Abstract
The presence of subclinical infection or clinical disease in laboratory zebrafish may have a significant impact on research results, animal health and welfare, and transfer of animals between institutions. As use of zebrafish as a model of disease increases, a harmonized method for monitoring and reporting the health status of animals will facilitate the transfer of animals, allow institutions to exclude diseases that may negatively impact their research programs, and improve animal health and welfare. All zebrafish facilities should implement a health monitoring program. In this study, we review important aspects of a health monitoring program, including choice of agents, samples for testing, available testing methodologies, housing and husbandry, cost, test subjects, and a harmonized method for reporting results. Facilities may use these recommendations to implement their own health monitoring program.
Introduction
Ethical and fiscal responsibilities require biomedical research be conducted and reported utilizing methods that produce valid, reproducible results. When reporting results from a study utilizing an animal research model, it is important to describe the health status of the animals, including the presence or absence of clinical or subclinical infection.1 Failure to report presence of disease can lead to misinterpretation of research results from zebrafish2,3 and mammalian models4–6 and result in published studies becoming unusable or nonreplicable.7 Subclinical infections in zebrafish can result in confounded experimental results, misinterpretation of experimental data, poor reproducibility, and the unnecessary utilization of more animals to demonstrate statistical significance.
At many institutions, the centralization of zebrafish facilities improves operational efficiencies, promotes better husbandry practices, fosters collaboration, and facilitates fish transfers among investigators. However, as zebrafish facilities increase in size and complexity, so does the risk associated with infectious disease. The large multirack recirculating aquaculture systems widely utilized by zebrafish core facilities may contain the colonies of multiple investigators, more than one species of fish, or numerous zebrafish lines that may differ in immune status. Therefore, the introduction of infectious agents is potentially devastating to research programs.
Despite earlier calls for standardized health monitoring programs and reporting formats, information on the health status of animals from many institutions remains difficult to obtain.8,9 The obstacles associated with implementing health monitoring programs for zebrafish colonies have been previously examined.8,9 These include: limited knowledge regarding transmission, diagnosis, and treatment of infectious agents of zebrafish, lack of awareness of the impact of infectious diseases on animal health and research results, paucity of personnel, and cost.8,9 Determining the health status of imported animals remains a challenge: reporting of zebrafish colony health information is sporadic, with no consensus on which agents should be monitored or testing frequency.
Knowledge of common zebrafish diseases and their impact on research outcomes has increased substantially, and it is recommended that all zebrafish facilities have a routine colony health monitoring program. Adoption of minimum reporting standards for health monitoring will make the transfer of fish between institutions less time consuming. A standardized reporting format (as described subsequently) will allow facilities to quickly and easily determine the risk to their facility from imported fish, to take measures to mitigate that risk, and to ensure that results achieved from research performed is free of uncontrolled variables.10
General Considerations
A health monitoring program should minimize or eliminate the impact of infectious diseases on animal health and research results, as well as protect human health.8,9,11 There are many factors to consider when designing a health monitoring program: size and diversity of the research program, frequency of importation, type of housing (facility design) and husbandry, use of live versus commercial feed, agents to be monitored, testing methodologies to be used, frequency of monitoring, and financial costs. Well-designed health monitoring programs will track the health of a zebrafish colony over time, as well as assist in the identification of biosecurity and husbandry issues as they arise. Exclusion of pathogens from zebrafish colonies is the most powerful tool for protecting zebrafish health. However, when breaks in biosecurity occur, detecting new pathogens as soon as possible allows for rapid assessment of risks to animal health and research programs, rapid action for biocontainment, treatment of infected animals if possible, and/or prevention of the spread of infectious agents within and between facilities or institutions.
Colony health monitoring programs must reflect the needs of the research being conducted at the institution and exhibit flexibility to accommodate changing needs. For example, research utilizing immunocompromised animal models or performing toxicology studies should exclude as many pathogens as possible, whereas opportunistic pathogens may be considered acceptable for other research objectives. Each facility or program must determine the frequency of testing and particular list of agents of concern and must review their health monitoring program as new information becomes available. Generally, agents, which may have a significant impact on the research program, should be tested for more frequently. Agents that present less risk to the program because of low prevalence or low pathogenicity can be tested for less frequently.
Choice of Agents
Several agents have been shown to infect zebrafish (Table 1), potentially altering physiology, immunity, histology, genetics, behavior and imaging results in zebrafish. These agents may be primary and/or opportunistic pathogens exacerbated by stress, poor husbandry techniques, and poor water quality.12,13 Many of these agents can cause subclinical infections, or remain subclinical for weeks or months following infection, rendering detection during routine daily health checks more difficult. Subtle signs of infection may include a slight decrease in reproductive efficiency, a reduction in appetite or growth, and/or a low increase in colony mortality over time. Other agents cause anorexia, dropsy, emaciation, exophthalmia, skin ulceration, and high mortality. All seven of the Mycobacterium spp. that are known to infect zebrafish are potentially zoonotic; of these, Mycobacterium marinum has been most frequently implicated in zoonotic infections of immunocompetent individuals.14–17
Table 1.
Select Infectious Agents of Zebrafish
| Agent | Classification | Pathogenicity | References |
|---|---|---|---|
| Aeromonas hydrophila | Bacterium | Usually an opportunist | 12,53 |
| Edwardsiella ictaluri | Bacterium | Primary pathogen | 49,50 |
| Flavobacterium columnare | Bacterium | Primary pathogen | 52 |
| Mycobacterium spp. | Bacterium | Primary or secondary pathogen | 13,25,26,28,29–39,82 |
| M. abscessus | |||
| M. chelonae | |||
| M. fortuitum | |||
| M. haemophilum | |||
| M. marinum | |||
| M. peregrinum | |||
| M. saopaulense | |||
| Coleps sp(p). | Ciliate | Facultative parasite of larvae | 55 |
| Ichthyophthirius multifiliis | Ciliate | Primary pathogen | 55 |
| Tetrahymena sp(p). | Ciliate | Facultative parasite of larvae | 54,55 |
| Trichodina sp(p). | Ciliate | Primary pathogen | 56 |
| Piscinoodinium pillulare | Dinoflagellate | Primary pathogen | 54 |
| Saprolegnia brachydanis | Oomycete | Opportunist | 79 |
| Saprolegnia ferax | Oomycete | Opportunist | 79 |
| Myxidium streisingeri | Myxozoan | Primary pathogen | 48 |
| Gyrodactylus sp(p). | Monogenean | Primary pathogen | 56 |
| Pleistophora hyphessobryconis | Microsporidium | Primary pathogen | 23,24 |
| Pseudoloma neurophilia | Microsporidium | Primary pathogen | 18–21,24,74,75 |
| Pseudocapillaria tomentosa | Nematode | Primary pathogen | 2,44,45,47 |
| Transversotrema patialense (sensu lato) | Trematode | Primary pathogen | 57 |
| Red-spotted grouper nervous necrosis virus (RGNNV) | Betanodavirus | Primary pathogen | 62 |
| Viral hemorrhagic septicemia virus (VHSV) | Rhabdovirus | Experimental infection | 63,65 |
| Infectious spleen and kidney necrosis virus (ISKNV) | Megalocytivirus | Experimental infection | 69,70 |
| Spring viremia of carp virus (SVCV) | Rhabdovirus | Experimental infection | 56,75 |
Facility managers, veterinarians, and animal handlers should also be aware of potential emerging agents that may pose an unidentified risk to the facility or may impact the international transfer of animals. When determining which agents to monitor, methods, frequency of testing, and knowledge of the biology of the agents are essential. Pathogenicity; virulence; the utilization of alternate, paratenic, or intermediate hosts; mode of transmission; persistence in the environment; effective drugs or disinfectants; host specificity; and geographic distribution, all warrant consideration. Our knowledge of some of these agents has increased over the past few years and will be briefly reviewed in this study.
Pseudoloma neurophilia, an obligate intracellular microsporidium, which preferentially infects neural tissue, is the most prevalent infectious agent of laboratory zebrafish. Infection may result in subclinical infection or cause clinical signs such as emaciation and spinal deformities. More commonly, it is associated with reduced growth and decreased reproductive fitness.18 Infection with this organism was recently shown to alter behavioral testing conducted with infected fry,19 and intramuscular granuloma development and myonecrosis associated with infection may display autofluorescence interfering with imaging studies.20 This agent can be vertically as well as horizontally transmitted, and most currently used protocols for embryo surface disinfection with sodium hypochlorite do not prevent horizontal transmission to offspring.21,22 There is currently no known treatment for this infection.
Zebrafish can become infected in <2 months under certain conditions and an entire colony may become infected within 6 months.18 Based on this information, fish may require exposure to this pathogen for at least 3 months before the identification of new infections of P. neurophilia, however zebrafish exposed for 6 months or more have a greater likelihood of testing positive. Therefore, quarterly testing of fish with at least 3 months of potential exposure may be a helpful strategy for identification of this pathogen. Sensitivity of P. neurophilia detection through sentinels can be improved by placing batches of sentinel fish with overlapping 6-month periods of effluent exposure.
Research involving behavioral analyses should import P. neurophilia-free fish, and exclude P. neurophilia to prevent nonexperimental behavioral variables associated with this infection. Similarly, given its persistence in the hindbrain and spinal cord, investigators performing neuroanatomical, neurodevelopmental, and neurophysiological studies should exclude this pathogen from their colonies. Pleistophora hyphessobryconis is another microsporidium that infects laboratory zebrafish; however, this pathogen is much less prevalent and primarily targets skeletal muscle, resulting in severe necrosis and expansion of myofibers, muscle deformity, and lethargy.23 It is likely that measures used to prevent or control infection for P. neurophilia will also be effective for Pleistophora hyphessobryconis.23,24
Mycobacteriosis is the most widely distributed and second most prevalent infectious disease of laboratory zebrafish. There are more than 170 recognized Mycobacterium spp., including both infectious and noninfectious environmental species, which may be found in zebrafish systems and have the potential to cause disease in fish and/or human handlers.25–27 Immunosuppressed handlers are particularly at risk. Seven different species have been reported to be pathogenic in zebrafish, including M. chelonae, M. marinum, M. haemophilum, M. abscessus, M. fortuitum and M. peregrinum and M. saopaulense.25,26 These mycobacterial species are facultative pathogens that can both proliferate in the system biofilms and infect immunocompetent zebrafish.25,27
Two of these, M. marinum and M. haemophilum, are slowly growing mycobacteria that cause obvious clinical disease and mortality in infected colonies.25,28 The five remaining species are all rapidly growing mycobacteria in culture. M. chelonae is the most widely distributed and prevalent mycobacterial infection of zebrafish. M. chelonae occasionally causes swim bladder necrosis and aerocystitis, but is usually subclinical—making its detection difficult if routine screening is not performed. Recent work has shown that Mycobacterium spp. may interfere with a wide variety of research results. The presence of granulomas confounds histopathology data in a number of studies.3 Parikka et al. showed that M. marinum upregulates various inflammatory cytokines that may confound immunological research.29 Infection with this species has been documented to alter gene expression in several studies.30–33 Finally, fast-growing M. chelonae has been observed to autofluoresce, which may confound studies using fluorescence to examine biological processes.34
M. abscessus is very close phylogenetically to M. chelonae, and similarly produces predominantly subclinical infections; the recently described M. saopaulense also belongs to this group.26 M. fortuitum is a widely distributed species associated with chronic subclinical granulomatous infections.35 M. peregrinum is relatively uncommon and is usually subclinical,36 although a high mortality epizootic has also been reported in association with this species.37,38 Mycobacterium spp. have been detected in embryos and larvae, as well as adults. Some authors have suggested that Tübingen (TU) fish may be more susceptible to mycobacterial infection.28,39
Although they influence research results and negatively impact animal health, Mycobacterium spp. are ubiquitous in the aquatic environment. Therefore, exclusion of all species of mycobacteria that infect zebrafish is extremely challenging and usually cost-prohibitive. Some individual laboratories have generated germ-free zebrafish and subsequently colonized them with known microbiota.40–43 Many zebrafish facilities, therefore, exclude some species (e.g., M. marinum), while allowing other species that are both costly to exclude and infrequently cause clinical disease, such as M. chelonae.
Pseudocapillaria tomentosa is a common nematode pathogen that has been associated with emaciation and decreased reproductive efficiency in zebrafish.2 Infected fish expel the eggs that embryonate in the environment and are then ingested by naive fish. Transmission can occur in as little as 1 month.44 P. tomentosa has also been associated with an increased incidence of tumor formation in zebrafish exposed to carcinogens.2 As more zebrafish are being used for cancer research, this is another possible confounding agent. Three recent publications described effective treatments for zebrafish infected with this parasite.44–47 These include use of fenbendazole, ivermectin, emamectin benzoate, mebendazole and praziquantel.
Once P. tomentosa infections become patent, the eggs embryonate and infect new hosts quickly, so an adequate sample of effluent sentinels in place for at least 3 months would likely reveal infection. If fish infected with P. tomentosa are imported into a facility, these fish can be quarantined and treated before research use or spawning. Treatment of recirculating systems during an epizootic are costly, labor intensive, and time consuming; thus, it is important to identify this pathogen during the quarantine period.
Myxidium streisingeri is a fairly common myxozoan parasite of the zebrafish urinary tract, with plasmodia occurring in the collecting ducts and mesonephric ducts.48 The plasmodia exhibit presporogonic stages and occasional mature myxospores that are shed into the environment.48 Nearly all myxozoans have complex life cycles that require both a vertebrate and an invertebrate host, with the infected invertebrate host shedding actinospores that infect the vertebrate host.48 It is likely that the invertebrate host of Myxidium streisingeri is an oligochaete worm, which is free-living in many zebrafish systems. However, an invertebrate host species for Myxidium streisingeri has not yet been identified, and it is possible that this parasite exhibits direct transmission.48
Edwardsiella ictaluri is a Gram-negative bacterium responsible for causing high mortality epizootics in zebrafish characterized by severe systemic infection with necrotizing inflammation in various visceral organs and associated morbidity and mortality.49,50 It is transmitted by direct contact, water, feces, and orally. Although this pathogen may be susceptible to several antibiotics, it is unknown if treatment eliminates the infection or induces a carrier state that could allow for further spread of disease. Several other Gram-negative bacteria, including Aeromonas hydrophila (causative agent of motile Aeromonas septicemia), Edwardsiella tarda, Edwardsiella piscicida, Flavobacterium columnare, and other Flavobacterium spp. have the potential to be primary or opportunistic pathogens in zebrafish.49,51–53
A number of other ciliate and helminth parasites are most likely to be introduced into laboratory colonies as a result of importing fish from pet stores, other facilities, which import fish from pet stores, commercial aquaculture facilities, or facilities that house other species of fish. Ichthyophthirius multifiliis and Tetrahymena species are related ciliate parasites that can infect zebrafish.54 I. multifiliis is an obligate parasite, whereas Tetrahymena spp. can be free-living or opportunistic parasites. The specific identity of the species that infect zebrafish is unknown, but outbreaks can result in high mortality of larvae and juvenile zebrafish.54
Piscinoodinium pillulare is a parasitic dinoflagellate that is common in the ornamental fish trade and can cause epizootics in laboratory zebrafish. These pathogens can damage the gills and epithelium and lead to osmoregulatory disturbances, hypoxia, and increased susceptibility to secondary infections.55
Helminth parasites, including monogenean ectoparasites such as Gyrodactylus sp(p). that can damage the skin and gills, have also been reported to naturally infect zebrafish.56 Zebrafish obtained from the pet trade may exhibit cestodes or metacercariae of digenean trematodes.55 To minimize the risk of introducing uncommon pathogens, zebrafish should be obtained from reputable sources with accompanying health monitoring reports. Transversotrema patialense (sensu lato), a digenean trematode that requires a snail as an intermediate host, was recently reported to cause naturally occurring infections in zebrafish that were sold as “laboratory-reared” and “specific pathogen-free.”57
With the exception of the microsporidia, fungal pathogens of zebrafish are poorly characterized. Fungal aerocystitis is relatively common in some zebrafish facilities, but the species involved have not yet been identified. Noninvasive fungal hyphae are sometimes observed on the skin of zebrafish, and in extreme cases may occlude the mouth and gill openings of zebrafish larvae. Lecythophora mutabilis was identified in one case,58 although it is possible that other fungi may present similarly. Aquatic fungi are common in zebrafish systems and controlling fungi usually requires improving water quality and sanitization practices.
Relatively little is known about naturally occurring viral infections in zebrafish.59 Multiple endogenous retroviruses, retrotransposons, and retroid agent sequences have been described in the zebrafish genome60,61; however, production of an infectious virion has not been reported. There is a single brief report of a naturally occurring infection of zebrafish by Red-spotted grouper nervous necrosis virus, a Betanodavirus.62 Many aquatic viruses are not host specific, which increases the risk that naive laboratory zebrafish may be susceptible to viral diseases transmitted from other fish species housed in the same facility or from zebrafish acquired from aquaculture facilities or pet stores.58
Zebrafish have been shown to be experimentally susceptible to a number of fish viruses,63–66 including infectious spleen and kidney necrosis virus,67–70 a Megalocytivirus, which infects a very broad range of fish hosts71,72 and is prevalent in the tropical ornamental fish trade.73,74 Zebrafish have also been shown to be experimentally susceptible to a commercially important Rhabdovirus, spring viremia of carp virus (SVCV),75,76 which has restricted importation of laboratory zebrafish into Canada and other nations. The relevance of testing for some viruses on a regular basis, such as SVCV, is questionable as these animals were infected under artificial housing conditions.56 However, testing may be mandatory for import/export of fish to countries with restrictions on importation. Further investigation into the prevalence and pathogenicity of viruses is needed before evidence-based recommendations for their testing can be provided. Zebrafish may be susceptible to many viruses with broad host specificity. To mitigate this risk, fish of different species should be housed on separate systems.
Samples for Testing
Samples that may be utilized to screen a colony include the animals themselves and environmental samples. Many different types of animals may be sampled for health monitoring: colony animals, aged zebrafish (>12 months), fish found in the system sumps, or intentionally placed sentinel zebrafish. Each of these types of fish provides some information on colony health.
Direct testing of colony animals will identify pathogens with a high prevalence, but may miss uncommon pathogens unless the sample size is very large. Other disadvantages of testing colony animals are a non-uniform genetic and experimental background of sampled fish and variable sampling time points based on the availability of animals after experiments. Because many infectious diseases of zebrafish are chronic subclinical infections that are not cleared by the immune system, some institutions use retired zebrafish breeders as sentinels because they have had the longest window of exposure.
Previous publications have shown that infection with Pseudoloma neurophilia is more prevalent in zebrafish over 1 year of age.18 Similarly, as female zebrafish age, they are more likely to have Pseudoloma spores within their ovaries, which can spread infection both vertically within the ovum as well as horizontally into the environment.77 Older zebrafish may also be more susceptible to reactivation of latent mycobacterial infections.29 It is recommended to euthanize fish older than 12 months of age to minimize the spread of infection through a colony. These animals may be submitted for routine screening for infectious diseases.
Sump fish may also provide information as to the infectious diseases present in a colony. They are typically exposed to all the effluent water from the system on which they were housed. In the sump, they may live off of uneaten food, tank debris, and feces. These fish also present a risk to the primary colonies, as they may develop and then propagate disease. Furthermore, health and welfare issues in these fish may go undetected as it is difficult to perform daily health checks on these animals. We recommend removing these animals from the sumps periodically. These animals may also be submitted for routine screening when removed from the sump.
Sentinel animals are a major component of rodent health monitoring programs.11 Zebrafish are usually housed on recirculating systems with many tanks plumbed in parallel. This arrangement reduces the likelihood of transmission of infectious agents from tank to tank, but also increases the necessary sample size to detect pathogens by testing colony animals directly. Sentinel programs are designed to maximize transmission of pathogens to a smaller group to maximize pathogen detection using fewer animals. Many zebrafish holding systems are now designed with a sentinel tank position; tanks in these positions may be exposed to effluent water from the entire system. Other advantages of sentinel zebrafish are that they facilitate meaningful comparisons of sentinel data over time, may include a known genetic background without experimental manipulation, and are housed on the system for a known period of time.
Ideally, sentinel fish should be introduced to the housing conditions in which they will be used as sentinels at the larval stage. This minimizes the risk of introducing new or unknown pathogens to the system from imported adult sentinels. If adult zebrafish are obtained from another system or facility, their health status should be known with regard to all infectious agents of interest to ensure that any positive result was acquired from the system being monitored and not introduced along with the imported fish to be used as sentinels.
Minimally, sentinel fish should be exposed to the water/housing system being tested for at least 3 months and should be screened every 3–6 months for pathogens. In rodent sentinel programs, outbred stock mice are often used because they can seroconvert to a number of pathogens. It is still unclear what line(s) of zebrafish may be the best for routine screening of pathogens. As stated above, TU fish exhibit increased susceptibility to M. chelonae.77 Transparent fish (e.g., Casper zebrafish) have also been suggested as a way to perform screening for mycobacterial infections.34 Some facilities may want to use whatever background strain is used most commonly on the system being monitored. As more is learned about the immune system of zebrafish and various lines, future recommendations may include a specific line that is susceptible to a variety of pathogens.
When performing routine screening, the minimum number of animals sampled to detect a pathogen with 95% confidence depends on the expected prevalence of a pathogen in the colony,8 but in practice the number of zebrafish sampled is usually limited by financial constraints, as in rodents. For pathogens of low prevalence, the number of fish that would be required to detect infection is often impractical. Moreover, the statistical formula presupposes that every fish is equally likely to infect every other fish, which is not the case for zebrafish systems. The exposure of fish to effluent water is a method that can be used to increase the chances of detecting a pathogen with the evaluation of fewer fish. Expected prevalence is often difficult to determine as housing method, water filtration, and breeding practices can have a significant impact on prevalence in a population.
Incidence of a pathogen may also vary based on animal genetics and immune status as well as husbandry procedures. For these reasons, prevalence of infection may differ substantially among colonies. Therefore, sentinel fish maintained in colony tanks or in a specially designed tank that receives effluent system water may be the most reliable detection method. If budget allows, aged colony animals may also be submitted to provide a more complete picture of colony health. The number of zebrafish sampled will reflect a balance of the risk of not detecting all infectious agents with the cost of the health monitoring program.
Environmental sampling is very useful for the detection of agents such as Mycobacterium spp. that proliferate in system biofilms and are thus common in the environment. The sensitivity of environmental sample type varies according to the life cycle and biology of the agent. Relying on environmental testing alone may be insufficient for detection of agents that are relatively uncommon in the environment or are intermittently or poorly shed by infected fish. Since there is limited information on environmental sampling for zebrafish pathogens, and since some pathogens may be more common in infected fish than in the environment, environmental sampling should be considered an adjunct to testing animals.
Testing Methodologies
Testing methodology is also an important factor to consider when designing a health monitoring program. The most appropriate diagnostic platform will depend on several factors, including the type of information needed, the pathogen of interest, the likely stage of infection, and cost-effectiveness. Historically, health monitoring for zebrafish colonies relied on postmortem necropsies and histopathology of whole adult zebrafish.78 Today, several methodologies are available for facilities to utilize, including real-time polymerase chain reaction (PCR), conventional PCR, microbiology with microbial with identification by matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS), histopathology, and simple direct exams, such as wet mounts.
For monitoring of external parasites, an external examination with wet mount preparations of fin clips and gill clips may be used. Molecular diagnostic techniques, such as real-time PCR are commercially available and provide exquisite sensitivity and specificity with rapid turnaround times. Real-time PCR offers significant advantages for detection of Mycobacterium spp., which are slowing growing bacteria and often require special conditions for growth.25 Bacterial culture can facilitate isolation and species-level identification of a wide array of microorganisms, including bacteria, yeast, filamentous fungi, and oomycetes. Sensitivity of microbiology for zebrafish varies according to the biology of the organisms cultivated and the experience of the laboratory with aquatics, since several fish pathogens have specific requirements, including unique incubation temperatures, specialized culture media, and/or longer incubation times.
The recent application of MALDI-TOF MS to laboratory animal diagnostics has dramatically increased specificity and the number of organisms that can be correctly identified to the species level, which is limited by the diversity of existing spectral databases.79,80 Histopathology is very specific for some pathogens, particularly parasites with unique morphologic characteristics, but cannot be used to identify Mycobacterium spp. to the species level, and often cannot be used to identify other bacterial and fungal pathogens, except to narrow the list of possibilities to broad categories. Similarly, histopathology is often insensitive relative to other methods.
The key advantage of histopathology for health monitoring is the capacity for simultaneous evaluation for a wide variety of infectious and noninfectious conditions. Macroscopic and microscopic evaluation of epithelial tissues can reveal pathogens, including ectoparasites and gliding bacteria. A combination of PCR, microbiological techniques, histopathology, and gross examination of animals contribute to a complete health profile of the colony.
We recommend that in addition to more frequent sampling by other methods, histopathology should be performed for routine screening. Histopathology is important for evaluating the colony for an increased incidence of noninfectious lesions, including tumors, hepatocytic megalocytosis, laminar epithelial hyperplasia of the gills, heart disease, egg-associated inflammation and fibroplasia, nephrocalcinosis, and other lesions. When possible, histopathology should be performed on euthanized sick or moribund fish, particularly if multiple fish are clinically affected.
Housing and Husbandry
The source of animals, water source and quality, and live feeds are important risk factors for the introduction of pathogens. Water quality, stocking density, and other husbandry parameters influence the spread of infectious agents and severity of clinical signs. Maintaining adequate water quality is important for preventing disease from opportunistic agents. A number of publications have described appropriate housing conditions and stocking densities for zebrafish.81 Poor water quality and stress may exacerbate infections13 and latent infections can become reactivated when the fish are stressed or irradiated.29 Live feed has also been associated with potential introduction of pathogens,82 so some facilities now exclusively utilize commercially manufactured feeds (Carrie Barton, SARL, pers. comm.).
Maintenance of appropriate stocking densities and water quality, quarantine of fish with clinical disease, prompt removal of dead fish, surface disinfection of embryos, culling fish over 12 months of age, periodic removal of sump fish, and feeding of commercial diets or routine verification that live feeds are free of infectious agents are all husbandry measures that can be taken to prevent infection with opportunistic pathogens in immunocompetent zebrafish lines. Excluding primary pathogens from a facility by evaluating health reports and using quarantine will also minimize the risk of pathogen introduction into the facility.
The use of large recirculating water systems supporting multiple research laboratories makes biocontainment more difficult, since most existing systems were not designed to be compartmentalized to control the spread of an introduced disease. In contrast, facilities that utilize flow-through systems or compartmentalized recirculating systems with multiple life support systems allow more options to control pathogen transmission following a break in biosecurity. Because pathogens are more likely to be detected in quarantine than on main systems, we recommend that flow-through systems be utilized for quarantine whenever possible. The increased risk of potentially exposing the colonies of multiple investigators with fish housed on the same system makes appropriate quarantine and routine health monitoring procedures even more crucial.
Cost
There are costs associated with performing regular health monitoring. However, as some outbreaks have shown, neglecting proper quarantine and inappropriate health monitoring can lead to loss of entire colonies,50 which could also result in the loss of genetically engineered models that have not been cryopreserved or disseminated to other facilities. Research results may also be irreproducible or invalid due to presence of infections.10 We propose that the risk of not performing routine health monitoring is much greater than the cost of implementing a program.
Test Subjects
Currently, there are limited sources for zebrafish with a known health background and limited agreement among researchers regarding which pathogens must be avoided or eliminated. As a result, each facility must determine the agents it would like to exclude and those it wishes to control and must take this into consideration when importing fish and placing sentinels. For example, if a facility wishes to control and evaluate for the presence of P. neurophilia, sentinel fish should be obtained from a source that is able to provide zebrafish that are free of this pathogen, such as The Sinnhuber Aquatic Resource Laboratory.83 As the zebrafish continues to grow in importance as a model organism, more centers should consider developing pathogen-free zebrafish lines.
Health Monitoring Report
A recent and specific health monitoring report should be available for all zebrafish colonies. Health reports are critically important for the transfer of fish between facilities and should provide a complete picture of the health status of the animals being shipped or received. Health reports should include a summary report of the most recent findings as well as a synopsis of recent history (e.g., the past 1–2 years). A complete report should also provide information regarding husbandry parameters.
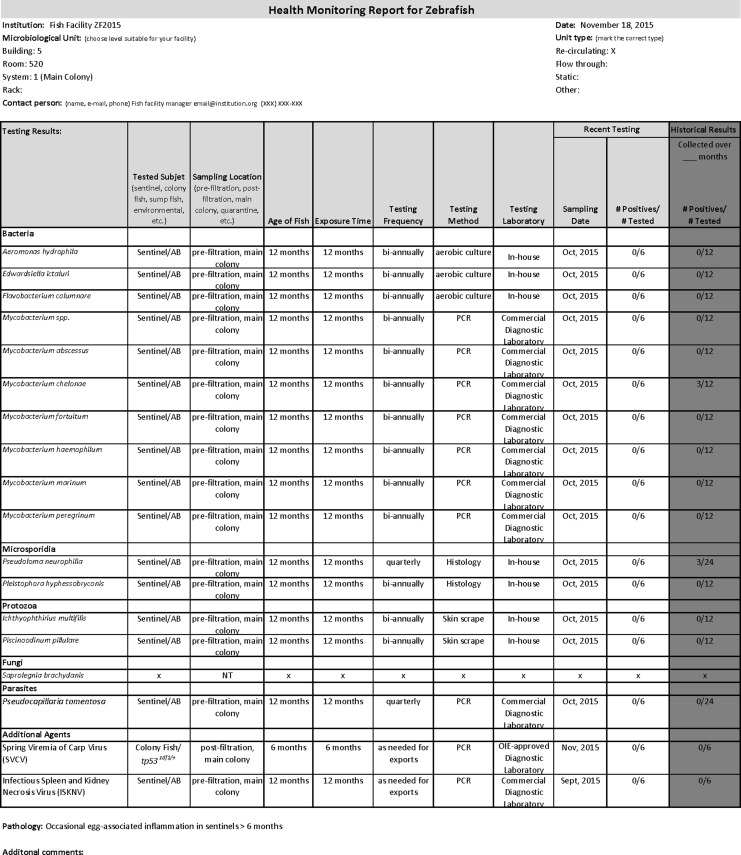
Facilities should designate a specific individual or individuals to oversee the health monitoring program and update the health monitoring reports. This person should be involved in all zebrafish imports and exports. See Figure 1 for an example of a health monitoring report that may be provided to a facility receiving animals from a noncommercial source. If a particular agent is not tested for, NOT TESTED (NT) should be indicated where that agent is listed. A positive result must be noted on the health monitoring report. A colony that was previously positive for an agent that has now been eradicated, should maintain a positive status for at least 1–2 years after the last negative result. Once at least a year has passed, the colony may be identified as negative.
FIG. 1.
Example of a health monitoring report.
Accompanying the health monitoring report, a 1–2-page description detailing housing and husbandry (Fig. 2) should be included that indicates the approximate facility size, and a description of what testing occurs at the tank level, rack level, room level, and/or facility level. Other important details include whether the colony is closed or animals are frequently imported, quarantine measures routinely applied to incoming animals, and embryo surface disinfection practices. If a colony has had a positive result, the letter should also indicate what steps were taken to identify the outbreak, control transmission, and/or eradicate the pathogen.
FIG. 2.
Example of a housing and husbandry description.
In addition to being utilized for zebrafish transfers, health monitoring reports should be made readily available to all researchers. The Animal Research: Reporting of In Vivo Experiments guidelines specify that the health status of animals and husbandry methods should be reported when publishing results obtained utilizing an animal model.1 The provision of this information is important for an adequate description of the methods used when conducting biomedical research, and thus should be made readily available to the researchers utilizing these models. (Supplementary Figs. S1 and S2 can be downloaded by individual facilities and used to facilitate transfer of relevant information; Supplementary Data are available online at www.liebertpub.com/zeb).
Limitations
One limitation of health monitoring programs is that the results are always retrospective and thus may not always reflect the current health status of the colony. There are still many challenges such as limited availability of pathogen-free zebrafish, limited knowledge of the impact of background genetics on disease detection, a lack of communication between researchers and veterinarians, and the costs involved with developing a health monitoring program. Continued work on zebrafish health and husbandry will ensure quality research results and positively impact animal health.
Conclusions
We propose that all facilities utilizing zebrafish should make a concerted effort to evaluate and report health status information more systematically to facilitate harmonization; including a summary of recent testing, a synopsis of recent history (e.g., the past 1–2 years), information regarding husbandry parameters, and appropriate contact details for questions regarding the report. A common method of reporting will facilitate transfer of animals, ensure the health of different colonies, and minimize the introduction of pathogens that may have devastating consequences on animal health and research objectives.
Supplementary Material
Acknowledgments
The authors thank Dr. Zoltan Varga for his input regarding the ZIRC health monitoring program, Carrie Barton for information on the use of commercial diets at SARL, and Dr. E. Kate Banks for critical review of the article.
Disclosure Statement
One of the three authors (M.J.C.) is employed by a diagnostic company with a financial interest in the subject matter.
References
- 1.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE Guidelines for reporting animal research. Osteoarthritis Cartilage 2012;20:256–260 [DOI] [PubMed] [Google Scholar]
- 2.Kent ML, Bishop-Stewart JK, Matthews JL, Spitsbergen JM. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplams of zebrafish (Danio rerio) kept in research colonies. Comp Med 2002;52:354–358 [PubMed] [Google Scholar]
- 3.Kent ML, Harper C, Wolf JC. Documented and potential research impacts of subclinical diseases in zebrafish. ILAR J 2012;53:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonakopoulos GN, Turton J, Whitfield P, Newman J. Host-parasite interface of the urinary bladder-inhabiting nematode Trichosomoides crassicauda: changes induced in the urothelium of infected rats. Int J Parasitol 1991;21:187–193 [DOI] [PubMed] [Google Scholar]
- 5.Baker DG. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev 1998;11:231–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvir E, Clift SJ. Evaluation of selected growth factor expression in canine spirocercosis (Spirocerca lupi)-associated non-neoplastic nodules and sarcomas. Vet Parasitol 2010;174:257–266 [DOI] [PubMed] [Google Scholar]
- 7.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12-and gamma interferon-dependent mechanism. Infect Immun 1998;66:5157–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Mac Law J, Sanchez-Morgado JM, et al. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comp Biochem Physiol Part C 2009;149:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence C, Ennis DG, Harper C, Kent ML, Murray K, Sanders GE. The challenges of implementing pathogen control strategies for fishes used in biomedical research. Comp Biochem Physiol Part C 2012;155:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen R, Needham JP, Colquhoun DJ, Poppe TT, Smith AJ. Guidelines for health and welfare monitoring of fish used in research. Lab Anim 2006;40:323–340 [DOI] [PubMed] [Google Scholar]
- 11.Mahler M, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, Pritchett-Corning K, et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim 2014:48:178–192 [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez I, Novoa B, Figueras A. Immune response of zebrafish (Danio rerio) against a newly isolated bacterial pathogen Aeromonas hydrophila. Fish Shellfish Immunol 2008;25:239–249 [DOI] [PubMed] [Google Scholar]
- 13.Ramsay JM, Watral V, Schreck CB, Kent ML. Husbandry stress exacerbates mycobacterial infections in adult zebrafish, Danio rerio (Hamilton). J Fish Dis 2009;32:931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang P, Rattana-Apiromyakij N, Goh C-l. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol 2000;39:343–347 [DOI] [PubMed] [Google Scholar]
- 15.Bartralot R, Pujol RM, Garcia-Patos V, Sitjas D, Martin-Casabona N, Coll P, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. Comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol 2000;27:124–129 [DOI] [PubMed] [Google Scholar]
- 16.Jolly HW, Seabury JH. Infections with Mycobacterium marinum. Arch Dermatol 1972;106:32–36 [PubMed] [Google Scholar]
- 17.Wu T-S, Chiu C-H, Yang C-H, Leu H-S, Huang C-T, Chen Y-C, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS ONE 2012;7:e41296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia infections in zebrafish Danio rerio: effects of stress on survival, growth, and reproduction. Dis Aquat Organ 2009;88:69–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spagnoli S, Xue L, Kent ML. The common neural parasite Pseudoloma neurophilia is associated with altered startle response habituation in adult zebrafish (Danio rerio): implications for the zebrafish as a model organism. Behav Brain Res 2015;291:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West K, Miles R, Kent ML, Kimble Fraser J. Unusual fluorescent granulomas and myonecrosis in Danio rerio infected by the microsporidian pathogen Pseudoloma neurophilia. Zebrafish 2014;11:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson JA, Watral V, Schwindt AR, Kent ML. Spores of two fish microsporidia (Pseudoloma neurophilia and Glugea anomala) are highly resistant to chlorine. Dis Aquat Organ 2007;76:205–214 [DOI] [PubMed] [Google Scholar]
- 22.Kent ML, Buchner C, Barton C, Tanguay RL. Toxicity of chlorine to zebrafish embryos. Dis Aquat Organ 2014;107:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders JL, Lawrence C, Nichols DK, Brubaker JF, Peterson TS. Murray KN, et al. Pleistophora hyphessobryconis (microsporidia) infecting zebrafish (Danio rerio) in research facilities. Dis Aquat Organ 2010;91:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders JL, Watral V, Kent ML. Microsporidiosis in zebrafish research facilities. ILAR J 2012;53:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in zebrafish colonies. ILAR J 2012;53:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogueira CL, Whipps CM, Matsumoto CK, Chimara E, Droz S, Tortoli E, et al. Description of Mycobacterium Saopaulense sp. Nov., a rapidly growing mycobacterium closely related with members of the Mycobacterium chelonae–M. abscessus group. Int J Syst Evol Microbiol 2015;65:4403–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauthier DT. Bacterial zoonoses of fishes: a review and appraisal of evidence for linkages between fish and human infections. Vet J 2015;103:27–35 [DOI] [PubMed] [Google Scholar]
- 28.Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish Danio rerio. Dis Aquat Organ 2008;82:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikka M, Hammaren MM, Harjula S-KE, Halfpenny NJA, Oksanen KE, Lahtinen MJ, et al. Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog 2012;8:e1002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer AH, Verbeek FJ, Salas-Vidal E, Corredor-Adamez M, Bussman J, van der Sar AM, et al. Transcriptome profiling of adult zebrafish at the late stage of chronic tuberculosis due to Mycobacterium marinum infection. Mol Immunol 2005;42:1185–1203 [DOI] [PubMed] [Google Scholar]
- 31.Meijer AH, Gabby Krens SF, Medina Rodriguez IA, He S, Bitter W, Snaar-Jagalska E, et al. Expression analysis of the toll-like receptor and TIR domain adaptor families of zebrafish. Mol Immunol 2004;40:773–783 [DOI] [PubMed] [Google Scholar]
- 32.Van der Sar AM, Spaink HP, Zakrzewska A, Bitter W, Meijer AH. Specificity of the zebrafish host transcriptome response to acute and chronic mycobacterial infection and the role of innate and adaptive immune components. Mol Immunol 2009;46:2317–2332 [DOI] [PubMed] [Google Scholar]
- 33.Weedenburg EM, Abdallah AM, Mitra S, de Punder K, van der Wel NN, Bird S, et al. ESX-5-deficient Mycobacterium marinum is hypervirulent in adult zebrafish. Cell Microbiol 2012;14:728–739 [DOI] [PubMed] [Google Scholar]
- 34.Whipps CM, Moss LG, Sisk DM, Murray KN, Tobin DM, Moss JB. Detection of autofluorescent Mycobacterim chelonae in living zebrafish. Zebrafish 2014;11:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Astrofsy KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comp Med 2000;50:666–672 [PubMed] [Google Scholar]
- 36.Harriff MJ, Bermudez LE, Kent ML. Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: a potential model for environmental mycobacterial infection. J Fish Dis 2007;30:587–600 [DOI] [PubMed] [Google Scholar]
- 37.Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, et al. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp Biochem Physiol Part C 2004;138:383–390 [DOI] [PubMed] [Google Scholar]
- 38.Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Comp Biochem Physiol Part C 2007;145:55–60 [DOI] [PubMed] [Google Scholar]
- 39.Murray KN, Bauer J, Matthews JL, Westerfield M, Varga ZM. Characterization and management of asymptomatic mycobacterium infections at the zebrafish international resource center. J Am Assoc Lab Anim Sci 2011;50:675–679 [PMC free article] [PubMed] [Google Scholar]
- 40.Pham LN, Kanther M, Semova I, Rawis JF. Methods for generating and colonizing gnotobiotic zebrafish. Nat Protoc 2008;3:1862–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 2006;127:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A 2004;101:4596–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rendueles O, Ferrieres L, Fretaud M, Begaud E, Herbomel P, Levraud JP, et al. A new zebrafish model of oro-intestinal pathogen colonization reveals a key role for adhesion in protection by probiotic bacteria. PLoS Pathog 2012;8:e1002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collymore C, Watral V, White JR, Colvin ME, Rasmussen S, Tolwani RJ, et al. Tolerance and efficacy of emamectin benzoate and ivermectin for the treatment of Pseudocapillaria tomentosa in laboratory zebrafish (Danio rerio). Zebrafish 2014;11:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maley D, Laird AS, Rinkwitz S, Becker TS. A simple and efficient protocol for the treatment of zebrafish colonies infected with parasitic nematodes. Zebrafish 2013;10:447–450 [DOI] [PubMed] [Google Scholar]
- 46.Maley (Correction). Zebrafish 2015;12:270. [DOI] [PubMed] [Google Scholar]
- 47.Samaee S-M. Experimental assessment of the efficacy of five veterinary broad-spectrum anthelminthics to control the intestinal capillariasis in zebrafish (Danio rerio). Zebrafish 2015;12:255–267 [DOI] [PubMed] [Google Scholar]
- 48.Whipps CM, Murray KN, Kent ML. Occurrence of a myxozoan parasite Myxidium streisingeri n. sp. in laboratory zebrafish Danio rerio. J Parasitol 2015;101:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hawke JP, Kent M, Rogge M, Baumgartner W, Wiles J, Shelley J, et al. Edwardiellosis caused by Edwardsiella ictaluri in laboratory populations of zebrafish Danio rerio. J Aquat Anim Health 2013;25:171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrie-Hanson L, Romano CL, Mackey RB, Khosravi P, Hohn CM, Boyle CR. Evaluation of zebrafish Danio rerio as a model for enteric septicemia of catfish (ESC). J Aquat Anim Health 2007;19:151–158 [DOI] [PubMed] [Google Scholar]
- 51.Abayneh T, Colquhoun DJ, Sorum H. Edwardsiella piscicida sp. nov., a Novel species pathogenic to fish. J Appl Microbiol 2012;114:644–654 [DOI] [PubMed] [Google Scholar]
- 52.Moyer TR, Hunnicutt DW. Susceptibility of zebra Fish Danio rerio to infection by Flavobacterium columnare and F. johnsoniae. Dis Aquat Organ 2007;76:39–44 [DOI] [PubMed] [Google Scholar]
- 53.Pullium JK, Dillehay DL, Webb S. High mortality in zebrafish (Danio rerio). Contemp Top Lab Anim Sci 1999;38:80–83 [PubMed] [Google Scholar]
- 54.Astrofsky KM, Schech JM, Sheppard BJ, Obenschain CA, Chin AM, Kacergis MC, et al. High mortality due to Tetrahymena sp. infection in laboratory-maintained zebrafish (Brachydanio rerio). Comp Med 2002;52:363–367 [PubMed] [Google Scholar]
- 55.Matthews JL: Common diseases of laboratory zebrafish. In: The Zebrafish: Genetics, Genomics and Informatics, 3rd edn. Dietrich HW, Westerfield M, and Zon LI. (eds), pp. 617–643, Elsevier, Waltham, MA, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Sanders GE, Battas WN, Winton JR. Susceptibility of zebrafish (Danio rerio) to a model pathogen, spring viremia of carp virus. Comp Med 2003;53:514–521 [PubMed] [Google Scholar]
- 57.Womble MR, Cox-Gardiner SJ, Cribb TH, Bullard SA. First record of Transversotrema Witenberg, 1944 (Digenea) from the Americas, with comments on the taxonomy of Transversotrema patialense (Soparkar, 1924) Crusz and Sathananthan, 1960, and an updated list of its hosts and geographic distribution. J Parasitol 2015;101:717–725 [DOI] [PubMed] [Google Scholar]
- 58.Dykstra MJ, Astrofsky KM, Schrenzel MD, Fox JG, Bullis RA, Farrington S, et al. High mortality in a large-scale zebrafish colony (Brachydanio rerio Hamilton & Buchanan, 1822) associated with Lecythophora mutabilis (van Beyma) W. Gams & McGinnis. Comp Med 2001;51:361–368 [PubMed] [Google Scholar]
- 59.Crim MJ, Riley LK. Viral diseases in zebrafish: what is known and unknown. ILAR J 2012: 53:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basta HA, Buzak AJ, McClure MA. Identification of novel retroid agents in Danio rerio, Oryzias latipes, Gasteroteurs aculeatus and Tetraodon nigroviridis. Evol Bioinform 2007;3:179–195 [PMC free article] [PubMed] [Google Scholar]
- 61.Shen C-H, Steiner LA. Genome structure and thymic expression of an endogenous retrovirus in zebrafish. J Virol 2004;78:899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Binesh CP. Mortality due to viral nervous necrosis in zebrafish Danio rerio and goldfish Carassius auratus. Dis Aquat Organ 2013;104:257–260 [DOI] [PubMed] [Google Scholar]
- 63.Encinas P, Rodriguez-Milla MA, Novoa B, Estepa A, Figueras A, Coll J. Zebrafish fin immune responses during high mortality infections with viral haemorrhagic septicemia rhabdovirus. a proteomic and transcriptomic approach. BMC Genomics 2010;11:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phelan PE, Pressley ME, Witten PE, Mellon MT, Blake S, Kim CH. Characterization of snakehead rhabdovirus infection in zebrafish (Danio rerio). J Virol 2005;79:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Novoa B, Romero A, Mulero V, Rodriguez I, Fernandez I, Figueras A. Zebrafish (Danio rerio) as a model for the study of vaccination against viral haemorrhagic septicemia virus (VHSV). Vaccine 2006;24:5806–5816 [DOI] [PubMed] [Google Scholar]
- 66.LaPatra SE, Barone L, Jones GR, Zon LI. Effects of infectious hematopoeitic necrosis virus and infectious pancreatic necrosis virus infection on hematopoietic precursors of the zebrafish. Blood Cells Mol Dis 2000;26:445–452 [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Xu X, Huang L, wu j, lu q, xiang z, et al. administration of recombinant ifn1 protects Zebrafish (Danio rerio) from ISKNV infection. Fish Shellfish Immunol 2010;29:399–406 [DOI] [PubMed] [Google Scholar]
- 68.Wang Z-L, Xu X-P, He B-L, Weng S-P, Xiao J, Lin T, et al. Infectious spleen and kidney necrosis virus ORF48R functions as a new viral vascular endothelial growth factor. J Virol 2008;82:4371–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong X-P, Dong C-F, Xu X, Weng S-P, Liu Z-Y, He J-G. Proteomic analysis of zebrafish (Danio rerio) infected with infectious spleen and kidney necrosis virus. Dev Comp Immunol 2011;35:431–440 [DOI] [PubMed] [Google Scholar]
- 70.Xu X, Zhang L, Weng S, Huang Z, Lu J, Lan D, et al. A zebrafish (Danio rerio) model of infectious spleen and kidney necrosis virus (ISKNV) infection. Virology 2008;376:1–12 [DOI] [PubMed] [Google Scholar]
- 71.Fu X, Li N, Liu L, Lin Q, Wang F, Lai Y, et al. Genotype and host range analysis of infectious spleen and kidney necrosis virus (ISKNV). Virus Genes 2011;42:97–109 [DOI] [PubMed] [Google Scholar]
- 72.Wang YQ, Lu L, Weng SP, Huang JN, Chan S-M, He JG. Molecular epidemiology and phylogenetic analysis of a marine fish infectious spleen and kidney necrosis virus-like (ISKNV-like) virus. Arch Virol 2007;152:763–773 [DOI] [PubMed] [Google Scholar]
- 73.Go J, Lancaster M, Deece K, Dhungyel, Whittington R. The molecular epidemiology of iridovirus in murray cod (Maccullochella peelii peelii) and dwarf gouramie (Colisa lalia) from distant biogeographical regions suggests a link between trade in ornamental fish and emerging iridoviral diseases. Mol Cell Probes 2006;20:212–222 [DOI] [PubMed] [Google Scholar]
- 74.Jeong JB, Kim HY, Jun LJ, Lyu JH, Park NG, Kim JK, et al. Outbreaks and risks of infectious spleen and kidney necrosis virus disease in freshwater ornamental fishes. Dis Aquat Organ 2008;209–215 [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Munoz A, Roca FJ, Sepulcre MP, Meseguer J, Mulero V. Zebrafish larvae are unable to mount a protective antiviral response against waterborne infection by spring viremia of carp virus. Dev Comp Immunol 2010;34:546–552 [DOI] [PubMed] [Google Scholar]
- 76.Sanders GE, Battas WN, Winton JR. Susceptibility of Zebrafish (Danio rerio) to a Model Pathogen, Spring Viremia of Carp Virus. Comparative Medicine 2003; 53:514–521 [PubMed] [Google Scholar]
- 77.Murray KN, Dreska M, Nasiadka A, Rinne M, Matthews JL, Carmichael C, et al. Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities. J Am Assoc Lab Anim Sci 2011;61:322–329 [PMC free article] [PubMed] [Google Scholar]
- 78.Chow FW, Xue L, Kent ML. Retrospective study of the prevalence of Pseudoloma neurophilia shows male sex bias in Zebrafish Danio rerio (Hamilton-Buchanan). J Fish Dis 2015; [Epub ahead of print]; DOI: 10.1111/jfd.12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goto K, Yamamoto M, Asahara M, Tamura T, Matsumura M, Hayashimoto N, et al. Rapid identification of Mycoplasma pulmonis isolated from laboratory mice and rats using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J Vet Med Sci 2012;74:1083–1086 [DOI] [PubMed] [Google Scholar]
- 80.Phillips BH, Crim MJ, Hankenson FC, Steffen EK, Klein PS, Brice AK, et al. Evaluation of presurgical skin preparation agents in african clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 2015;54:1–11 [PMC free article] [PubMed] [Google Scholar]
- 81.Harper C, Lawrence C: The Laboratory Zebrafish. CRC Press, Boca Raton, FL, 2011 [Google Scholar]
- 82.Peterson TS, Ferguson JA, Watral VG, Mutoji KN, Ennis DG, Kent ML. Paramecium caudatum enhances transmission and infectivity of Mycobacterium marinum and Mycobacterium chelonae in zebrafish (Danio rerio). Dis Aquat Organ 2013;106:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kent ML, Buchner C, Watral VG, Sanders JL, LaDu J, Peterson TS, et al. Development and maintenance of a specific pathogen free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Organ 2011;95:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.