 2 mm wide and were immersed in neutrally buffered formaldehyde. The formaldehyde fixed rings were visualized under microscope (Zeiss, Stemi 2000-C). The views were frame grabbed, displayed on a monitor and the wall thickness was measured at four locations of each ring. Since there was no variation in thickness along the circumference, the thickness was given as the average of these four measurements (h0).
Figure 1.
Schematic drawing of the in vitro setup. The bile duct is placed in the organ bath and attached to the volume infusion/pressure measurement system. A video camera connected to a monitor and VCR provides pictures of the bile duct for external bile duct morphometric measurements.
Validation of system
Pump infusion and withdrawal precision were tested as the repeatability in the range from 0.5 to 1.5 ml min-1 by infusing and withdrawing over 2 minutes for each volume setting 10 times [13]. The coefficient of variance was between 2.2% and 6.3%, which was found acceptable. No difference in volume was found between when the pump was set at infusing or withdrawing volume (p > 0.2).
Repeatability of the morphometric measurements done using the digital image analyzing software SigmaScan Pro (Jandel Scientific, Germany) was tested by measuring a precision scale 10 times and the result was given as the coefficient of variance [13,14]. The resolution was evaluated as the two-point discrimination. Evaluation was done with magnifications corresponding to the experimental conditions. When the video camera was not mounted on the microscope, a calibration scale of 10 mm was measured ten times. The measured value was 9.97 ± 0.03 mm with 0.86% coefficient of variance. The two point discrimination was 0.096 ± 0.001 mm evaluated over ten experiments. When the video camera was mounted on the microscope (magnification 20×), a calibration scale of 5 mm was measured as 4.99 ± 0.01 mm with 0.74% coefficient of variance. The two point discrimination was 0.023 ± 0.002 mm over ten experiments.
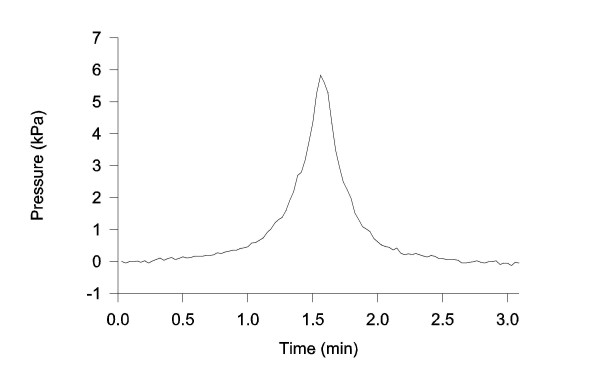
The dynamic parameters of the pressure measuring system were evaluated by a pressure chamber test [15]. The pressure recording system behaved as an overdamped second order system. The rise time was 0.08 sec (10 – 90% of the pressure drop from 9 to 0 kPa) meaning that the system was capable of measuring a pressure rise of 56 kPa sec-1. This value was magnitudes higher than the pressure changes measured in the experiments (between 0.21 and 0.67 kPa sec-1 close to the end of the inflation where the pressure rise was steepest (Figure 2)).
Figure 2.
Pressure recording versus time during volume infusion. The pump was reversed at a pressure of 5 kPa. The overshoot may be caused by inertia in the infusion system.
Morphometric and mechanical data analysis
Still pictures from the VCR were frame grabbed at pressures 0, 0.5, 1, 1.5, 2, 3, 4 and 5 kPa for each segment during infusion. The length and diameter were measured from the digitized images using SigmaScan Pro. The length was measured between the 20 and 80% locations to avoid edge effects. The outer diameter was calculated by measuring the area of the segment between 20 and 80% length and dividing it by this length to obtain a mean diameter of the segment. The internal diameter and wall thickness could not be measured experimentally with this preparation but was calculated based on the following assumptions [9]: 1) the wall was incompressible, i.e. the volume of the wall did not change during distension, 2) the shape of the common bile duct was cylindrical, 3) the wall thickness at no-load conditions and outer diameter and length at various inflation pressures were measurable, and 4) the wall thickness-to-radius ratio was small. The volume of the wall (V) at pressure 0 kPa was calculated as
V = (ro02 - (ro0 - h0)2)πl0 (1)
where ro0, h0 and l0 were the measured outer radius, the wall thickness and the length at pressure 0 kPa. The wall thickness was calculated for all pressure steps as:
![]()
where l and ro were the measured length and outer diameter during loading.
Calculations and measurements as described above were used for computation of the mechanical parameters assuming equilibrium conditions [9,12]. The Circumferential stress was defined as:
![]()
where ΔP was the transmural pressure and ri the inner radius (ri = ro - h). As the segment was isolated and the surface of the Krebs-Ringer in the organ bath was the zero pressure reference, the transmural pressure was equal to the applied pressure in the segment.
The Longitudinal stress was defined as:
![]()
Cauchy strains were calculated for simplicity, though the deformation was quite large [9].
Circumferential strain was defined as:
![]()
where rm was the midwall radius (by subtracting 1/2h from the outer radius) at the various pressure loads. r0m was the reference midwall radius calculated from the radius measured at a pressure of 0 kPa.
The Longitudinal strain was defined as:
![]()
where l was the measured segment length under the imposed pressure loads and l0 the reference length measured at a pressure of 0 kPa.
The Radial strain was defined as:
![]()
where h was the calculated wall thickness of the imposed pressure loads and h0 the measured wall thickness of the formaldehyde fixed rings.
The circumferential and longitudinal stress-strain relations were compared using curve-fitting software (TableCurve 1.12®, Jandel Scientific, Germany). The exponential equation σ = α . (exp(βε) - 1) was used since the elasticity of biological tissues often exhibits exponential behavior [9]. The α and β values were derived from the curve fit regression done for each segment separately. The slope of the non-linear stress-strain curves is called the incremental elastic modulus and is a measure of wall stiffness. The exponential equation was differentiated, yielding σ' = (αβ) . exp (βε), to define the incremental elastic modulus. The circumferential incremental elastic modulus at high and low strain was correlated to the diameter at 0 kPa and to the wall thickness-to-radius ratio (determinant for the stress). The circumferential incremental elastic modulus was calculated at the highest and lowest strain in common for all bile ducts.
Statistical analysis
Results are expressed as mean ± SEM unless otherwise stated. The data distribution was tested for normality by inspecting probability plots and for variance homogeneity by Bartlett's test. Student's t-test and in case of non-parametric distribution of data Mann-Whitney Rank sum test was used for statistical analysis. The α and β values were compared statistically by using one-way analysis of variance (ANOVA) or Kruskal-Wallis one-way analysis of variance on rank if the normality test or the test of equal variance failed. Association between the morphometric and biomechanical parameters was evaluated by Pearson Product Moment correlation. The results were considered significant when p < 0.05.
Results
The pressure increased only slightly during the first minute of inflation. This was followed by a gradually steeper increase until the maximum pressure of 5 kPa was reached. Thus, the inflation curve had an exponential course with a long toe region and a steep region (Figure 2). Though the pump was reversed at a pressure of 5 kPa, overshoot was observed before the pressure decreased again.
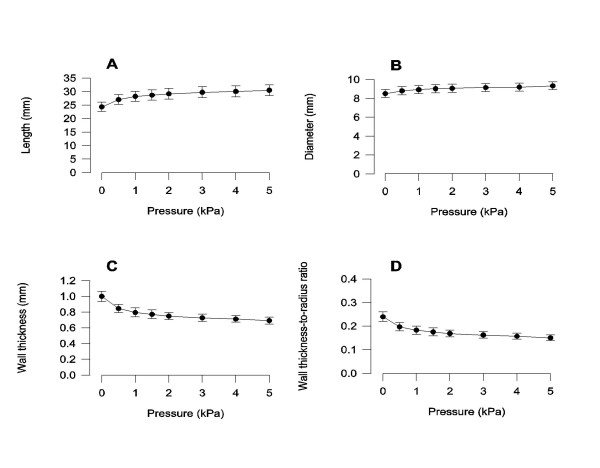
The variation of length, diameter and wall thickness of the bile duct as function of pressure were non-linear. The most pronounced change occurred in the pressure range from 0 to 2 kPa (Figure 3). The length increased by 25% from 24.39 ± 1.75 mm at 0 kPa to 30.54 ± 2.08 mm at 5 kPa (p < 0.01). In the same pressure range the diameter increased less than 10% from 8.61 ± 0.40 mm to 9.33 ± 0.41 mm (p < 0.01). The wall thickness decreased by 31% from 1.00 ± 0.06 mm at 0 kPa to 0.69 ± 0.04 mm at 5 kPa (p < 0.01). The wall thickness-to-radius ratio decreased by 38% from 0.24 ± 0.02 at 0 kPa to 0.15 ± 0.01 at 5 kPa (p < 0.01).
Figure 3.
Bile duct morphometric parameters as a function of pressure. All parameters varied as function of pressure (p < 0.01). n = 10. Mean ± SEM is shown.
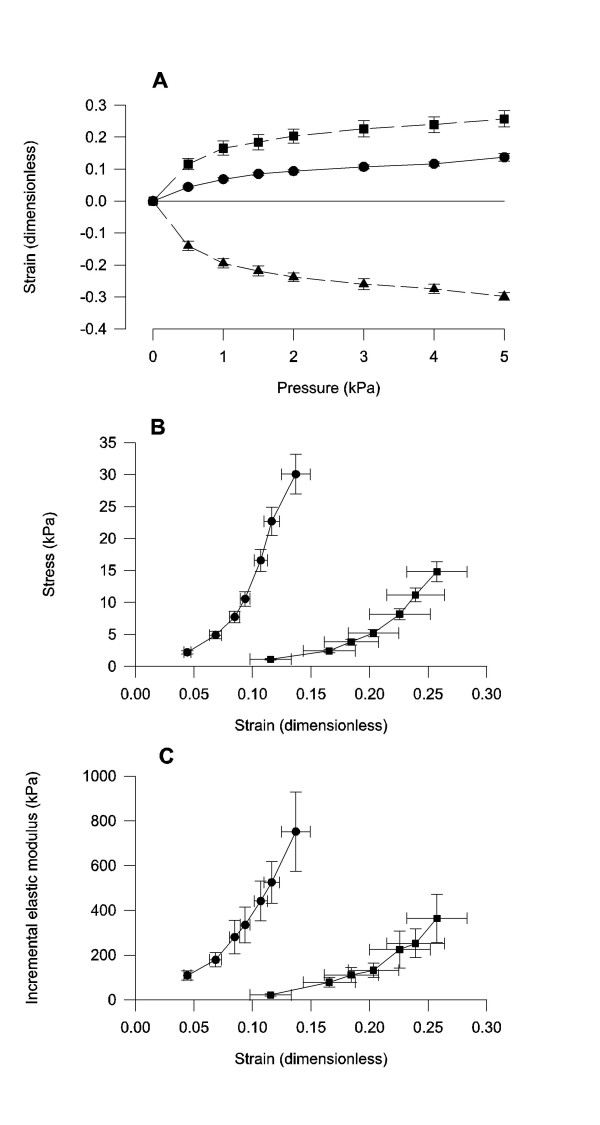
The longitudinal and circumferential strains were positive and with the largest strain in the longitudinal direction (Figure 4A). The radial strain was negative. All three strains were non-linear when expressed as a function of pressure. The longitudinal and circumferential stress-strain relations were non-linear (Figure 4B). The determination coefficients for the equation: σ = α . (exp(βε) - 1) were 0.90 ± 0.05 and 0.92 ± 0.01 in circumferential and longitudinal directions, respectively. The circumferential stress-strain curve was shifted to the left when compared to the longitudinal stress-strain curve. The linear constant differed (p < 0.01) whereas the exponential constant did not differ (p > 0.5) between the circumferential and longitudinal direction. The incremental elastic modulus as a function of strain was non-linear and the circumferential curve was shifted to the left of the longitudinal curve (Figure 4C).
Figure 4.
Bile duct mechanical parameters. The strain-pressure data are from circumferential (circles), longitudinal (squares) and radial (triangles) directions. Note in the stress strain graph (B) and the elastic modulus graph (C) that the circumferential curves are shifted to the left of the longitudinal indicating that the bile duct is stiffest in circumferential direction. n = 10. Mean ± SEM is shown.
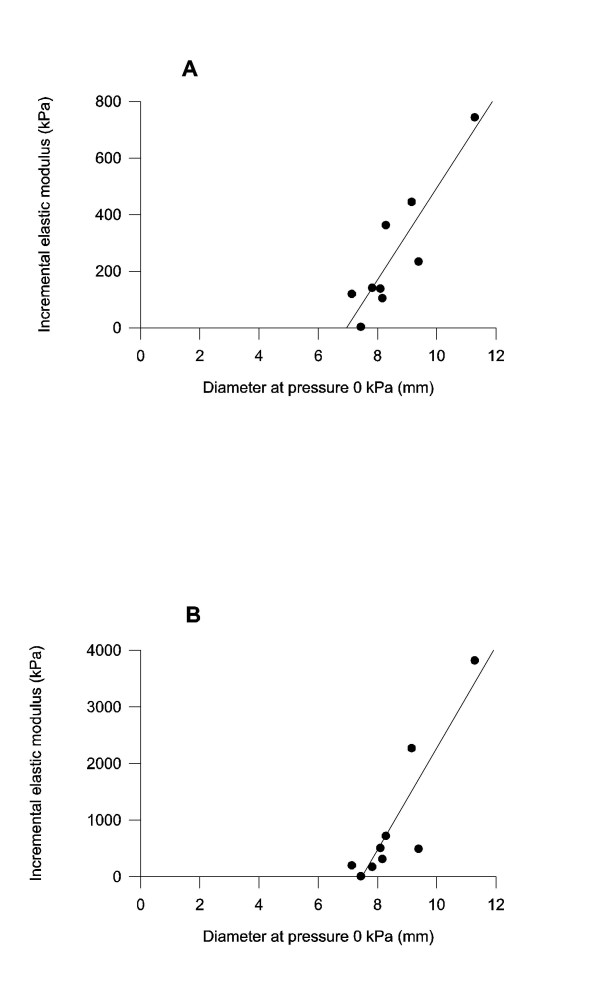
An association was found between the circumferential incremental elastic modulus and the diameter at a pressure of 0 kPa. The association was significant for the incremental elastic modulus at both high and low strain values (r = 0.884 and 0.894, p < 0.01 for both, figures 5A and 5B). An inverse association was found between the circumferential incremental elastic modulus at low strain and wall thickness-to-radius ratio at 0 and 5 kPa (r = -0.764 and -0.677, p < 0.05). No association was found between the elastic modulus at high strain value and the wall thickness-to-radius ratio.
Figure 5.
Regression curves between the unloaded diameter at 0 kPa and the circumferential incremental elastic modulus at low (A) and high (B) strains. An association was found at both low strains (r = 0.894) and high strains (r = 0.884) (p < 0.01).
Discussion
The major findings were that in the pressure range studied 1) the increase in bile duct length was much larger than the increase in bile duct diameter, 2) the circumferential and longitudinal stress-strain relations and the incremental elastic modulus showed an exponential behavior and the circumferential curves were shifted to the left of the longitudinal curves, and 3) an association was found between the circumferential incremental elastic modulus and the diameter at the initial unpressurised state and between the circumferential incremental elastic modulus and wall thickness-to-radius ratio.
Methodological aspects
Data on bile duct stress-strain relations were obtained by volume infusion into the common bile duct under simultaneous pressure recordings. At the same time outer dimensional changes were recorded. Analysis of diameter and length showed a high degree of accuracy based on the evaluations performed in this study. One of the assumptions made in this study for the stress calculations was that the geometric configuration of the lumen was circular. This was confirmed by vision and further validated by determining a second diameter in 90° planes by a prism in a few pilot experiments (unpublished data). Other assumptions made in this study were that the bile duct wall thickness-to-radius ratio was relatively small in order to use thin shell theory for analysis and that the wall was incompressible. Such assumptions are commonly made in biomechanical studies because it simplifies the analysis. Thin shell theory can be applied as long as the membrane is so thin that bending rigidity can be neglected [9]. The wall thickness-to-radius ratio should be around 0.10–0.20. In this study wall thickness was measured at the unpressurized state and then calculated for the pressurized states. At the unpressurized state the wall thickness-to-radius ratio was 0.24 and decreased to 0.15 at 5 kPa. The stress-strain relation under the circumstances in this study reflects mainly the passive elastic properties. Preconditioning was done to obtain repeatable results [9]. Thus, after the specimen was mounted in the organ bath, the loading cycles were repeated until the stress-strain relationship became stabilized. The interpretation of preconditioning is that the tissues are disturbed in the preparation process by cutting, temperature changes, chemical environment, hypoxia and smooth muscle contractions and need to be restored to a stabile condition [22].
Physiological and mechanical aspects
Exponential mechanical behavior has been observed in the bile duct in vitro [10] as well as in other tissues in vivo and in vitro [16-19]. The exponential behavior is expedient for organs with reservoir function since low wall stiffness at physiological pressures facilitate wall stretch to accommodate the gall. The steep increase in wall stiffness with higher loads provides a mechanism to avoid overstretch and damage to the tissue. This is in agreement with a previous study of compliance in the intact bile duct where high compliance was found at low pressures and low compliance at high pressures [20]. The normal pressure range in the bile duct is associated with the migrating motor complex (MMC) of the intestine and the pressure waves are transmitted from the sphincter of Oddi [1,21]. In phase I and II of the MMC the pressure range in the bile duct is between 0.6 and 1 kPa and rise to 1.3 kPa under phase III [21]. With bile duct obstruction the pressure stabilizes at about 3 kPa [4]. The most pronounced changes in dimension seen in this material were in the pressure range from 0 to 2 kPa, i.e. in the physiological pressure area. As commonly done in biomechanical studies, these experiments also superseded the physiological range.
Positive association between the incremental elastic modulus and the diameter of the segment at 0 kPa pressure at both low and high strain values was demonstrated. Hence, the bigger the initial diameter, the bigger the elastic modulus. This is likely due to that a bigger duct needs to be stiffer to counteract the higher force exerted by the pressure. A thicker wall or a stiffer material in the wall can contribute to the increased stiffness.
The common bile duct shows an anisotropic behavior with a much larger capacity to stretch in the longitudinal direction compared to the circumferential direction. A previous study in the intact bile duct showed that stress in the longitudinal direction was lower than in the circumferential direction [11] which supports our finding. Anisotropy also characterize other biomaterials such as arteries [12,23] where it was found that the elastic modulus in the circumferential direction was less than in longitudinal direction [12]. The fact that the bile duct more readily elongates than increases its diameter may have several, yet hypothetical, functions. First, elongation may mechanically affect the sphincter of Oddi, facilitating leakage of gall through it at high biliary tract pressures. Second, a rather stiffwalled organ in circumferential direction reduce the wall stress but increases the shear stress and the resistance to flow. Elongation further contributes to higher shear stress and resistance to flow. Hence, during obstruction a pressure will build up faster, resulting in inhibition of bile production. These hypotheses obviously need further study using more advanced biomechanical approaches.
Authors' contributions
Authors BUD and HA carried out all the experimental work, took part in the design of the study, analyzed the data and wrote the first draft of the manuscript. Author HG took part in the design and guided the experimental and analytical work. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgments
This study was supported by grants from the Institute of Experimental Clinical Research, Aarhus University, NOVO Nordisk Foundation and Karen Elise Jensen's Foundation.
Contributor Information
Birgitte U Duch, Email: duch@dadlnet.dk.
Helle Andersen, Email: hag@smi.auc.dk.
Hans Gregersen, Email: hag@aas.nja.dk.
References
- Hauge CW, Mark JB. Common bile duct motility and sphincter mechanism. I. Pressure measurements with multiple-lumen catheter in dogs. Ann Surg. 1965;162:1028–38. doi: 10.1097/00000658-196512000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frierson-HG J. The gross anatomy and histology of the gallbladder, extrahepatic bile ducts, Vaterian system, and minor papilla. Am J Surg Pathol. 1989;13:146–62. doi: 10.1097/00000478-198902000-00008. [DOI] [PubMed] [Google Scholar]
- Ludwick JR. Observations on the smooth muscle and contractile activity of the common bile duct. Ann Surg. 1966;164:1041–50. doi: 10.1097/00000658-196612000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PA, Poston GJ, Williamson RC. Biliary motility. Gut. 1990;31:571–82. doi: 10.1136/gut.31.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsoli A, Corazziari E, Habib FI, Cicala M. Pressure relationships within the human bile tract. Normal and abnormal physiology. Scand J Gastroenterol Suppl. 1990;175:52–7. doi: 10.3109/00365529009093127. [DOI] [PubMed] [Google Scholar]
- Minker E, Varkonyi T, Tamaskovits E. The pharmacological reactivity of an isolated circular smooth muscle strip of the rabbit common bile duct. Acta Pharm Hung. 1993;63:163–75. [PubMed] [Google Scholar]
- Walsh TH, Akoglu T. The muscle content and contractile capability of the common bile duct. Ann R Coll Surg Engl. 1979;61:206–9. [PMC free article] [PubMed] [Google Scholar]
- Patacchini R, Bartho L, Maggi CA. Characterization of recptors mediating contraction induced by tachykinins in the guinea-pig isolated common bile duct. Br J Pharmacol. 1997;122:1633–8. doi: 10.1038/sj.bjp.0701560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung YC. Biomechanics, mechanical properties of living tissues. 2. New York, Springer-Verlag; 1993. [Google Scholar]
- Duch BU, Petersen JA, Gregersen H. Luminal crosse-sectional area and tension-strain relation of the porcine bile duct. Neurogastroenterol Motil. 1998;10:203–9. doi: 10.1046/j.1365-2982.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- Jian C, Wang G. Biomechanical study of the bile duct system outside the liver. Biomed Mater Eng. 1991;1:105–13. [PubMed] [Google Scholar]
- Dobrin PB. Mechnical properties of arteries. Physiol Rev. 1978;58:397–460. doi: 10.1152/physrev.1978.58.2.397. [DOI] [PubMed] [Google Scholar]
- British Standards Institution Precision of test methods Guide for the determination of repeatability and reproducibility for a standard test method by inter-laboratory tests; 1987.
- Armitage P, Berry G. Statistical methods in medical research. 3. Oxford, Blackwall Science Ltd; 1994. [Google Scholar]
- Andersen MB, Bergsten O. Blood pressure – measurements and methods. Simonsen & Weel Medico teknik A/S, Denmark; 1982. [Google Scholar]
- Fung YC. Biomechanics Motion, flow, stress, and growth. New York, Springer-Verlag; 1990. [Google Scholar]
- Villadsen GE, Storkholm JH, Hendel L, Vilstrup H, Gregersen H. Impedance planimetric characterization of esophagus in systemic sclerosis patients with severe involvement of esophagus. Dig Dis Sci. 1997;42:2317–26. doi: 10.1023/A:1018831104549. [DOI] [PubMed] [Google Scholar]
- Frobert O, Gregersen H, Bagger JP. Mechanics of porcine coronary arteries ex vivo employing impedance planimetry: a new intravascular technique. Ann Biomed Eng. 1996;24:148–55. doi: 10.1007/BF02771003. [DOI] [PubMed] [Google Scholar]
- Duch BU, Petersen JA, Vinter JL, Gregersen H. Elastic properties in the circumferential direction in isolated rat small intestine. Acta Physiol Scand. 1996;157:157–63. doi: 10.1046/j.1365-201X.1996.503248000.x. [DOI] [PubMed] [Google Scholar]
- Slater G, Tartter P, Delman D, Aufses-AH J, Dreiling DA, Rudick J. Compliance of the extramural portion of the canine common bile duct. Proc Soc Exp Biol Med. 1983;173:344–8. doi: 10.3181/00379727-173-41654. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Tanaka M. Biliary pressure variation in coordination with migrating motor complex of duodenum in patients with cholecystectomy and effects of morphine and cerulean. Dig Dis Sci. 1992;37:1531–6. doi: 10.1007/BF01296498. [DOI] [PubMed] [Google Scholar]
- Deng SX, Tomioka J, Debes JC, Fung YC. New experiments on shear modulus of elasticity of arteries. Am J Physiol. 1994;266:H1–10. doi: 10.1152/ajpheart.1994.266.1.H1. [DOI] [PubMed] [Google Scholar]
- Zhou J, Fung YC. The degree of non-linearity and anisotropy of blood vessel elasticity. Proc Natl Acad Sci USA. 1997;94:14255–60. doi: 10.1073/pnas.94.26.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]